化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3336-3344.DOI: 10.16085/j.issn.1000-6613.2024-0662
• 化工过程与装备 • 上一篇
氢原子转移反应活化能垒预测研究进展
李想( ), 李佳莹, 倪恒, 孙浩然, 曹家伟, 陈宇轩, 刘凤娇(
), 李佳莹, 倪恒, 孙浩然, 曹家伟, 陈宇轩, 刘凤娇( )
)
- 上海工程技术大学化学化工学院,上海 201620
-
收稿日期:2024-04-19修回日期:2024-06-22出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:刘凤娇 -
作者简介:李想(2000—),男,硕士研究生,研究方向为计算化学。E-mail:sdnylx129@163.com。 -
基金资助:上海市科学技术委员会“扬帆计划”(20YF1416000);上海工程技术大学校级大学生创新训练项目(cx2304008)
Advances in the prediction of activation energy barriers for hydrogen atom transfer reactions
LI Xiang( ), LI Jiaying, NI Heng, SUN Haoran, CAO Jiawei, CHEN Yuxuan, LIU Fengjiao(
), LI Jiaying, NI Heng, SUN Haoran, CAO Jiawei, CHEN Yuxuan, LIU Fengjiao( )
)
- School of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China
-
Received:2024-04-19Revised:2024-06-22Online:2025-06-25Published:2025-07-08 -
Contact:LIU Fengjiao
摘要:
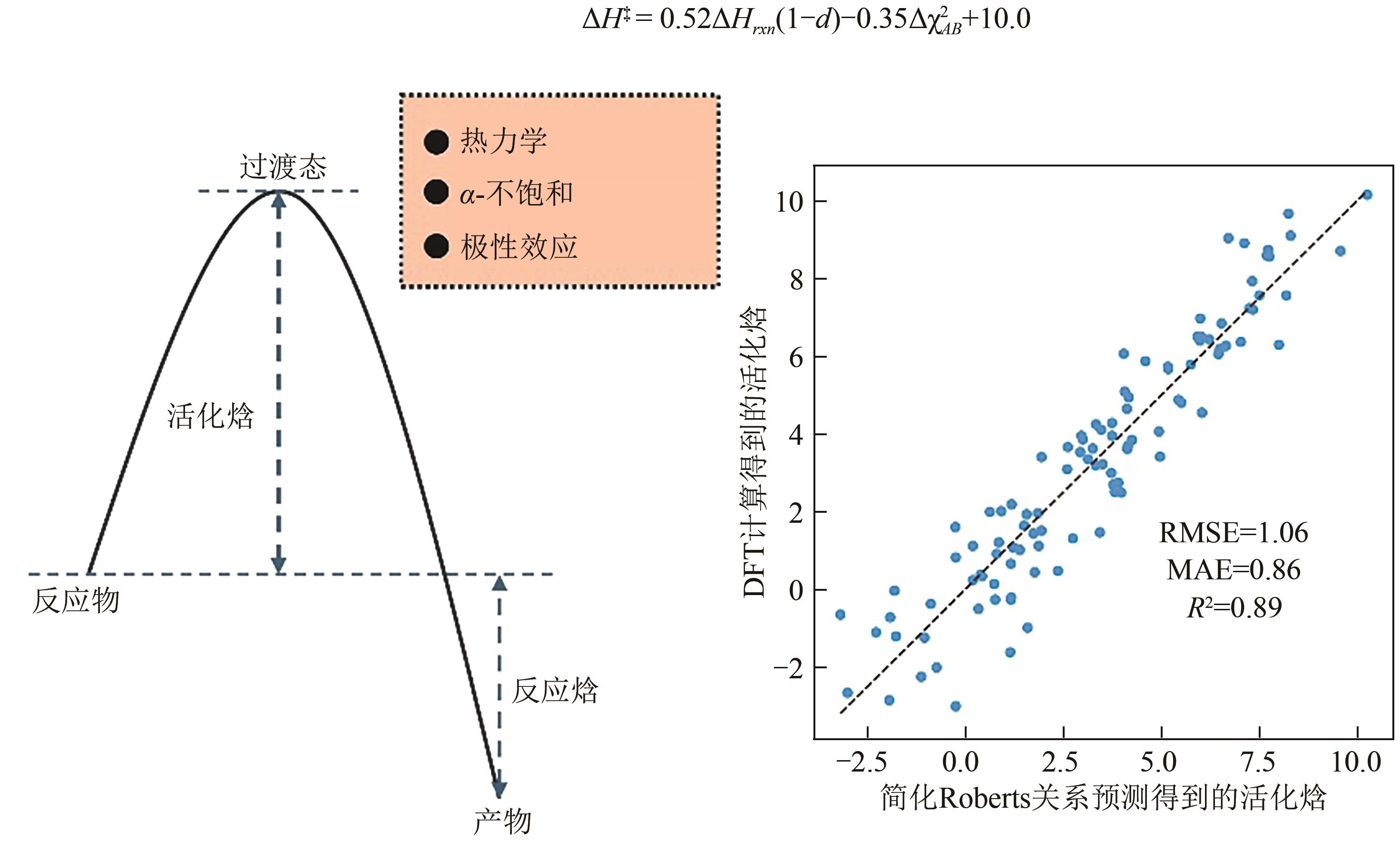
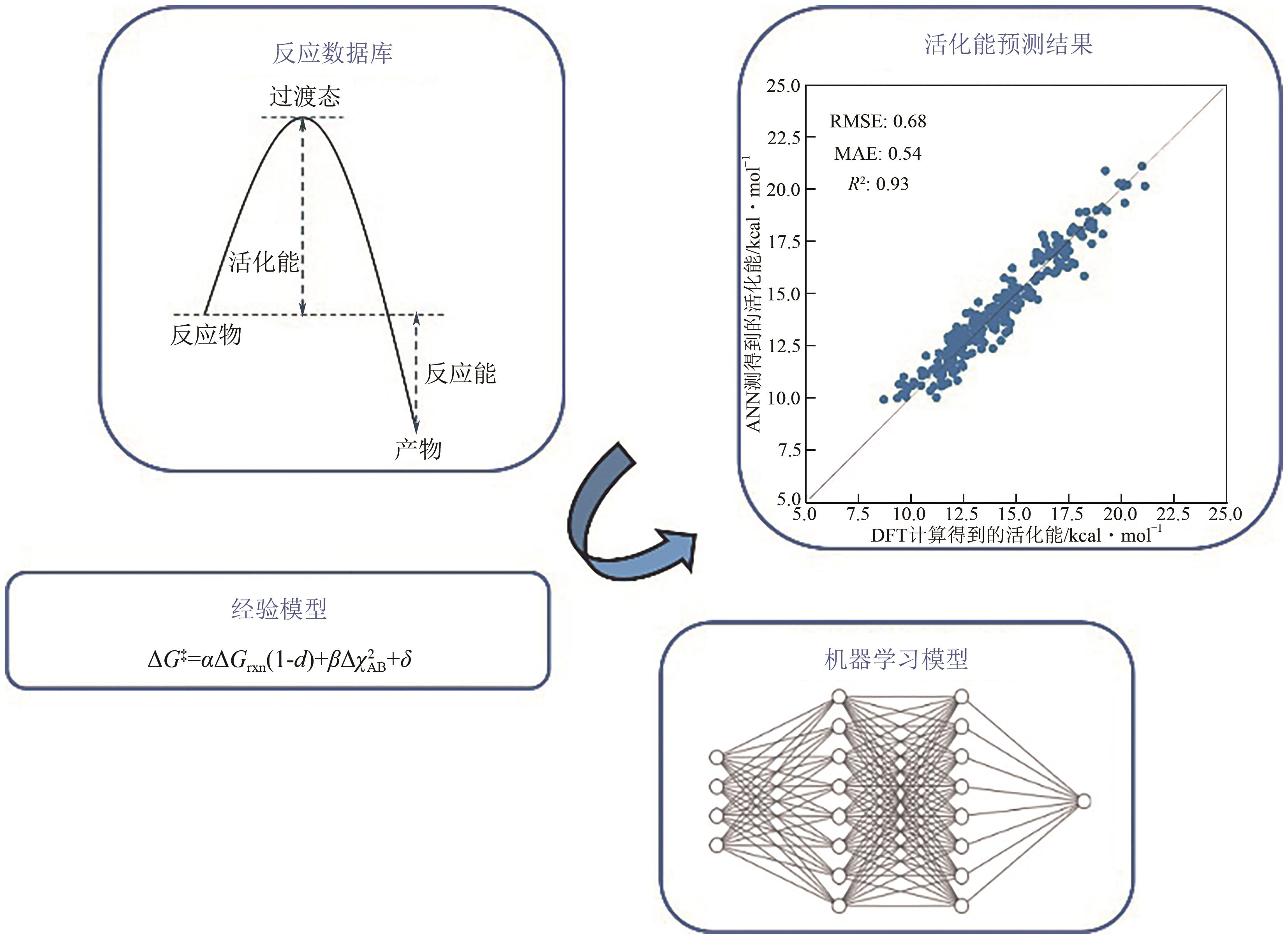
氢原子转移(hydrogen atom transfer,HAT)是自然界中的基本化学反应之一,准确预测其反应性和选择性对于合理设计相关化学反应至关重要。其中一种重要方法是通过预测反应的活化能垒来研究其反应性和选择性。本文从经验模型和机器学习模型两个角度综述了当前预测活化能垒的研究进展。经验模型基于已知反应的实验数据和化学规律,采用经验公式(如线性方程)进行拟合,具有较好的可解释性,但在适用性和准确性方面存在一定局限性。而机器学习模型则能够处理更大量级的数据和更复杂的反应机理,在准确预测活化能垒方面更有潜力,但是预测效果依赖于数据的质量,并且可解释性较弱。最后,本文对未来如何开发更准确且可解释的活化能垒预测模型进行了展望,并且期待通过提高活化能垒预测模型的可解释性进而提高人们对反应活性影响因素的理解。
中图分类号:
引用本文
李想, 李佳莹, 倪恒, 孙浩然, 曹家伟, 陈宇轩, 刘凤娇. 氢原子转移反应活化能垒预测研究进展[J]. 化工进展, 2025, 44(6): 3336-3344.
LI Xiang, LI Jiaying, NI Heng, SUN Haoran, CAO Jiawei, CHEN Yuxuan, LIU Fengjiao. Advances in the prediction of activation energy barriers for hydrogen atom transfer reactions[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3336-3344.
| [1] | CAO Hui, TANG Xinxin, TANG Haidi, et al. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis[J]. Chem Catalysis, 2021, 1(3): 523-598. |
| [2] | WANG Yong, CARDER Hayden M, WENDLANDT Alison E. Synthesis of rare sugar isomers through site-selective epimerization[J]. Nature, 2020, 578(7795): 403-408. |
| [3] | VASILOPOULOS Aristidis, KRSKA Shane W, STAHL Shannon S. C(sp3)—H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling[J]. Science, 2021, 372(6540): 398-403. |
| [4] | SARKAR Sumon, CHEUNG Kelvin Pak Shing, GEVORGYAN Vladimir. C—H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals[J]. Chemical Science, 2020, 11(48): 12974-12993. |
| [5] | LI Jiayuan, ZHANG Zhihan, WU Lianqian, et al. Site-specific allylic C-H bond functionalization with a copper-bound N-centred radical[J]. Nature, 2019, 574(7779): 516-521. |
| [6] | LI Hanning, YANG Yang, JING Xu, et al. Triarylamine-based porous coordination polymers performing both hydrogen atom transfer and photoredox catalysis for regioselective α-amino C(sp3)-H arylation[J]. Chemical Science, 2021, 12(24): 8512-8520. |
| [7] | HUANG Chia-Yu, LI Jianbin, LI Chaojun. A cross-dehydrogenative C(sp3)-H heteroarylation via photo-induced catalytic chlorine radical generation[J]. Nature Communications, 2021, 12: 4010. |
| [8] | CAO Hui, KONG Degong, YANG Licheng, et al. Brønsted acid-enhanced direct hydrogen atom transfer photocatalysis for selective functionalization of unactivated C(sp3)-H bonds[J]. Nature Synthesis, 2022, 1(10): 794-803. |
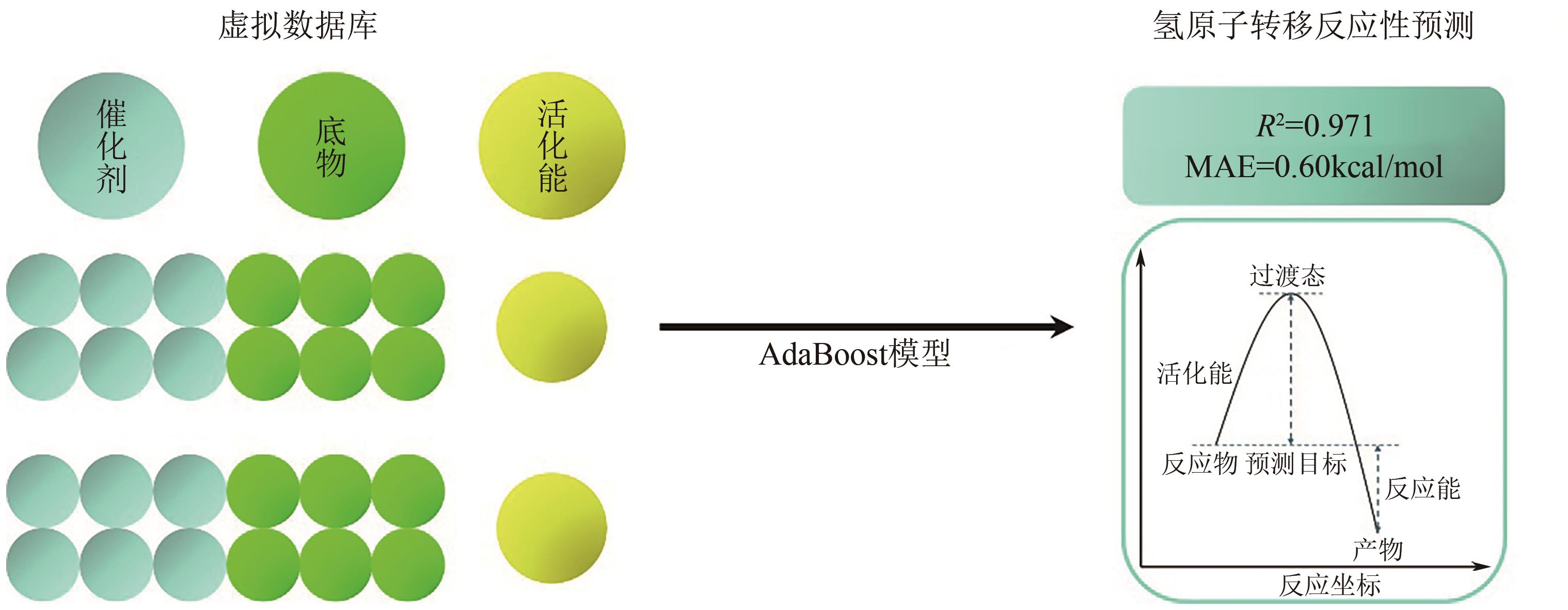
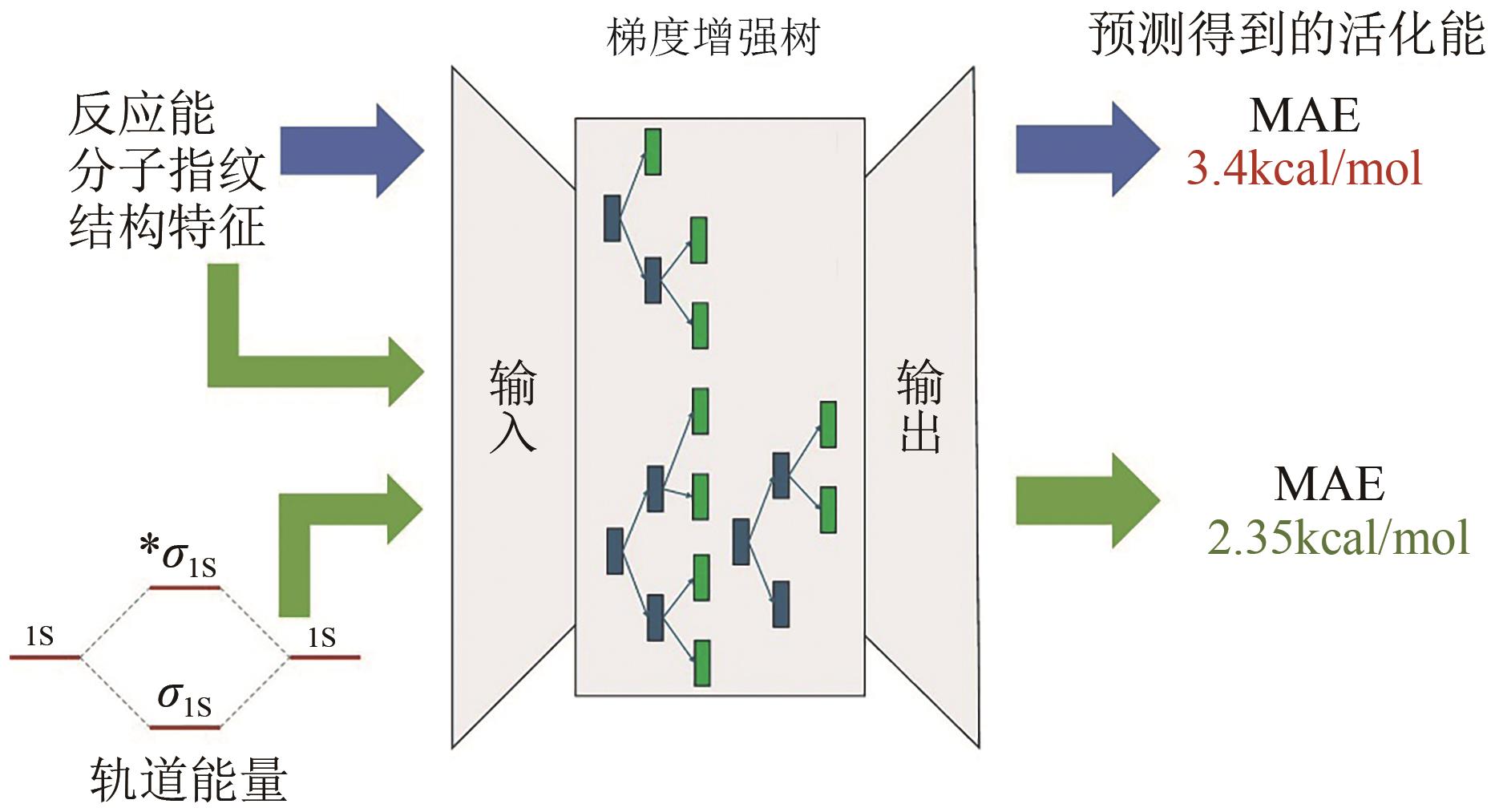
| [9] | MA Siqi, WANG Shipeng, CAO Jiawei, et al. Rapid and accurate estimation of activation free energy in hydrogen atom transfer-based C-H activation reactions: From empirical model to artificial neural networks[J]. ACS Omega, 2022, 7(39): 34858-34867. |
| [10] | BELL Ronald Percy. The theory of reactions involving proton transfers[J]. Proceedings of the Royal Society of London Series A - Mathematical and Physical Sciences, 1936, 154(882): 414-429. |
| [11] | EVANS Meredith Gwynne, POLANYI Michael. Further considerations on the thermodynamics of chemical equilibria and reaction rates[J]. Transactions of the Faraday Society, 1936, 32(0): 1333-1360. |
| [12] | EVANS Meredith Gwynne, POLANYI Michael. Inertia and driving force of chemical reactions[J]. Transactions of the Faraday Society, 1938, 34: 11-24. |
| [13] | TEDDER John Michael. Which factors determine the reactivity and regioselectivity of free radical substitution and addition reactions?[J]. Angewandte Chemie International Edition, 1982, 21(6): 401-410. |
| [14] | ROBERTS Brian Peter, STEEL Andrew J. An extended form of the Evans-Polanyi equation: A simple empirical relationship for the prediction of activation energies for hydrogen-atom transfer reactions[J]. Journal of the Chemical Society, Perkin Transactions 2, 1994(10): 2155-2162. |
| [15] | BIETTI Massimo, CUCINOTTA Erica, DILABIO Gino A, et al. Evaluation of polar effects in hydrogen atom transfer reactions from activated phenols[J]. The Journal of Organic Chemistry, 2019, 84(4): 1778-1786. |
| [16] | SALAMONE Michela, BIETTI Massimo. Tuning reactivity and selectivity in hydrogen atom transfer from aliphatic C-H bonds to alkoxyl radicals: Role of structural and medium effects[J]. Accounts of Chemical Research Journal, 2015, 48(11): 2895-2903. |
| [17] | GROFF Benjamin D, KORONKIEWICZ Brian, MAYER James M. Polar effects in hydrogen atom transfer reactions from a proton-coupled electron transfer (PCET) perspective: Abstractions from toluenes[J]. The Journal of Organic Chemistry, 2023, 88(23): 16259-16269. |
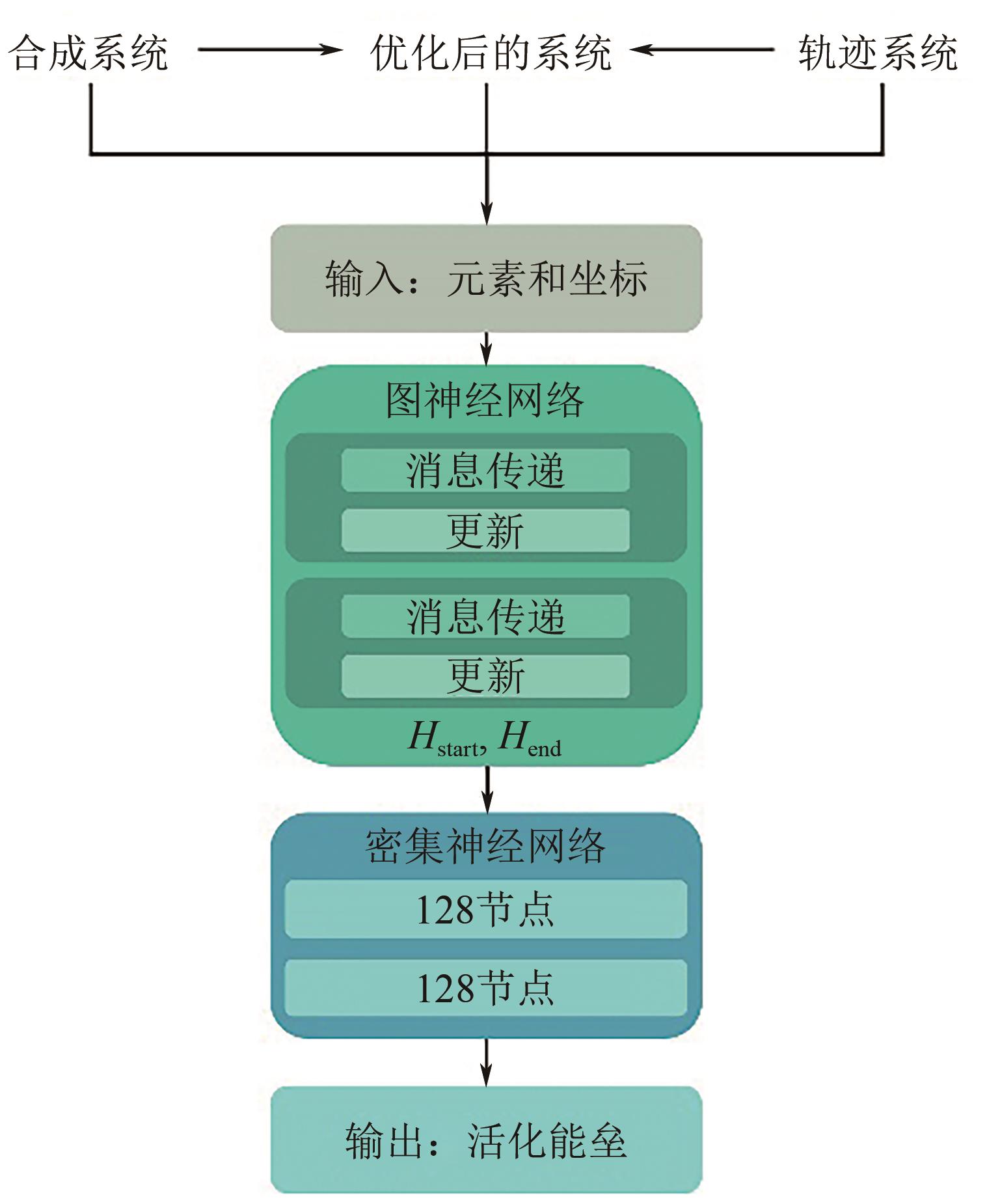
| [18] | Béatrice QUICLET-SIRE, ZARD Samir Z. An unusual route to deoxysugars by hydrogen atom transfer from cyclohexane. possible manifestation of polar effects in a radical process[J]. Journal of the American Chemical Society, 1996, 118(38): 9190-9191. |
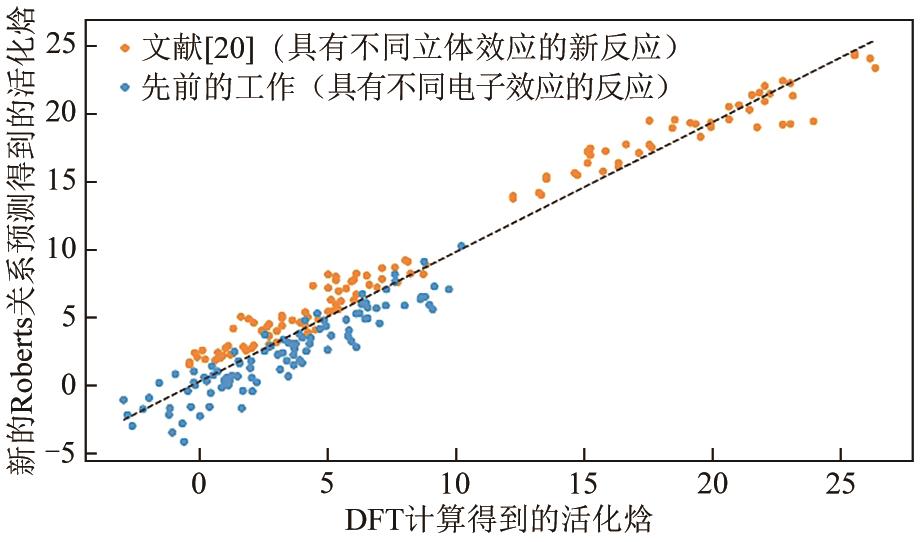
| [19] | LIU Fengjiao, MA Siqi, LU Zeying, et al. Hydrogen abstraction by alkoxyl radicals: Computational studies of thermodynamic and polarity effects on reactivities and selectivities[J]. Journal of the American Chemical Society, 2022, 144(15): 6802-6812. |
| [20] | SUN Yi, LIU Fengjiao, SANDERS Jacob N, et al. Role of steric effects on rates of hydrogen atom transfer reactions[J]. The Journal of Organic Chemistry, 2023, 88(17): 12668-12676. |
| [21] | MARCUS Rudolph Arthur. On the theory of oxidation-reduction reactions involving electron transfer. Ⅰ[J]. The Journal of Chemical Physics, 1956, 24(5): 966-978. |
| [22] | MAYER James M. Understanding hydrogen atom transfer: From bond strengths to Marcus theory[J]. Accounts of Chemical Research Journal, 2011, 44(1): 36-46. |
| [23] | LI Jilai, ZHOU Shaodong, ZHANG Jun, et al. Mechanistic variants in gas-phase metal-oxide mediated activation of methane at ambient conditions[J]. Journal of the American Chemical Society, 2016, 138(35): 11368-11377. |
| [24] | DE VISSER Sam P, KUMAR Devesh, COHEN Shimrit, et al. A predictive pattern of computed barriers for C-H hydroxylation by compound I of cytochrome P450[J]. Journal of the American Chemical Society, 2004, 126(27): 8362-8363. |
| [25] | LI Jilai, ZHOU Shaodong, ZHANG Jun, et al. Electronic origins of the variable efficiency of room-temperature methane activation by homo- and heteronuclear cluster oxide cations [XYO2]+ (X, Y = Al, Si, Mg): Competition between proton-coupled electron transfer and hydrogen-atom transfer[J]. Journal of the American Chemical Society, 2016, 138(25): 7973-7981. |
| [26] | PROSS Addy, SHAIK Sason S. A qualitative valence-bond approach to organic reactivity[J]. Accounts of Chemical Research, 1983, 16(10): 363-370. |
| [27] | LAI Wenzhen, LI Chunsen, CHEN Hui, et al. Hydrogen-abstraction reactivity patterns from A to Y: The valence bond way[J]. Angewandte Chemie International Edition, 2012, 51(23): 5556-5578. |
| [28] | USHARANI Dandamudi, LACY David C, BOROVIK A S, et al. Dichotomous hydrogen atom transfer vs. proton-coupled electron transfer during activation of X-H bonds (X = C, N, O) by nonheme iron-oxo complexes of variable basicity[J]. Journal of the American Chemical Society, 2013, 135(45): 17090-17104. |
| [29] | USHARANI Dandamudi, LAI Wenzhen, LI Chunsen, et al. A tutorial for understanding chemical reactivity through the valence bond approach[J]. Chemical Society Reviews, 2014, 43(14): 4968-4988. |
| [30] | Daniel H ESS, HOUK Kendall Newcomb. Distortion/interaction energy control of 1,3-dipolar cycloaddition reactivity[J]. Journal of the American Chemical Society, 2007, 129(35): 10646-10647. |
| [31] | LIU Siqi, SU Yongliang, SUN Tianyu, et al. Precise introduction of the -CH n X3- n (X = F, Cl, Br, I) moiety to target molecules by a radical strategy: A theoretical and experimental study[J]. Journal of the American Chemical Society, 2021, 143(33): 13195-13204. |
| [32] | TAO L, ZHANG Peng, QIN Chu, et al. Recent progresses in the exploration of machine learning methods as in-silico ADME prediction tools[J]. Advanced Drug Delivery Reviews, 2015, 86: 83-100. |
| [33] | LU Mingkun, YIN Jiayi, ZHU Qi, et al. Artificial intelligence in pharmaceutical sciences[J]. Engineering, 2023, 27: 37-69. |
| [34] | Stephen GOW, NIRANJAN Mahesan, KANZA Samantha, et al. A review of reinforcement learning in chemistry[J]. Digital Discovery, 2022, 1(5): 551-567. |
| [35] | OUYANG Yulou, ZHANG Zhongwei, YU Cuiqian, et al. Accuracy of machine learning potential for predictions of multiple-target physical properties[J]. Chinese Physics Letters, 2020, 37(12): 126301. |
| [36] | YANG Qi, LIU Yidi, CHENG Junjie, et al. An ensemble structure and physicochemical (SPOC) descriptor for machine-learning prediction of chemical reaction and molecular properties[J]. ChemPhySchem, 2022, 23(14): e202200255. |
| [37] | INOKUCHI Takuya, LI Na, MOROHOSHI Kei, et al. Multiscale prediction of functional self-assembled materials using machine learning: High-performance surfactant molecules[J]. Nanoscale, 2018, 10(34): 16013-16021. |
| [38] | GROVEN Steven D, DESGRANGES Caroline, DELHOMMELLE Jérôme. Prediction of the boiling and critical points of polycyclic aromatic hydrocarbons via Wang-Landau simulations and machine learning[J]. Fluid Phase Equilibria, 2019, 484: 225-231. |
| [39] | YANG Licheng, LI Xin, ZHANG Shuoqing, et al. Machine learning prediction of hydrogen atom transfer reactivity in photoredox-mediated C-H functionalization[J]. Organic Chemistry Frontiers, 2021, 8(22): 6187-6195. |
| [40] | MARQUES Esteban, DE GENDT Stefan, POURTOIS Geoffrey, et al. Improving accuracy and transferability of machine learning chemical activation energies by adding electronic structure information[J]. Journal of Chemical Information and Modeling, 2023, 63(5): 1454-1461. |
| [41] | PAN Wenxiao, CHANG Jiamin, HE Shuming, et al. Machine learning strategy on activation energy of environmental heterogeneous reactions and its application to atmospheric formation of typical montmorillonite-bound phenoxy radicals[J]. Science of the Total Environment, 2023, 895: 165117. |
| [42] | Daniel PLATERO-ROCHART, KRIVOBOKOVA Tatyana, GASTEGGER Michael, et al. Prediction of enzyme catalysis by computing reaction energy barriers via steered QM/MM molecular dynamics simulations and machine learning[J]. Journal of Chemical Information and Modeling, 2023, 63(15): 4623-4632. |
| [43] | RIEDMILLER Kai, REISER Patrick, BOBKOVA Elizaveta, et al. Substituting density functional theory in reaction barrier calculations for hydrogen atom transfer in proteins[J]. Chemical Science, 2024, 15(7): 2518-2527. |
| [1] | 王御豪, 蒋沁利, 徐西蒙. 表面修饰FeOCl活化过硫酸盐引发有机污染物非自由基降解[J]. 化工进展, 2025, 44(6): 3101-3111. |
| [2] | 陈少伟, 陈奕, 牛江奇, 刘天奇, 黄建国, 陈焕浩, 范晓雷. 介质阻挡放电等离子体催化反应器研究进展及应用展望[J]. 化工进展, 2025, 44(6): 3175-3189. |
| [3] | 李明, 周依, 南兰, 叶晓生. 自动优化连续合成研究进展[J]. 化工进展, 2025, 44(6): 3190-3198. |
| [4] | 吴展华, 孔德宝, 田均均, 梁汝军, 卢朋慧, 盛敏. “1+N”模式的反应安全技术体系探讨[J]. 化工进展, 2025, 44(6): 3199-3207. |
| [5] | 王家慧, 李培雅, 杨福胜, 王斌, 方涛. 有机液态储氢载体甲基环己烷脱氢研究进展[J]. 化工进展, 2025, 44(6): 3208-3223. |
| [6] | 余子昱, 陈晓飞, 侯春光, 岳殿鹤, 彭跃莲, 安全福. 渗透汽化和真空膜蒸馏在氨基甲酸酯脱水中的比较[J]. 化工进展, 2025, 44(6): 3247-3257. |
| [7] | 石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052. |
| [8] | 范晓娅, 赵镇, 彭强. 电催化二氧化碳和硝酸根共还原合成尿素研究进展[J]. 化工进展, 2025, 44(5): 2856-2869. |
| [9] | 马梓轩, 施瑞晨, 刘明杰, 杨莹杰, 宋子瑜, 梅晓鹏, 高晓峰, 洪龙城, 姚思宇, 张治国, 任其龙. 环烷烃催化制氢反应器的设计与性能优化: 前沿进展与挑战[J]. 化工进展, 2025, 44(5): 2919-2937. |
| [10] | 孟凡志, 孙冰, 杨哲. 原料替代对化工生产过程新工艺安全的影响与风险评估[J]. 化工进展, 2025, 44(5): 2955-2971. |
| [11] | 王水众, 宋国勇. 木质素选择性氢解制备高功能化单酚及其高值利用[J]. 化工进展, 2025, 44(5): 2535-2540. |
| [12] | 王嘉, 孙丹卉, 乔一凡, 范秀方, 赵立东, 贺雷, 陆安慧. 乙醇催化转化制高值化学品研究进展[J]. 化工进展, 2025, 44(5): 2587-2597. |
| [13] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [14] | 付东龙, 冯冠晴, 徐心泉, 陆振谱, 裴春雷, 巩金龙. 塑料催化资源化利用研究进展[J]. 化工进展, 2025, 44(5): 2758-2766. |
| [15] | 王笑楠, 傅思维, 刘宽, 林琮盛, 林晓风. 能源材料替代与转型中的机器学习方法[J]. 化工进展, 2025, 44(5): 2767-2776. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||