化工进展 ›› 2025, Vol. 44 ›› Issue (1): 341-353.DOI: 10.16085/j.issn.1000-6613.2023-2275
黑色二氧化钛纳米材料的构筑及其应用现状
刘炜1( ), 张敏2, 朱照琪1, 王毅1, 梁卫东1(
), 张敏2, 朱照琪1, 王毅1, 梁卫东1( ), 孙寒雪1(
), 孙寒雪1( )
)
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃东方钛业有限公司,甘肃 白银 730900
-
收稿日期:2023-12-27修回日期:2024-01-30出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:梁卫东,孙寒雪 -
作者简介:刘炜(1999—),男,硕士研究生,研究方向为微纳孔功能材料。E-mail:2497865718@qq.com。 -
基金资助:甘肃省教育科技创新项目(2021CYZC-10);兰州市人才创新创业项目(2023-RC-11);甘肃省科技计划(22CX8GA121)
Preparation and current applications of black titanium dioxide nanomaterials
LIU Wei1( ), ZHANG Min2, ZHU Zhaoqi1, WANG Yi1, LIANG Weidong1(
), ZHANG Min2, ZHU Zhaoqi1, WANG Yi1, LIANG Weidong1( ), SUN Hanxue1(
), SUN Hanxue1( )
)
- 1.School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Gansu Dongfang Titanium Co. , Ltd. , Baiyin 730900, Gansu, China
-
Received:2023-12-27Revised:2024-01-30Online:2025-01-15Published:2025-02-13 -
Contact:LIANG Weidong, SUN Hanxue
摘要:
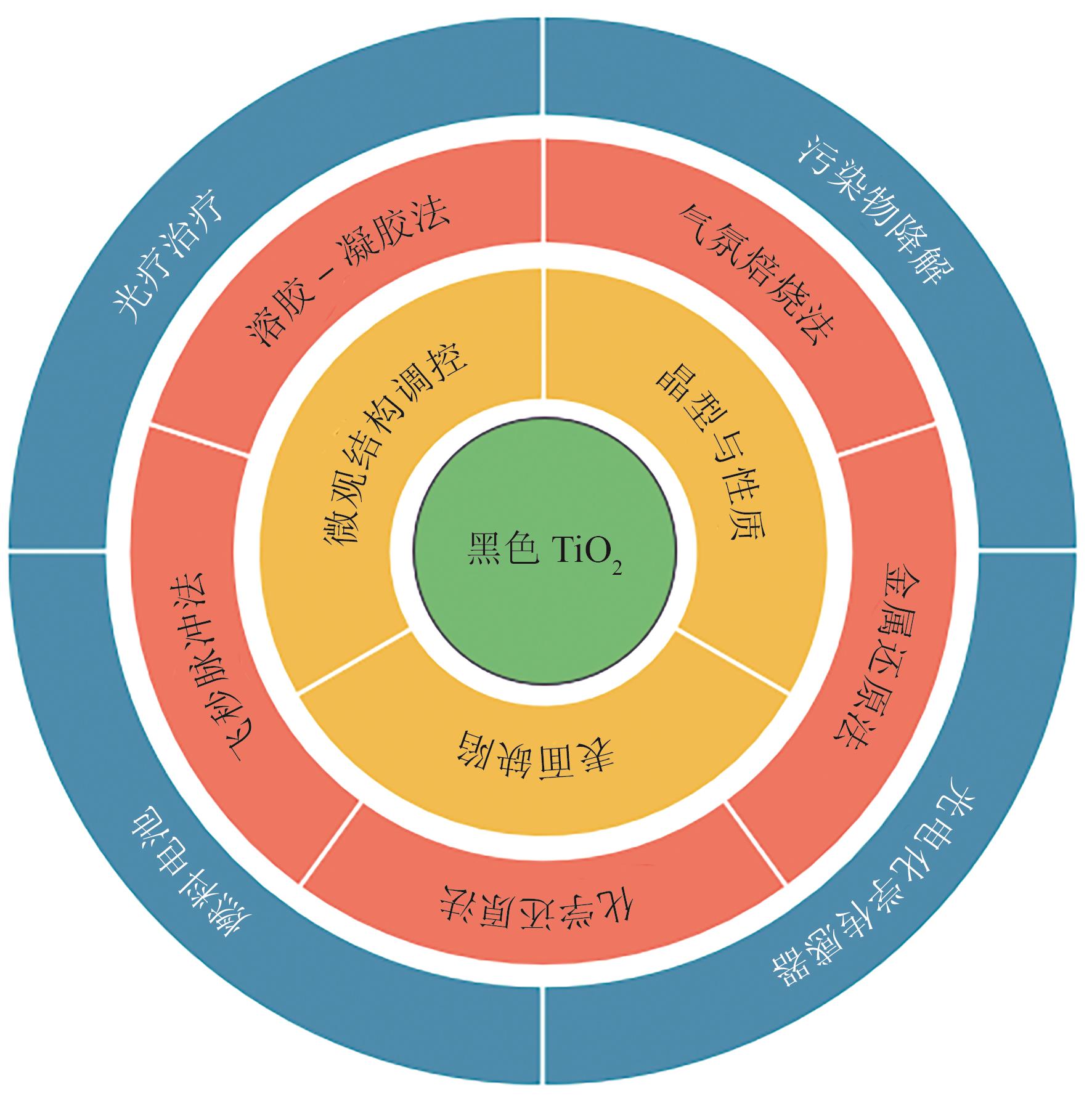
二氧化钛(TiO2)是典型的氧化物半导体材料,广泛应用于光催化领域,但其较宽的能量带隙限制了其在实际中的应用。黑色TiO2(B-TiO2)纳米材料通过在TiO2表面构筑结构缺陷降低带隙宽度,能够有效提高其可见光响应性能和量子效率。本文总结了B-TiO2的基本特性,包括光学性质、晶型、缺陷与微观结构性质,并对近年来B-TiO2的制备与缺陷构筑方法(如气氛焙烧法、溶胶-凝胶法、化学还原法等)进行了系统总结。同时,本文梳理了黑色TiO2性能提升策略及其在水体净化、有机物降解、能源转换及医疗等领域的应用现状。最后指出B-TiO2研究领域未来需要重点关注的几个方面为:结合理论和实验深入探讨其结构-性能之间的构效关系是合理设计B-TiO2表面缺陷、开发高效合成方法的基础,先进的表征和分析手段有待加强;在保持高活性的同时,开发结构更加稳定的B-TiO2是将其推向实际应用的重要前提;此外,交叉学科的发展应推动B-TiO2的应用范围向更多领域延伸。
中图分类号:
引用本文
刘炜, 张敏, 朱照琪, 王毅, 梁卫东, 孙寒雪. 黑色二氧化钛纳米材料的构筑及其应用现状[J]. 化工进展, 2025, 44(1): 341-353.
LIU Wei, ZHANG Min, ZHU Zhaoqi, WANG Yi, LIANG Weidong, SUN Hanxue. Preparation and current applications of black titanium dioxide nanomaterials[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 341-353.
| 样品名称 | 合成方法 | 带隙宽度 /eV | 吸光波长 /nm | 微观结构 | 缺陷类型 | 参考文献 |
|---|---|---|---|---|---|---|
| 黑色 TiO2 纳米晶体 | 气氛焙烧法(H2) | 1.26 | — | 无序工程纳米晶体结构 | Ti3+,氧空位 | [ |
| 黑色 TiO2 纳米粒子 | 气氛焙烧法(真空) | 2.07 | 200~1000 | 介孔颗粒结构 | Ti3+,氧空位 | [ |
| 掺杂氮的黑色TiO2 | 气氛焙烧法(N2) | 1.47 | 400~500 | 锐钛矿相晶体结构 | N元素,氧空位,Ti3+ | [ |
| 黑色TiO2 | 铝热还原法 | 3.02 | — | 超薄空心球 | 表面积,氧空位 | [ |
| 黑色TiO2 | 铝热还原法 盐酸酸洗法 | 2.4~2.8 | 600~800 | 纯锐钛矿晶体结构 | 结构无序、氧空位 | [ |
| 高度畸变S/C/Ti3+掺杂的黑色TiO2 | 溶胶-凝胶法 | 2.53 | — | 球形纳米结构 | Ti3+,氧空位 | [ |
| 具有核壳结构的黑色TiO2 | 溶胶-凝胶法 | 2.96 | 400~600 | 核壳结构 | Ti3+ | [ |
| 具有可控晶格无序的黑色TiO2 | 溶剂挥发诱导自组装法 化学还原法 | 2.65 | 400~800 | 空心球结构 | Ti3+,比较大的比表面积,较高的无序度 | [ |
| 具有反蛋白石结构的负载镍的 黑色TiO2 | 模板法 气氛焙烧法(H2) | 1.71 | 400~800 | IO结构 | Ti3+,掺杂元素Ni | [ |
| 黑色TiO2纳米胶体 | 脉冲激光辐照法 | 1.9 | 400~500 | 球形核结构 | 氧空位,晶格缺陷 | [ |
| 掺杂碳黑的锐钛矿型TiO2纳米棒 | 气氛焙烧法(N2) | 2.4~3.2 | 350~450 | 短纳米棒结构 | Ti3+,氧空位,杂质掺杂 | [ |
| 黑色TiO2纳米板 | 气氛焙烧法(Ar2) | 1.5 | 500~600 | 非晶态结构 | 氧空位,无序结构,Ti3+ | [ |
表1 黑色TiO2的制备方法及特性
| 样品名称 | 合成方法 | 带隙宽度 /eV | 吸光波长 /nm | 微观结构 | 缺陷类型 | 参考文献 |
|---|---|---|---|---|---|---|
| 黑色 TiO2 纳米晶体 | 气氛焙烧法(H2) | 1.26 | — | 无序工程纳米晶体结构 | Ti3+,氧空位 | [ |
| 黑色 TiO2 纳米粒子 | 气氛焙烧法(真空) | 2.07 | 200~1000 | 介孔颗粒结构 | Ti3+,氧空位 | [ |
| 掺杂氮的黑色TiO2 | 气氛焙烧法(N2) | 1.47 | 400~500 | 锐钛矿相晶体结构 | N元素,氧空位,Ti3+ | [ |
| 黑色TiO2 | 铝热还原法 | 3.02 | — | 超薄空心球 | 表面积,氧空位 | [ |
| 黑色TiO2 | 铝热还原法 盐酸酸洗法 | 2.4~2.8 | 600~800 | 纯锐钛矿晶体结构 | 结构无序、氧空位 | [ |
| 高度畸变S/C/Ti3+掺杂的黑色TiO2 | 溶胶-凝胶法 | 2.53 | — | 球形纳米结构 | Ti3+,氧空位 | [ |
| 具有核壳结构的黑色TiO2 | 溶胶-凝胶法 | 2.96 | 400~600 | 核壳结构 | Ti3+ | [ |
| 具有可控晶格无序的黑色TiO2 | 溶剂挥发诱导自组装法 化学还原法 | 2.65 | 400~800 | 空心球结构 | Ti3+,比较大的比表面积,较高的无序度 | [ |
| 具有反蛋白石结构的负载镍的 黑色TiO2 | 模板法 气氛焙烧法(H2) | 1.71 | 400~800 | IO结构 | Ti3+,掺杂元素Ni | [ |
| 黑色TiO2纳米胶体 | 脉冲激光辐照法 | 1.9 | 400~500 | 球形核结构 | 氧空位,晶格缺陷 | [ |
| 掺杂碳黑的锐钛矿型TiO2纳米棒 | 气氛焙烧法(N2) | 2.4~3.2 | 350~450 | 短纳米棒结构 | Ti3+,氧空位,杂质掺杂 | [ |
| 黑色TiO2纳米板 | 气氛焙烧法(Ar2) | 1.5 | 500~600 | 非晶态结构 | 氧空位,无序结构,Ti3+ | [ |
| B-TiO2 | 合成方法 | 目标分子 | 降解率 | 降解机理 | 参考文献 |
|---|---|---|---|---|---|
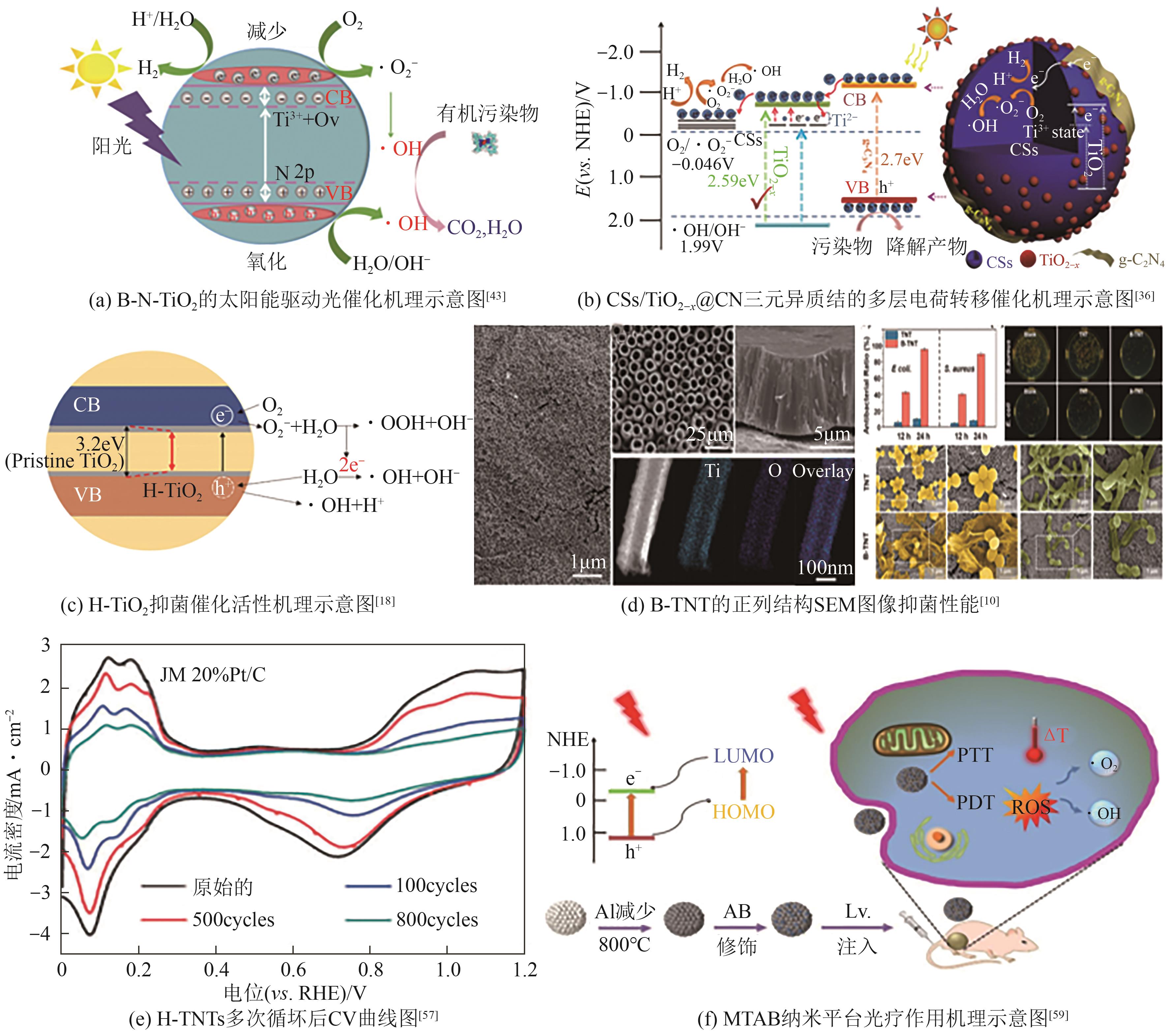
| 金红石型黑色TiO2 | 化学还原法、气氛焙烧法(Ar) | 亚甲基蓝 | 100%(10-5mol/L,20min) | 较窄的带隙显示出较宽的吸光度,且降低了光电子的重组速率,增强了载流子的迁移,产生的H+和·OH是降解亚甲基蓝分子的主要活性物种 | [ |
| 三元碳球(CSs)/ TiO2-x @碳化氮(CN) | 化学还原法 | 2,4,6-三氯苯酚 | >90%(10mg/L,150min) | TiO2-x /g-C3N4异质结与CSs结构中Ti-O-C桥之间多层转移的有效电子传递,使其具有良好的可见光收集能力,同时高的比表面积为吸附和表面反应提供有效反应位点,反应生成·OH和电子空穴有效降解2,4,6-三氯苯酚 | [ |
| 氢化的黑色TiO2 | 气氛烘焙法(H2) | 蚁醛 | 57%(100mg/L,4h) | 表面存在氧空位,并通过杂质缺陷电离产生大量电子;吸附在H-TiO2/H-C-TiO2表面的O2与电子相互作用,转化为活性氧降解甲醛 | [ |
| 黑色TiO2纳米粒子 | 化学还原法、 气氛焙烧法(N2) | 二甲苯 | 35.64%(60mg/L) | CuOTiO2(mb)中Ti4+、Ti3+、Cu2+和Cu+的共存降低了e--h+的复合速率,Cu-MOF前体引入的晶粒最小使其带隙变窄为2.75eV,显著提高了可见光下对二甲苯的去除效率 | [ |
| 黑色TiO2 | 气氛烘焙法 (真空) | 乙酰氨基酚 | 100% (1mg/L,3h) | 黑色TiO2样品的价带比OH-/·OH的氧化还原电位更正,在光催化过程中具有较高的生成·OH自由基的电位 | [ |
| 磁性γ-Fe2O3超薄纳米片修饰的介孔黑色TiO2空心球 | 化学还原法、 气氛烘焙法(H2) | 四环素 | 99.3%(10mg/L,50min) | 辐照后的氧化活性物质主要有四种:羟基自由基、空穴、超氧阴离子和过氧化氢,这些活性氧化物在降解过程中攻击四环素的官能团使其分解 | [ |
| 氮掺杂的黑色TiO2 | 化学还原法、 气氛烘焙法(Ar) | 环丙沙星 | 100%(500mg/L,70min) | 样品具有更高的光致电子空穴对分离效率,通过·OH自由基氧化分解环丙沙星 | [ |
| 磁性Fe2O3修饰的介孔黑色TiO2空心球 | 化学还原法、 气氛烘焙法(H2) | 赛克津 | 99%(10mg /L,60min) | 由于氧空位、Ti3+和Fe2+的形成,使TiO2带隙明显缩小,有利于光生载流子的分离,在水介质中捕获溶解氧,从而形成超氧自由基和H2O2,孔洞与水或OH-反应,使赛克津分子完全矿化 | [ |
表2 B-TiO2在水体污染物去除方面的应用
| B-TiO2 | 合成方法 | 目标分子 | 降解率 | 降解机理 | 参考文献 |
|---|---|---|---|---|---|
| 金红石型黑色TiO2 | 化学还原法、气氛焙烧法(Ar) | 亚甲基蓝 | 100%(10-5mol/L,20min) | 较窄的带隙显示出较宽的吸光度,且降低了光电子的重组速率,增强了载流子的迁移,产生的H+和·OH是降解亚甲基蓝分子的主要活性物种 | [ |
| 三元碳球(CSs)/ TiO2-x @碳化氮(CN) | 化学还原法 | 2,4,6-三氯苯酚 | >90%(10mg/L,150min) | TiO2-x /g-C3N4异质结与CSs结构中Ti-O-C桥之间多层转移的有效电子传递,使其具有良好的可见光收集能力,同时高的比表面积为吸附和表面反应提供有效反应位点,反应生成·OH和电子空穴有效降解2,4,6-三氯苯酚 | [ |
| 氢化的黑色TiO2 | 气氛烘焙法(H2) | 蚁醛 | 57%(100mg/L,4h) | 表面存在氧空位,并通过杂质缺陷电离产生大量电子;吸附在H-TiO2/H-C-TiO2表面的O2与电子相互作用,转化为活性氧降解甲醛 | [ |
| 黑色TiO2纳米粒子 | 化学还原法、 气氛焙烧法(N2) | 二甲苯 | 35.64%(60mg/L) | CuOTiO2(mb)中Ti4+、Ti3+、Cu2+和Cu+的共存降低了e--h+的复合速率,Cu-MOF前体引入的晶粒最小使其带隙变窄为2.75eV,显著提高了可见光下对二甲苯的去除效率 | [ |
| 黑色TiO2 | 气氛烘焙法 (真空) | 乙酰氨基酚 | 100% (1mg/L,3h) | 黑色TiO2样品的价带比OH-/·OH的氧化还原电位更正,在光催化过程中具有较高的生成·OH自由基的电位 | [ |
| 磁性γ-Fe2O3超薄纳米片修饰的介孔黑色TiO2空心球 | 化学还原法、 气氛烘焙法(H2) | 四环素 | 99.3%(10mg/L,50min) | 辐照后的氧化活性物质主要有四种:羟基自由基、空穴、超氧阴离子和过氧化氢,这些活性氧化物在降解过程中攻击四环素的官能团使其分解 | [ |
| 氮掺杂的黑色TiO2 | 化学还原法、 气氛烘焙法(Ar) | 环丙沙星 | 100%(500mg/L,70min) | 样品具有更高的光致电子空穴对分离效率,通过·OH自由基氧化分解环丙沙星 | [ |
| 磁性Fe2O3修饰的介孔黑色TiO2空心球 | 化学还原法、 气氛烘焙法(H2) | 赛克津 | 99%(10mg /L,60min) | 由于氧空位、Ti3+和Fe2+的形成,使TiO2带隙明显缩小,有利于光生载流子的分离,在水介质中捕获溶解氧,从而形成超氧自由基和H2O2,孔洞与水或OH-反应,使赛克津分子完全矿化 | [ |
| 1 | RAJARAMAN T S, PARIKH Sachin P, GANDHI Vimal G. Black TiO2: A review of its properties and conflicting trends[J]. Chemical Engineering Journal, 2020, 389: 123918. |
| 2 | LIAO Lijun, WANG Mingtao, LI Zhenzi, et al. Recent advances in black TiO2 nanomaterials for solar energy conversion[J]. Nanomaterials, 2023, 13(3): 468. |
| 3 | DETTE Christian, PÉREZ-OSORIO Miguel A, KLEY Christopher S, et al. TiO2 anatase with a bandgap in the visible region[J]. Nano Letters, 2014, 14(11): 6533-6538. |
| 4 | 张甄, 王宝冬, 徐文强, 等. 黑色二氧化钛纳米材料研究进展[J]. 材料导报, 2019, 33(S1): 8-15. |
| ZHANG Zhen, WANG Baodong, XU Wenqiang, et al. Research progress of black titanium dioxide nanomaterials[J]. Materials Reports, 2019, 33(S1): 8-15. | |
| 5 | CHEN Xiaobo, LIU Lei, YU Peter Y, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331(6018): 746-750. |
| 6 | WANG Xiangdong, FU Rong, YIN Qianqian, et al. Black TiO2 synthesized via magnesiothermic reduction for enhanced photocatalytic activity[J]. Journal of Nanoparticle Research, 2018, 20(4): 89. |
| 7 | YU Xiaomei, KIM Boseong, KIM Yu Kwon. Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation-induced surface defects[J]. ACS Catalysis, 2013, 3(11): 2479-2486. |
| 8 | SINHAMAHAPATRA Apurba, JEON Jong-Pil, YU Jong-Sung. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production[J]. Energy & Environmental Science, 2015, 8(12): 3539-3544. |
| 9 | SONG Hui, LI Chenxi, LOU Zirui, et al. Effective formation of oxygen vacancies in black TiO2 nanostructures with efficient solar-driven water splitting[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 8982-8987. |
| 10 | LI Zhe, WANG Engui, ZHANG Yingzi, et al. Antibacterial ability of black titania in dark: Via oxygen vacancies mediated electron transfer[J]. Nano Today, 2023, 50: 101826. |
| 11 | 张开富. 多孔黑二氧化钛及其复合体的制备与光催化性能研究[D]. 哈尔滨: 黑龙江大学, 2016. |
| ZHANG Kaifu. Preparation and photocatalytic properties of porous black titanium dioxide and its complex[D]. Harbin: Helongjiang University, 2016. | |
| 12 | KATAL Reza, SALEHI Mojtaba, FARAHANI Mohammad Hossein Davood Abadi, et al. Preparation of a new type of black TiO2 under a vacuum atmosphere for sunlight photocatalysis[J]. ACS Applied Materials & Interfaces, 2018, 10(41): 35316-35326. |
| 13 | YE Jin, HE Jiahui, WANG Shuang, et al. Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light[J]. Separation and Purification Technology, 2019, 220: 8-15. |
| 14 | ZHOU Li, CAI Min, ZHANG Xu, et al. In-situ nitrogen-doped black TiO2 with enhanced visible-light-driven photocatalytic inactivation of Microcystis aeruginosa cells: Synthesization, performance and mechanism[J]. Applied Catalysis B: Environmental, 2020, 272: 119019. |
| 15 | PATIL Shivaraj B, PHATTEPUR Harish, NAGARAJU G, et al. Highly distorted mesoporous S/C/Ti3+ doped black TiO2 for simultaneous visible light degradation of multiple dyes[J]. New Journal of Chemistry, 2020, 44(23): 9830-9836. |
| 16 | YANG Chongyin, WANG Zhou, LIN Tianquan, et al. Core-shell nanostructured “black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping[J]. Journal of the American Chemical Society, 2013, 135(47): 17831-17838. |
| 17 | WU Tong, FAN Jinchen, LI Qiaoxia, et al. Palladium nanoparticles anchored on anatase titanium dioxide-black phosphorus hybrids with heterointerfaces: Highly electroactive and durable catalysts for ethanol electrooxidation[J]. Advanced Energy Materials, 2018, 8(1): 1701799. |
| 18 | AN Ha-Rim, PARK So Young, Jin Young HUH, et al. Nanoporous hydrogenated TiO2 photocatalysts generated by underwater discharge plasma treatment for solar photocatalytic applications[J]. Applied Catalysis B: Environmental, 2017, 211: 126-136. |
| 19 | ZHENG Peiru, ZHANG Lishu, ZHANG Xingfan, et al. Hydrogenation of TiO2 nanosheets and nanoparticles: Typical reduction stages and orientation-related anisotropic disorder[J]. Journal of Materials Chemistry A, 2021, 9(39): 22603-22614. |
| 20 | HAN Lijuan, AN Xingcai, ZHANG Ping, et al. The removal of formaldehyde via visible photocatalysis using the black TiO2 nanoparticles with mesoporous[J]. ChemistrySelect, 2020, 5(1): 97-103. |
| 21 | ZHANG Xu, CAI Min, CUI Naxin, et al. Defective black TiO2: Effects of annealing atmospheres and urea addition on the properties and photocatalytic activities[J]. Nanomaterials, 2021, 11(10): 2648. |
| 22 | LI Jun, WU Enhui, HOU Jing, et al. A facile method for the preparation of black TiO2 by Al reduction of TiO2 and their visible light photocatalytic activity[J]. RSC Advances, 2020, 10(57): 34775-34780. |
| 23 | HAIDER Sajjad, NAWAZ Rab, ANJUM Muzammil, et al. Property-performance relationship of core-shell structured black TiO2 photocatalyst for environmental remediation[J]. Frontiers of Environmental Science & Engineering, 2023, 17(9): 111. |
| 24 | JIANG Xiongrui, YAN Zhiyao, ZHANG Jing, et al. Mesoporous hollow black TiO2 with controlled lattice disorder degrees for highly efficient visible-light-driven photocatalysis[J]. RSC Advances, 2019, 9(63): 36907-36914. |
| 25 | RAVEENDRAN NAIR Pooja, ROSA SANTIAGO RAMIREZ Claudia, ANGEL GRACIA PINILLA Miguel, et al. Black titanium dioxide nanocolloids by laser irradiation in liquids for visible light photo-catalytic/electrochemical applications[J]. Applied Surface Science, 2023, 623: 157096. |
| 26 | LI Wenjuan, LIANG Robert, ZHOU Norman Y, et al. Carbon black-doped anatase TiO2 nanorods for solar light-induced photocatalytic degradation of methylene blue[J]. ACS Omega, 2020, 5(17): 10042-10051. |
| 27 | KANG Jianxin, ZHANG Yan, CHAI Ziwei, et al. Amorphous domains in black titanium dioxide[J]. Advanced Materials, 2021, 33(23): e2100407. |
| 28 | GAMBOA Julio Andrade, PASQUEVICH Daniel M. Effect of chlorine atmosphere on the anatase-rutile transformation[J]. Journal of the American Ceramic Society, 1992, 75(11): 2934-2938. |
| 29 | Joohyun LIM, KIM Se-Ho, AYMERICH ARMENGOL Raquel Aymerich, et al. Atomic-scale mapping of impurities in partially reduced hollow TiO2 nanowires[J]. Angewandte Chemie International Edition, 2020, 59(14): 5651-5655. |
| 30 | ZHU Guilian, YIN Hao, YANG Chongyin, et al. Black titania for superior photocatalytic hydrogen production and photoelectrochemical water splitting[J]. ChemCatChem, 2015, 7(17): 2614-2619. |
| 31 | XIAO Bo, YU Fang, XIA Yue, et al. Wood-based, bifunctional, mulberry-like nanostructured black titania evaporator for solar-driven clean water generation[J]. Energy Technology, 2022, 10(3): 2100679. |
| 32 | LI Zebiao, BIAN Haidong, XU Zhengtao, et al. Solution-based comproportionation reaction for facile synthesis of black TiO2 nanotubes and nanoparticles[J]. ACS Applied Energy Materials, 2020, 3(7): 6087-6092. |
| 33 | ZHAN Xuepeng, XU Huailiang, LI Chunhao, et al. Remote and rapid micromachining of broadband low-reflectivity black silicon surfaces by femtosecond laser filaments[J]. Optics Letters, 2017, 42(3): 510-513. |
| 34 | SU Yue, ZHANG Wei, CHEN Shanming, et al. Engineering black titanium dioxide by femtosecond laser filament[J]. Applied Surface Science, 2020, 520: 146298. |
| 35 | GRABSTANOWICZ Lauren R, GAO Shanmin, LI Tao, et al. Facile oxidative conversion of TiH2 to high-concentration Ti3+-self-doped rutile TiO2 with visible-light photoactivity[J]. Inorganic Chemistry, 2013, 52(7): 3884-3890. |
| 36 | ZHU Chengzhang, CHEN Xiao, MA Jian, et al. Carbon nitride-modified defective TiO2– x @Carbon spheres for photocatalytic H2 evolution and pollutants removal: Synergistic effect and mechanism insight[J]. The Journal of Physical Chemistry C, 2018, 122(35): 20444-20458. |
| 37 | ZENG Lei, SONG Wulin, LI Minghui, et al. Catalytic oxidation of formaldehyde on surface of H-TiO2/H-C-TiO2 without light illumination at room temperature[J]. Applied Catalysis B: Environmental, 2014, 147: 490-498. |
| 38 | ZHOU Wenjun, SHEN Boxiong, WANG Fumei, et al. Enhanced photocatalytic degradation of xylene by blackening TiO2 nanoparticles with high dispersion of CuO[J]. Journal of Hazardous Materials, 2020, 391: 121642. |
| 39 | REN Liping, ZHOU Wei, SUN Bojing, et al. Defects-engineering of magnetic γ-Fe2O3 ultrathin nanosheets/mesoporous black TiO2 hollow sphere heterojunctions for efficient charge separation and the solar-driven photocatalytic mechanism of tetracycline degradation[J]. Applied Catalysis B: Environmental, 2019, 240: 319-328. |
| 40 | SARAFRAZ Mansour, SADEGHI Morteza, YAZDANBAKHSH Ahmadreza, et al. Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: Kinetic, energy consumption, degradation pathway, and toxicity assessment[J]. Process Safety and Environmental Protection, 2020, 137: 261-272. |
| 41 | SUN Bojing, ZHOU Wei, LI Haoze, et al. Magnetic Fe2O3/mesoporous black TiO2 hollow sphere heterojunctions with wide-spectrum response and magnetic separation[J]. Applied Catalysis B: Environmental, 2018, 221: 235-242. |
| 42 | CAREY John H, LAWRENCE John, TOSINE Helle M. Photodechlorination of PCB in the presence of titanium dioxide in aqueous suspensions[J].Bull of Environ Contam Toxic,1976,16(6): 697-701. |
| 43 | CAO Yan, XING Zipeng, SHEN Yingcai, et al. Mesoporous black Ti3+/N-TiO2 spheres for efficient visible-light-driven photocatalytic performance[J]. Chemical Engineering Journal, 2017, 325: 199-207. |
| 44 | JIANG Jiaojiao, XING Zipeng, LI Meng, et al. In situ Ti3+/N-codoped three-dimensional (3D) urchinlike black TiO2 architectures as efficient visible-light-driven photocatalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(28): 7948-7956. |
| 45 | XUE Chaorui, LI Dong, LI Yangsen, et al. 3D-carbon dots decorated black TiO2 nanotube Array@Ti foam with enhanced photothermal and photocatalytic activities[J]. Ceramics International, 2019, 45(14): 17512-17520. |
| 46 | DU Jimin, WANG Huiming, CHEN Huijuan, et al. Synthesis and enhanced photocatalytic activity of black porous Zr-doped TiO2 monoliths[J]. Nano, 2016, 11(6): 1650068. |
| 47 | ZHANG Hang, XING Zipeng, ZHANG Yan, et al. Ni2+ and Ti3+ Co-doped porous black anatase TiO2 with unprecedented-high visible-light-driven photocatalytic degradation performance[J]. RSC Advances, 2015, 5(129): 107150-107157. |
| 48 | YANG Jingxia, ZHANG Jingjin, ZOU Bingjie, et al. Black SnO2-TiO2 nanocomposites with high dispersion for photocatalytic and photovoltalic applications[J]. ACS Applied Nano Materials, 2020, 3(5): 4265-4273. |
| 49 | CHEN Ping. A novel synthesis of Ti3+ self-doped Ag2O/TiO2 (p-n) nanoheterojunctions for enhanced visible photocatalytic activity[J]. Materials Letters, 2016, 163: 130-133. |
| 50 | LI Kai, HUANG Zhenyu, ZENG Xiaoqiao, et al. Synergetic effect of Ti3+ and oxygen doping on enhancing photoelectrochemical and photocatalytic properties of TiO2/g-C3N4 heterojunctions[J]. ACS Applied Materials & Interfaces, 2017, 9(13): 11577-11586. |
| 51 | WANG Shanchi, CAI Jingsheng, MAO Jiajun, et al. Defective black Ti3+ self-doped TiO2 and reduced graphene oxide composite nanoparticles for boosting visible-light driven photocatalytic and photoelectrochemical activity[J]. Applied Surface Science, 2019, 467/468: 45-55. |
| 52 | WILKINSON John L, BOXALL Alistair B A, KOLPIN Dana W, et al. Pharmaceutical pollution of the world’s rivers[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(8): e2113947119. |
| 53 | WU Suqing, LI Xueyan, TIAN Yanqin, et al. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light[J]. Chemical Engineering Journal, 2021, 406: 126747. |
| 54 | ANDRONIC Luminita, GHICA Daniela, STEFAN Mariana, et al. Visible-light-active black TiO2 nanoparticles with efficient photocatalytic performance for degradation of pharmaceuticals[J]. Nanomaterials, 2022, 12(15): 2563. |
| 55 | GAO Peiru, NOOR Nor Qhairul Izzreen Mohd, SHAARANI Sharifudin Md. Current status of food safety hazards and health risks connected with aquatic food products from Southeast Asian Region[J]. Critical Reviews in Food Science and Nutrition, 2022, 62(13): 3471-3489. |
| 56 | WANG Gongming, WANG Hanyu, LING Yichuan, et al. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting[J]. Nano Letters, 2011, 11(7): 3026-3033. |
| 57 | ZHANG Changkun, YU Hongmei, LI Yongkun, et al. Supported noble metals on hydrogen-treated TiO2 nanotube arrays as highly ordered electrodes for fuel cells[J]. ChemSusChem, 2013, 6(4): 659-666. |
| 58 | TOUNI Aikaterini, LIU Xin, KANG Xiaolan, et al. Methanol oxidation at platinum coated black titania nanotubes and titanium felt electrodes[J]. Molecules, 2022, 27(19): 6382. |
| 59 | LIU Ning, ZHU Ming, NIU Niu, et al. Aza-BODIPY probe-decorated mesoporous black TiO2 nanoplatform for the highly efficient synergistic phototherapy[J]. ACS Applied Materials & Interfaces, 2020, 12(37): 41071-41078. |
| 60 | DAI Ting, HE Wenming, TU Shuangshuang, et al. Black TiO2 nanoprobe-mediated mild phototherapy reduces intracellular lipid levels in atherosclerotic foam cells via cholesterol regulation pathways instead of apoptosis[J]. Bioactive Materials, 2022, 17: 18-28. |
| [1] | 李洪慧, 李青云, 李梅, 方艺燕, 沈慧婷, 林宏飞. 生物法处理典型难降解铁氰络合物[J]. 化工进展, 2025, 44(2): 1163-1169. |
| [2] | 闫鹏程, 高卓凡, 周志辉, 吴红丹, 陈霞, 周显, 范泽宇, 邓闪闪, 鲁麒, 向媛. 聚酰胺/聚醚醚酮复合膜的制备及其有机溶剂纳滤性能[J]. 化工进展, 2025, 44(2): 1147-1156. |
| [3] | 赵珂, 张恒, 翟倩, 甄琪, 苏天阳, 崔景强. PLA/PEG@SDS超细纤维水蒸发器的复合结构设计及其液体传输行为[J]. 化工进展, 2025, 44(2): 1014-1024. |
| [4] | 游小银, 汪楚乔, 刘才华, 彭小明. Z型CN/NGBO/BV催化剂体系的构筑及光类芬顿降解四环素性能[J]. 化工进展, 2025, 44(1): 286-296. |
| [5] | 吴迪, 彭明国, 谷宇, 毛林强, 张文艺. 磁性纳米破乳剂的制备及其稠油乳状液破乳性能[J]. 化工进展, 2025, 44(1): 436-444. |
| [6] | 蒋莉萍, 张雪乔, 钟晓娟, 魏于凡, 肖利, 郭旭晶, 羊依金. 钒渣酸浸提铁工艺优化及复合光催化剂的制备[J]. 化工进展, 2025, 44(1): 538-548. |
| [7] | 杜小聪, 辛春福, 赵钰. 路用复合相变材料及相变改性沥青性能评价[J]. 化工进展, 2024, 43(S1): 419-430. |
| [8] | 马桂璇, 徐子桐, 肖志华, 宁国庆, 魏强, 徐春明. 氧硫双掺杂CNTs水系导电剂辅助构筑高性能石墨/SiO负极[J]. 化工进展, 2024, 43(S1): 443-456. |
| [9] | 高觊兴, 丁玉梅, 张超, 谭晶, 丁熙, 李好义, 杨卫民. 熔体微分电纺PLA/PCL微纳米纤维膜的制备及其性能[J]. 化工进展, 2024, 43(S1): 457-468. |
| [10] | 慕铭, 赵伟伟, 陈光孟, 刘小青. 基于激光诱导石墨烯的应变传感器研究进展[J]. 化工进展, 2024, 43(9): 4970-4979. |
| [11] | 申纯宇, 李翠利, 汤建伟, 刘咏, 刘鹏飞, 丁俊祥, 申博, 王保明. 纳米氢氧化镁制备及其阻燃应用进展[J]. 化工进展, 2024, 43(9): 4980-4995. |
| [12] | 李镇武, 蒲迪, 熊亚春, 吴定莹, 金诚, 郭拥军. 驱油用纳米材料在提高采收率方面研究进展[J]. 化工进展, 2024, 43(9): 5035-5048. |
| [13] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| [14] | 李美萱, 成建凤, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王俊莲, 王春霞, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 高电压镍锰酸锂正极材料的合成与电化学机理[J]. 化工进展, 2024, 43(9): 5086-5094. |
| [15] | 楼高波, 姚潇翎, 倪静雯, 傅深渊, 刘丽娜. 离子络合物改性二维云母环氧树脂复合材料的制备及性能[J]. 化工进展, 2024, 43(9): 5142-5156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||