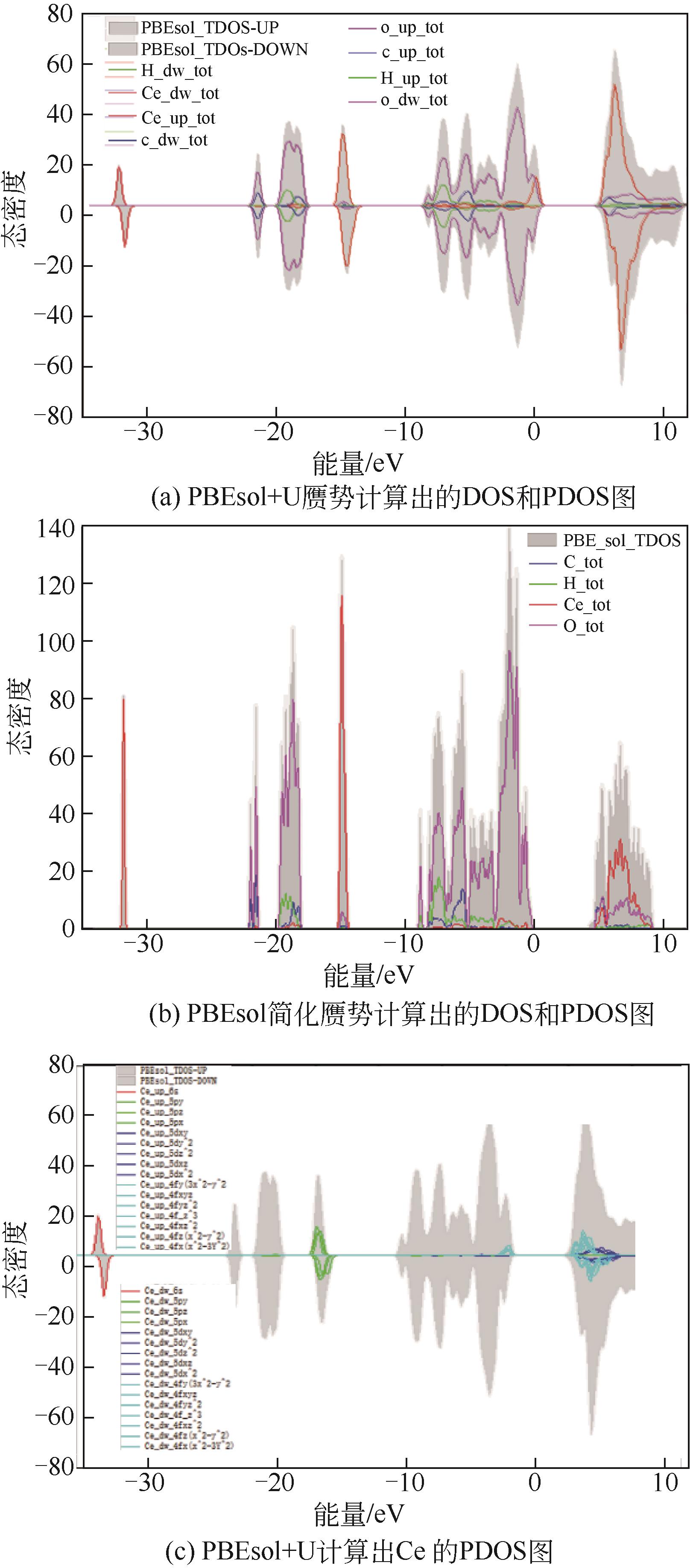
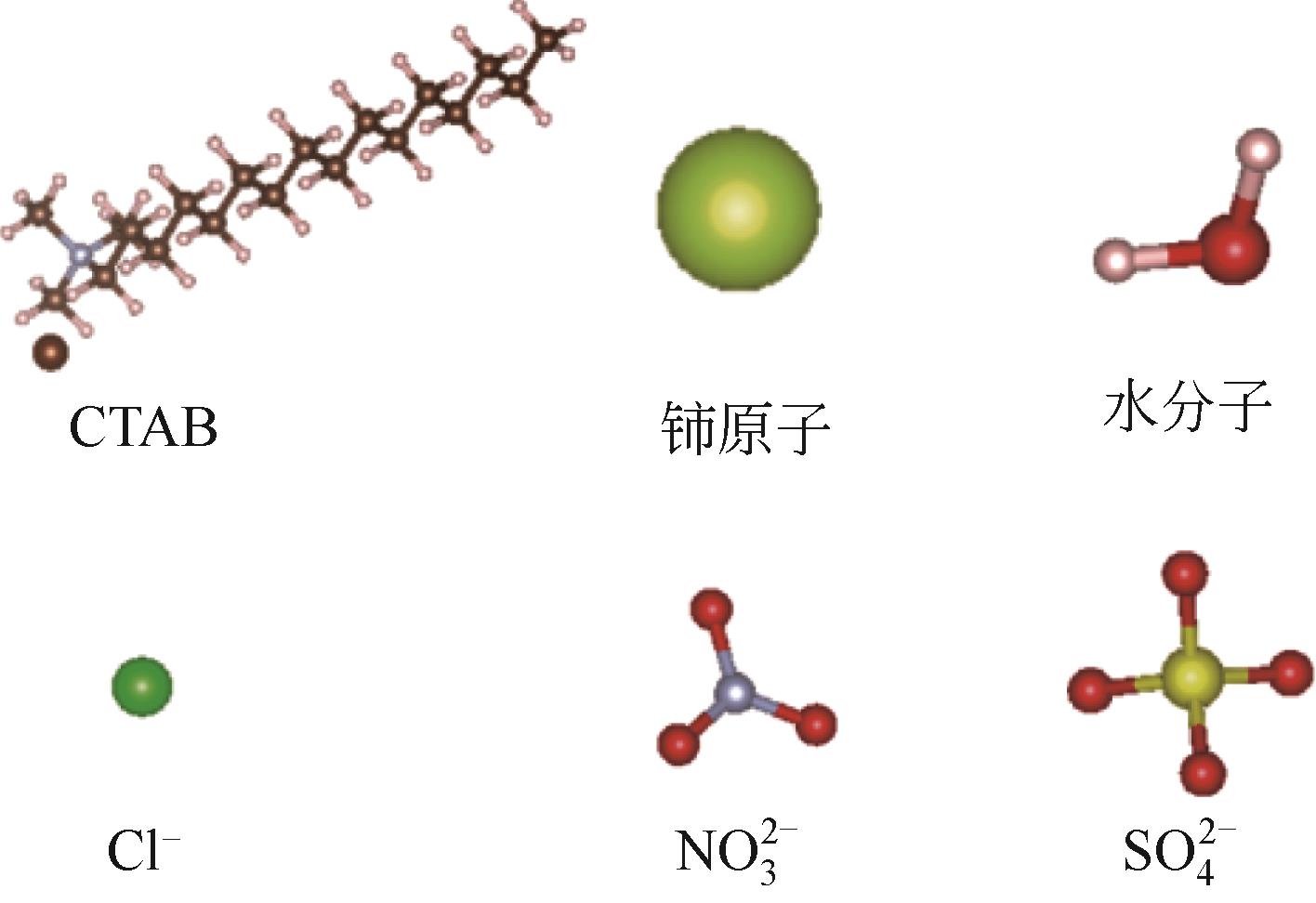
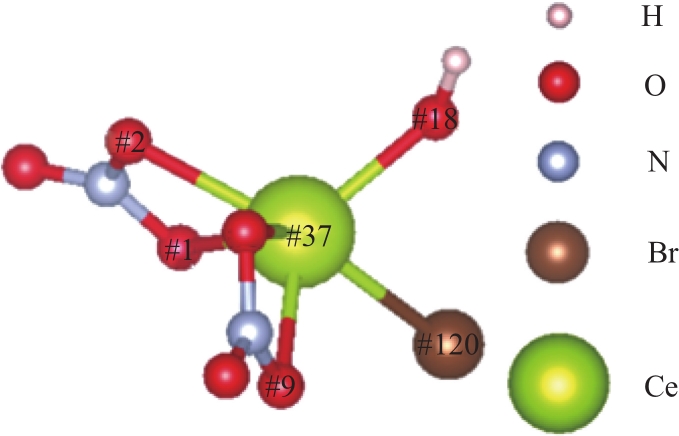
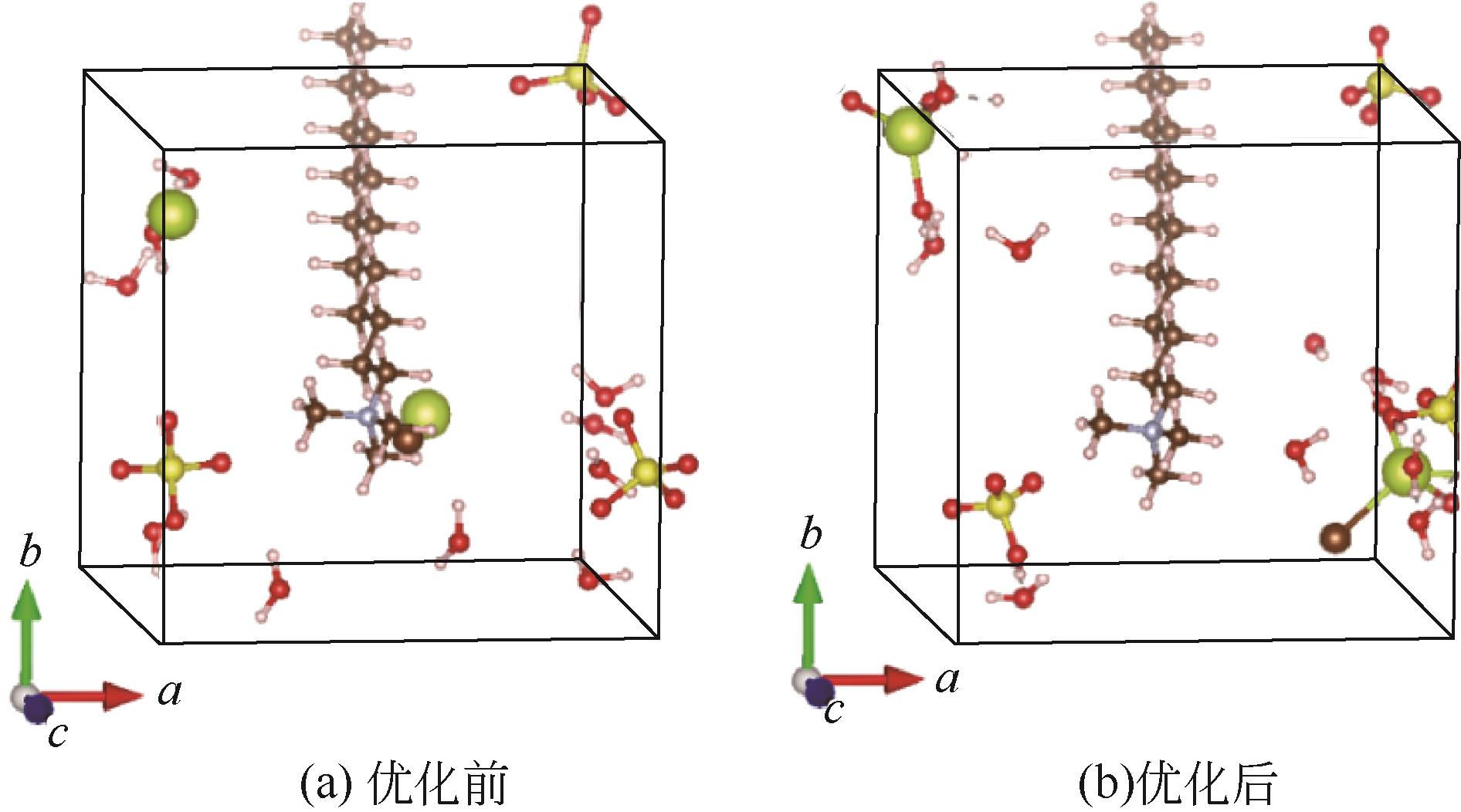
| 1 |
高天佐, 于晓丽, 张玉玺, 等. 不同形貌二氧化铈的制备及其紫外屏蔽性能研究[J]. 湿法冶金, 2018, 37(6): 497-500.
|
|
GAO Tianzuo, YU Xiaoli, ZHANG Yuxi, et al. Preparation and UV-shielding properties of ceria with different morphologies[J]. Hydrometallurgy of China, 2018, 37(6): 497-500.
|
| 2 |
王竹梅, 朱晓玲, 李月明, 等. 片状和球状纳米CeO2的可控制备及其光催化性能[J]. 人工晶体学报, 2017, 46(8): 1559-1563, 1586.
|
|
WANG Zhumei, ZHU Xiaoling, LI Yueming, et al. Controllable preparation and photocatalytic properties of nano-CeO2 sphere and sheet[J]. Journal of Synthetic Crystals, 2017, 46(8): 1559-1563, 1586.
|
| 3 |
冯雅楠, 甘俊珍, 陈星晖, 等. 形貌和粒度对纳米二氧化铈光催化降解盐基品红的研究[J]. 应用化工, 2019, 48(1): 14-17.
|
|
FENG Yanan, GAN Junzhen, CHEN Xinghui, et al. Study of morphology and particle size of nano-CeO2 on basic fuchsine photocatalytic degradation[J]. Applied Chemical Industry, 2019, 48(1): 14-17.
|
| 4 |
黄绍东, 杨国胜, 张存瑞, 等. 稀土抛光粉及其制备方法: CN112080207A[P]. 2020-12-15.
|
|
HUANG Shaodong, YANG Guosheng, ZHANG Cunrui, et al. Rare earth polishing powder and its preparation method: CN112080207A[P]. 2020-12-15.
|
| 5 |
梁恩武, 王滨, 张双双, 等. 稀土抛光粉的制备工艺及显色机理研究[J]. 稀土, 2020, 41(4): 102-110.
|
|
LIANG Enwu, WANG Bin, ZHANG Shuangshuang, et al. Preparation and color development mechanism of rare earth polishing powder[J]. Chinese Rare Earths, 2020, 41(4): 102-110.
|
| 6 |
温宁, 伊元夫, 张薇薇, 等. 氧化铈与氧化铒对玻璃渗透氧化铝陶瓷颜色的影响[C]//第十六届全国高技术陶瓷学术年会. 江西景德镇, 2010.
|
|
WEN Ning, YI Yuanfu, ZHANG Weiwei, et al. Cerium oxide on the color of glass-infiltrated alumina ceramics[C]//16th National High-tech Ceramics Conference. Jingdezhen, Jiangxi, 2010.
|
| 7 |
谷广锋, 曹兴涛, 刘铭辉, 等. Pd-M/CeO2催化剂对甲苯催化性能研究[J]. 环境科学与技术, 2020, 43(11): 110-115.
|
|
GU Guangfeng, CAO Xingtao, LIU Minghui, et al. Catalytic performance of Pd-M/CeO2 for toluene combustion[J]. Environmental Science & Technology, 2020, 43(11): 110-115.
|
| 8 |
YANG Z X, LU Z S, LUO G X, et al. Oxygen vacancy formation energy at the Pd/CeO2(111) interface[J]. Physics Letters A, 2007, 369(1/2): 132-139.
|
| 9 |
MAI H X, SUN L D, ZHANG Y W, et al. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes[J]. The Journal of Physical Chemistry B, 2005, 109(51): 24380-24385.
|
| 10 |
YANG Z J, YANG Y Z, LIANG H, et al. Hydrothermal synthesis of monodisperse CeO2 nanocubes[J]. Materials Letters, 2009, 63(21): 1774-1777.
|
| 11 |
李钿. 碳酸铈反应结晶过程及晶体形貌调控研究[D]. 广州: 华南理工大学, 2020.
|
|
LI Dian. Research on the reactive crystallization process and morphology control of cerium carbonate[D]. Guangzhou: South China University of Technology, 2020.
|
| 12 |
DRONSKOWSKI R, BLOECHL P E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations[J]. The Journal of Physical Chemistry, 1993, 97(33): 8617-8624.
|
| 13 |
DERINGER V L, TCHOUGRÉEFF A L, DRONSKOWSKI R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets[J]. The Journal of Physical Chemistry A, 2011, 115(21): 5461-5466.
|
| 14 |
KRESSE G, HAFNER J. Ab initio molecular dynamics for liquid metals[J]. Physical Review B: Condensed Matter, 1993, 47(1): 558-561.
|
| 15 |
KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50.
|
| 16 |
KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758-1775.
|
| 17 |
MAINTZ S, DERINGER V L, TCHOUGRÉEFF A L, et al. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids[J]. Journal of Computational Chemistry, 2013, 34(29): 2557-2567.
|
| 18 |
MAINTZ S, DERINGER V L, TCHOUGRÉEFF A L, et al. LOBSTER: a tool to extract chemical bonding from plane-wave based DFT[J]. Journal of Computational Chemistry, 2016, 37(11): 1030-1035.
|
| 19 |
WANG G J, ZHOU M F, GOETTEL J T, et al. Identification of an iridium-containing compound with a formal oxidation state of Ⅸ[J]. Nature, 2014, 514(7523): 475-477.
|
 ), 胡艳宏1,2,3(
), 胡艳宏1,2,3( ), 刘鹏1,2,3, 唐茂1,2,3, 胡泽1,2,3, 柳召刚1,2,3, 吴锦绣1,2,3
), 刘鹏1,2,3, 唐茂1,2,3, 胡泽1,2,3, 柳召刚1,2,3, 吴锦绣1,2,3
 ), HU Yanhong1,2,3(
), HU Yanhong1,2,3( ), LIU Peng1,2,3, TANG Mao1,2,3, HU Ze1,2,3, LIU Zhaogang1,2,3, WU Jinxiu1,2,3
), LIU Peng1,2,3, TANG Mao1,2,3, HU Ze1,2,3, LIU Zhaogang1,2,3, WU Jinxiu1,2,3