化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5612-5632.DOI: 10.16085/j.issn.1000-6613.2023-1691
• 材料科学与技术 • 上一篇
氢气纯化吸附材料研究进展
- 1.中石化石油化工科学研究院有限公司新能源研究所,北京 100083
2.中国石油大学(北京)化学工程与环境学院,北京 102249
-
收稿日期:2023-09-25修回日期:2023-11-27出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:郑金玉 -
作者简介:苏士焜(1991—),男,博士,研究方向为气体纯化分离、多孔催化材料等。E-mail:tmacssk@163.com。
Advances of adsorption materials for hydrogen purification
SU Shikun1( ), LIU Tang1,2, JIN Ye1, ZHENG Jinyu1(
), LIU Tang1,2, JIN Ye1, ZHENG Jinyu1( )
)
- 1.New Energy Research Institute, RIPP, SINOPEC, Beijing 100083, China
2.School of Chemical and Environmental Engineering, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2023-09-25Revised:2023-11-27Online:2024-10-15Published:2024-10-29 -
Contact:ZHENG Jinyu
摘要:
氢能作为未来重要的能源组成,其来源具有多样性。其中工业制氢和副产氢过程通常会伴随多种杂质,如H2O、CO2、CO、N2、烃类、硫化物等,而杂质对氢气的实际应用影响较大,因此对工业粗氢和副产氢进行纯化处理是满足各类合格用氢需求的关键环节。吸附分离是目前工业上最为常用的氢气纯化技术之一,其中吸附材料的性能直接影响分离效率和工艺操作成本,研发高性能、低成本吸附材料是吸附分离技术应用于氢气纯化的重点研究方向。本文简述了常见的氢气纯化技术及吸附分离机理,重点归纳总结了活性炭、沸石分子筛和金属有机骨架材料(MOFs)对氢气中所含CH4、CO2、CO杂质的吸附脱除行为,讨论了吸附材料性能优化的研究现状,总结了上述吸附材料在工业应用中的优缺点,并认为未来氢气纯化吸附材料的研究应在吸附机理和计算方面有所侧重。
中图分类号:
引用本文
苏士焜, 刘唐, 金也, 郑金玉. 氢气纯化吸附材料研究进展[J]. 化工进展, 2024, 43(10): 5612-5632.
SU Shikun, LIU Tang, JIN Ye, ZHENG Jinyu. Advances of adsorption materials for hydrogen purification[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5612-5632.
| 气源 | H2 | CO | CO2 | CH4 | N2 | H2O | 其他 |
|---|---|---|---|---|---|---|---|
| 煤气化气 | 25~35 | 35~45 | 15~25 | 0.1~0.3 | 0.5~1 | 15~20 | 0.2~1 |
| 甲烷重整气 | 70~75 | 10~15 | 10~15 | 1~3 | 0.1~0.5 | — | — |
| 甲醇重整气 | 75~80 | 0.5~2 | 20~25 | — | — | — | — |
| 焦炉煤气 | 45~60 | 5~10 | 2~5 | 25~30 | 2~5 | — | 2.5~5 |
| 合成氨尾气 | 60~75 | — | — | — | 15~20 | 1~3 | 11~18 |
| 生物质气 | 25~35 | 30~40 | 10~15 | 10~20 | 1 | — | 0.5~2 |
表1 部分工业粗氢的组成(质量分数)[4] ( 单位:%)
| 气源 | H2 | CO | CO2 | CH4 | N2 | H2O | 其他 |
|---|---|---|---|---|---|---|---|
| 煤气化气 | 25~35 | 35~45 | 15~25 | 0.1~0.3 | 0.5~1 | 15~20 | 0.2~1 |
| 甲烷重整气 | 70~75 | 10~15 | 10~15 | 1~3 | 0.1~0.5 | — | — |
| 甲醇重整气 | 75~80 | 0.5~2 | 20~25 | — | — | — | — |
| 焦炉煤气 | 45~60 | 5~10 | 2~5 | 25~30 | 2~5 | — | 2.5~5 |
| 合成氨尾气 | 60~75 | — | — | — | 15~20 | 1~3 | 11~18 |
| 生物质气 | 25~35 | 30~40 | 10~15 | 10~20 | 1 | — | 0.5~2 |
| 方法 | 原理 | 典型进料气 | 产品氢纯度/% | 技术难点 |
|---|---|---|---|---|
| 低温分离 | 相对挥发度的差别 | 石化废气,含氢在30%~80%内 | 90~98 | 不易得到高纯度氢气 |
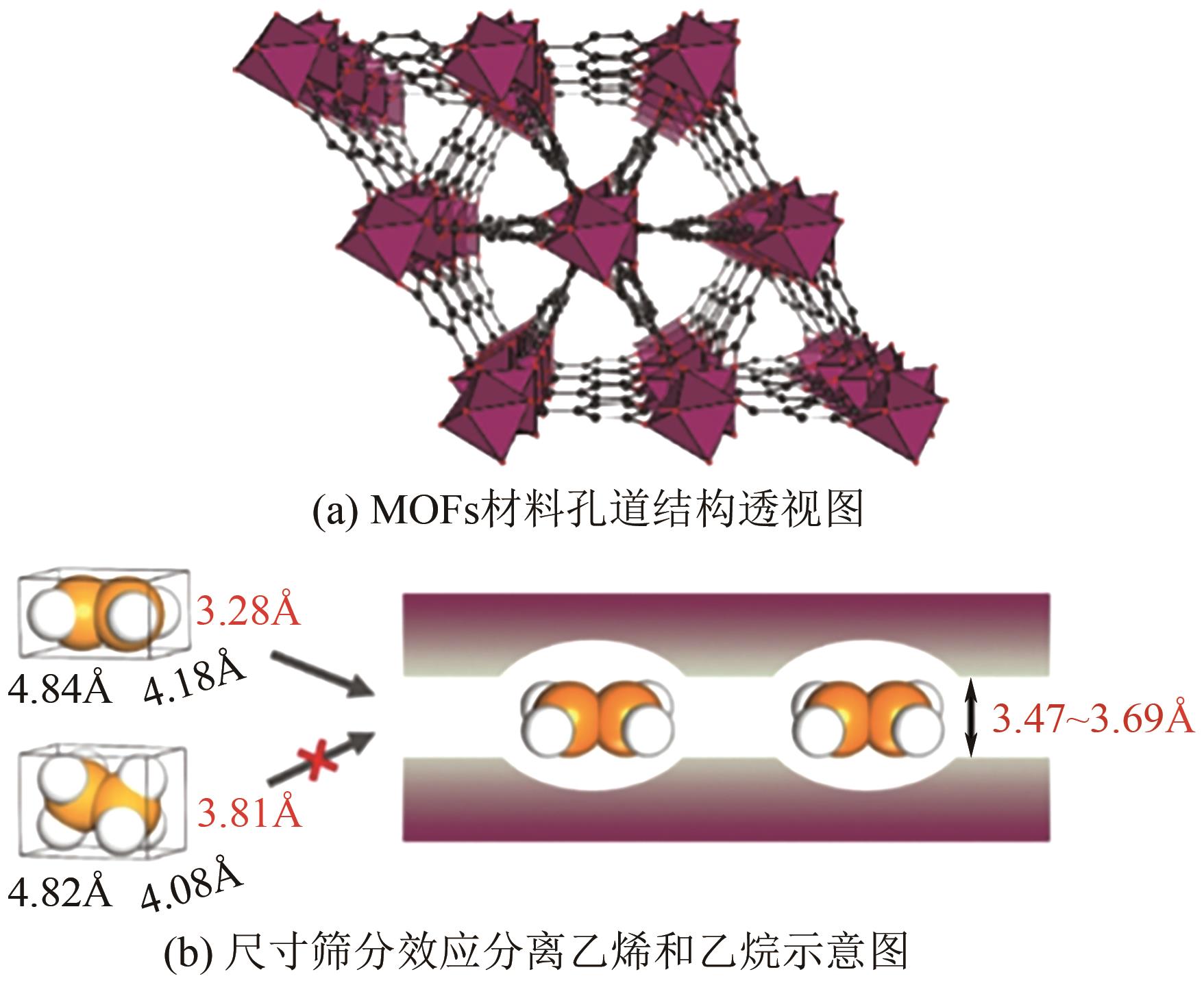
| 聚合物膜分离 | 穿过膜的扩散速率差别 | 石化废气和氨吹扫气 | 92~98 | He、CO2、H2O也可能会穿过膜 |
| 钯膜分离 | 氢气选择性渗透 | 任何含氢气体 | ≥99.999 | 硫化物和不饱和烃会削弱膜的渗透性 |
| 金属氢化物分离 | 氢与金属形成金属氢化物的可逆反应 | 氨吹扫气 | 99.999 | O2、CO、硫化物会使材料中毒 |
| 变压吸附 | 吸附剂选择性吸附杂质 | 任何富氢气体 | 99.999 | 吹扫气阶段有氢气损失,回收率相对较低 |
表2 常见的氢气纯化方法[10]
| 方法 | 原理 | 典型进料气 | 产品氢纯度/% | 技术难点 |
|---|---|---|---|---|
| 低温分离 | 相对挥发度的差别 | 石化废气,含氢在30%~80%内 | 90~98 | 不易得到高纯度氢气 |
| 聚合物膜分离 | 穿过膜的扩散速率差别 | 石化废气和氨吹扫气 | 92~98 | He、CO2、H2O也可能会穿过膜 |
| 钯膜分离 | 氢气选择性渗透 | 任何含氢气体 | ≥99.999 | 硫化物和不饱和烃会削弱膜的渗透性 |
| 金属氢化物分离 | 氢与金属形成金属氢化物的可逆反应 | 氨吹扫气 | 99.999 | O2、CO、硫化物会使材料中毒 |
| 变压吸附 | 吸附剂选择性吸附杂质 | 任何富氢气体 | 99.999 | 吹扫气阶段有氢气损失,回收率相对较低 |
| 气体名称 | 动力学直径/Å | 极化率/Å3 | 偶极矩/Å | 四极矩/Å2 |
|---|---|---|---|---|
| H2 | 2.83~2.90 | 0.8042 | 0 | 0.662 |
| CH4 | 3.76 | 2.593 | 0 | 0 |
| CO2 | 3.30 | 2.911 | 0 | 4.30 |
| CO | 3.69~3.76 | 1.950 | 0.11 | 2.50 |
| N2 | 3.64 | 1.740 | 0 | 1.52 |
表3 氢气及主要杂质的物理性质[38]
| 气体名称 | 动力学直径/Å | 极化率/Å3 | 偶极矩/Å | 四极矩/Å2 |
|---|---|---|---|---|
| H2 | 2.83~2.90 | 0.8042 | 0 | 0.662 |
| CH4 | 3.76 | 2.593 | 0 | 0 |
| CO2 | 3.30 | 2.911 | 0 | 4.30 |
| CO | 3.69~3.76 | 1.950 | 0.11 | 2.50 |
| N2 | 3.64 | 1.740 | 0 | 1.52 |
| 吸附模型 | 模型表达式 | 参数及意义 |
|---|---|---|
| Langmuir | q为吸附量,qm为最大吸附量,b为Langmuir平衡常数,p为压力 | |
| 扩展Langmuir | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 为纯组的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Toth | q为吸附量,qm为最大吸附量,b为Toth平衡常数,n是和吸附剂不均匀性相关的量纲为1参数,p为压力 | |
| 扩展Toth | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 、ti 为纯组分i的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Virial | p为压力,q为吸附量,KH为Henry常数,S为吸附剂比表面积,A、B为Virial系数 |
表4 用于吸附拟合常见的吸附模型[59-61]
| 吸附模型 | 模型表达式 | 参数及意义 |
|---|---|---|
| Langmuir | q为吸附量,qm为最大吸附量,b为Langmuir平衡常数,p为压力 | |
| 扩展Langmuir | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 为纯组的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Toth | q为吸附量,qm为最大吸附量,b为Toth平衡常数,n是和吸附剂不均匀性相关的量纲为1参数,p为压力 | |
| 扩展Toth | qi 和pi 为气体混合物吸附量和分压,qmi 、bi 、ti 为纯组分i的对应方程拟合参数,j为混合物中各纯组分,n为混合物中的气体种类数 | |
| Virial | p为压力,q为吸附量,KH为Henry常数,S为吸附剂比表面积,A、B为Virial系数 |
| 交换离子 | 分子筛 | 交换条件 | 吸附气体 | 参考文献 |
|---|---|---|---|---|
| 碱金属离子 | 13X | 0.5mol/L盐溶液,固液比1∶80,353K下反应4h | CO、CH4、N2 | [ |
| 碱金属离子和H+ | RHO | 1mol/L盐溶液,固液比1∶10,353K下反应4h | CO2 | [ |
| Na+、K+、Cs+、NH4+、Ca2+、Mg2+、Ba2+ | 天然斜发沸石分子筛 | 2mol/L盐溶液,固液比1∶10,微沸下反应84h | CO、CH4、O2、N2 | [ |
| 碱金属离子 | 13X、NaY | 1mol/L盐溶液,固液比1∶10,350K下反应5h | CO2 | [ |
| Ca2+、Mg2+ | 13X | 1mol/L盐溶液,固液比1∶10,353K微波下反应0.5h | CO、CH4、CO2、H2 | [ |
| NH4+、Li+、Cu2+ | 13X | 1mol/L盐溶液,固液比1∶5,353K下反应4h | CH4、CO2、N2 | [ |
| Li+、Pd2+、Ag+ | 13X | 0.4mol/L的Li+溶液,343K下反应3h得到LiX;依次于 PdCl2、AgNO3、LiCl2中交换得到LiPdAgX | CO2 | [ |
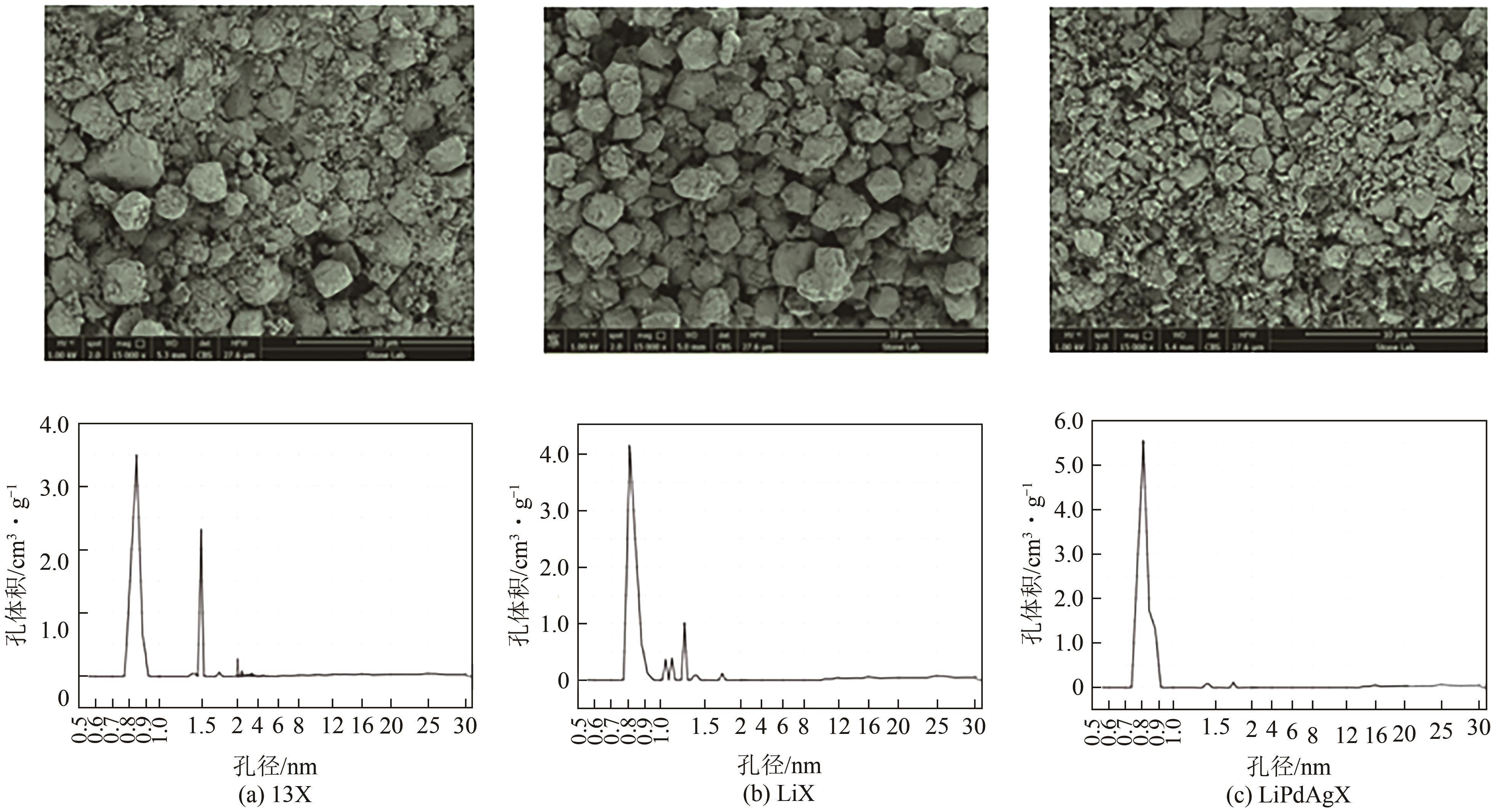
表5 分子筛离子交换改性总结
| 交换离子 | 分子筛 | 交换条件 | 吸附气体 | 参考文献 |
|---|---|---|---|---|
| 碱金属离子 | 13X | 0.5mol/L盐溶液,固液比1∶80,353K下反应4h | CO、CH4、N2 | [ |
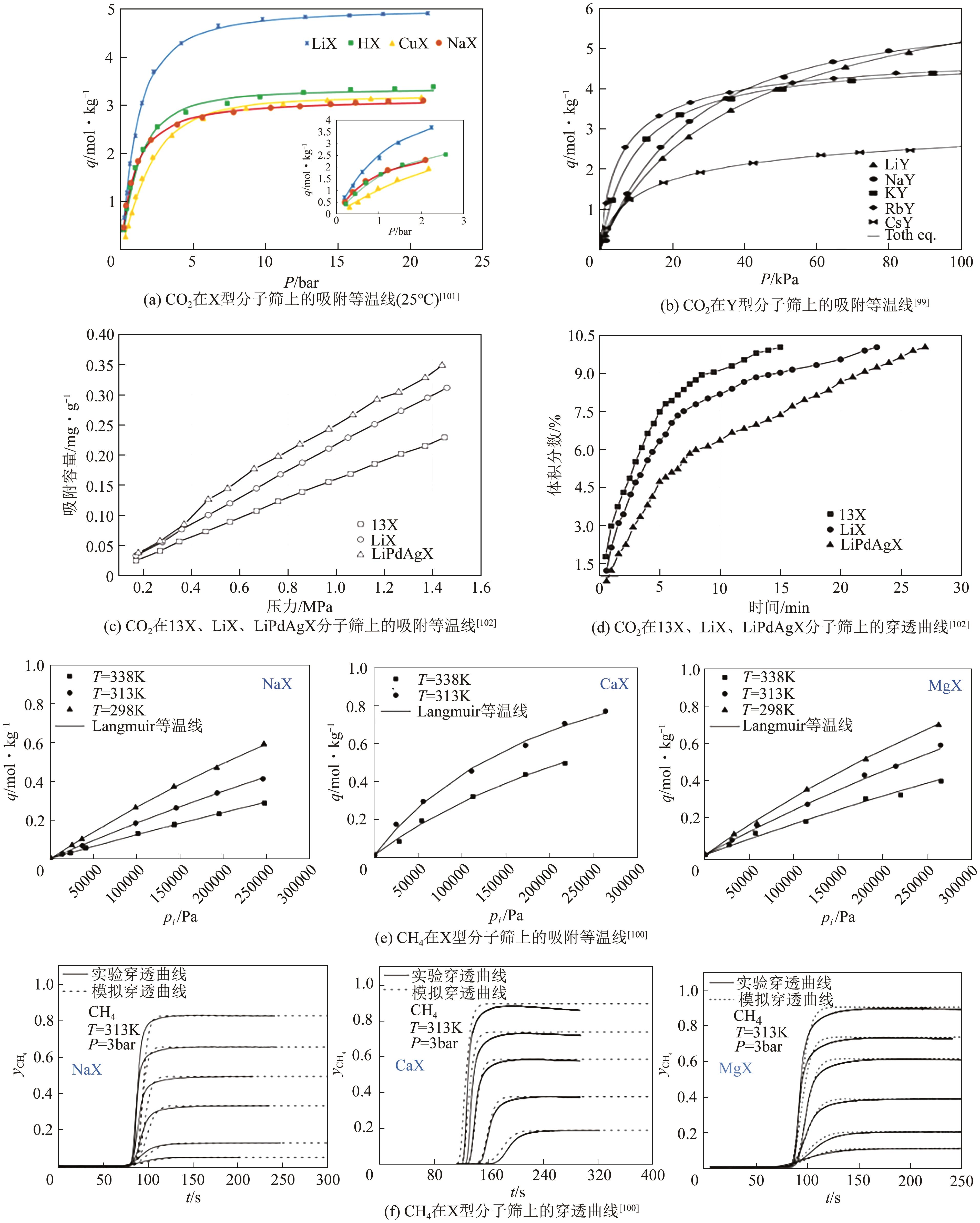
| 碱金属离子和H+ | RHO | 1mol/L盐溶液,固液比1∶10,353K下反应4h | CO2 | [ |
| Na+、K+、Cs+、NH4+、Ca2+、Mg2+、Ba2+ | 天然斜发沸石分子筛 | 2mol/L盐溶液,固液比1∶10,微沸下反应84h | CO、CH4、O2、N2 | [ |
| 碱金属离子 | 13X、NaY | 1mol/L盐溶液,固液比1∶10,350K下反应5h | CO2 | [ |
| Ca2+、Mg2+ | 13X | 1mol/L盐溶液,固液比1∶10,353K微波下反应0.5h | CO、CH4、CO2、H2 | [ |
| NH4+、Li+、Cu2+ | 13X | 1mol/L盐溶液,固液比1∶5,353K下反应4h | CH4、CO2、N2 | [ |
| Li+、Pd2+、Ag+ | 13X | 0.4mol/L的Li+溶液,343K下反应3h得到LiX;依次于 PdCl2、AgNO3、LiCl2中交换得到LiPdAgX | CO2 | [ |
| MOFs | 吸附气体 | 吸附条件 | 吸附量/mmol·g-1 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| MOF-5 | CO2 CH4 H2 | 298K、4MPa | 22.5 10 0.8 | — | [ |
| MOF-5 | CH4 H2 | 300K、3MPa | 9 0.58 | — | [ |
| MOF-74 | CO2 H2 CO2∶H2=1∶4 | 313K、4MPa | 13 2 — | — 380 | [ |
| MOF-74 | CO2∶H2=1∶4 CH4∶H2=1∶1 CO2∶CH4∶H2=4∶1∶20 | 313K、4MPa | — | 380 15 300 | [ |
| Cu-BTC | CO2 CH4 CO H2 | 308K、0.6MPa 308K、0.6MPa 303K、0.08MPa 303K、0.5MPa | 9.2 3.1 0.65 0.41 | — | [ |
| Cu-TDPAT | CO2∶H2= 1:4 CH4∶H2= 1:1 | 298K、4MPa | 12.5 8 | 80 — | [ |
| UiO-66 | CO2;CO2∶H2= 3:7 CH4;CH4∶H2=3:7 CO;CO∶H2=3:7 H2 | 298K、4MPa | 8.2 6.7 5 1.4 | 100 18 12 — | [ |
| UiO-66-Br | CO2;CO2∶H2=3∶7 CH4;CH4∶H2=3∶7 CO;CO∶H2=3∶7 H2 | 298K、4MPa | 7 5 4.5 1.2 | 130 21 15 — | [ |
| UTSA-16 | CO2 CH4 CO H2 | 298K、4MPa 298K、4MPa 298K、0.5MPa 298K、4MPa | 4.9 2.4 0.9 0.5 | — — — | [ |
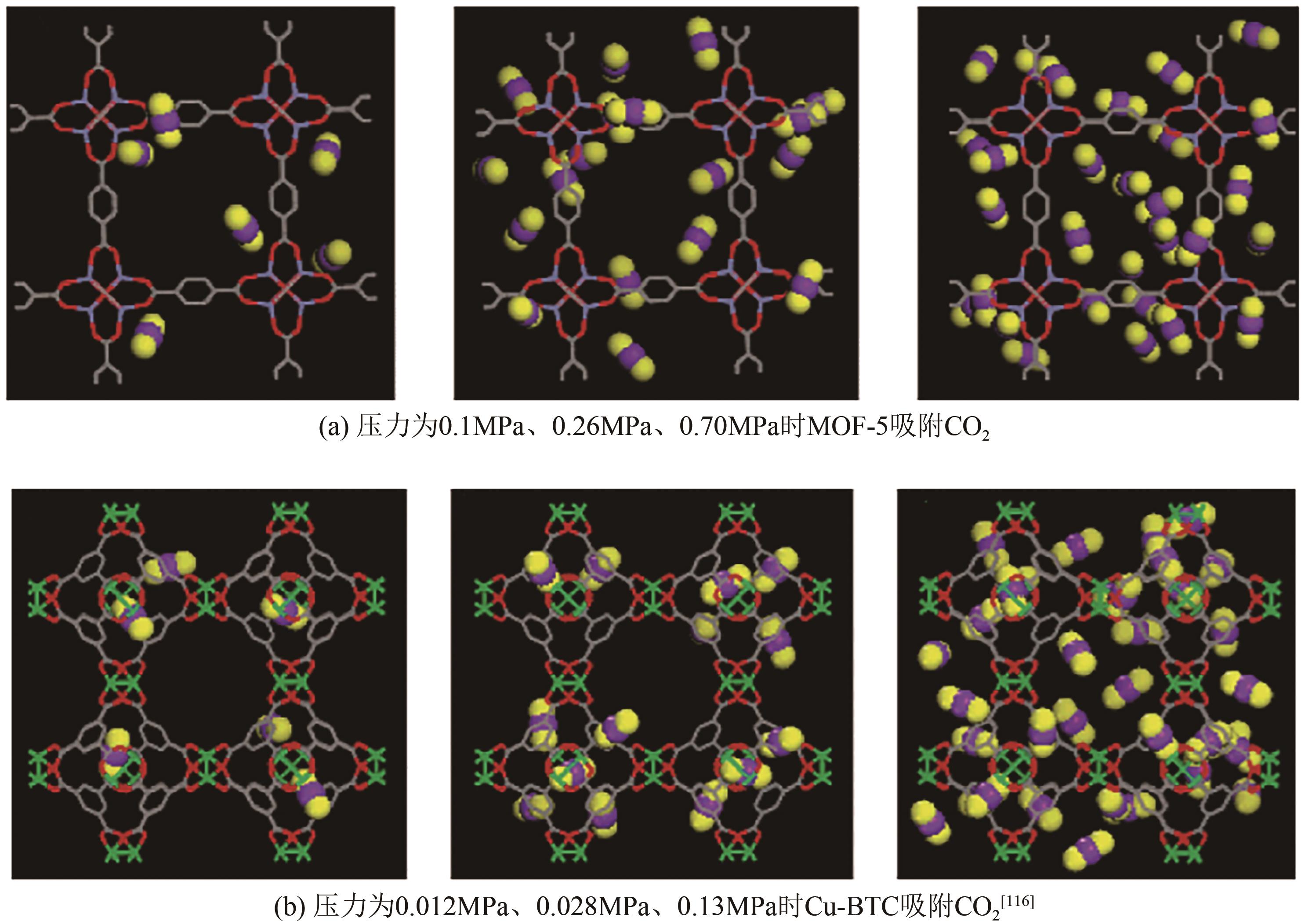
表6 MOFs对H2及杂质的吸附数据
| MOFs | 吸附气体 | 吸附条件 | 吸附量/mmol·g-1 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| MOF-5 | CO2 CH4 H2 | 298K、4MPa | 22.5 10 0.8 | — | [ |
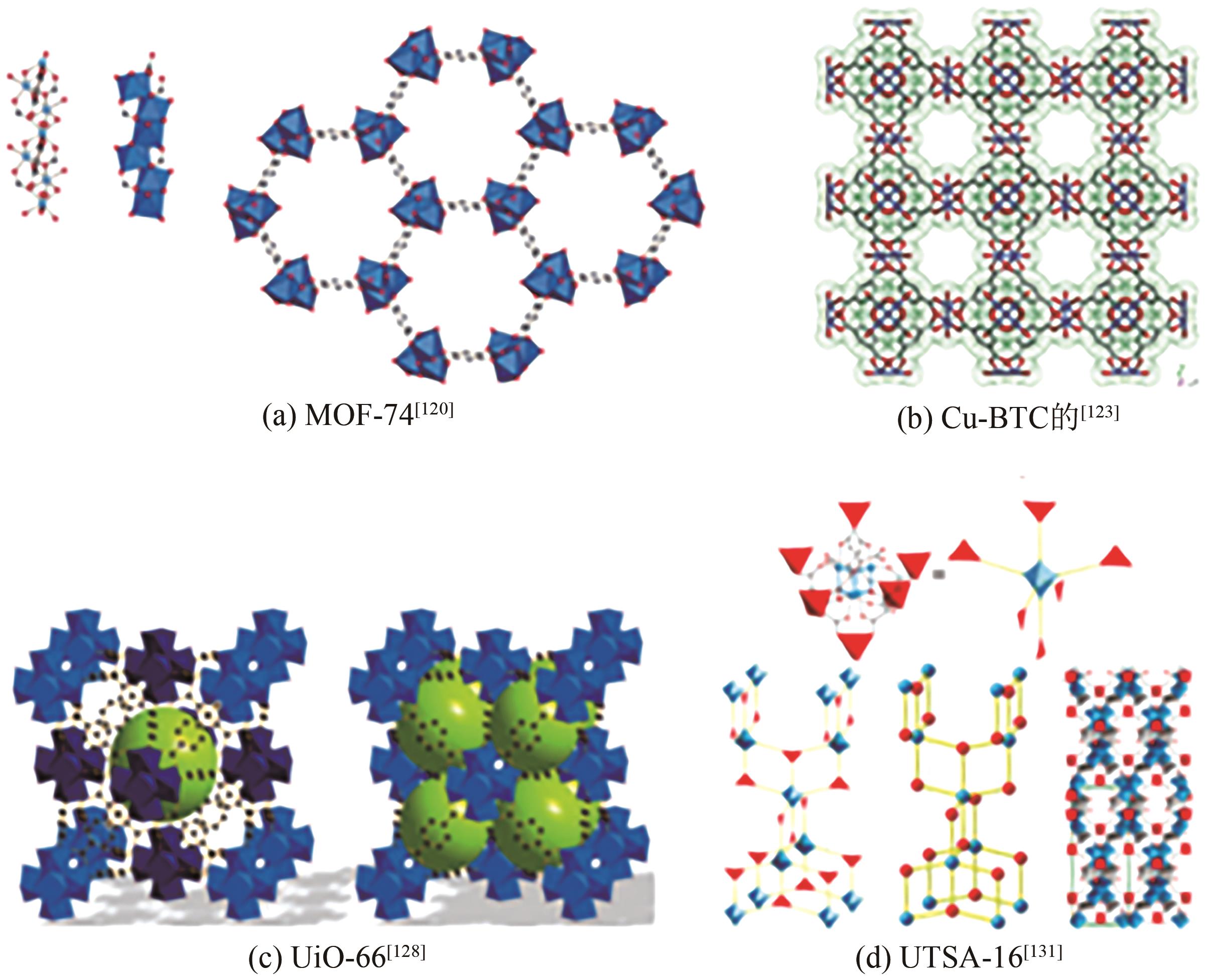
| MOF-5 | CH4 H2 | 300K、3MPa | 9 0.58 | — | [ |
| MOF-74 | CO2 H2 CO2∶H2=1∶4 | 313K、4MPa | 13 2 — | — 380 | [ |
| MOF-74 | CO2∶H2=1∶4 CH4∶H2=1∶1 CO2∶CH4∶H2=4∶1∶20 | 313K、4MPa | — | 380 15 300 | [ |
| Cu-BTC | CO2 CH4 CO H2 | 308K、0.6MPa 308K、0.6MPa 303K、0.08MPa 303K、0.5MPa | 9.2 3.1 0.65 0.41 | — | [ |
| Cu-TDPAT | CO2∶H2= 1:4 CH4∶H2= 1:1 | 298K、4MPa | 12.5 8 | 80 — | [ |
| UiO-66 | CO2;CO2∶H2= 3:7 CH4;CH4∶H2=3:7 CO;CO∶H2=3:7 H2 | 298K、4MPa | 8.2 6.7 5 1.4 | 100 18 12 — | [ |
| UiO-66-Br | CO2;CO2∶H2=3∶7 CH4;CH4∶H2=3∶7 CO;CO∶H2=3∶7 H2 | 298K、4MPa | 7 5 4.5 1.2 | 130 21 15 — | [ |
| UTSA-16 | CO2 CH4 CO H2 | 298K、4MPa 298K、4MPa 298K、0.5MPa 298K、4MPa | 4.9 2.4 0.9 0.5 | — — — | [ |
| 1 | 刘美琴, 李奠础, 乔建芬, 等. 氢能利用与碳质材料吸附储氢技术[J]. 化工时刊, 2013, 27(11): 35-38. |
| LIU Meiqin, LI Dianchu, QIAO Jianfen, et al. The use of hydrogen energy and hydrogen adsorption storage technology of carbonaceous materials[J]. Chemical Industry Times, 2013, 27(11): 35-38. | |
| 2 | 王欣, 王苏礼, 刘伟, 等. 日本和德国的氢能产业发展现状综述[J]. 现代商业, 2019(24): 26-27. |
| WANG Xin, WANG Suli, LIU Wei, et al. Summary of the development status of hydrogen energy industry in Japan and Germany[J]. Modern Business, 2019(24): 26-27. | |
| 3 | 吴善略, 张丽娟. 世界主要国家氢能发展规划综述[J]. 科技中国, 2019(7): 91-97. |
| WU Shanlue, ZHANG Lijuan. Summary of hydrogen energy development planning in major countries in the world[J]. China Scitechnology Business, 2019(7): 91-97. | |
| 4 | DU Zhemin, LIU Congmin, ZHAI Junxiang, et al. A review of hydrogen purification technologies for fuel cell vehicles[J]. Catalysts, 2021, 11(3): 393. |
| 5 | DAWOOD Furat, ANDA Martin, SHAFIULLAH G M. Hydrogen production for energy: An overview[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3847-3869. |
| 6 | 孙强, 贺玉刚, 严大洲, 等. 高纯氢制备工艺研究进展[J]. 精细石油化工进展, 2020, 21(3): 42-44, 57. |
| SUN Qiang, HE Yugang, YAN Dazhou, et al. Progress of research on study of high purity hydrogen production technology[J]. Advances in Fine Petrochemicals, 2020, 21(3): 42-44, 57. | |
| 7 | DECOSTE Jared B, PETERSON Gregory W, SCHINDLER Bryan J, et al. The effect of water adsorption on the structure of the carboxylate containing metal-organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66[J]. Journal of Materials Chemistry A, 2013, 1(38): 11922-11932. |
| 8 | Pia KÜSGENS, ROSE Marcus, SENKOVSKA Irena, et al. Characterization of metal-organic frameworks by water adsorption[J]. Microporous and Mesoporous Materials, 2009, 120(3): 325-330. |
| 9 | AASADNIA Majid, MEHRPOOYA Mehdi, GHORBANI Bahram. A novel integrated structure for hydrogen purification using the cryogenic method[J]. Journal of Cleaner Production, 2021, 278: 123872. |
| 10 | 陈绍华, 邢丕峰, 陈文梅. 稀贵金属在氢气纯化中的应用[J]. 稀有金属, 2003, 27(1): 8-17. |
| CHEN Shaohua, XING Pifeng, CHEN Wenmei. The application of rare-noble metals to the purification of hydrogen[J]. Chinese Journal of Rare Metals, 2003, 27(1): 8-17. | |
| 11 | SONG Chunfeng, LIU Qingling, DENG Shuai, et al. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges[J]. Renewable and Sustainable Energy Reviews, 2019, 101: 265-278. |
| 12 | LI Panyuan, WANG Zhi, QIAO Zhihua, et al. Recent developments in membranes for efficient hydrogen purification[J]. Journal of Membrane Science, 2015, 495: 130-168. |
| 13 | 李忠于, 黄伟, 张楚璠. 燃料电池用高纯氢纯化技术研究进展[J]. 能源化工, 2020, 41(5): 1-7. |
| LI Zhongyu, HUANG Wei, ZHANG Chufan. Research progress on high purity hydrogen purification technology for fuel cell[J]. Energy Chemical Industry, 2020, 41(5): 1-7. | |
| 14 | 栾永超, 熊亚林, 何广利, 等. 氢气分离膜研究进展[J]. 中国工程科学, 2022, 24(3): 140-152. |
| LUAN Yongchao, XIONG Yalin, HE Guangli, et al. Research progress of hydrogen separation membrane[J]. Strategic Study of CAE, 2022, 24(3): 140-152. | |
| 15 | YUN Samhun, OYAMA S TED. Correlations in palladium membranes for hydrogen separation: A review[J]. Journal of Membrane Science, 2011, 375(1/2): 28-45. |
| 16 | WARD Timothy L, Tien DAO. Model of hydrogen permeation behavior in palladium membranes[J]. Journal of Membrane Science, 1999, 153(2): 211-231. |
| 17 | TSUCHIMOTO K, YUKAWA H, NAMBU T, et al. Design of Nb-W-Mo alloy membrane for hydrogen separation and purification[J]. Journal of Alloys and Compounds, 2013, 580: S391-S396. |
| 18 | RAHIMPOUR M R, SAMIMI F, BABAPOOR A, et al. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review[J]. Chemical Engineering and Processing: Process Intensification, 2017, 121: 24-49. |
| 19 | NAYEBOSSADRI S, SPEIGHT J, BOOK David. University of Birmingham Hydrogen separation from blended natural gas and hydrogen by Pd-based membranes[J]. International Journal Hydrogen Energy, 2019, 44: 29092-29099. |
| 20 | ZHAO Chenyang, SUN Bing, JIANG Jie, et al. H2 purification process with double layer BCC-PdCu alloy membrane at ambient temperature[J]. International Journal of Hydrogen Energy, 2020, 45(35): 17540-17547. |
| 21 | NAVARRO R M, GUIL R, FIERRO J L G. Introduction to hydrogen production[M]//Compendium of Hydrogen Energy. Amsterdam: Elsevier, 2015: 21-61. |
| 22 | KONG Seong Young, KIM Da Hye, HENKENSMEIER Dirk, et al. Ultrathin layered Pd/PBI-HFA composite membranes for hydrogen separation[J]. Separation and Purification Technology, 2017, 179: 486-493. |
| 23 | KIADEHI Afshin Dehghani, TAGHIZADEH Majid. Fabrication, characterization, and application of palladium composite membrane on porous stainless steel substrate with NaY zeolite as an intermediate layer for hydrogen purification[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2889-2904. |
| 24 | IULIANELLI A, GHASEMZADEH K, MARELLI M, et al. A supported Pd-Cu/Al2O3 membrane from solvated metal atoms for hydrogen separation/purification[J]. Fuel Processing Technology, 2019, 195: 106141. |
| 25 | María YÁÑEZ, ORTIZ Alfredo, GORRI Daniel, et al. Comparative performance of commercial polymeric membranes in the recovery of industrial hydrogen waste gas streams[J]. International Journal of Hydrogen Energy, 2021, 46(33): 17507-17521. |
| 26 | Neha PAL, AGARWAL Madhu, MAHESHWARI Karishma, et al. A review on types, fabrication and support material of hydrogen separation membrane[J]. Materials Today: Proceedings, 2020, 28: 1386-1391. |
| 27 | STRUGOVA D V, M Yu ZADOROZHNYY, BERDONOSOVA E A, et al. Novel process for preparation of metal-polymer composite membranes for hydrogen separation[J]. International Journal of Hydrogen Energy, 2018, 43(27): 12146-12152. |
| 28 | REZAKAZEMI Mashallah, SHAHIDI Kazem, MOHAMMADI Toraj. Hydrogen separation and purification using crosslinkable PDMS/zeolite A nanoparticles mixed matrix membranes[J]. International Journal of Hydrogen Energy, 2012, 37(19): 14576-14589. |
| 29 | PEYDAYESH Mohammad, MOHAMMADI Toraj, BAKHTIARI Omid. Effective hydrogen purification from methane via polyimide Matrimid® 5218- Deca-dodecasil 3R type zeolite mixed matrix membrane[J]. Energy, 2017, 141: 2100-2107. |
| 30 | XIAO Jinsheng, TONG Liang, YANG Tianqi, et al. Lumped parameter simulation of hydrogen storage and purification systems using metal hydrides[J]. International Journal of Hydrogen Energy, 2017, 42(6): 3698-3707. |
| 31 | DUNIKOV D, BORZENKO V, BLINOV D, et al. Biohydrogen purification using metal hydride technologies[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21787-21794. |
| 32 | YANG F S, CHEN X Y, WU Z, et al. Experimental studies on the poisoning properties of a low-plateau hydrogen storage alloy LaNi4.3Al0.7 against CO impurities[J]. International Journal of Hydrogen Energy, 2017, 42(25): 16225-16234. |
| 33 | HANADA Nobuko, ASADA Hirotaka, NAKAGAWA Tessui, et al. Effect of CO2 on hydrogen absorption in Ti-Zr-Mn-Cr based AB2 type alloys[J]. Journal of Alloys and Compounds, 2017, 705: 507-516. |
| 34 | 王春燕, 杨莉娜, 王念榕, 等. 变压吸附技术在天然气脱除CO2上的应用探讨[J]. 石油规划设计, 2013, 24(1): 52-55. |
| WANG Chunyan, YANG Lina, WANG Nianrong, et al. Discussion on application of pressure swing adsorption technology in CO2 removal from natural gas[J]. Petroleum Planning & Engineering, 2013, 24(1): 52-55. | |
| 35 | SONG C.Hydrogen and syngas production and purification technologies[M]. New York: John Wiley & Sons, Inc. 2009. |
| 36 | 寇丹. 变压吸附制氢装置改进及工艺优化研究[D]. 北京: 北京理工大学, 2016. |
| KOU Dan. PSA hydrogen plant improved and research of the process optimization[D]. Beijing: Beijing Institute of Technology, 2016. | |
| 37 | SIRCAR S, GOLDEN T C. Purification of hydrogen by pressure swing adsorption[J]. Separation Science and Technology, 2000, 35(5): 667-687. |
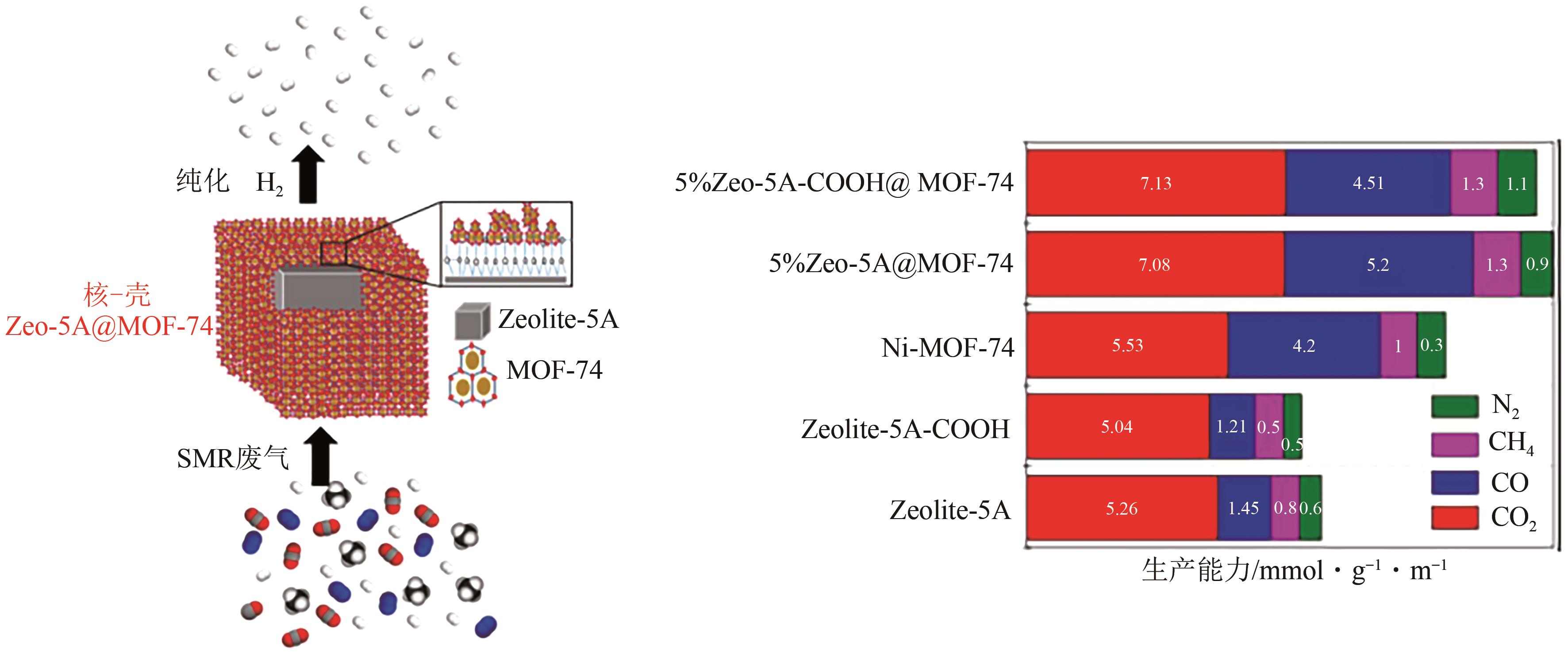
| 38 | Qasim AL-NADDAF, ROWNAGHI Ali A, REZAEI Fateme. Multicomponent adsorptive separation of CO2, CO, CH4, N2, and H2 over core-shell zeolite-5A@MOF-74 composite adsorbents[J]. Chemical Engineering Journal, 2020, 384: 123251. |
| 39 | 刘珊珊, 柴玉超, 关乃佳, 等. 分子筛材料在小分子吸附分离中的应用[J]. 高等学校化学学报, 2021, 42(1): 268-288. |
| LIU Shanshan, CHAI Yuchao, GUAN Naijia, et al. Small molecule adsorption and separation on zeolites[J]. Chemical Journal of Chinese Universities, 2021, 42(1): 268-288. | |
| 40 | KELLER J U, STAUDT R, Gas adsorption equilibria : experimental methods and adsorptive isotherms[M]. Springer Science & Business Media, 2005 |
| 41 | LIN Jerry Y S. Molecular sieves for gas separation[J]. Science, 2016, 353(6295): 121-122. |
| 42 | KUZNICKI Steven M, BELL Valerie A, NAIR Sankar, et al. A titanosilicate molecular sieve with adjutsable pores for size-selective adsorption of molecules[J]. Nature, 2001, 412(6848): 720-724. |
| 43 | 赵振国. 吸附作用应用原理[M]. 北京: 化学工业出版社, 2005: 123-134. |
| ZHAO Zhenguo. Application principle of adsorption[M]. Beijing: Chemical Industry Press, 2005: 123-134. | |
| 44 | BAO Zongbi, WANG Jiawei, ZHANG Zhiguo, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 45 | LI Kunhao, OLSON David H, SEIDEL Jonathan, et al. Zeolitic imidazolate frameworks for kinetic separation of propane and propene[J]. Journal of the American Chemical Society, 2009, 131(30): 10368-10369. |
| 46 | LI Jianrong, KUPPLER Ryan J, ZHOU Hongcai. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
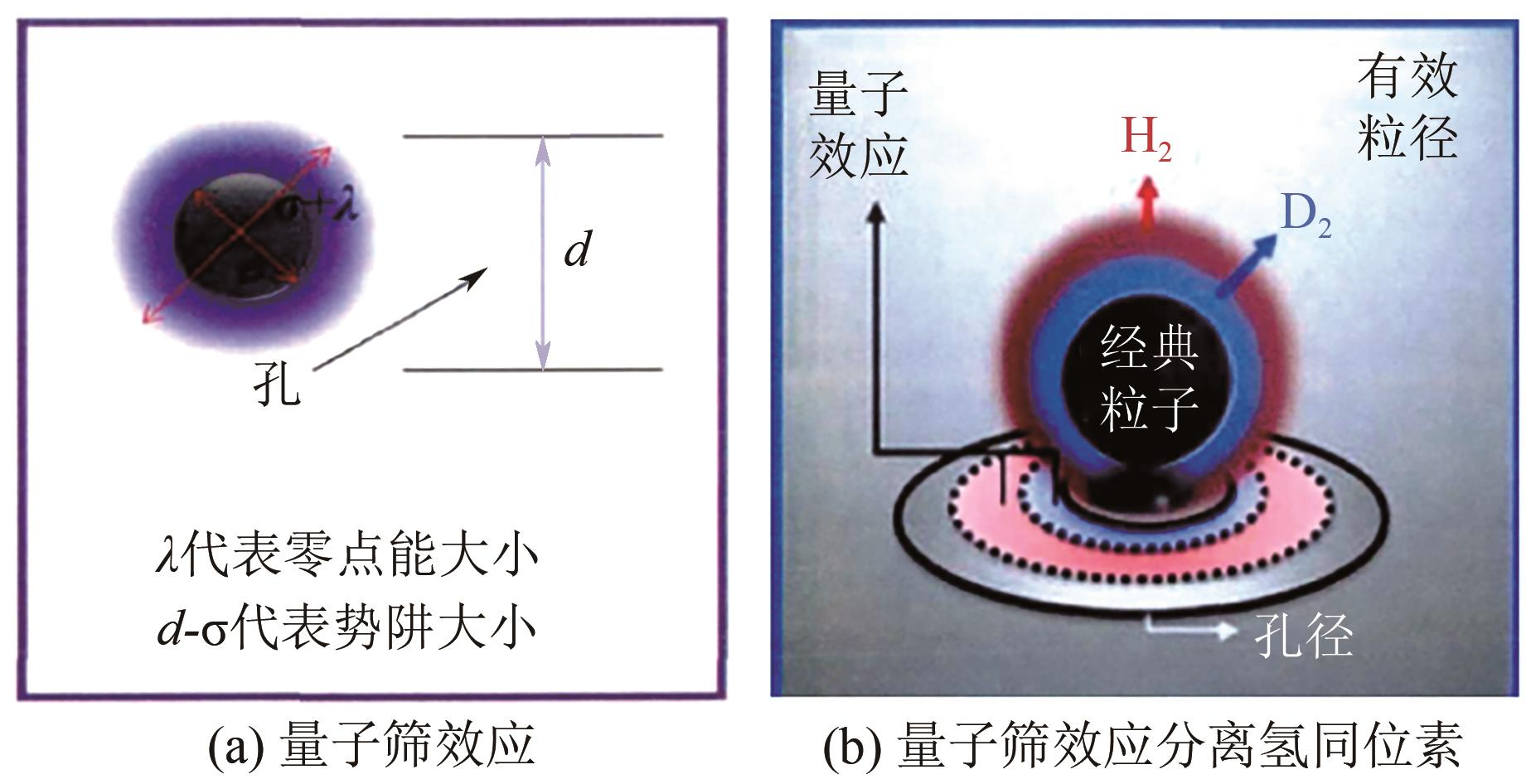
| 47 | BEENAKKER J J M, BORMAN V D, S Yu KRYLOV. Molecular transport in subnanometer pores: Zero-point energy, reduced dimensionality and quantum sieving[J]. Chemical Physics Letters, 1995, 232(4): 379-382. |
| 48 | TEUFEL Julia, Hyunchul OH, HIRSCHER Michael, et al. MFU-4—A metal-organic framework for highly effective H2/D2 separation[J]. Advanced Materials, 2013, 25(4): 635-639. |
| 49 | 曹大伟. 微孔金属有机框架材料氢同位素热力学量子筛效应研究[D]. 绵阳: 中国工程物理研究院, 2017. |
| CAO Dawei. Study on quantum screen effect of hydrogen isotope thermodynamics in porous metal-organic frame materials[D].Mianyang: China Academy of Engineering Physics, 2017. | |
| 50 | 张延鹏, 张胜中, 范得权, 等. 用于甲烷/氮气分离的活性炭吸附剂研究进展[J]. 当代化工, 2021, 50(10): 2466-2470, 2474. |
| ZHANG Yanpeng, ZHANG Shengzhong, FAN Dequan, et al. Research progress of activated carbon for CH4/N2 separation[J]. Contemporary Chemical Industry, 2021, 50(10): 2466-2470, 2474. | |
| 51 | 彭育志, 明越, 肖金生, 等. 活性炭/沸石层状床氢气纯化穿透曲线热效应[J]. 工程热物理学报, 2016, 37(7): 1511-1518. |
| PENG Yuzhi, MING Yue, XIAO Jinsheng, et al. Thermal effects on hydrogen purification breakthrough curves of activated carbon/zeolite layered bed[J]. Journal of Engineering Thermophysics, 2016, 37(7): 1511-1518. | |
| 52 | RIBEIRO Ana M, GRANDE Carlos A, LOPES Filipe V S, et al. A parametric study of layered bed PSA for hydrogen purification[J]. Chemical Engineering Science, 2008, 63(21): 5258-5273. |
| 53 | YANG Se-Il, CHOI Do-Young, JANG Seong-Cheol, et al. Hydrogen separation by multi-bed pressure swing adsorption of synthesis gas[J]. Adsorption, 2008, 14(4): 583-590. |
| 54 | CHO Soon Haeng, BHAT Sodankoor Garadi Thirumaleshwara, HAN Sang Sup, et al. Pressure swing adsorption apparatus and method for hydrogen purification using the same: US8298319[P]. 2012-10-30. |
| 55 | Sol AHN, YOU Young-Woo, LEE Dong-Geun, et al. Layered two- and four-bed PSA processes for H2 recovery from coal gas[J]. Chemical Engineering Science, 2012, 68(1): 413-423. |
| 56 | DELGADO José A, ÁGUEDA V I, UGUINA M A, et al. Adsorption and diffusion of H2, CO, CH4, and CO2 in BPL activated carbon and 13X zeolite: Evaluation of performance in pressure swing adsorption hydrogen purification by simulation[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15414-15426. |
| 57 | BAILEY Susan E, OLIN Trudy J, Mark BRICKA R, et al. A review of potentially low-cost sorbents for heavy metals[J]. Water Research, 1999, 33(11): 2469-2479. |
| 58 | GUO Nannan, ZHANG Su, WANG Luxiang, et al. Application of plant-based porous carbon for supercapacitors[J]. Acta Physico-Chimica Sinica, 2020, 36(2): 1903055-. |
| 59 | YAVARY Milad, ALE EBRAHIM Habib, FALAMAKI Cavus. Competitive adsorption equilibrium isotherms of CO, CO2, CH4, and H2 on activated carbon and zeolite 5A for hydrogen purification[J]. Journal of Chemical & Engineering Data, 2016, 61(10): 3420-3427. |
| 60 | YI Honghong, LI Fenrong, NING Ping, et al. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon[J]. Chemical Engineering Journal, 2013, 215/216: 635-642. |
| 61 | LOPES Filipe V S, GRANDE Carlos A, RIBEIRO Ana M, et al. Enhancing capacity of activated carbons for hydrogen purification[J]. Industrial & Engineering Chemistry Research, 2009, 48(8): 3978-3990. |
| 62 | LOPES Filipe V S, GRANDE Carlos A, RODRIGUES Alírio E. Activated carbon for hydrogen purification by pressure swing adsorption: Multicomponent breakthrough curves and PSA performance[J]. Chemical Engineering Science, 2011, 66(3): 303-317. |
| 63 | HE Bojun, LIU Jinglei, ZHANG Ying, et al. Comparison of structured activated carbon and traditional adsorbents for purification of H2 [J]. Separation and Purification Technology, 2020, 239: 116529. |
| 64 | PAN Hongyan, ZHAO Jingyun, LIN Qian, et al. Preparation and characterization of activated carbons from bamboo sawdust and its application for CH4 selectivity adsorption from a CH4/N2 system[J]. Energy & Fuels, 2016, 30(12): 10730-10738. |
| 65 | LI Dawei, WANG Yu, ZHANG Xiaoxiao, et al. Effects of compacting activated carbons on their volumetric CO2 adsorption performance[J]. Fuel, 2020, 262: 116540. |
| 66 | 袁翠翠. CO2活化法制备煤基微孔活性炭的研究[D]. 徐州: 中国矿业大学, 2016. |
| YUAN Cuicui. Research on preparation of coal-based microporous activated carbon with CO2 as activating agent[D]. Xuzhou: China University of Mining and Technology, 2016. | |
| 67 | 赵敏, 潘红艳, 郑蓓蕾, 等. 碱改性活性炭富集低浓度含氧煤层气甲烷的研究[J]. 天然气化工(C1化学与化工), 2016, 41(3): 37-42. |
| ZHAO Min, PAN Hongyan, ZHENG Beilei, et al. Methane enrichment from low concentration of coal mine methane by alkali-modified activated carbons[J]. Natural Gas Chemical Industry, 2016, 41(3): 37-42. | |
| 68 | 张梦竹, 李琳, 刘俊新, 等. 碱改性活性炭表面特征及其吸附甲烷的研究[J]. 环境科学, 2013, 34(1): 39-44. |
| ZHANG Mengzhu, LI Lin, LIU Junxin, et al. Surface characteristics of alkali modified activated carbon and the adsorption capacity of methane[J]. Environmental Science, 2013, 34(1): 39-44. | |
| 69 | ZHAO Guofeng, BAI Peng, ZHU Hongmei, et al. The modification of activated carbons and the pore structure effect on enrichment of coal-bed methane[J]. Asia-Pacific Journal of Chemical Engineering, 2008, 3(3): 284-291. |
| 70 | PAN Hongyan, YI Yun, LIN Qian, et al. Effect of surface chemistry and textural properties of activated carbons for CH4 selective adsorption through low-concentration coal bed methane[J]. Journal of Chemical & Engineering Data, 2016, 61(6): 2120-2127. |
| 71 | SHEN Wenzhong, LI Zhijie, LIU Yihong. Surface chemical functional groups modification of porous carbon[J]. Recent Patents on Chemical Engineeringe, 2008, 1(1): 27-40. |
| 72 | SHAMSUDIN I K, ABDULLAH A, IDRIS I, et al. Hydrogen purification from binary syngas by PSA with pressure equalization using microporous palm kernel shell activated carbon[J]. Fuel, 2019, 253: 722-730. |
| 73 | KWON Sunil, YOU Youngwoo, Hyungseob LIM, et al. Selective CO adsorption using sulfur-doped Ni supported by petroleum-based activated carbon[J]. Journal of Industrial and Engineering Chemistry, 2020, 83: 289-296. |
| 74 | HAROUN M F, MOUSSOUNDA P S, LÉGARÉ P. Theoretical study of methane adsorption on perfect and defective Ni(111) surfaces[J]. Catalysis Today, 2008, 138(1/2): 77-83. |
| 75 | Silvia GONZÁLEZ, Francesc VIÑES, GARCÍA Juan Fernando, et al. A DF-vdW study of the CH4 adsorption on different Ni surfaces[J]. Surface Science, 2014, 625: 64-68. |
| 76 | 郑蓓蕾, 林倩, 潘红艳, 等. 响应面法优化甲烷吸附用活性炭的镍改性工艺[J]. 过程工程学报, 2016, 16(3): 431-437. |
| ZHENG Beilei, LIN Qian, PAN Hongyan, et al. Optimization of the nickel modified active carbon used for adsorption of methane by response surface method[J]. The Chinese Journal of Process Engineering, 2016, 16(3): 431-437. | |
| 77 | Frank ABILD-PEDERSEN, LYTKEN Ole, Jakob ENGBÆK, et al. Methane activation on Ni(111): Effects of poisons and step defects[J]. Surface Science, 2005, 590(2/3): 127-137. |
| 78 | LIU Hongyan, TENG Botao, FAN Maohong, et al. CH4 dissociation on the perfect and defective MgO(001) supported Ni4 [J]. Fuel, 2014, 123: 285-292. |
| 79 | LANZINI A, GUERRA C, LEONE P, et al. Influence of the microstructure on the catalytic properties of SOFC anodes under dry reforming of methane[J]. Materials Letters, 2016, 164: 312-315. |
| 80 | ABDELJAOUED Amna, RELVAS Frederico, MENDES Adélio, et al. Simulation and experimental results of a PSA process for production of hydrogen used in fuel cells[J]. Journal of Environmental Chemical Engineering, 2018, 6: 338-355. |
| 81 | GOLMAKANI Ayub, FATEMI Shohreh, TAMNANLOO Javad. Investigating PSA, VSA, and TSA methods in SMR unit of refineries for hydrogen production with fuel cell specification[J]. Separation and Purification Technology, 2017, 176: 73-91. |
| 82 | KHAN Nazmul Abedin, JHUNG Sung Hwa. Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of π-complexation[J]. Journal of Hazardous Materials, 2017, 325: 198-213. |
| 83 | YANG R T, Adsorbents fundamentals and applications[M]. Hoboken: John Wiley & Sons, 2004: 3-4. |
| 84 | HUANG Helen Y, PADIN Joel, YANG Ralph T. Comparison of π-complexations of ethylene and carbon monoxide with Cu+ and Ag+ [J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2720-2725. |
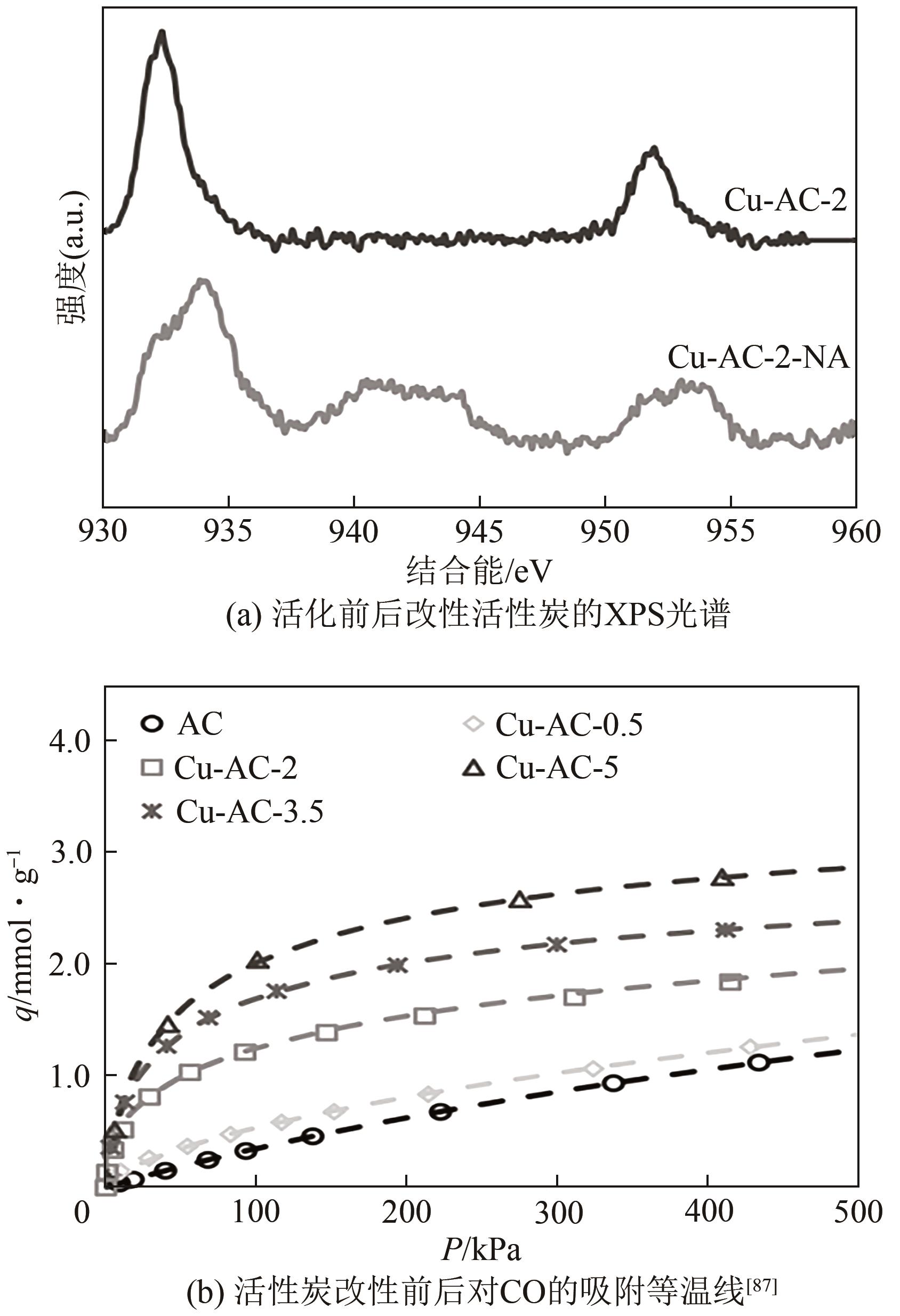
| 85 | The Ky VO, Youn-Sang BAE, CHANG Bong-Jun, et al. Highly CO selective Cu(Ⅰ)-doped MIL-100(Fe) adsorbent with high CO/CO2 selectivity due to π complexation: Effects of Cu(Ⅰ) loading and activation temperature[J]. Microporous and Mesoporous Materials, 2019, 274: 17-24. |
| 86 | 陈鸿雁, 涂晋林, 施亚钧. 载铜活性炭吸附分离氢气中的微量CO[J]. 华东理工大学学报(自然科学版), 1995, 21(1): 1-6. |
| CHEN Hongyan, TU Jinlin, SHI Yajun. Removal of trace CO from hydrogen by adsorption on active carbon supported copper[J]. Journal of East China University of Science and Technology, 1995, 21(1): 1-6. | |
| 87 | RELVAS Frederico, WHITLEY Roger D, SILVA Carlos, et al. Single-stage pressure swing adsorption for producing fuel cell grade hydrogen[J]. Industrial & Engineering Chemistry Research, 2018, 57(14): 5106-5118. |
| 88 | GAO Fei, WANG Yaquan, WANG Xiao, et al. Selective CO adsorbent CuCl/AC prepared using CuCl2 as a precursor by a facile method[J]. RSC Advances, 2016, 6(41): 34439-34446. |
| 89 | XUE Cailong, HAO Wenming, CHENG Wenping, et al. CO adsorption performance of CuCl/activated carbon by simultaneous Reduction-Dispersion of mixed Cu(Ⅱ) salts[J]. Materials, 2019, 12(10): 1605. |
| 90 | MA Jinghong, LI Li, REN Jin, et al. CO adsorption on activated carbon-supported Cu-based adsorbent prepared by a facile route[J]. Separation and Purification Technology, 2010, 76(1): 89-93. |
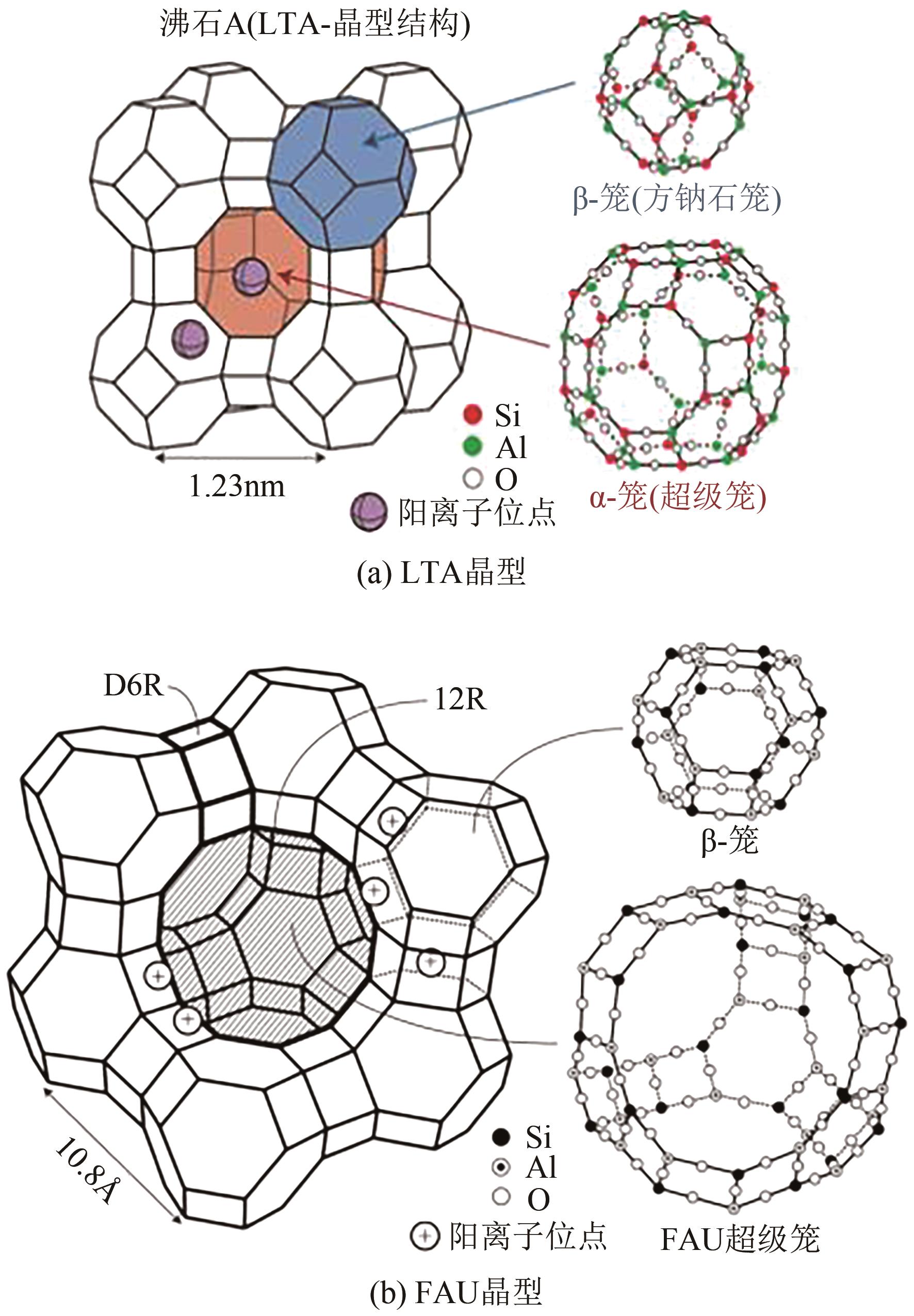
| 91 | 徐如人, 庞文琴. 分子筛与多孔材料化学[M]. 北京: 科学出版社, 2004: 69-70, 420-436. |
| XU Ruren, PANG Wenqin. Molecular sieves and porous materials chemistry[M]. Beijing: Science Press, 2004: 69-70, 420-436. | |
| 92 | PETROV I, MICHALEV Todor. Synthesis of zeolite A: A review[J]. НАУЧНИ ТРУДОВЕ НА РУСЕНСКИЯ УНИВЕРСИТЕТ, 2012, 51: 31-35. |
| 93 | NOZUE Yasuo, AMAKO Yusaku, KAWANO Ryoko, et al. Insulating state and metallic phase transition of heavily sodium-doped low-silica X (LSX) zeolites[J]. Journal of Physics and Chemistry of Solids, 2012, 73(12): 1538-1541. |
| 94 | ZHANG Junfang, BURKE N, ZHANG Shuichang, et al. Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites[J]. Chemical Engineering Science, 2014, 113: 54-61. |
| 95 | MONTANARI Tania, SALLA Isabel, BUSCA Guido. Adsorption of CO on LTA zeolite adsorbents: An IR investigation[J]. Microporous and Mesoporous Materials, 2008, 109(1/2/3): 216-222. |
| 96 | PILLAI Renjith S, SETHIA Govind, JASRA Raksh V. Sorption of CO, CH4, and N2 in alkali metal ion exchanged zeolite-X: Grand canonical Monte Carlo simulation and volumetric measurements[J]. Industrial & Engineering Chemistry Research, 2010, 49(12): 5816-5825. |
| 97 | LOZINSKA Magdalena M, MANGANO Enzo, MOWAT John P S, et al. Understanding carbon dioxide adsorption on univalent cation forms of the flexible zeolite Rho at conditions relevant to carbon capture from flue gases[J]. Journal of the American Chemical Society, 2012, 134(42): 17628-17642. |
| 98 | ARCOYA A, GONZÁLEZ J A, LLABRE G, et al. Role of the countercations on the molecular sieve properties of a clinoptilolite[J]. Microporous Materials, 1996, 7(1): 1-13. |
| 99 | WALTON Krista S, ABNEY Morgan B, DOUGLAS LEVAN M. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange[J]. Microporous and Mesoporous Materials, 2006, 91(1/2/3): 78-84. |
| 100 | BREA P, DELGADO J A, ÁGUEDA Vicente I, et al. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification[J]. Microporous and Mesoporous Materials, 2019, 286: 187-198. |
| 101 | GOLIPOUR Haleh, MOKHTARANI Babak, MAFI Morteza, et al. Systematic measurements of CH4 and CO2 adsorption isotherms on cation-exchanged zeolites 13X[J]. Journal of Chemical & Engineering Data, 2019, 64(10): 4412-4423. |
| 102 | CHEN S J, ZHU M, FU Y, et al. Using 13X, LiX, and LiPdAgX zeolites for CO2 capture from post-combustion flue gas[J]. Applied Energy, 2017, 191: 87-98. |
| 103 | XIE Youchang, ZHANG Jiaping, QIU Jianguo, et al. Zeolites modified by CuCl for separating CO from gas mixtures containing CO2 [J]. Adsorption, 1997, 3(1): 27-32. |
| 104 | GAO Fei, WANG Yaquan, WANG Shuhai. Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: Equilibrium and thermodynamics[J]. Chemical Engineering Journal, 2016, 290: 418-427. |
| 105 | 李淑娜, 张东辉, 鲁东东. CO吸附剂制备及其吸附脱除微量CO的性能[J]. 化学工业与工程, 2014, 31(5): 1-7. |
| LI Shuna, ZHANG Donghui, LU Dongdong. Preparation of carbon monoxide adsorbent and its adsorption performance for removing trace carbon monoxide[J]. Chemical Industry and Engineering, 2014, 31(5): 1-7. | |
| 106 | HERNÁNDEZ-MALDONADO Arturo J, YANG Ralph T. Desulfurization of commercial liquid fuels by selective adsorption via π-complexation with Cu(Ⅰ)-Y zeolite[J]. Industrial & Engineering Chemistry Research, 2003, 42(13): 3103-3110. |
| 107 | 张子宝. Cu-π络合吸附剂及其脱除CO研究[D]. 大连: 大连理工大学, 2021. |
| ZHANG Zibao. Study on Cu π-complexed adsorbent and its removal of CO[D]. Dalian: Dalian University of Technology, 2021. | |
| 108 | QIN Juxiang, WANG Zhimin, LIU Xiaoqin, et al. Low-temperature fabrication of Cu(Ⅰ) sites in zeolites by using a vapor-induced reduction strategy[J]. Journal of Materials Chemistry A, 2015, 3(23): 12247-12251. |
| 109 | LI Hailian, EDDAOUDI Mohamed, GROY Thomas L, et al. Establishing microporosity in open metal-organic frameworks: Gas sorption isotherms for Zn(BDC) (BDC=1,4-benzenedicarboxylate)[J]. Journal of the American Chemical Society, 1998, 120(33): 8571-8572. |
| 110 | GHANBARI Taravat, ABNISA Faisal, WAN DAUD Wan Mohd Ashri. A review on production of metal organic frameworks (MOF) for CO2 adsorption[J]. Science of the Total Environment, 2020, 707: 135090. |
| 111 | 边青敏, 忻睦迪, 邹亢, 等. 分子筛和金属有机骨架材料用于乙烯/乙烷分离的研究进展[J]. 石油学报(石油加工), 2020, 36(4): 866-877. |
| BIAN Qingmin, XIN Mudi, ZOU Kang, et al. Advances in separation of ethylene and ethane with molecular sieve and metal-organic frameworks[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(4): 866-877. | |
| 112 | GUO Ya, HU Jiangliang, LIU Xiaowei, et al. Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2 [J]. Chemical Engineering Journal, 2017, 327: 564-572. |
| 113 | GALLO Marco, Daniel GLOSSMAN-MITNIK. Fuel gas storage and separations by metal-organic frameworks: Simulated adsorption isotherms for H2 and CH4 and their equimolar mixture[J]. The Journal of Physical Chemistry C, 2009, 113(16): 6634-6642. |
| 114 | TYLIANAKIS E, FROUDAKIS G E. Grand canonical Monte Carlo method for gas adsorption and separation[J]. Journal of Computational and Theoretical Nanoscience, 2009, 6(2): 335-348. |
| 115 | GUO Haichao, SHI Fan, MA Zhengfei, et al. Molecular simulation for adsorption and separation of CH4/H2 in zeolitic imidazolate frameworks[J]. The Journal of Physical Chemistry C, 2010, 114(28): 12158-12165. |
| 116 | YANG Qingyuan, ZHONG Chongli. Molecular simulation of carbon dioxide/methane/hydrogen mixture adsorption in metal-organic frameworks[J]. The Journal of Physical Chemistry B, 2006, 110(36): 17776-17783. |
| 117 | FISCHER Michael, HOFFMANN Frank, Michael FRÖBA. Metal-organic frameworks and related materials for hydrogen purification: Interplay of pore size and pore wall polarity[J]. RSC Advances, 2012, 2(10): 4382-4396. |
| 118 | 刘秀英, 袁俊鹏, 李晓东, 等. 甲烷/氢气在超微孔金属有机骨架中的吸附与分离性能研究[J]. 化工新型材料, 2021, 49(2): 178-181, 186. |
| LIU Xiuying, YUAN Junpeng, LI Xiaodong, et al. Study on adsorption and separation of UM-MOFs for CH4/H2 [J]. New Chemical Materials, 2021, 49(2): 178-181, 186. | |
| 119 | MENG Zhaoshun, LU Ruifeng, RAO Dewei, et al. Catenated metal-organic frameworks: Promising hydrogen purification materials and high hydrogen storage medium with further lithium doping[J]. International Journal of Hydrogen Energy, 2013, 38(23): 9811-9818. |
| 120 | ROSI Nathaniel L, KIM Jaheon, EDDAOUDI Mohamed, et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units[J]. Journal of the American Chemical Society, 2005, 127(5): 1504-1518. |
| 121 | HERM Zoey R, SWISHER Joseph A, SMIT Berend, et al. Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture[J]. Journal of the American Chemical Society, 2011, 133(15): 5664-5667. |
| 122 | HERM Zoey R, KRISHNA Rajamani, LONG Jeffrey R. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc)[J]. Microporous and Mesoporous Materials, 2012, 151: 481-487. |
| 123 | CHUI Stephen S-Y, LO Samuel M-F, CHARMANT Jonathan P H, et al. A chemically functionalizable nanoporous material[Cu3(TMA)2(H2O)3] n [J]. Science, 1999, 283(5405): 1148-1150. |
| 124 | SILVA Bruna, SOLOMON Ioan, RIBEIRO Ana M, et al. H2 purification by pressure swing adsorption using CuBTC[J]. Separation and Purification Technology, 2013, 118: 744-756. |
| 125 | JAMALI Sadegh, MOFARAHI Masoud, RODRIGUES Alirio E. Investigation of a novel combination of adsorbents for hydrogen purification using Cu-BTC and conventional adsorbents in pressure swing adsorption[J]. Adsorption, 2018, 24(5): 481-498. |
| 126 | WU Haohan, YAO Kexin, ZHU Yihan, et al. Cu-TDPAT, an Rht-type dual-functional metal-organic framework offering significant potential for use in H2 and natural gas purification processes operating at high pressures[J]. The Journal of Physical Chemistry C, 2012, 116(31): 16609-16618. |
| 127 | ASGARI Mehrdad, STREB Anne, VAN DER SPEK Mijndert, et al. Synergistic material and process development: Application of a metal-organic framework, Cu-TDPAT, in single-cycle hydrogen purification and CO2 capture from synthesis gas[J]. Chemical Engineering Journal, 2021, 414: 128778. |
| 128 | WIERSUM Andrew D, Estelle SOUBEYRAND-LENOIR, YANG Qingyuan, et al. An evaluation of UiO-66 for gas-based applications[J]. Chemistry: an Asian Journal, 2011, 6(12): 3270-3280. |
| 129 | RAMSAHYE, N A, GAO, J, JOBIC, H, et al. Adsorption and diffusion of light hydrocarbons in UiO-66(Zr): A combination of experimental and modeling tools[J]. The Journal of Physical Chemistry C: Nanomaterials and Interfaces, 2014, 118(47): 27470-27482. |
| 130 | BANU Ana-Maria, FRIEDRICH Daniel, BRANDANI Stefano, et al. A multiscale study of MOFs as adsorbents in H2 PSA purification[J]. Industrial & Engineering Chemistry Research, 2013, 52(29): 9946-9957. |
| 131 | XIANG Shengchang, WU Xintao, ZHANG Jianjun, et al. A 3D canted antiferromagnetic porous metal-organic framework with anatase topology through assembly of an analogue of polyoxometalate[J]. Journal of the American Chemical Society, 2005, 127(47): 16352-16353. |
| 132 | XIANG Shengchang, HE Yabing, ZHANG Zhangjing, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions[J]. Nature Communications, 2012, 3: 954. |
| 133 | AGUEDA Vicente I, DELGADO José A, UGUINA María A, et al. Adsorption and diffusion of H2, N2, CO, CH4 and CO2 in UTSA-16 metal-organic framework extrudates[J]. Chemical Engineering Science, 2015, 124: 159-169. |
| 134 | BREA P, DELGADO J A, ÁGUEDA Vicente I, et al. Comparison between MOF UTSA-16 and BPL activated carbon in hydrogen purification by PSA[J]. Chemical Engineering Journal, 2019, 355: 279-289. |
| 135 | DELGADO José A, ÁGUEDA Vicente I, UGUINA María A, et al. Comparison and evaluation of agglomerated MOFs in biohydrogen purification by means of pressure swing adsorption (PSA)[J]. Chemical Engineering Journal, 2017, 326: 117-129. |
| 136 | BOSCH Mathieu, ZHANG Muwei, ZHOU Hongcai. Increasing the stability of metal-organic frameworks[J]. Advances in Chemistry, 2014, 2014: 182327. |
| 137 | Mircea DINCǍ, YU Anta F, LONG Jeffrey R. Microporous metal-organic frameworks incorporating 1,4-benzeneditetrazolate: Syntheses, structures, and hydrogen storage properties[J]. Journal of the American Chemical Society, 2006, 128(27): 8904-8913. |
| 138 | NANDI Shyamapada, DE LUNA Phil, DAFF Thomas D, et al. A single-ligand ultra-microporous MOF for precombustion CO2 capture and hydrogen purification[J]. Science Advances, 2015, 1(11): e1500421. |
| 139 | Qasim AL-NADDAF, THAKKAR Harshul, REZAEI Fateme. Novel zeolite-5A@MOF-74 compositeadsorbents with core-shell structure for H2 purification[J]. ACS Applied Materials & Interfaces, 2018, 10(35): 29656-29666. |
| 140 | 董秀婷, 张文, 赵颂, 等. 金属有机骨架材料的复合成型[J]. 化学进展, 2021, 33(12): 2173-2187. |
| DONG Xiuting, ZHANG Wen, ZHAO Song, et al. Shaping methods for metal-organic framework composites[J]. Progress in Chemistry, 2021, 33(12): 2173-2187. | |
| 141 | MA Qinglang, ZHANG Teng, WANG Bo. Shaping of metal-organic frameworks, a critical step toward industrial applications[J]. Matter, 2022, 5(4): 1070-1091. |
| [1] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [2] | 王涛, 高翔, 高继峰, 邓海全, 余显涌, 周振华, 唐玲, 吕航. 改性Cu-BTC基混合基质膜在CO2分离中的应用[J]. 化工进展, 2024, 43(6): 3240-3246. |
| [3] | 黄坤, 许明, 吴秀娟, 裴思佳, 刘大伟, 马晓迅, 徐龙. 生物质活性炭的制备与微结构特性调控研究进展[J]. 化工进展, 2024, 43(5): 2475-2493. |
| [4] | 周逸寰, 解强, 周红阳, 梁鼎成, 刘金昌. 基于分子模拟的多孔炭材料结构模型构建方法研究进展[J]. 化工进展, 2024, 43(3): 1535-1551. |
| [5] | 郭迎春, 梁晓怿. 柠檬酸改性球形活性炭对氨气吸附性能的影响[J]. 化工进展, 2024, 43(2): 1082-1088. |
| [6] | 汪尚, 姚瑶, 王佳, 董迪迪, 常刚刚. CoZn-MOF衍生多级孔取向碳载CoP及其析氢性能[J]. 化工进展, 2024, 43(1): 447-454. |
| [7] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [8] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [9] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [10] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [11] | 常晓青, 彭东来, 李东洋, 张延武, 王景, 张亚涛. MOFs基丙烯/丙烷高效分离混合基质膜研究进展[J]. 化工进展, 2023, 42(4): 1961-1973. |
| [12] | 邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539. |
| [13] | 陈飞, 丁玉栋, 马丽娇, 朱恂, 程旻, 廖强. 微波合成MOF-808及其水蒸气捕集特性[J]. 化工进展, 2023, 42(12): 6461-6468. |
| [14] | 周红阳, 周逸寰, 张连秀, 梁鼎成, 解强. VOCs在活性炭中的堆积:形成机制及影响因素[J]. 化工进展, 2023, 42(11): 5969-5980. |
| [15] | 田月, 董晓涵, 苏毅. SiO2-CTAB复合材料的制备及其对PNP的吸附性能[J]. 化工进展, 2023, 42(11): 6064-6075. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||