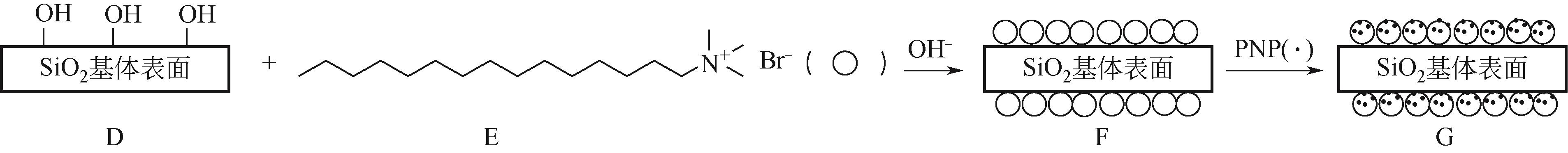化工进展 ›› 2023, Vol. 42 ›› Issue (11): 6064-6075.DOI: 10.16085/j.issn.1000-6613.2022-2370
• 资源与环境化工 • 上一篇
SiO2-CTAB复合材料的制备及其对PNP的吸附性能
- 昆明理工大学化学工程学院,云南 昆明 650500
-
收稿日期:2022-12-28修回日期:2023-02-19出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:苏毅 -
作者简介:田月(1996—),女,硕士研究生,研究方向为资源综合利用。E-mail:1561388678@qq.com。 -
基金资助:国家自然科学基金(21666015)
Preparation of the SiO2-CTAB composite material and its adsorption properties for PNP
TIAN Yue( ), DONG Xiaohan, SU Yi(
), DONG Xiaohan, SU Yi( )
)
- School of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2022-12-28Revised:2023-02-19Online:2023-11-20Published:2023-12-15 -
Contact:SU Yi
摘要:
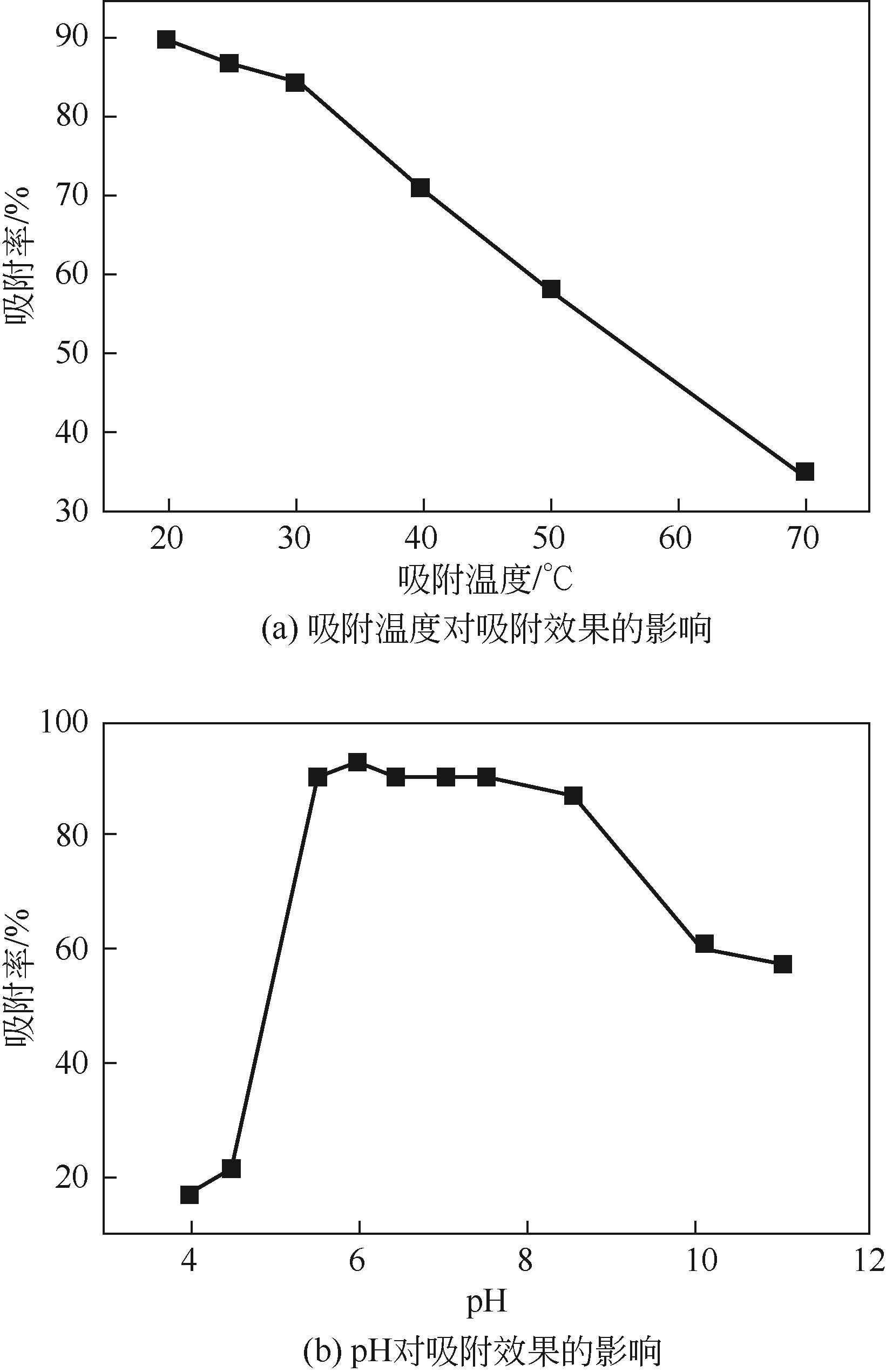
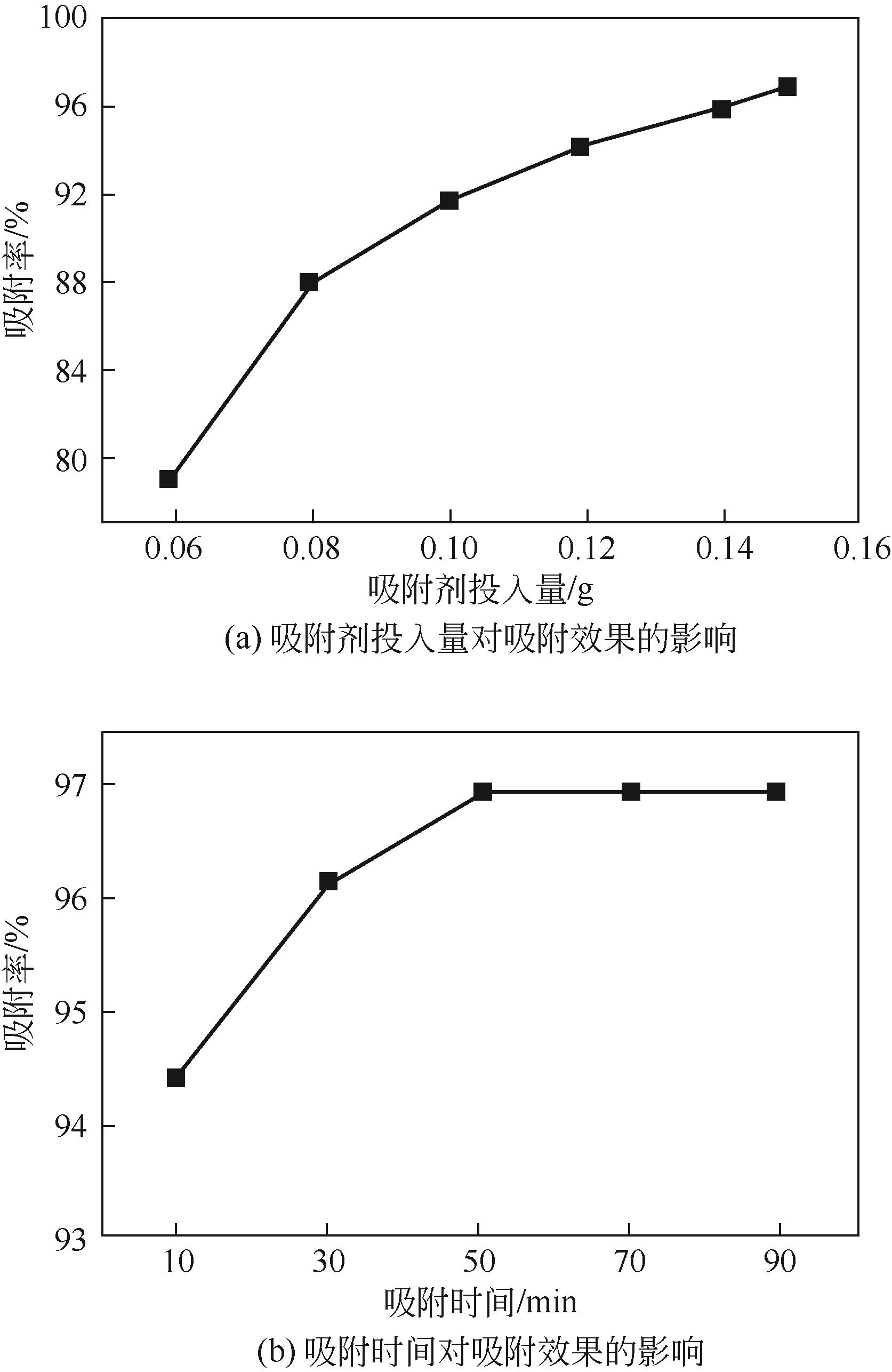
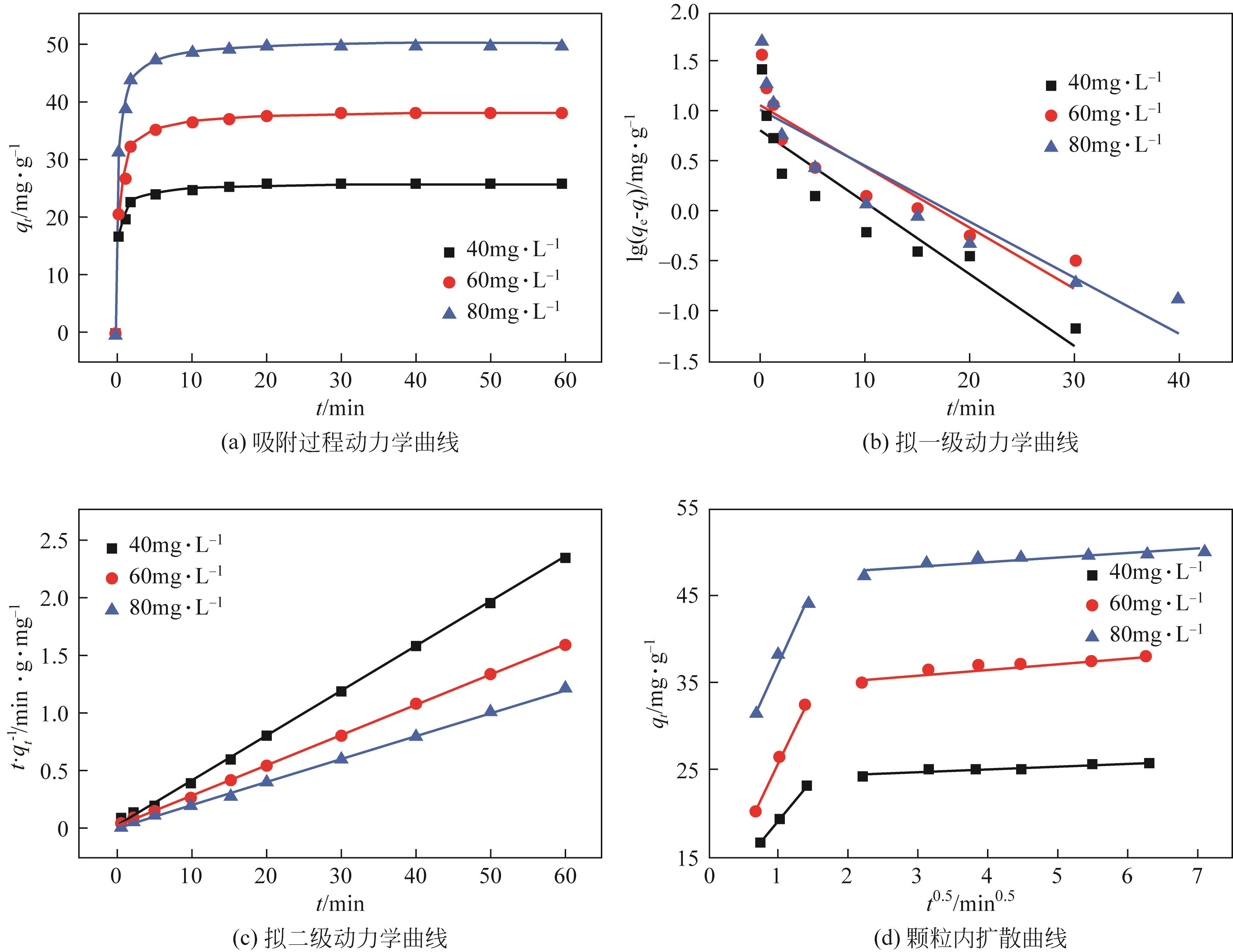
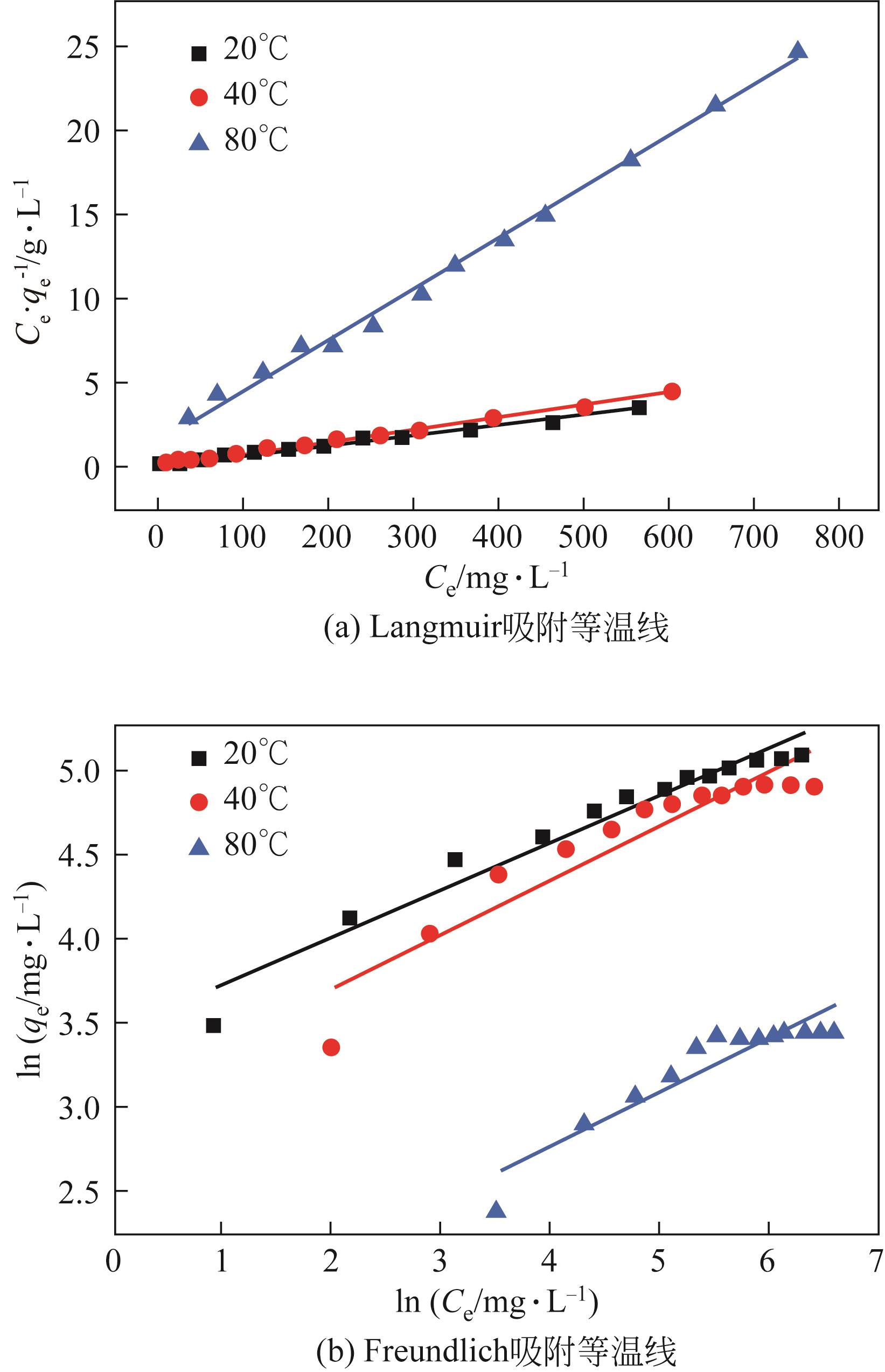
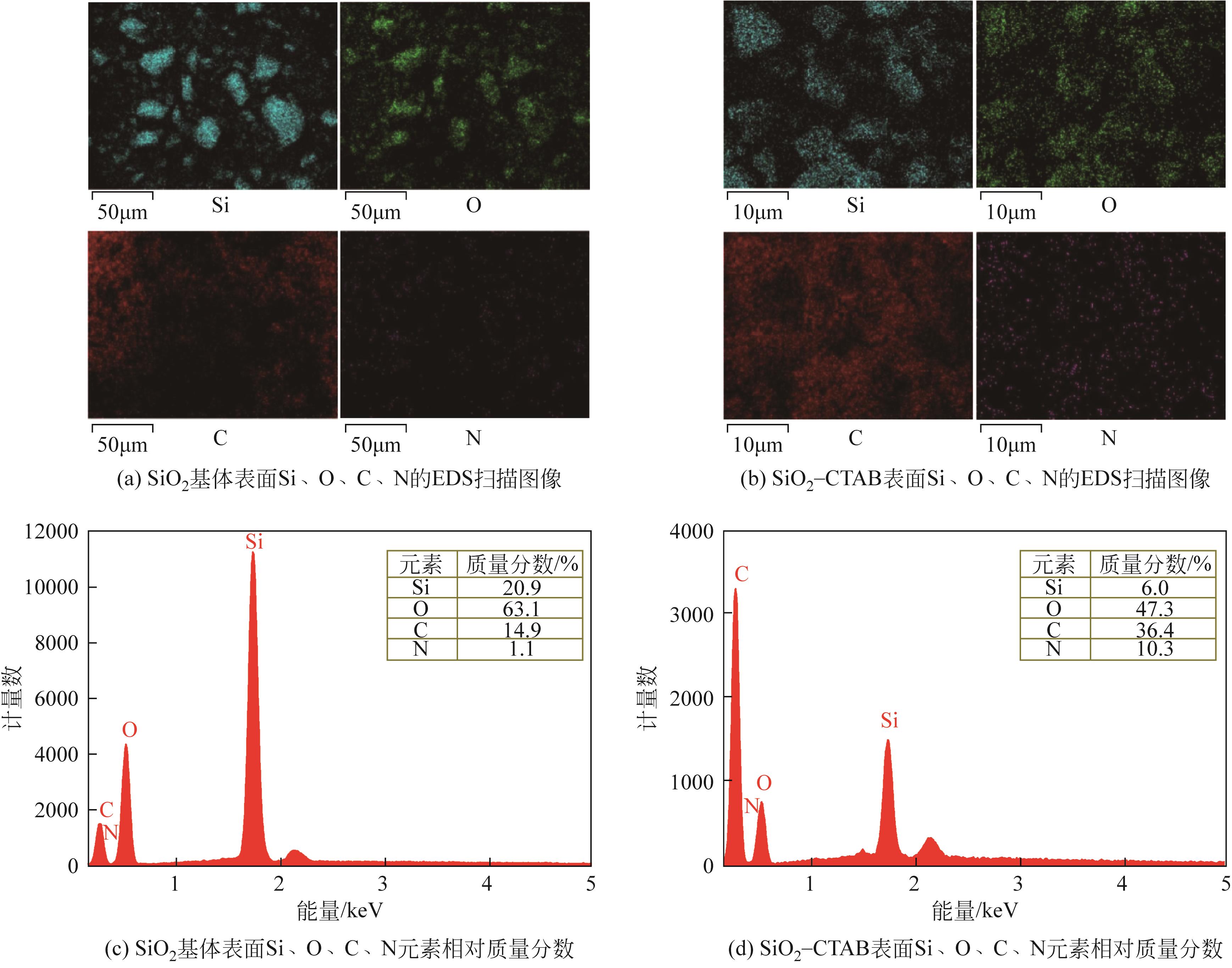
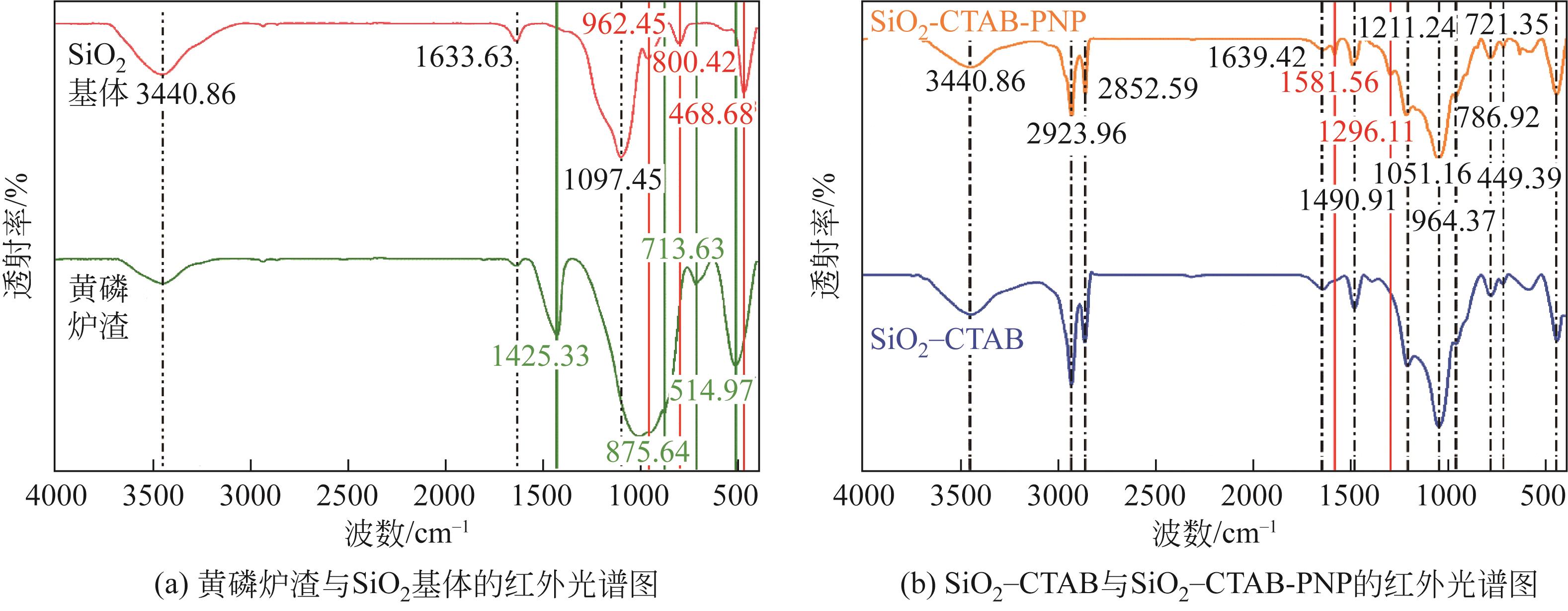
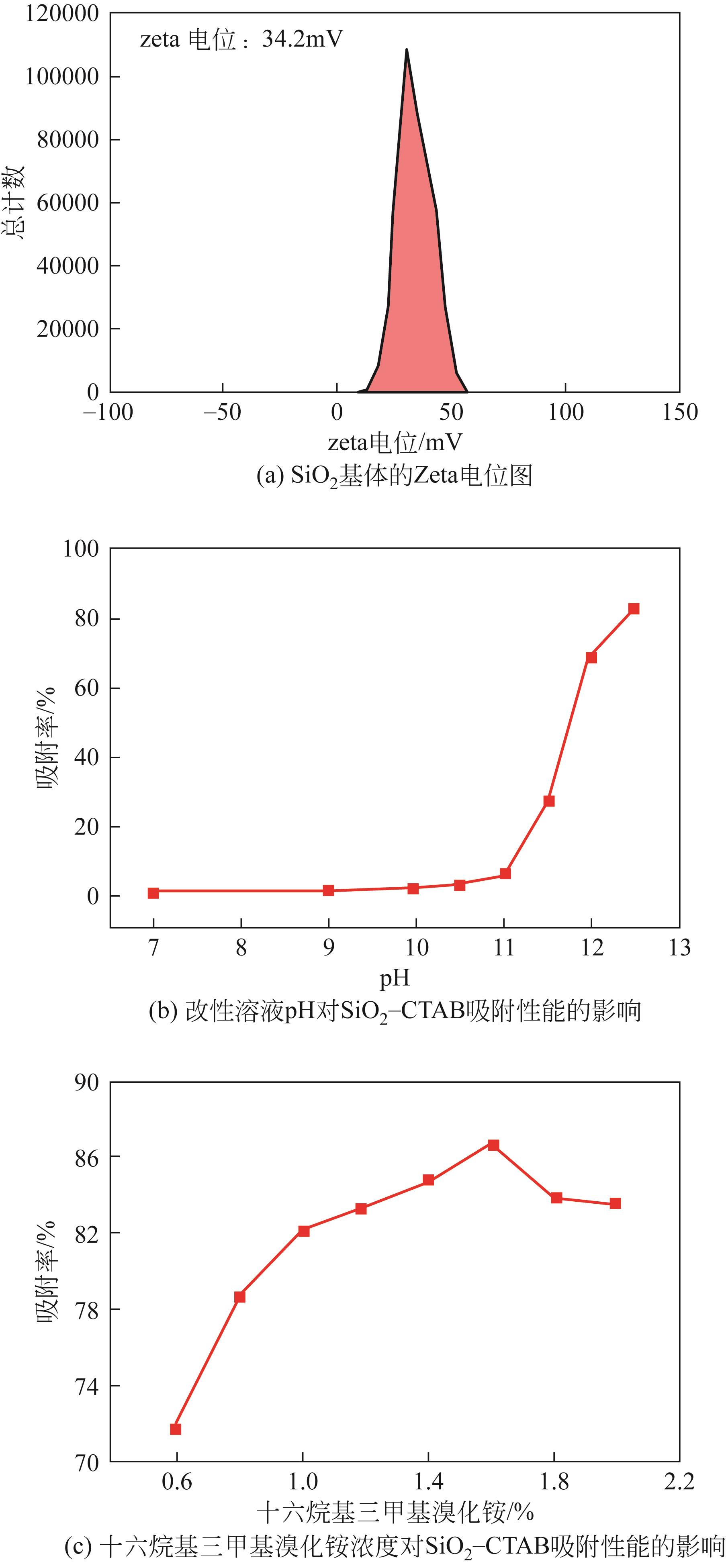
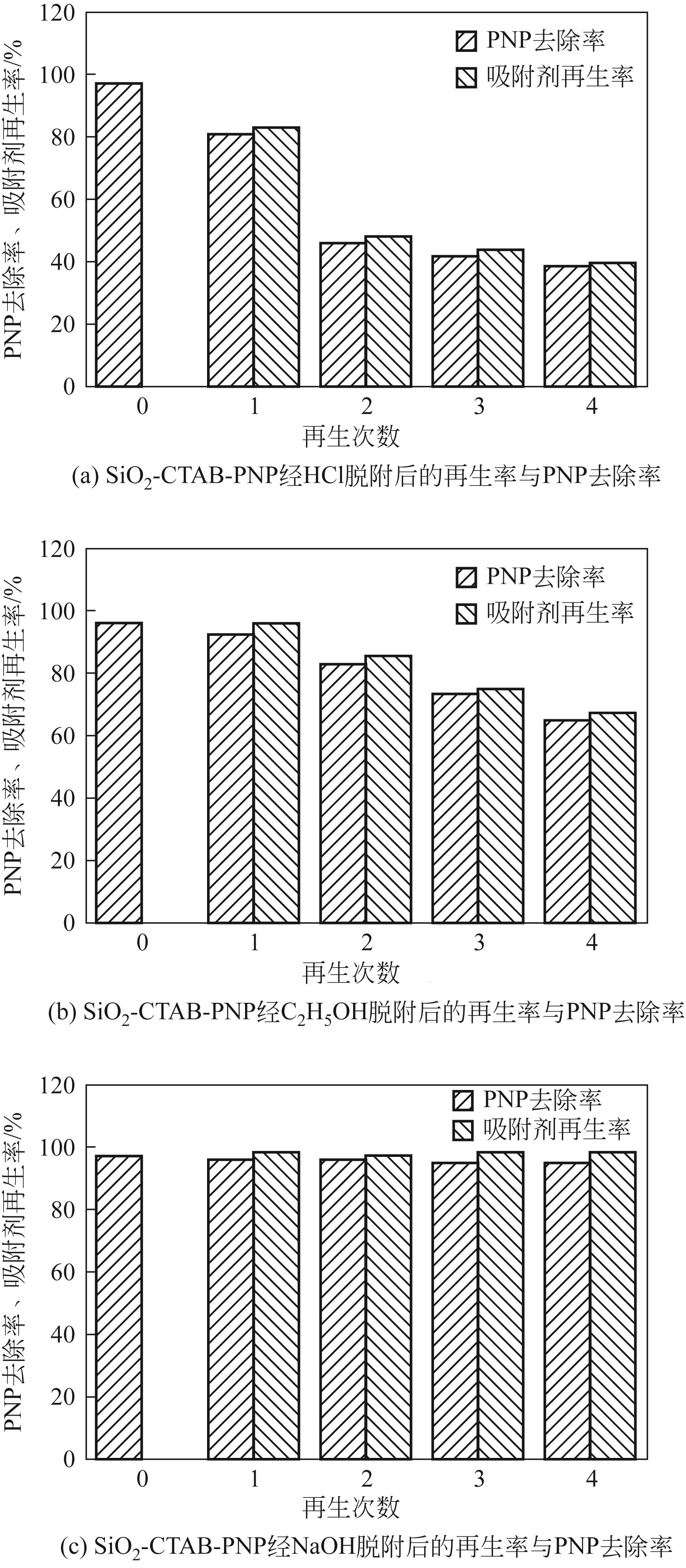
以黄磷炉渣为硅源,十六烷基三甲基溴化铵(CTAB)为负载剂,制备二氧化硅基复合吸附剂(SiO2-CTAB)并研究其对废水中对硝基苯酚(PNP)的吸附性能。考察吸附过程中吸附温度、溶液pH、吸附剂投入量、PNP初始浓度和吸附时间对吸附效果的影响;探究吸附过程动力学、热力学及吸附剂再生性能并采用扫描电子显微镜、能量色散光谱仪、X射线光电子能谱、傅里叶变换红外光谱仪等手段对相关物质进行表征。结果表明:在实验研究范围内,最佳吸附条件为温度20℃、pH=6、吸附剂投入量1.5g/L。该条件下,对PNP最大吸附容量为157.2mg/g;对50mg/L的PNP模拟废水去除率可达96.95%。该吸附剂对PNP的吸附过程符合拟二级动力学模型,Langmuir吸附等温线模型能很好地描述该吸附行为。
中图分类号:
引用本文
田月, 董晓涵, 苏毅. SiO2-CTAB复合材料的制备及其对PNP的吸附性能[J]. 化工进展, 2023, 42(11): 6064-6075.
TIAN Yue, DONG Xiaohan, SU Yi. Preparation of the SiO2-CTAB composite material and its adsorption properties for PNP[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6064-6075.
| 项目 | SiO2 | CaO | MgO | P2O5 | Al2O3 | Fe2O3 | 氟化物 |
|---|---|---|---|---|---|---|---|
| 黄磷炉渣 | 38.33 | 44.21 | 3.93 | 2.68 | 1.92 | 0.65 | 2.57 |
| SiO2基体 | 89.90 | 0.18 | 0.16 | 0.69 | 0.50 | 0.36 | 0.26 |
表1 黄磷炉渣与SiO2基体成分组成(质量分数,%)
| 项目 | SiO2 | CaO | MgO | P2O5 | Al2O3 | Fe2O3 | 氟化物 |
|---|---|---|---|---|---|---|---|
| 黄磷炉渣 | 38.33 | 44.21 | 3.93 | 2.68 | 1.92 | 0.65 | 2.57 |
| SiO2基体 | 89.90 | 0.18 | 0.16 | 0.69 | 0.50 | 0.36 | 0.26 |
| 模型 | C0/mg·L-1 | qe,exp/mg·g-1 | qe,cal/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|---|---|
| 拟一级动力学 | 40 | 25.53 | 6.496 | 7.144×10-2 | 0.8576 |
| 60 | 37.86 | 11.69 | 6.134×10-2 | 0.8338 | |
| 80 | 49.93 | 10.97 | 5.706×10-2 | 0.8453 |
表2 SiO2-CTAB吸附PNP的拟一级动力学拟合参数
| 模型 | C0/mg·L-1 | qe,exp/mg·g-1 | qe,cal/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|---|---|
| 拟一级动力学 | 40 | 25.53 | 6.496 | 7.144×10-2 | 0.8576 |
| 60 | 37.86 | 11.69 | 6.134×10-2 | 0.8338 | |
| 80 | 49.93 | 10.97 | 5.706×10-2 | 0.8453 |
| 模型 | C0/mg·L-1 | qe,exp/mg·g-1 | qe,cal/mg·g-1 | k2/g·mg-1·min-1 | R2 |
|---|---|---|---|---|---|
| 拟二级动力学 | 40 | 25.53 | 25.68 | 1.363×10-1 | 0.9999 |
| 60 | 37.86 | 38.17 | 5.872×10-2 | 0.9999 | |
| 80 | 49.93 | 50.20 | 6.505×10-2 | 0.9999 |
表3 SiO2-CTAB吸附PNP的拟二级动力学拟合参数
| 模型 | C0/mg·L-1 | qe,exp/mg·g-1 | qe,cal/mg·g-1 | k2/g·mg-1·min-1 | R2 |
|---|---|---|---|---|---|
| 拟二级动力学 | 40 | 25.53 | 25.68 | 1.363×10-1 | 0.9999 |
| 60 | 37.86 | 38.17 | 5.872×10-2 | 0.9999 | |
| 80 | 49.93 | 50.20 | 6.505×10-2 | 0.9999 |
| 模型 | C0/mg·L-1 | 阶段 | Kid/mg·g-1·min-0.5 | N/mg·g-1 | R2 |
|---|---|---|---|---|---|
| 颗粒内扩散 | 40 | 1 | 9.484 | 9.938 | 0.9810 |
| 2 | 2.984×10-1 | 23.80 | 0.8565 | ||
| 60 | 1 | 17.40 | 8.286 | 0.9901 | |
| 2 | 6.246×10-1 | 34.18 | 0.8976 | ||
| 80 | 1 | 18.07 | 19.10 | 0.9715 | |
| 2 | 4.974×10-1 | 46.80 | 0.7797 |
表4 SiO2-CTAB吸附PNP的颗粒内扩散拟合参数
| 模型 | C0/mg·L-1 | 阶段 | Kid/mg·g-1·min-0.5 | N/mg·g-1 | R2 |
|---|---|---|---|---|---|
| 颗粒内扩散 | 40 | 1 | 9.484 | 9.938 | 0.9810 |
| 2 | 2.984×10-1 | 23.80 | 0.8565 | ||
| 60 | 1 | 17.40 | 8.286 | 0.9901 | |
| 2 | 6.246×10-1 | 34.18 | 0.8976 | ||
| 80 | 1 | 18.07 | 19.10 | 0.9715 | |
| 2 | 4.974×10-1 | 46.80 | 0.7797 |
| T/℃ | 拟合方程 | qm/mg·g-1 | KL/L·mg-1 | RL | R2 |
|---|---|---|---|---|---|
| 20 | y=0.0061x+0.1717 | 164.0 | 0.0355 | 0.0340~0.3602 | 0.9967 |
| 40 | y=0.0071x+0.2158 | 140.9 | 0.0329 | 0.0366~0.3781 | 0.9997 |
| 80 | y=0.0298x+1.6559 | 33.56 | 0.0180 | 0.0649~0.5264 | 0.9961 |
表5 SiO2-CTAB吸附PNP的Langmuir相关拟合参数
| T/℃ | 拟合方程 | qm/mg·g-1 | KL/L·mg-1 | RL | R2 |
|---|---|---|---|---|---|
| 20 | y=0.0061x+0.1717 | 164.0 | 0.0355 | 0.0340~0.3602 | 0.9967 |
| 40 | y=0.0071x+0.2158 | 140.9 | 0.0329 | 0.0366~0.3781 | 0.9997 |
| 80 | y=0.0298x+1.6559 | 33.56 | 0.0180 | 0.0649~0.5264 | 0.9961 |
| T/℃ | 拟合方程 | KF | 1/a | R2 |
|---|---|---|---|---|
| 20 | y=0.2792x+3.4273 | 30.80 | 0.2792 | 0.9520 |
| 40 | y=0.3198x+3.0461 | 21.03 | 0.3198 | 0.8846 |
| 80 | y=0.3176x+1.4819 | 4.401 | 0.3176 | 0.8535 |
表6 SiO2-CTAB吸附PNP的Freundlich相关拟合参数
| T/℃ | 拟合方程 | KF | 1/a | R2 |
|---|---|---|---|---|
| 20 | y=0.2792x+3.4273 | 30.80 | 0.2792 | 0.9520 |
| 40 | y=0.3198x+3.0461 | 21.03 | 0.3198 | 0.8846 |
| 80 | y=0.3176x+1.4819 | 4.401 | 0.3176 | 0.8535 |
| T/K | KT | ΔGθ/J·mol-1 | ΔHθ/J·mol-1 | ΔSθ/J·mol-1·K-1 |
|---|---|---|---|---|
| 293 | 4.942×103 | -20.72×103 | -10.16×103 | 36.63 |
| 313 | 4.577×103 | -21.93×103 | ||
| 353 | 2.503×103 | -22.97×103 |
表7 SiO2-CTAB吸附PNP的热力学参数
| T/K | KT | ΔGθ/J·mol-1 | ΔHθ/J·mol-1 | ΔSθ/J·mol-1·K-1 |
|---|---|---|---|---|
| 293 | 4.942×103 | -20.72×103 | -10.16×103 | 36.63 |
| 313 | 4.577×103 | -21.93×103 | ||
| 353 | 2.503×103 | -22.97×103 |
| 样品 | 比表面积/m2·g-1 | Vt/cm3·g-1 | Da/nm |
|---|---|---|---|
| SiO2基体 | 519.2 | 0.7603 | 5.051 |
| 黄磷炉渣 | 1.307 | 6.683×10-3 | 34.35 |
表8 SiO2基体与黄磷炉渣表面特性
| 样品 | 比表面积/m2·g-1 | Vt/cm3·g-1 | Da/nm |
|---|---|---|---|
| SiO2基体 | 519.2 | 0.7603 | 5.051 |
| 黄磷炉渣 | 1.307 | 6.683×10-3 | 34.35 |
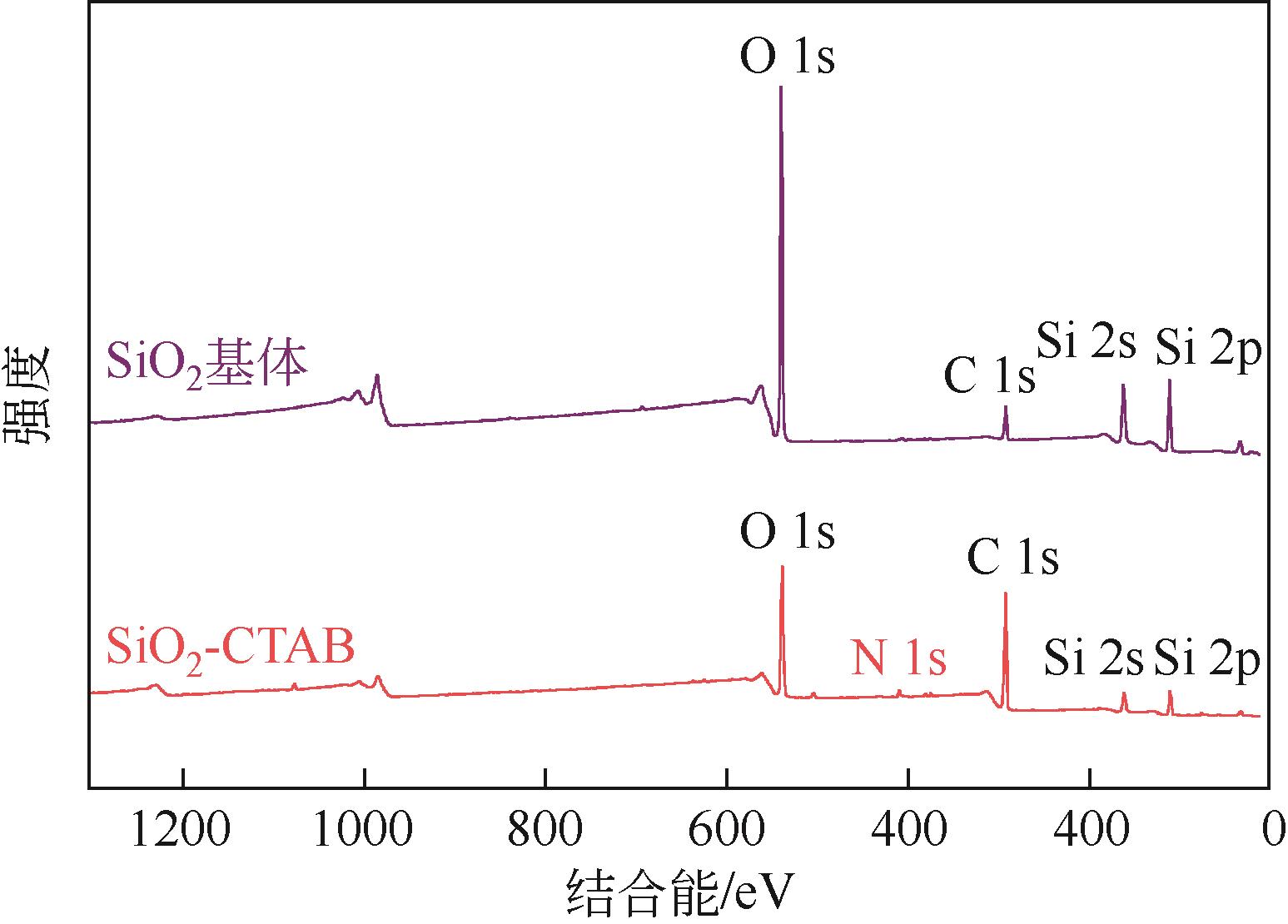
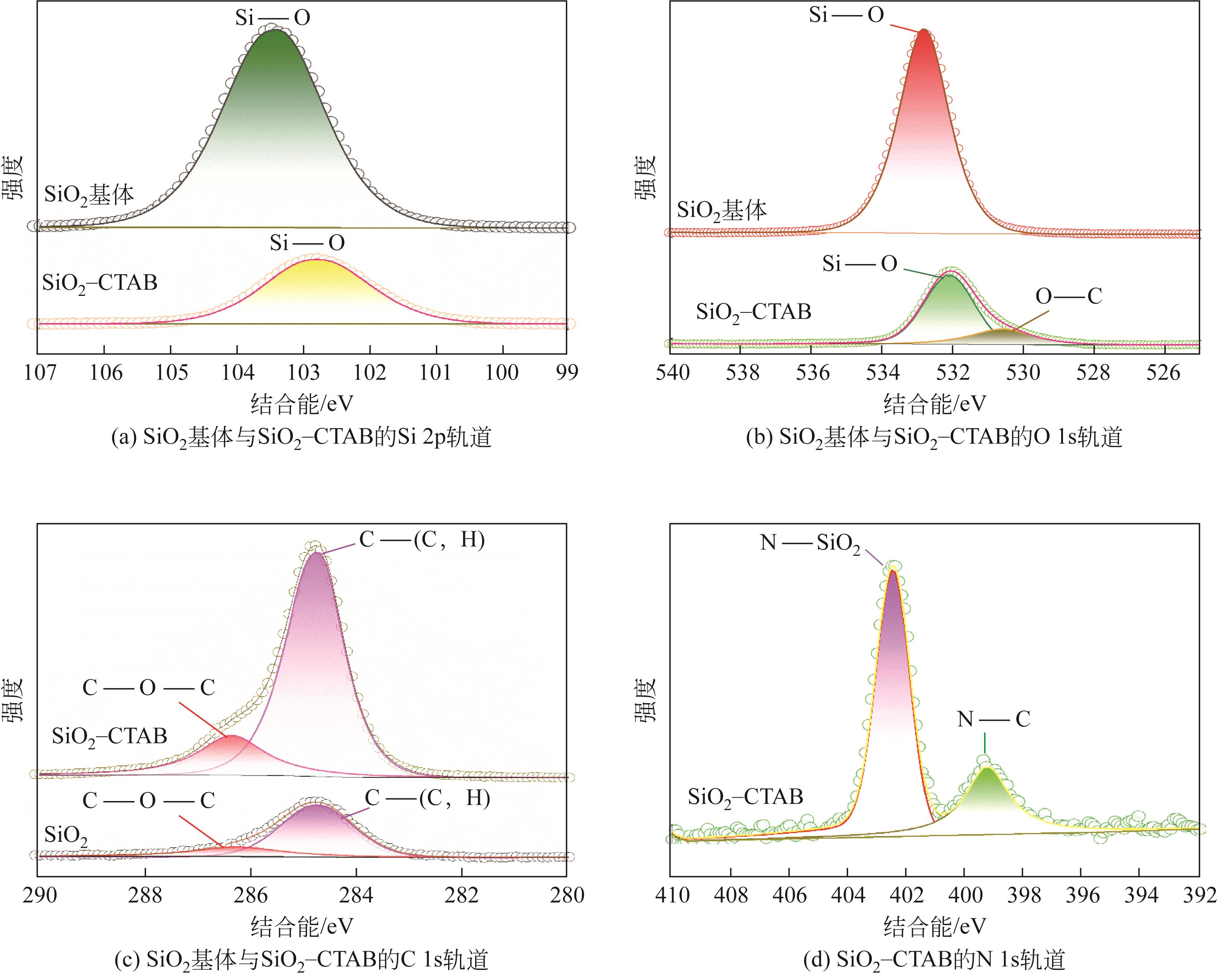
| 项目 | C 1s | Si 2p | O 1s | |
|---|---|---|---|---|
| C—(C,H) | C—O—C | Si—O | O—Si | |
| 结合能/eV | 284.74 | 286.26 | 103.39 | 532.84 |
| 质量分数/% | 10.39 | 3.20 | 28.41 | 58.00 |
表9 SiO2基体所含化学键结合能及相对质量分数
| 项目 | C 1s | Si 2p | O 1s | |
|---|---|---|---|---|
| C—(C,H) | C—O—C | Si—O | O—Si | |
| 结合能/eV | 284.74 | 286.26 | 103.39 | 532.84 |
| 质量分数/% | 10.39 | 3.20 | 28.41 | 58.00 |
| 项目 | C 1s | Si 2p | O 1s | N 1s | |||
|---|---|---|---|---|---|---|---|
| C—(C,H) | C—O—C | Si—O | O—Si | O—C | N—SiO2 | N—C | |
| 结合能/eV | 284.74 | 286.36 | 102.76 | 532.11 | 530.59 | 402.46 | 399.24 |
| 质量分数/% | 45.40 | 11.10 | 11.44 | 22.21 | 6.68 | 2.21 | 0.95 |
表10 SiO2-CTAB所含化学键结合能及相对质量分数
| 项目 | C 1s | Si 2p | O 1s | N 1s | |||
|---|---|---|---|---|---|---|---|
| C—(C,H) | C—O—C | Si—O | O—Si | O—C | N—SiO2 | N—C | |
| 结合能/eV | 284.74 | 286.36 | 102.76 | 532.11 | 530.59 | 402.46 | 399.24 |
| 质量分数/% | 45.40 | 11.10 | 11.44 | 22.21 | 6.68 | 2.21 | 0.95 |
| 1 | DIMITROVSKA Olgica, MARKOSKI Blagoja, TOSHEVSKA Biljana Apostolovska, et al. Surface water pollution of major rivers in the republic of macedonia[J]. Procedia Environmental Sciences, 2012, 14: 32-40. |
| 2 | ZHENG Zexiao, Irene MC LO. Multifunctional photoelectrochemical systems for coupled water treatment and high-value product generation: Current status, mechanisms, remaining challenges, and future opportunities[J]. Current Opinion in Chemical Engineering, 2021, 34: 100711. |
| 3 | PENG Xiaohui, WANG Ya, LUO Zhen, et al. Facile synthesis of fluorescent sulfur quantum dots for selective detection of p-nitrophenol in water samples[J]. Microchemical Journal, 2021, 170: 106735. |
| 4 | GERENT Giles G, SPINELLI Almir. Magnetite-platinum nanoparticles-modified glassy carbon electrode as electrochemical detector for nitrophenol isomers[J]. Journal of Hazardous Materials, 2017, 330: 105-115. |
| 5 | Ganga Ram Chaudhary, Singh Prabjot, Kaur Gurpreet, et al. Multifaceted approach for the fabrication of metallomicelles and metallic nanoparticles using solvophobic bisdodecylaminepalladium (Ⅱ) chloride as precursor[J]. Inorganic Chemistry, 2015, 54(18): 9002-9012. |
| 6 | NIU Baitong, LI Xinlou, LIN Da, et al. Highly efficient noble metal-free g-C3N4@Ni x Sy nanocomposites for catalytic reduction of nitrophenol, azo dyes and Cr( Ⅵ )[J]. Inorganic Chemistry Communications, 2022, 142: 109589. |
| 7 | YAN Kunyun, CHEN Jiayi, LI Xinyu, et al. Carboxylic acid enriched porous organic polymer as a platform for highly efficient removal of methylene blue from aqueous solution[J]. Macromolecular Chemistry and Physics, 2020, 221(5): 1900553. |
| 8 | 夏丞垚, 陈琼珍, 沈文静, 等. 对硝基苯酚的生物降解研究进展[J]. 生物加工过程, 2021, 19(4): 387-395. |
| XIA Chengyao, CHEN Qiongzhen, SHEN Wenjing, et al. Advancement inmicrobial degradation of para-nitrophenol[J]. Chinese Journal of Bioprocess Engineering, 2021, 19(4): 387-395. | |
| 9 | YANG Xiupei, WANG Dan, LUO Na, et al. Green synthesis of fluorescent N, S-carbon dots from bamboo leaf and the interaction with nitrophenol compounds[J]. Spectrochimica Acta A, Molecular and Biomolecular Spectroscopy, 2020, 239: 118462. |
| 10 | ZHU Guodong, TANG Qian, HUANG Manhong, et al. Polyaniline nanoconical array on carbon nanofiber for supersensitive determination of nitrophenol[J]. Sensors and Actuators B: Chemical, 2020, 320: 128593. |
| 11 | HAO Xiaoyan, DIAO Xiaogao, YU Shengchen, et al. Nutrient digestibility, rumen microbial protein synthesis, and growth performance in sheep consuming rations containing sea buckthorn pomace[J]. Journal of Animal Science, 2018, 96(8): 3412-3419. |
| 12 | KAVLOCK R J, DASTON G P, DEROSA C, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop[J]. Environmental Health Perspectives, 1996, 104(): 715-740. |
| 13 | KHATAMIAN M, KHANDAR A A, DIVBAND B, et al. Heterogeneous photocatalytic degradation of 4-nitrophenol in aqueous suspension by Ln (La3+, Nd3+ or Sm3+) doped ZnO nanoparticles[J]. Journal of Molecular Catalysis A: Chemical, 2012, 365: 120-127. |
| 14 | CLARK James H, FARMER Thomas J, Lorenzo HERRERO-DAVILA, et al. Circular economy design considerations for research and process development in the chemical sciences[J]. Green Chemistry, 2016, 18(14): 3914-3934. |
| 15 | SAMSAMI Shakiba, MOHAMADIZANIANI Maryam, SARRAFZADEH Mohammad-Hossein, et al. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives[J]. Process Safety and Environmental Protection, 2020, 143: 138-163. |
| 16 | WANG Xiaohong, JIANG Chenglong, HOU Bingxia, et al. Carbon composite lignin-based adsorbents for the adsorption of dyes[J]. Chemosphere, 2018, 206: 587-596. |
| 17 | ZHANG Ting, ZHOU Fa, HUANG Jianhan, et al. Ethylene glycol dimethacrylate modified hyper-cross-linked resins: Porogen effect on pore structure and adsorption performance[J]. Chemical Engineering Journal, 2018, 339: 278-287. |
| 18 | CHEN Jie, SHENG Ye, SONG Yanhua, et al. Multimorphology mesoporous silica nanoparticles for dye adsorption and multicolor luminescence applications[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3533-3545. |
| 19 | 田月, 董晓涵, 蒋宇, 等. 改性吸附法去除废水中对硝基苯酚研究进展[J]. 化工新型材料, 2022, 50(10): 76-80. |
| TIAN Yue, DONG Xiaohan, JIANG Yu, et al. Research progress in the removal of PNP from wastewater by modified adsorption[J]. New Chemical Materials, 2022, 50(10): 76-80. | |
| 20 | Jung-Yeol JO, CHOI Jeong-Hwan, TSANG Yiu Fai, et al. Pelletized adsorbent of alum sludge and bentonite for removal of arsenic[J]. Environmental Pollution, 2021, 277: 116747. |
| 21 | Petra ZAHAJSKÁ, OPFERGELT Sophie, FRITZ Sherilyn C, et al. What is diatomite?[J]. Quaternary Research, 2020, 96: 48-52. |
| 22 | DAOU Alan S S, FINDLEY John M, FANG Hanjun, et al. Quantifying impact of intrinsic flexibility on molecular adsorption in zeolites[J]. The Journal of Physical Chemistry C, 2021, 125(9): 5296-5305. |
| 23 | Facundo BARRAQUÉ, MONTES María L, FERNÁNDEZ Mariela A, et al. Arsenate removal from aqueous solution by montmorillonite and organo-montmorillonite magnetic materials[J]. Environmental Research, 2021, 192: 110247. |
| 24 | 韩乐. 黄磷炉渣制备SiO2基复合吸附剂及其铜吸附性能研究[D]. 昆明: 昆明理工大学, 2021. |
| HAN Le|Yue). Preparation of SiO2-based composite adsorbent from yellow phosphorus slag and its copper adsorption performance[D]. Kunming: Kunming University of Science and Technology, 2021. | |
| 25 | 郭俊元, 王彬. HDTMA改性沸石的制备及吸附废水中对硝基苯酚的性能和动力学[J]. 环境科学, 2016, 37(5): 1852-1857. |
| GUO Junyuan, WANG Bin. Preparation of HDTMA-modified zeolite and its performance in nitro-phenol adsorption from wastewaters[J]. Environmental Science, 2016, 37(5): 1852-1857. | |
| 26 | 蒋智慧. 固体废弃物热解制备吸附剂的实验研究[D]. 北京: 北京化工大学, 2020. |
| JIANG Zhihui. Study on preparation of adsorbent by pyrolysis of solid waste[D]. Beijing: Beijing University of Chemical Technology, 2020. | |
| 27 | 那立艳, 张丽影, 张凤杰, 等. 固液界面吸附热力学参数的计算[J]. 材料导报, 2020, 34(22): 22030-22035. |
| NA Liyan, ZHANG Liying, ZHANG Fengjie, et al. Calculation of adsorption thermodynamic parameters at solid-liquid interfaces[J]. Materials Reports, 2020, 34(22): 22030-22035. |
| [1] | 王尚彬, 欧红香, 薛洪来, 曹海珍, 王钧奇, 毕海普. 黄原胶和纳米二氧化硅对无氟泡沫性能的影响[J]. 化工进展, 2023, 42(9): 4856-4862. |
| [2] | 杨程瑞雪, 黄琪媛, 冉建速, 崔耘通, 王健健. 磷酸修饰二氧化硅负载钯催化剂用于木质素衍生物高效水相低温加氢脱氧[J]. 化工进展, 2023, 42(10): 5179-5190. |
| [3] | 潘月磊, 程旭东, 闫明远, 何盼, 张和平. 二氧化硅气凝胶及其在保温隔热领域应用进展[J]. 化工进展, 2023, 42(1): 297-309. |
| [4] | 郭振雪, 于海斌, 张国辉, 张景成, 卢雁飞, 何艳贞, 孙彦民, 韩恩山. Si改性对NiMo/Al2O3催化剂加氢脱硫性能的影响[J]. 化工进展, 2022, 41(S1): 210-220. |
| [5] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [6] | 单清雯, 张娟, 王亚娟, 刘文强. 聚合离子液体的合成及其吸附脱硫性能[J]. 化工进展, 2022, 41(8): 4571-4579. |
| [7] | 王光绪, 金晶, 张云鹏, 刘薄鉴治, 梁诗雨, 翟中媛. 硅钙摩尔比对准东煤燃烧过程中矿物演变及灰熔融特性的影响[J]. 化工进展, 2022, 41(8): 4140-4146. |
| [8] | 池成龙, 贾爱忠, 孙道来, 赵新强, 王延吉. 表面离子印迹聚合物金属离子吸附材料研究进展[J]. 化工进展, 2022, 41(7): 3758-3769. |
| [9] | 孙德贇, 胡艳宏, 刘鹏, 唐茂, 胡泽, 柳召刚, 吴锦绣. 不同铈盐体系(硝酸盐、硫酸盐、氯化盐)中CTAB与Ce3+的相互作用机理[J]. 化工进展, 2022, 41(6): 3212-3220. |
| [10] | 武晨浩, 李昆锋, 李肖华, 费志方, 张震, 杨自春. 二氧化硅气凝胶常压干燥工艺的研究进展[J]. 化工进展, 2022, 41(2): 837-847. |
| [11] | 章雪莹, 马俊, 何林, 隋红, 李鑫钢. 沥青岩中界面活性沥青质分子结构及其在矿物表面吸附特征[J]. 化工进展, 2022, 41(2): 628-636. |
| [12] | 李群艳, 孙路瑶, 常其飞, 周运炉. 磁性Fe3O4@SiO2@介孔SiO2空心微球的制备及漆酶固定化[J]. 化工进展, 2022, 41(10): 5494-5500. |
| [13] | 付欣, 张玉苍, 李瑞松, 刘群, 郭佳益. 气溶胶辅助自组装制备中空球形二氧化硅材料的机理及应用[J]. 化工进展, 2022, 41(1): 327-335. |
| [14] | 王亮才, 陈宇, 赵曼淇, 吴杰龙, 王哲, 马欢欢, 周建斌. 稻壳基SiO2的提取及其含量对铜基摩擦材料性能的影响[J]. 化工进展, 2021, 40(8): 4397-4405. |
| [15] | 闫文杰, 熊源泉, 杨思源, 何珊珊. 稻壳白炭黑负载Fe2O3的气相芬顿反应NO预氧化[J]. 化工进展, 2021, 40(7): 4027-4035. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||