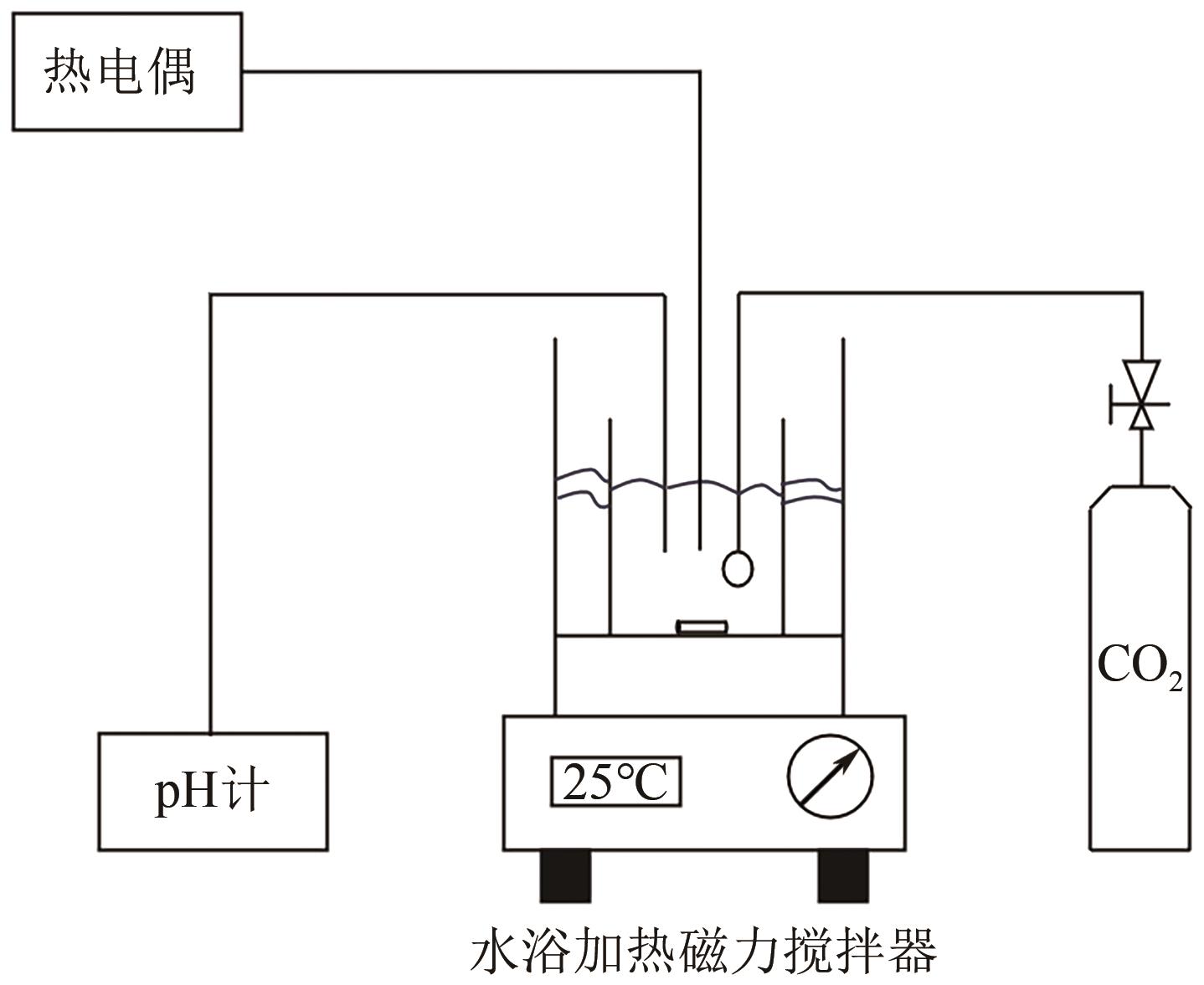
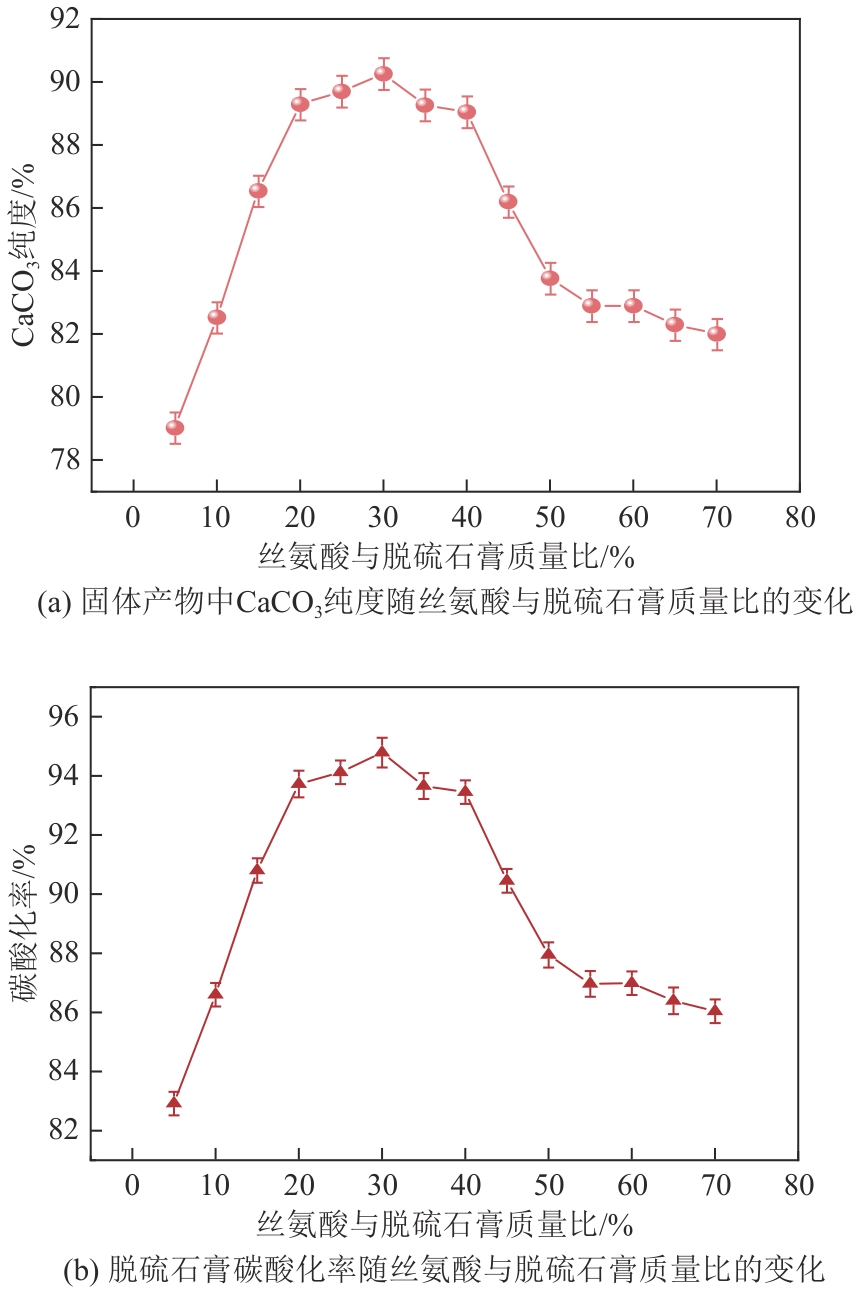
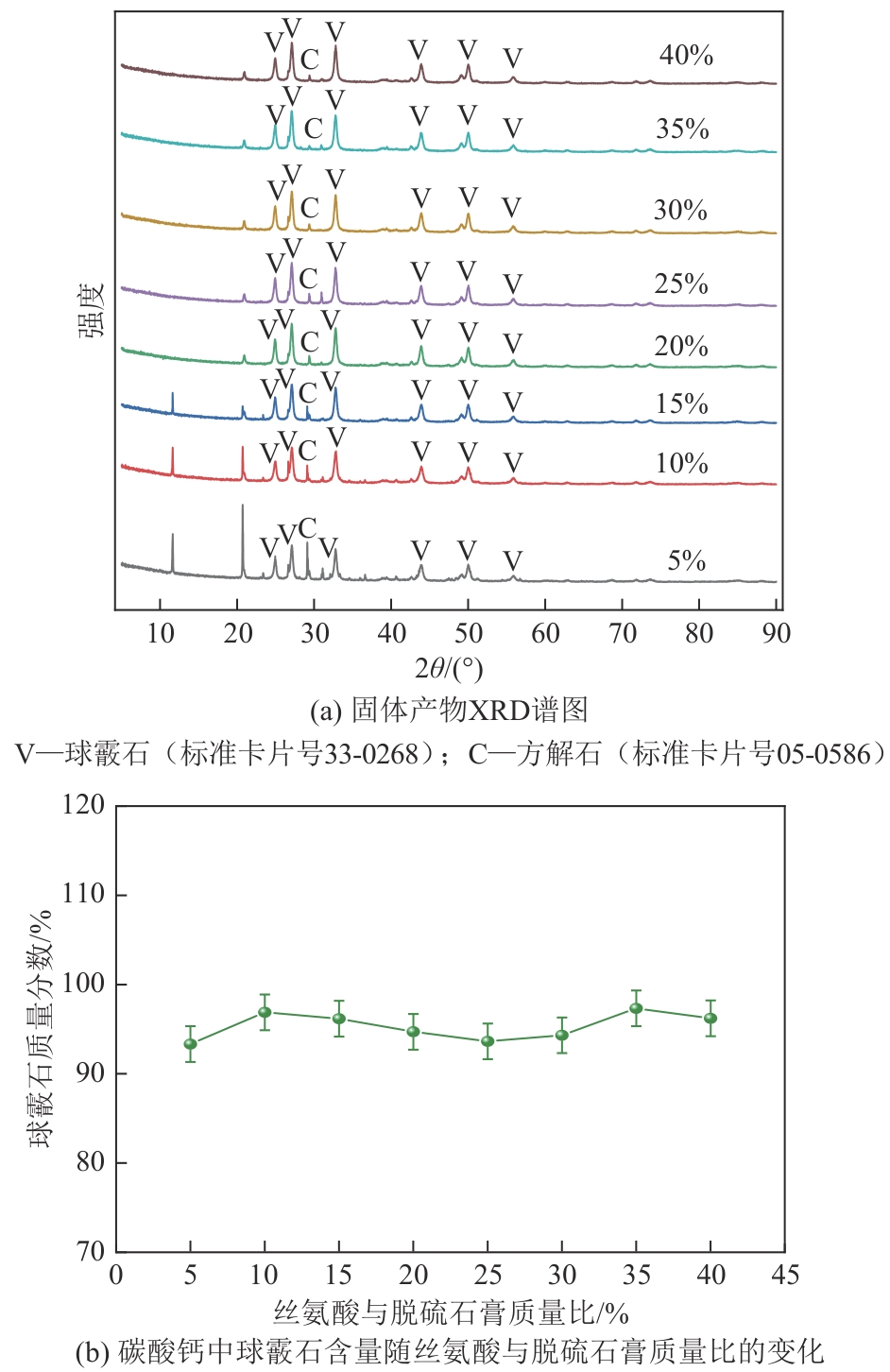
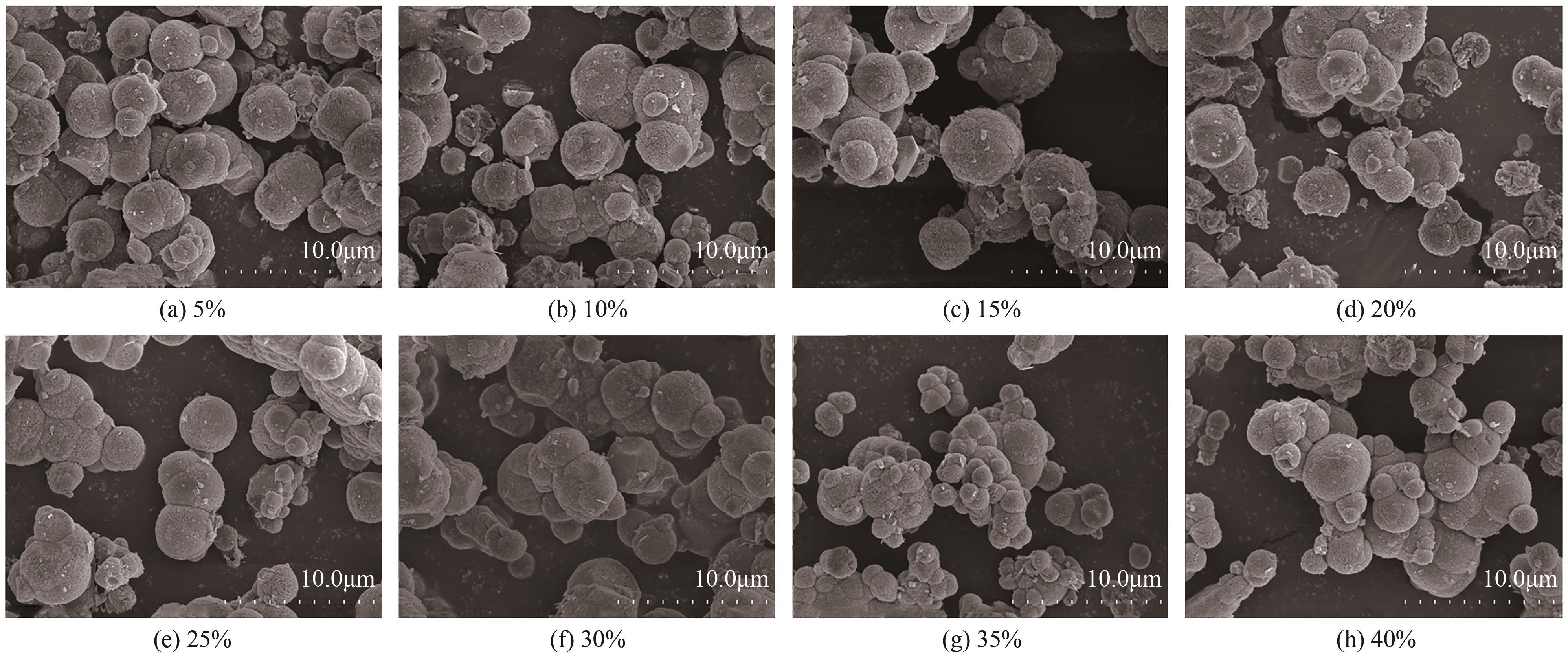
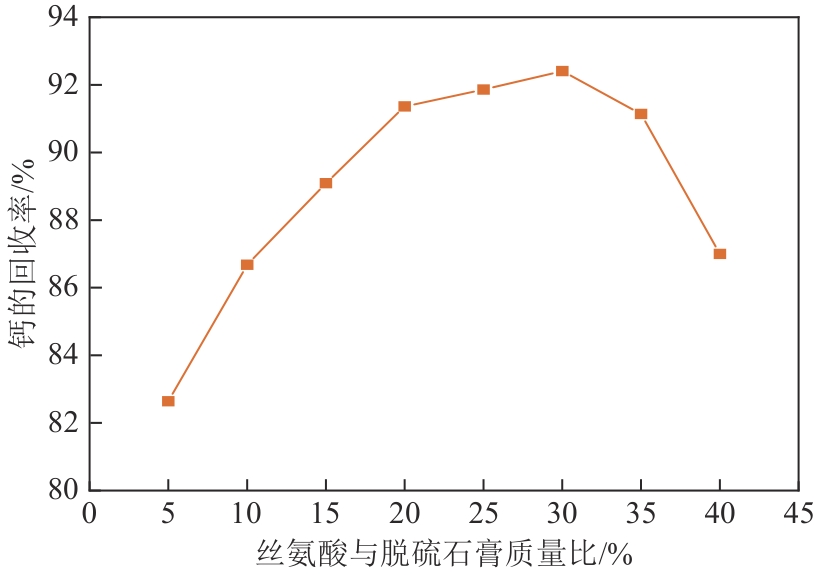
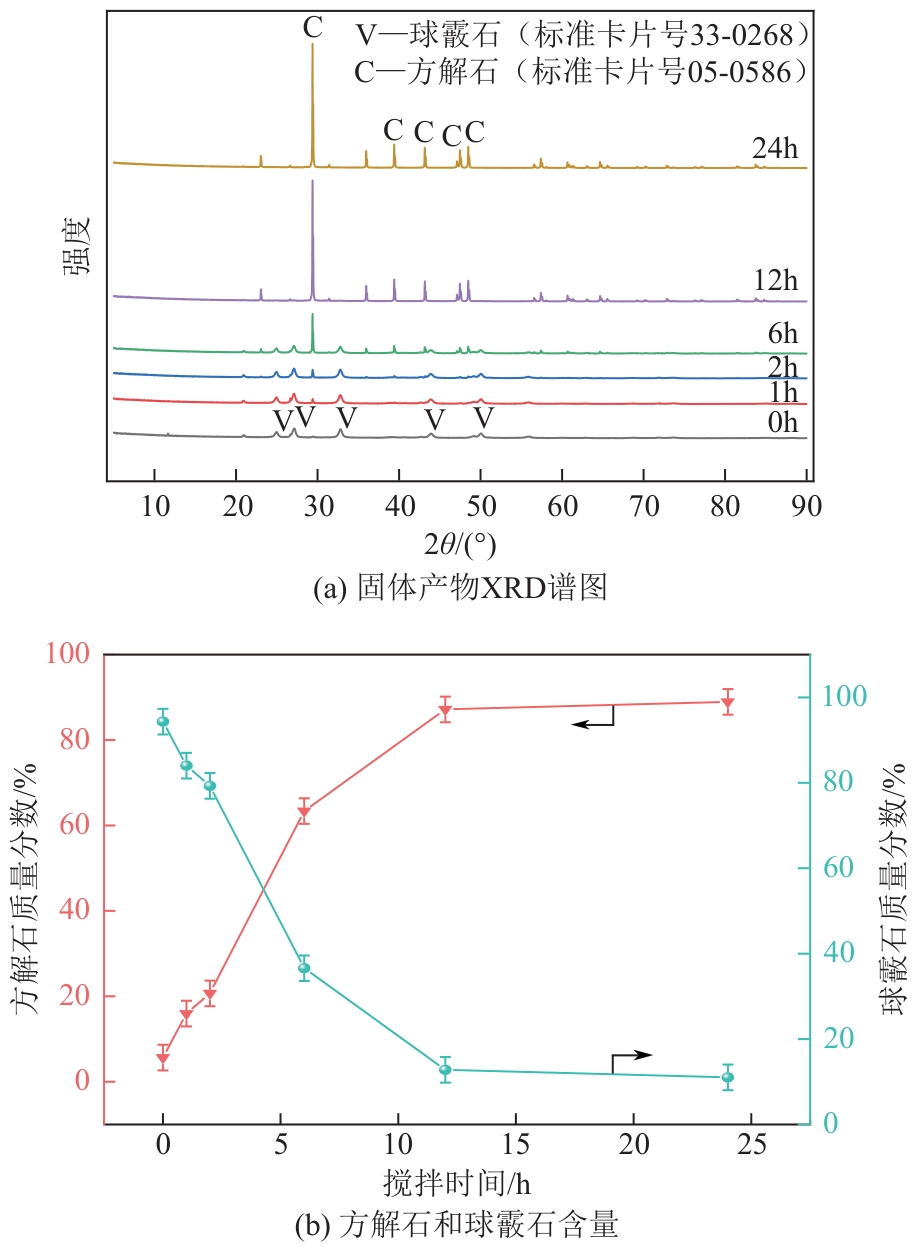
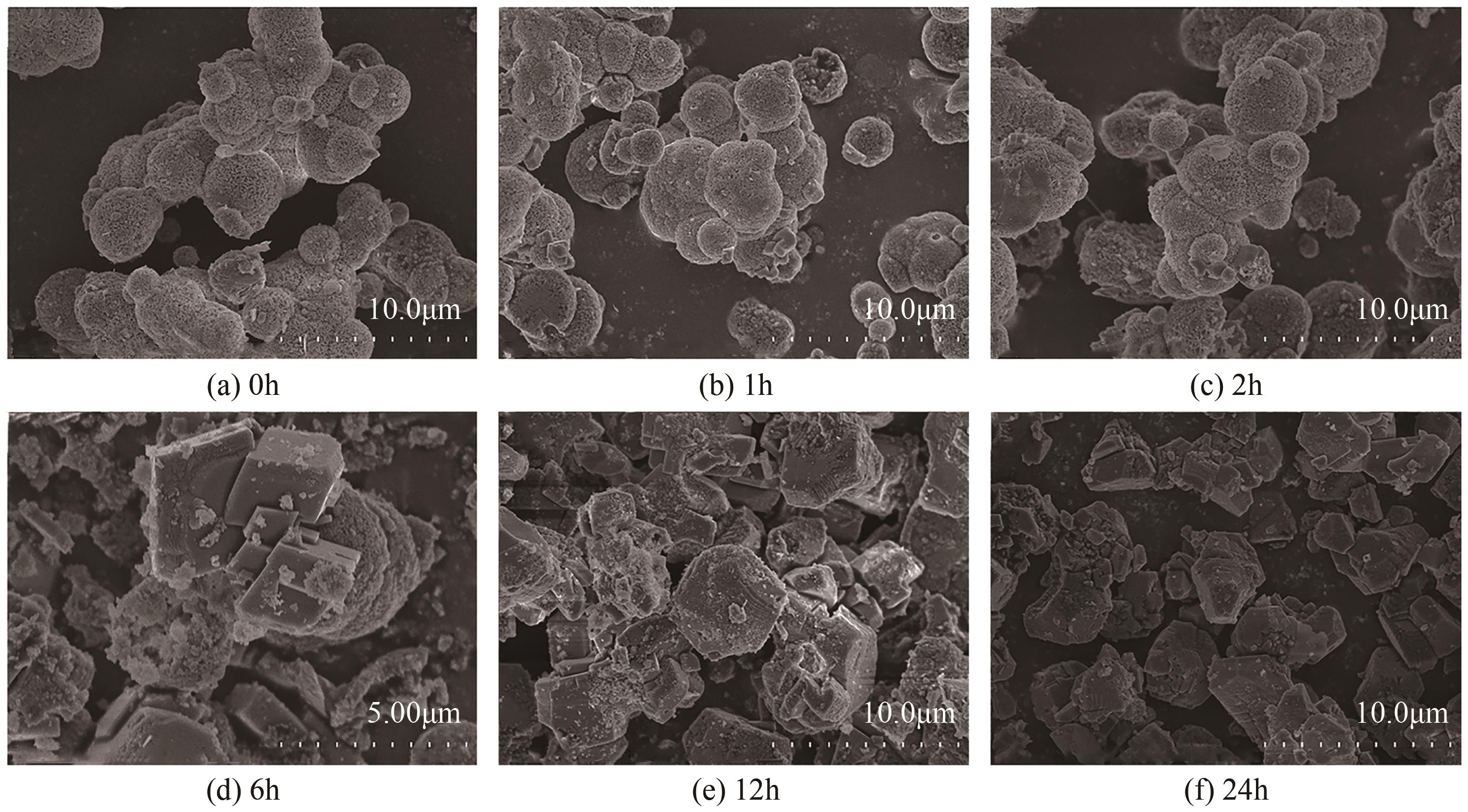
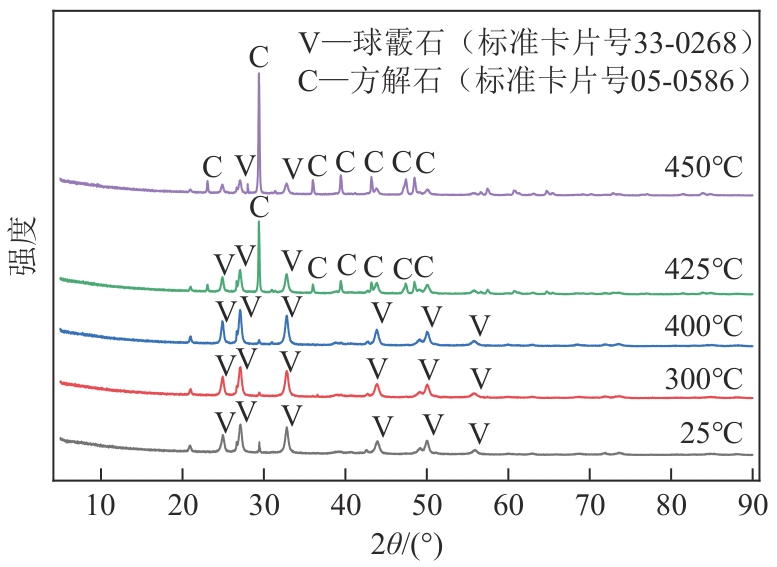
| 1 |
曹建宗, 刘琦, 陈文通,等. 典型湿法脱硫系统存在的问题及人工智能在优化运行中的应用[J]. 化工进展, 2020, 39(S1): 242-249
|
|
CAO Jianzong, LIU Qi, CHEN Wentong, et al. Problems of typical wet desulfurization system and application of artificial intelligence in optimal operation[J]. Chemical Industry and Engineering Progress, 2020, 39(S1): 242-249.
|
| 2 |
AAKRITI, MAITI Soumitra, JAIN Neeraj, et al. A comprehensive review of flue gas desulphurized gypsum: Production, properties, and applications[J]. Construction and Building Materials, 2023, 393: 131918.
|
| 3 |
王文飚, 许月阳, 薛建明,等. 燃煤电厂脱硫技术研究进展及建议[J]. 电力科技与环保, 2020, 36(3): 1-5.
|
|
WANG Wenbiao, XU Yueyang, XUE Jianming, et al. The research progress and suggestions of desulfurization technology in coal-fired power plants[J]. Electric Power Technology and Environmental Protection, 2020, 36(3): 1-5.
|
| 4 |
郭程程. 小型热电厂烟气脱硫系统改造设计优化与实践[J]. 电力科技与环保, 2020, 36(3): 42-44.
|
|
GUO Chengcheng. Optimization and practice about desulfurization reform in small thermal power plants[J]. Electric Power Technology and Environmental Protection, 2020, 36(3): 42-44.
|
| 5 |
WANG Guodong, HOU Zhiwei, ZHAN Li, et al. Integrated numerical simulation of CO2 flooding, heat recovery and storage in high temperature reservoir[J]. Petroleum Science and Technology, 2023, 41(12): 1250-1271.
|
| 6 |
FREEMAN C L, HARDING J H. The transformation of amorphous calcium carbonate to calcite and classical nucleation theory[J]. Journal of Crystal Growth, 2023, 603: 126978.
|
| 7 |
Anett LÁZÁR, Zsombor MOLNÁR, Attila DEMÉNY, et al. Insights into the amorphous calcium carbonate (ACC) → ikaite → calcite transformations[J]. CrystEngComm, 2023, 25(5): 738-750.
|
| 8 |
ZHANG Shuheng, NAHI Ouassef, CHEN Li, et al. Magnesium ions direct the solid-state transformation of amorphous calcium carbonate thin films to aragonite, magnesium-calcite, or dolomite[J]. Advanced Functional Materials, 2022, 32(25): 2201394.
|
| 9 |
LUO Mingzhi, ZHANG Guoquan, FANG Yuguo, et al. Calcium carbonate crystallization process from the mineralization of calcium chloride waste[J]. Separation and Purification Technology, 2023, 319: 124066.
|
| 10 |
MARMO Vitor Luca Moura, AMBRÓSIO Jéssica A R, GONÇALVES Erika Peterson, et al. Vaterite microparticle-loaded methylene blue for photodynamic activity in macrophages infected with Leishmania braziliensis [J]. Photochemical & Photobiological Sciences, 2023, 22(8): 1977-1989.
|
| 11 |
WILLIAMS Jonah M, ZHAO Diandian, MOON Seokyoon, et al. Stabilization of pure vaterite during carbon mineralization: Defining critical activities, additive concentrations, and gas flow conditions for carbon utilization[J]. Crystal Growth & Design, 2023, 23(11): 8103-8115.
|
| 12 |
Xingyuan SAN, HU Junwei, CHEN Mingyi, et al. Unlocking the mysterious polytypic features within vaterite CaCO3 [J]. Nature Communications, 2023, 14(1): 7858.
|
| 13 |
丁文金, 姚金, 乔静怡, 等. 磷石膏矿化CO2制备高纯碳酸钙及产物调控[J]. 矿产保护与利用, 2022, 42(4): 104-112.
|
|
DING Wenjin, YAO Jin, QIAO Jingyi, et al. Preparation of high-purity CaCO3 from phosphogypsum by CO2 mineralization and product control[J]. Conservation and Utilization of Mineral Resources, 2022, 42(4): 104-112.
|
| 14 |
李文秀, 杨宇航, 黄艳, 等. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057.
|
|
LI Wenxiu, YANG Yuhang, HUANG Yan, et al. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057.
|
| 15 |
SASAMOTO Ryo, KANDA Yasuharu, YAMANAKA Shinya. Difference in cadmium chemisorption on calcite and vaterite porous particles[J]. Chemosphere, 2022, 297: 134057.
|
| 16 |
FEBRIDA Renny, CAHYANTO Arief, HERDA Ellyza, et al. Synthesis and characterization of porous CaCO3 vaterite particles by simple solution method[J]. Materials, 2021, 14(16): 4425.
|
| 17 |
TRUSHINA Daria B, BORODINA Tatiana N, BELYAKOV Sergei, et al. Calcium carbonate vaterite particles for drug delivery: Advances and challenges[J]. Materials Today Advances, 2022, 14: 100214.
|
| 18 |
LUO Wenli, LI Zhaojian, ZHANG Ling, et al. Polyethylenimine-CO2 adduct templated CaCO3 nanoparticles as anticancer drug carrier[J]. Cancer Nanotechnology, 2023, 14(1): 7.
|
| 19 |
ABAKUMOVA Tatiana O, GUSLIAKOVA Olga I, CVJETINOVIC Julijana, et al. Barnase-loaded vaterite nanoparticles functionalized by EpCAM targeting vectors for the treatment of lung diseases[J]. ACS Applied Nano Materials, 2022, 5(8): 10744-10754.
|
| 20 |
Donata KONOPACKA-ŁYSKAWA. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review[J]. Crystals, 2019, 9(4): 223.
|
| 21 |
王宇轩, 徐颖, 王东平, 等. 球霰石的性质及其应用进展[J]. 安徽理工大学学报(自然科学版), 2017, 37(2): 76-80.
|
|
WANG Yuxuan, XU Ying, WANG Dongping, et al. Properties and applications of vaterite[J]. Journal of Anhui University of Science and Technology (Natural Science), 2017, 37(2): 76-80.
|
| 22 |
LAI Yonghua, CHEN Liangsen, BAO Weichao, et al. Glycine-mediated, selective preparation of monodisperse spherical vaterite calcium carbonate in various reaction systems[J]. Crystal Growth & Design, 2015, 15(3): 1194-1200.
|
| 23 |
GUO Yuming, WANG Feifei, ZHANG Jie, et al. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids[J]. Research on Chemical Intermediates, 2013, 39(6): 2407-2415.
|
| 24 |
王波. 脱硫石膏矿化CO2制备均一球霰石型CaCO3机理研究[D]. 太原: 山西大学, 2021.
|
|
WANG Bo. Study on mechanism of preparation of uniform vaterite-type CaCO3 via CO2 mineralization by flue gas desulfurization gypsum[D]. Taiyuan: Shanxi University, 2021.
|
| 25 |
SARKAR Arpita, DUTTA Kingshuk, MAHAPATRA Samiran. Polymorph control of calcium carbonate using insoluble layered double hydroxide[J]. Crystal Growth & Design, 2013, 13(1): 204-211.
|
| 26 |
ZHAO Diandian, WILLIAMS Jonah M, HOU Pengkun, et al. Stabilizing mechanisms of metastable vaterite in cement systems[J]. Cement and Concrete Research, 2024, 178: 107441.
|
| 27 |
NAKANISHI Yurie, CHENG Bohan, RICHARDSON Joseph J, et al. Using phenolic polymers to control the size and morphology of calcium carbonate microparticles[J]. RSC Advances, 2023, 13(43): 30539-30547.
|
| 28 |
SONG Kyungsun, JANG Young-Nam, KIM Wonbaek, et al. Precipitation of calcium carbonate during direct aqueous carbonation of flue gas desulfurization gypsum[J]. Chemical Engineering Journal, 2012, 213: 251-258.
|
| 29 |
LI Qing, DING Yi, LI Fanqing, et al. Solvothermal growth of vaterite in the presence of ethylene glycol, 1, 2-propanediol and glycerin[J]. Journal of Crystal Growth, 2002, 236(1/2/3): 357-362.
|
| 30 |
POLAT Sevgi. Experimental investigations on the effects of asparagine and serine on the polymorphism of calcium carbonate[J]. Advanced Powder Technology, 2020, 31(10): 4282-4291.
|
| 31 |
MENAHEM Tali, MASTAI Yitzhak. Controlled crystallization of calcium carbonate superstructures in macroemulsions[J]. Journal of Crystal Growth, 2008, 310(15): 3552-3556.
|
| 32 |
XU Meng, YUAN Liang, ZHANG Shuiqin, et al. Effects of a preparation containing amino acids on pakchoi nutrient absorption, yield, and quality when grown in saline-alkali soil[J]. Agriculture, 2023, 13(4): 863.
|
| 33 |
ZIMMERMANN Sandra E, BENSTEIN Ruben M, María FLORES-TORNERO, et al. The phosphorylated pathway of serine biosynthesis links plant growth with nitrogen metabolism[J]. Plant Physiology, 2021, 186(3): 1487-1506.
|
 ), 王文选3, 范春雷4, 马吉亮1,2, 梁财1,2, 陈晓平1,2(
), 王文选3, 范春雷4, 马吉亮1,2, 梁财1,2, 陈晓平1,2( )
)
 ), WANG Wenxuan3, FAN Chunlei4, MA Jiliang1,2, LIANG Cai1,2, CHEN Xiaoping1,2(
), WANG Wenxuan3, FAN Chunlei4, MA Jiliang1,2, LIANG Cai1,2, CHEN Xiaoping1,2( )
)