化工进展 ›› 2024, Vol. 43 ›› Issue (9): 4980-4995.DOI: 10.16085/j.issn.1000-6613.2023-1298
• 材料科学与技术 • 上一篇
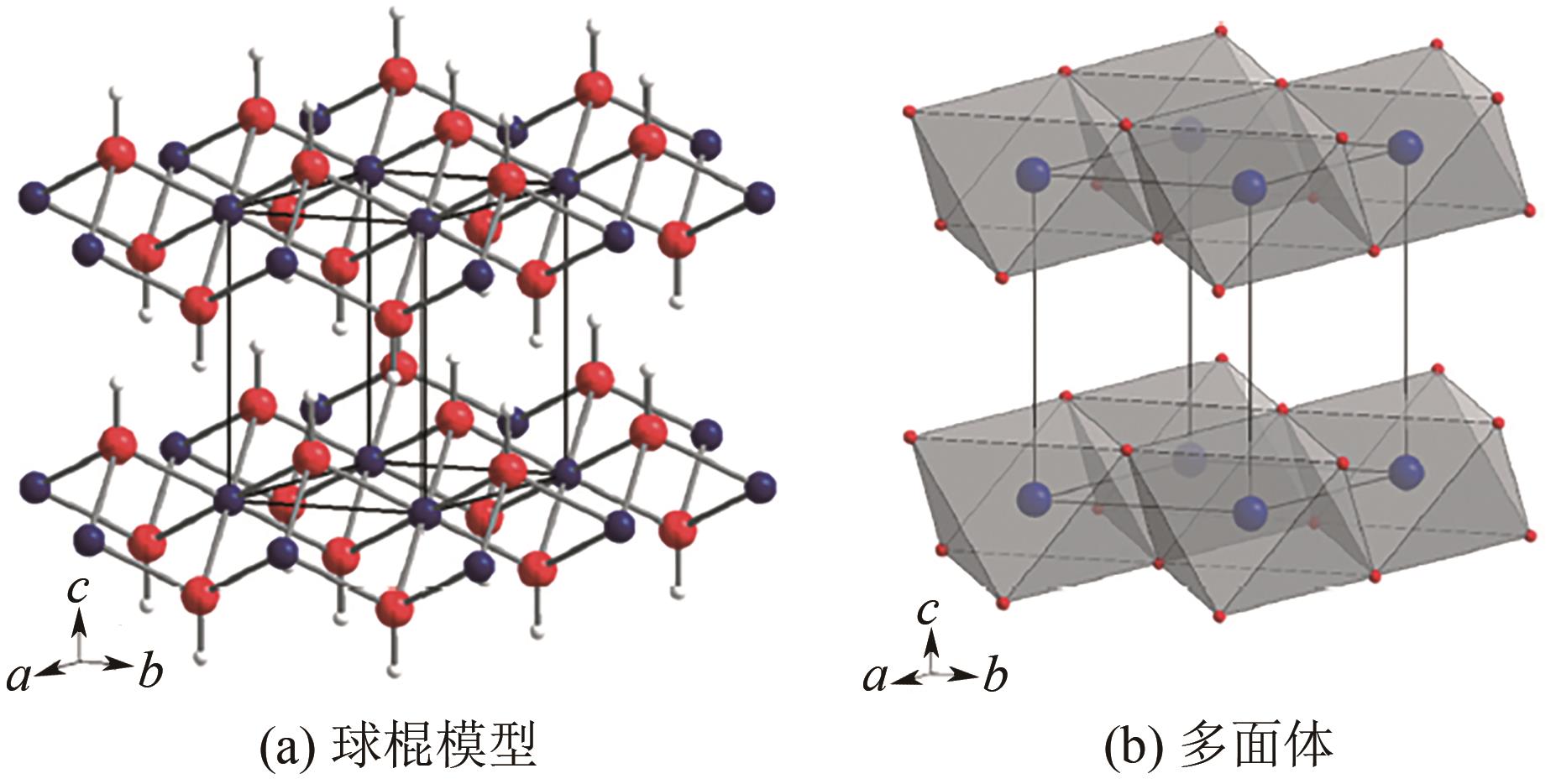
纳米氢氧化镁制备及其阻燃应用进展
申纯宇1( ), 李翠利2, 汤建伟1,3,4, 刘咏2,3, 刘鹏飞1,3,4, 丁俊祥1,3,4, 申博1,3,4, 王保明1,3,4(
), 李翠利2, 汤建伟1,3,4, 刘咏2,3, 刘鹏飞1,3,4, 丁俊祥1,3,4, 申博1,3,4, 王保明1,3,4( )
)
- 1.郑州大学生态与环境学院,河南 郑州 450001
2.郑州大学化工学院,河南 郑州 450001
3.郑州大学国家钙镁磷复合肥技术研究推广中心,河南 郑州 450001
4.郑州大学河南省减污降碳协同工程 技术研究中心,河南 郑州 450001
-
收稿日期:2023-07-30修回日期:2023-09-25出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:王保明 -
作者简介:申纯宇(1999—),男,硕士研究生,研究方向为纳米氢氧化镁制备与结构调控。E-mail:1395036067@qq.com。 -
基金资助:国家自然科学基金面上项目(22278381);郑州大学青年骨干教师培养计划(2021ZDGGJS015)
Progress in preparation and flame retardant application of nano magnesium hydroxide
SHEN Chunyu1( ), LI Cuili2, TANG Jianwei1,3,4, LIU Yong2,3, LIU Pengfei1,3,4, DING Junxiang1,3,4, SHEN Bo1,3,4, WANG Baoming1,3,4(
), LI Cuili2, TANG Jianwei1,3,4, LIU Yong2,3, LIU Pengfei1,3,4, DING Junxiang1,3,4, SHEN Bo1,3,4, WANG Baoming1,3,4( )
)
- 1.School of Ecology and Environment, Zhengzhou University, Zhengzhou 450001, Henan, China
2.School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, Henan, China
3.National Center for Research & Popularization on Calcium, Magnesium, Phosphate and Compound Fertilizer Technology, Zhengzhou University, Zhengzhou 450001, Henan, China
4.Research Centre of Engineering and Technology for Synergetic Control of Environmental Pollution and Carbon Emissions of Henan Province, Zhengzhou University, Zhengzhou 450001, Henan, China
-
Received:2023-07-30Revised:2023-09-25Online:2024-09-15Published:2024-09-30 -
Contact:WANG Baoming
摘要:
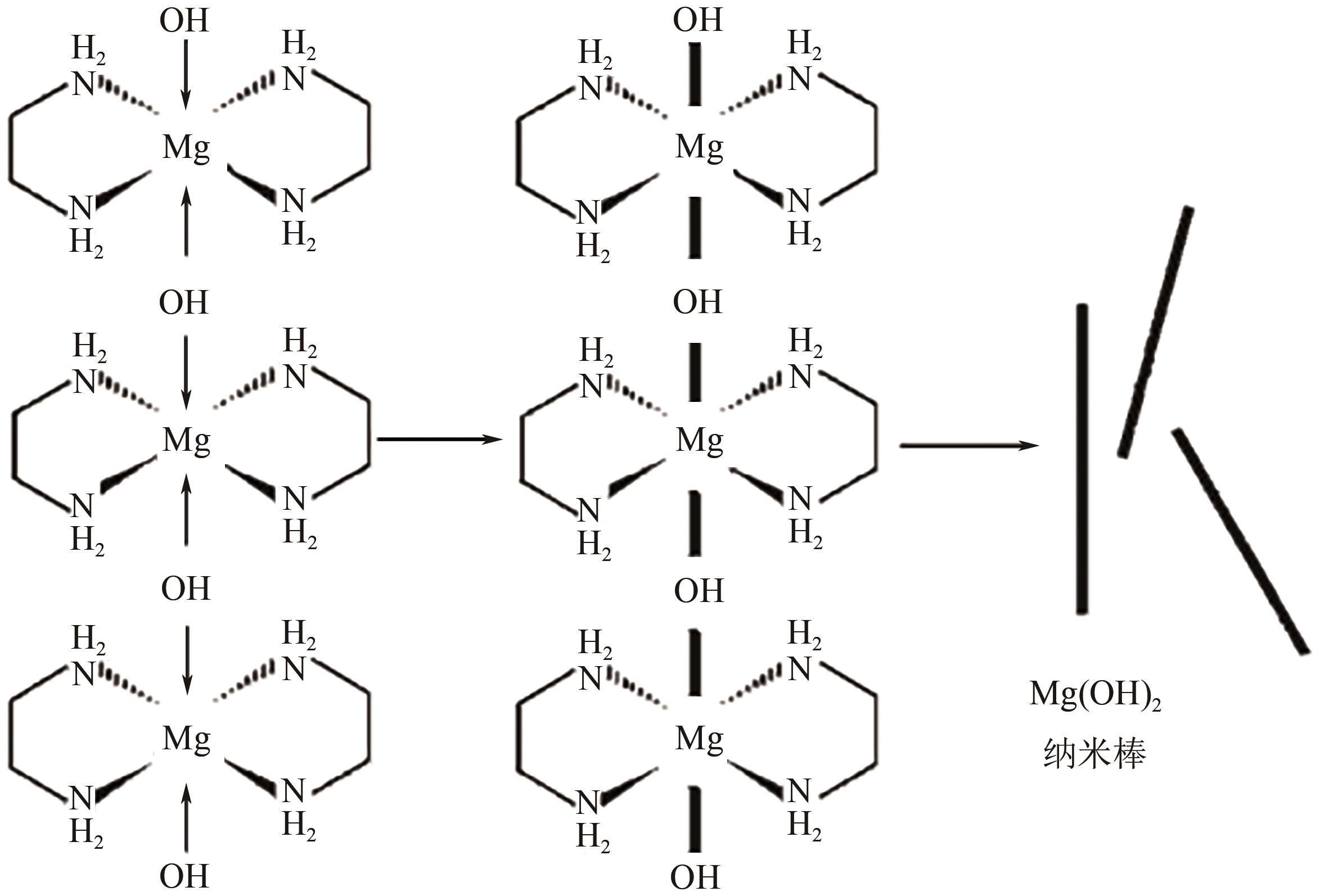
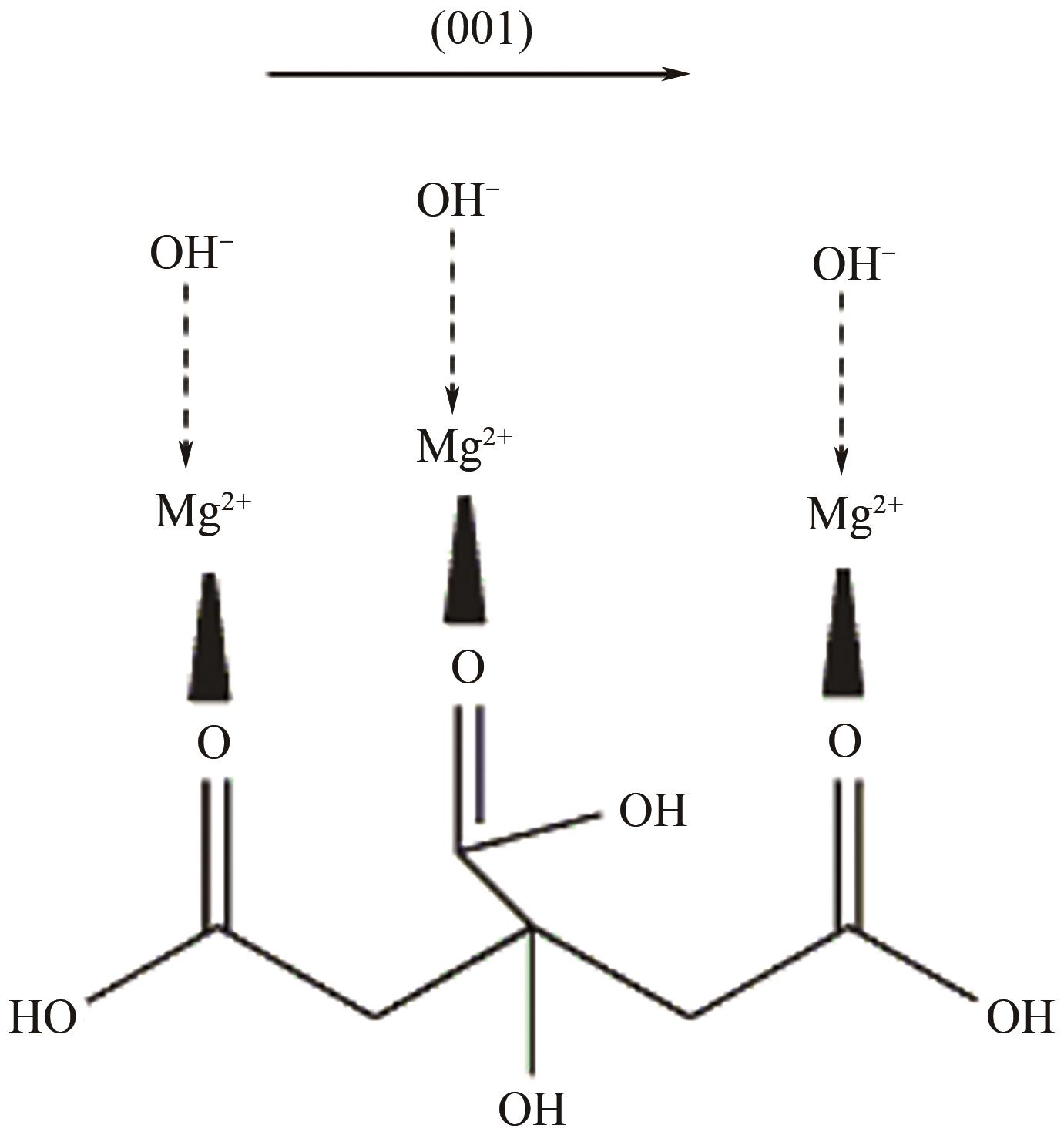
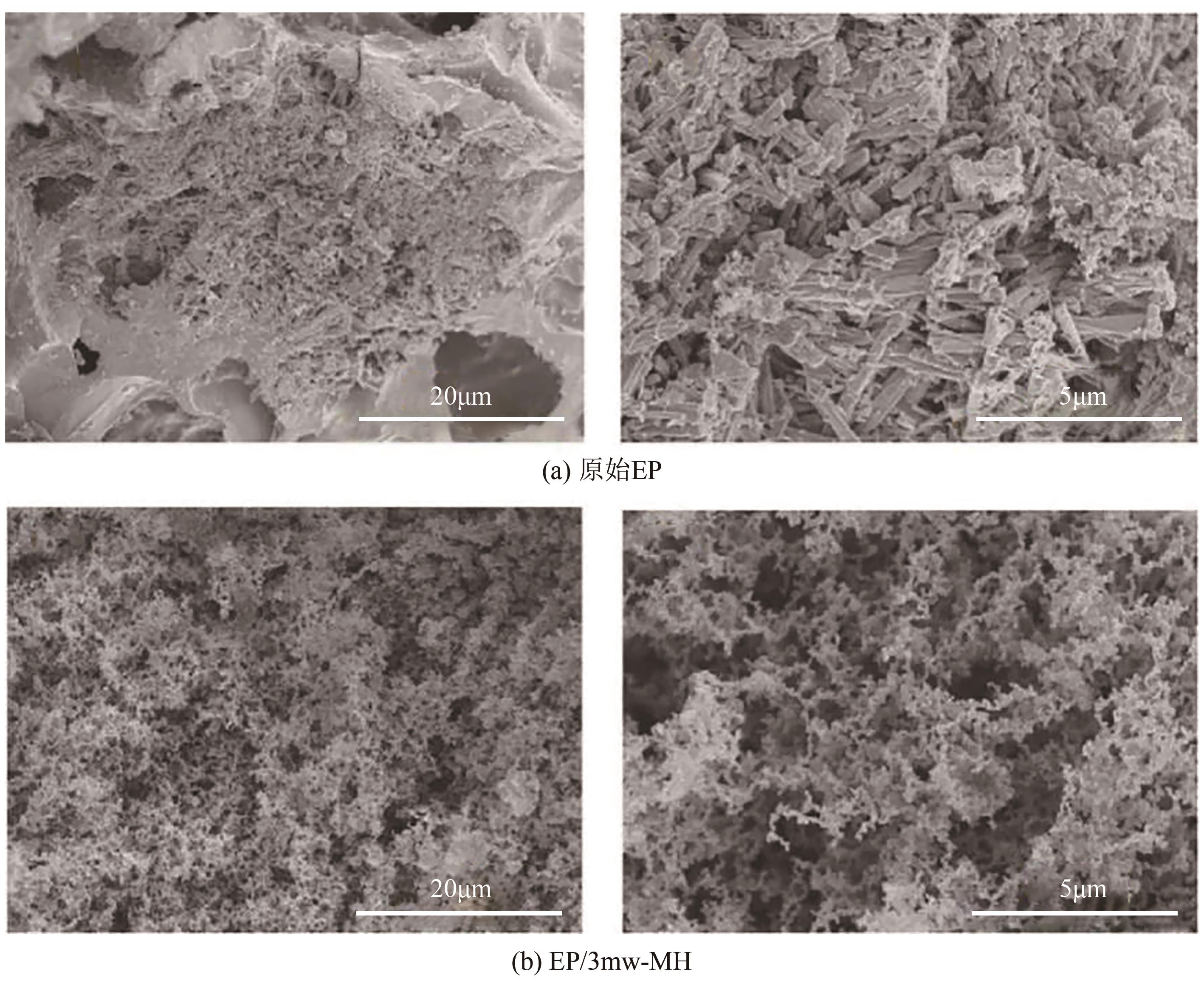
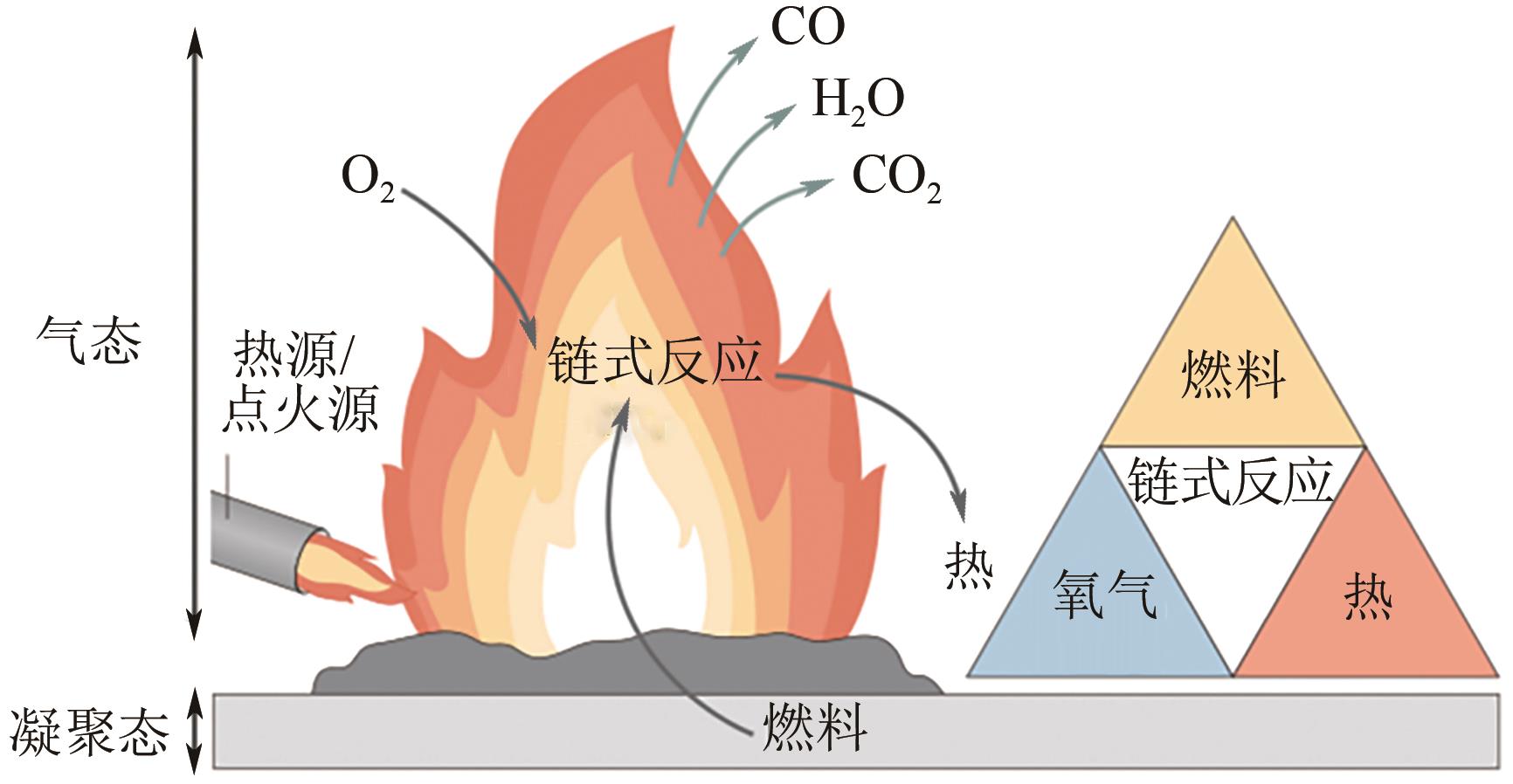
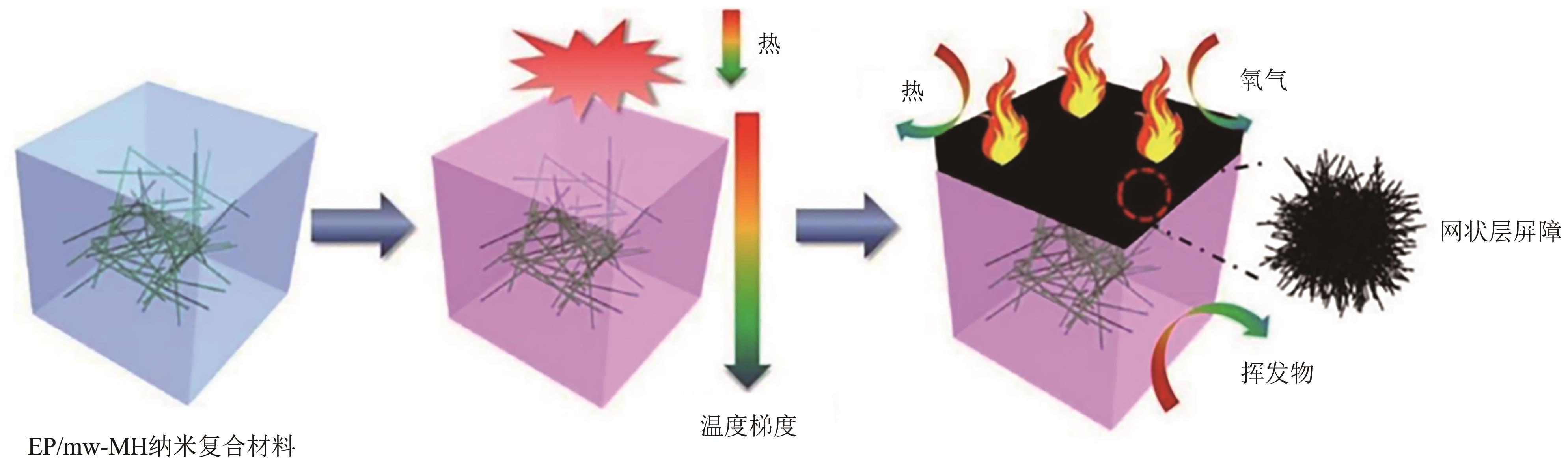
纳米氢氧化镁因其绿色、高效等优势在塑料、橡胶等高分子材料的阻燃方面有广泛的应用前景。随着研究的深入,沉淀法、水热/溶剂热法和微波辅助法等制备方法已在纳米氢氧化镁的制备中占据主导。本文首先从纳米氢氧化镁形貌与粒径的控制方面介绍了这3种主流制备方法,对部分制备过程中的作用机理进行了探究,对每种制备方法的优劣进行了阐述,进而对制备方法进行对比总结。其次讨论了纳米氢氧化镁在应用领域存在的问题及解决办法,概述了其在多种高分子材料阻燃领域的应用,并以理论结合实例从多个角度探究了纳米氢氧化镁的阻燃机理。最后对目前纳米氢氧化镁制备方法和应用方面遇到的机遇和挑战进行了展望,希望能为纳米氢氧化镁的进一步深入研究提供有益的启发和参考。
中图分类号:
引用本文
申纯宇, 李翠利, 汤建伟, 刘咏, 刘鹏飞, 丁俊祥, 申博, 王保明. 纳米氢氧化镁制备及其阻燃应用进展[J]. 化工进展, 2024, 43(9): 4980-4995.
SHEN Chunyu, LI Cuili, TANG Jianwei, LIU Yong, LIU Pengfei, DING Junxiang, SHEN Bo, WANG Baoming. Progress in preparation and flame retardant application of nano magnesium hydroxide[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4980-4995.
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间/h | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|
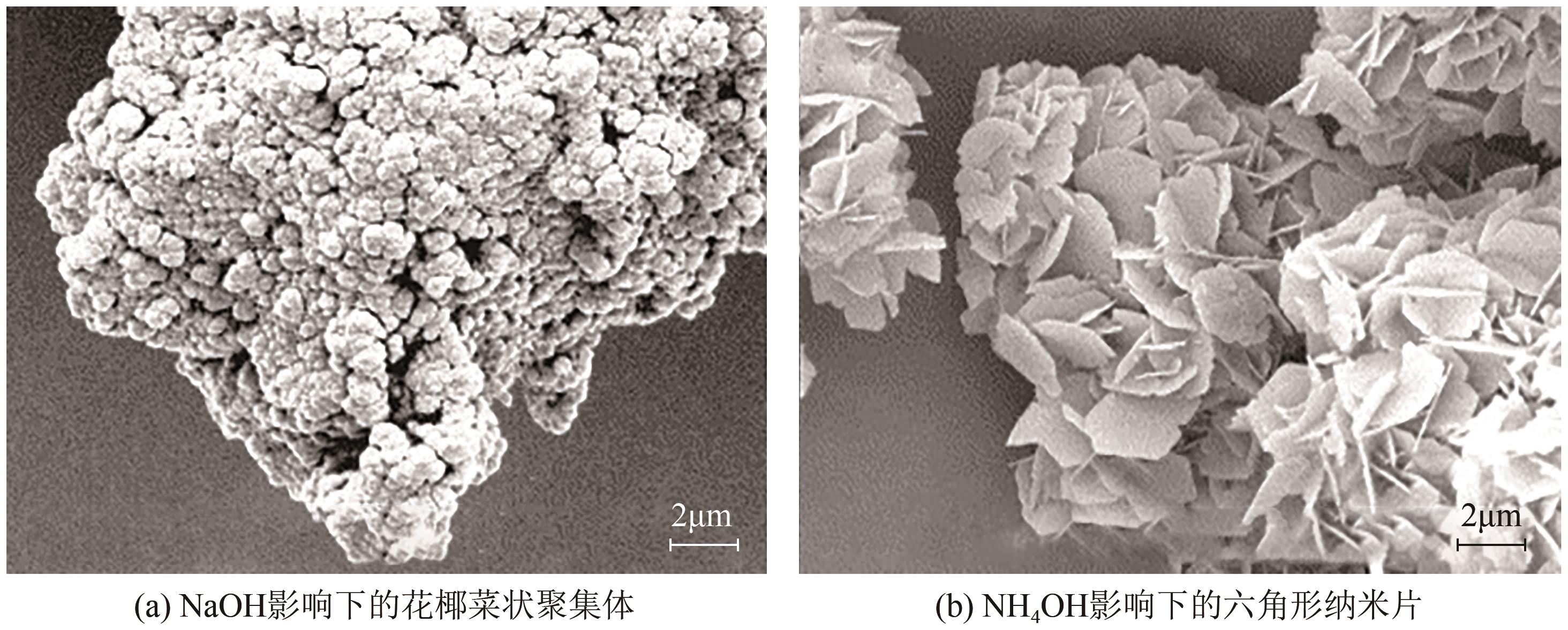
| MgCl2 | NaOH、H2O | — | 60 | 24 | 花椰菜状 | 300 | [ |
| MgCl2 | NH3·H2O | — | 60 | 24 | 片状 | 302×26 | [ |
| Mg(NO3)2 | NH3·H2O | — | 25 | 24 | 片状 | 351×10 | [ |
| MgSO4 | NH3·H2O | — | 25 | 24 | 片状 | 250×15 | [ |
| MgCl2 | NaOH、H2O | — | 60 | 1 | 片状 | 30~150(正向沉淀法) 20~50(反向沉淀法) 10~70(双向沉淀法) 30~50(超重力沉淀法) | [ |
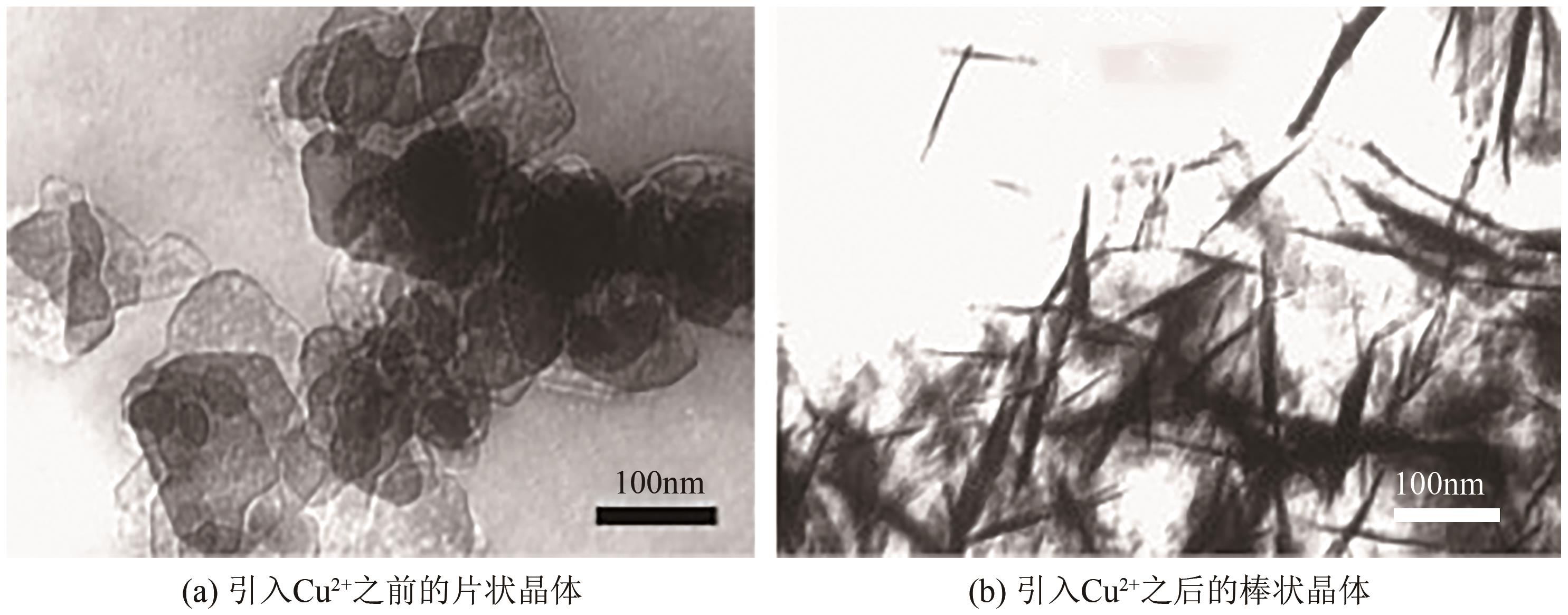
| MgSO4 | NaOH、H2O | 硬脂酸镁(MgSA)、 五水硫酸铜 | 50 | 4.5 | 棒状 | 150×12 | [ |
| MgCl2 | NaOH、H2O | NaCl | 60~100 | 50~200min | 球状 | 6~30μm | [ |
| Mg(NO3)2 | NaOH、H2O | 海藻提取物 | — | 4 | 片状 | 7.75×16.29 | [ |
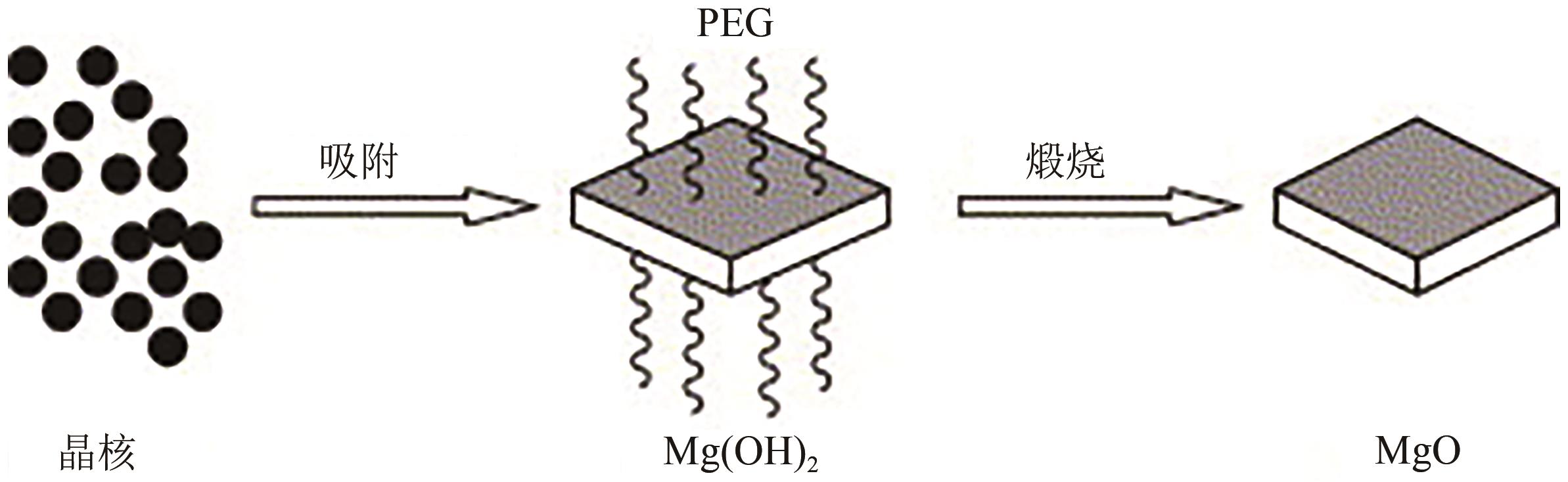
| MgCl2 | NH3·H2O、无水乙醇 | 聚乙二醇(PEG400) | 50 | 3 | — | — | [ |
| MgSO4 | NaOH、H2O | PEG200、PEG8000、PEG20000 | 80 | — | 不规则状 | 28~79 | [ |
| MgCl2 | NH3·H2O | PEG1000 | 60 | 1.5 | 管状 | 管径6~34、长度63~200 | [ |
| MgCl2 | NaOH、H2O | PEG4000、CaCl2、NaCl、KCl | 20 | 1 | 片状 | 19.22×10 | [ |
表1 通过沉淀法在不同条件下制备的纳米氢氧化镁
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间/h | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|
| MgCl2 | NaOH、H2O | — | 60 | 24 | 花椰菜状 | 300 | [ |
| MgCl2 | NH3·H2O | — | 60 | 24 | 片状 | 302×26 | [ |
| Mg(NO3)2 | NH3·H2O | — | 25 | 24 | 片状 | 351×10 | [ |
| MgSO4 | NH3·H2O | — | 25 | 24 | 片状 | 250×15 | [ |
| MgCl2 | NaOH、H2O | — | 60 | 1 | 片状 | 30~150(正向沉淀法) 20~50(反向沉淀法) 10~70(双向沉淀法) 30~50(超重力沉淀法) | [ |
| MgSO4 | NaOH、H2O | 硬脂酸镁(MgSA)、 五水硫酸铜 | 50 | 4.5 | 棒状 | 150×12 | [ |
| MgCl2 | NaOH、H2O | NaCl | 60~100 | 50~200min | 球状 | 6~30μm | [ |
| Mg(NO3)2 | NaOH、H2O | 海藻提取物 | — | 4 | 片状 | 7.75×16.29 | [ |
| MgCl2 | NH3·H2O、无水乙醇 | 聚乙二醇(PEG400) | 50 | 3 | — | — | [ |
| MgSO4 | NaOH、H2O | PEG200、PEG8000、PEG20000 | 80 | — | 不规则状 | 28~79 | [ |
| MgCl2 | NH3·H2O | PEG1000 | 60 | 1.5 | 管状 | 管径6~34、长度63~200 | [ |
| MgCl2 | NaOH、H2O | PEG4000、CaCl2、NaCl、KCl | 20 | 1 | 片状 | 19.22×10 | [ |
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间/h | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|
| Mg | H2O | EDA | 180 | 20 | 棒状 | 200×20 | [ |
| Mg10(OH)18Cl2·5H2O纳米线 | H2O | EDA | 180 | 6 | 管状 | 外径80~150、壁厚30~50、长度5~10μm | [ |
| MgCl2 | NaOH、H2O | CTAB | 180 | 18 | 片状 | 60~100 | [ |
| PEG500 | 板状 | 80~90 | |||||
| 明胶 | 球状 | 30~45 | |||||
| 油酸 | 圆盘状 | 90 | |||||
| MgCl2 | NH3·H2O | MEA、柠檬酸 | 180 | 6 | 片状 | 246 | [ |
| Mg(NO3)2 | N2H4·H2O | — | 150 | 24 | 片状 | — | [ |
| Mg(NO3)2·6H2O | N2H4·H2O | — | 180 | 12 | 片状 | 150~260 | [ |
| MgCl2 | NaOH、H2O | PVP | 200 | 8 | 片状 | 134(微波) | [ |
| 局部高镁镍渣(HMNS) | NaOH、H2O | — | 200 | 24 | 片状 | 189×13 | [ |
表2 使用水热/溶剂热法在不同条件下制备纳米氢氧化镁
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间/h | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|
| Mg | H2O | EDA | 180 | 20 | 棒状 | 200×20 | [ |
| Mg10(OH)18Cl2·5H2O纳米线 | H2O | EDA | 180 | 6 | 管状 | 外径80~150、壁厚30~50、长度5~10μm | [ |
| MgCl2 | NaOH、H2O | CTAB | 180 | 18 | 片状 | 60~100 | [ |
| PEG500 | 板状 | 80~90 | |||||
| 明胶 | 球状 | 30~45 | |||||
| 油酸 | 圆盘状 | 90 | |||||
| MgCl2 | NH3·H2O | MEA、柠檬酸 | 180 | 6 | 片状 | 246 | [ |
| Mg(NO3)2 | N2H4·H2O | — | 150 | 24 | 片状 | — | [ |
| Mg(NO3)2·6H2O | N2H4·H2O | — | 180 | 12 | 片状 | 150~260 | [ |
| MgCl2 | NaOH、H2O | PVP | 200 | 8 | 片状 | 134(微波) | [ |
| 局部高镁镍渣(HMNS) | NaOH、H2O | — | 200 | 24 | 片状 | 189×13 | [ |
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间 /min | 其他条件 | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Mg(NO3)2 | NaOH、H2O | — | 25 | 5d | 半透膜,微波20W、2.45GHz | 纤维状 | (20~40)×(100~150) | [ |
| MgCl2 | NaOH、H2O | 尿素 | 220 | 30 | 微波1000W | 片状 | 厚度95±10、长度几微米 | [ |
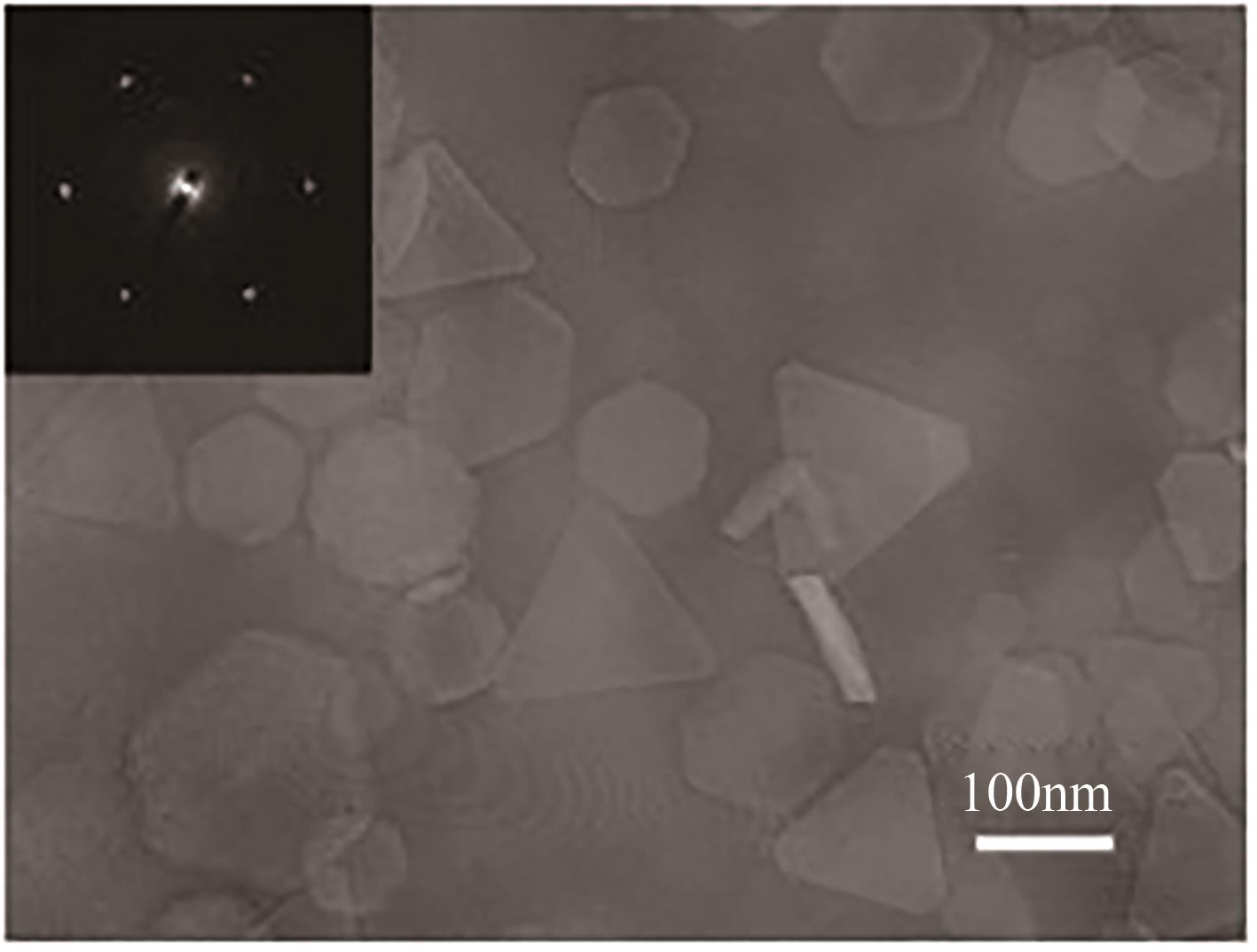
| Mg | H2O | — | — | — | 微波2.45GHz、20kPa | 三角形、截断三角形和六边形片状 | 50、80、70 | [ |
| Mg | H2O | — | — | 8 | 微波1000W | 颗粒状、片状 | 43、32 | [ |
| Mg | NaOH、H2O | — | 25~220 | 10 | 微波1000W | 片状 | 250~500 | [ |
| MgCl2 | H2O | 尿素 | 25~220 | 10 | 微波1000W | 玫瑰花团簇状 | 50μm | |
| Mg(C2O2H4)2 | H2O | 尿素 | 25~220 | 10 | 微波1000W | 玫瑰花团簇状 | 20μm | |
| MgO | H2O | — | — | 6 | 微波800W、2.45GHz | 片状 | 长度100~300、厚度10~60 | [ |
| Mg | H2O | NaCl | 20 | 30 | 微波1000W、20kHz | 片状 | 长度70~200、厚度20 | [ |
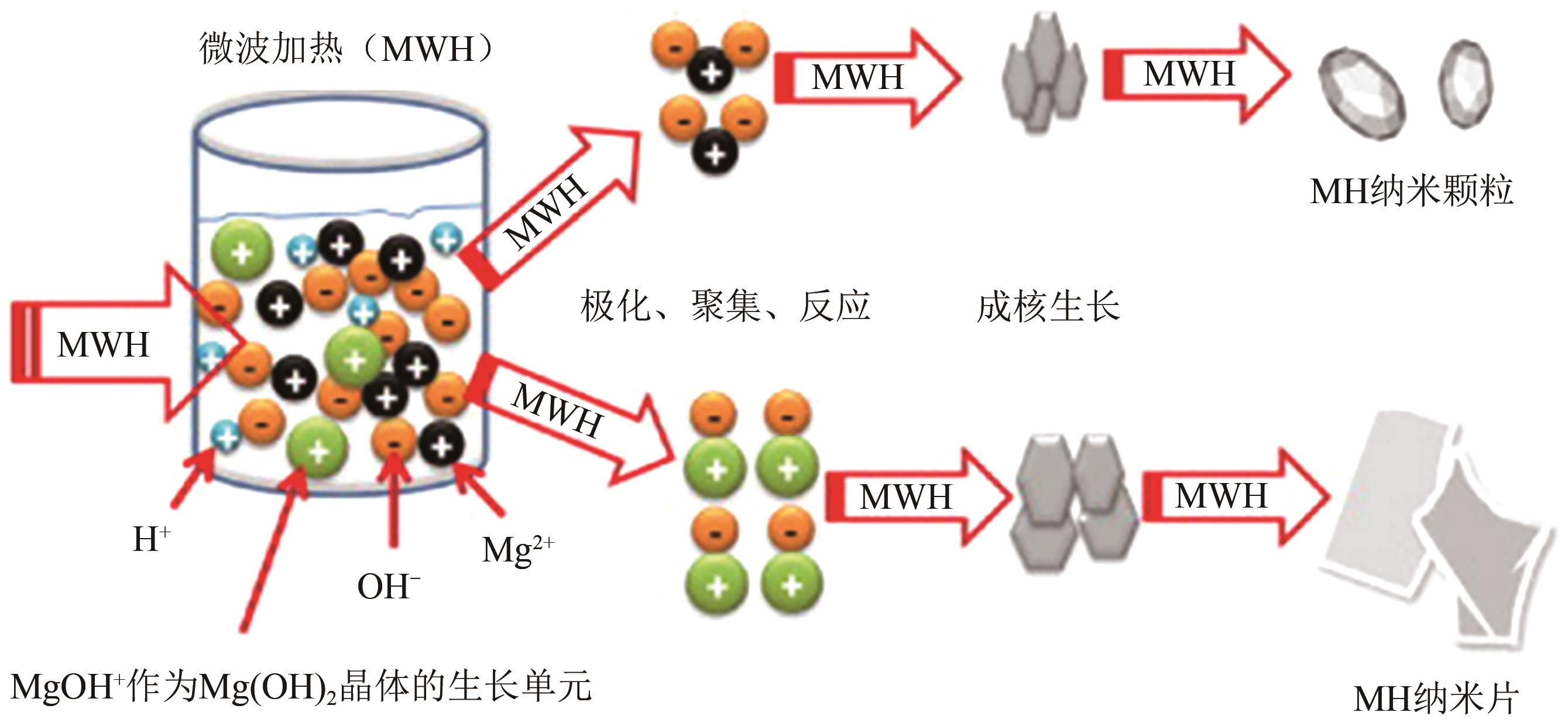
表3 通过微波辅助法在不同条件下制备纳米氢氧化镁
| 镁源 | 溶剂 | 添加剂 | 温度/℃ | 时间 /min | 其他条件 | 形貌 | 颗粒尺寸/nm | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Mg(NO3)2 | NaOH、H2O | — | 25 | 5d | 半透膜,微波20W、2.45GHz | 纤维状 | (20~40)×(100~150) | [ |
| MgCl2 | NaOH、H2O | 尿素 | 220 | 30 | 微波1000W | 片状 | 厚度95±10、长度几微米 | [ |
| Mg | H2O | — | — | — | 微波2.45GHz、20kPa | 三角形、截断三角形和六边形片状 | 50、80、70 | [ |
| Mg | H2O | — | — | 8 | 微波1000W | 颗粒状、片状 | 43、32 | [ |
| Mg | NaOH、H2O | — | 25~220 | 10 | 微波1000W | 片状 | 250~500 | [ |
| MgCl2 | H2O | 尿素 | 25~220 | 10 | 微波1000W | 玫瑰花团簇状 | 50μm | |
| Mg(C2O2H4)2 | H2O | 尿素 | 25~220 | 10 | 微波1000W | 玫瑰花团簇状 | 20μm | |
| MgO | H2O | — | — | 6 | 微波800W、2.45GHz | 片状 | 长度100~300、厚度10~60 | [ |
| Mg | H2O | NaCl | 20 | 30 | 微波1000W、20kHz | 片状 | 长度70~200、厚度20 | [ |
| 1 | ZHANG Tiefeng, WANG Chunfeng, WANG Yongliang, et al. Enhanced flame retardancy in ethylene-vinyl acetate copolymer/magnesium hydroxide/polycarbosilane blends[J]. Polymers, 2021, 14(1): 36. |
| 2 | WANG Qilei. The thermal resistance, flame retardance, and smoke control mechanism of nano MH/GF/NBR composite material[J]. Science and Engineering of Composite Materials, 2014, 21(3): 309-314. |
| 3 | ZHANG Chen, CHENG Xianwei, GUAN Jinping, et al. Preparation of nano-Mg(OH)2 for surface coating of silk fabric with improved flame retardancy and smoke suppression[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 625: 126868. |
| 4 | CHENG Liangsong, REN Shaobo, BROSSE Nicolas, et al. Synergic flame-retardant effect of cellulose nanocrystals and magnesium hydroxide in polyurethane wood coating[J]. Journal of Wood Chemistry and Technology, 2022, 42(4): 297-304. |
| 5 | COSTES L, LAOUTID F, BROHEZ S, et al. Bio-based flame retardants: When nature meets fire protection[J]. Materials Science & Engineering, 2017, 117: 1-25. |
| 6 | VAN DER VEEN Ike, DE BOER Jacob. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis[J]. Chemosphere, 2012, 88(10): 1119-1153. |
| 7 | HE Wentao, SONG Pingan, YU Bin, et al. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants[J]. Progress in Materials Science, 2020, 114: 100687. |
| 8 | LI Baojun, CAO Huaqiang, YIN Gui. Mg(OH)2@reduced graphene oxide composite for removal of dyes from water[J]. Journal of Materials Chemistry, 2011, 21(36): 13765-13768. |
| 9 | PIERO Baglioni, RODORICO Giorgi. Soft and hard nanomaterials for restoration and conservation of cultural heritage[J]. Soft Matter, 2006, 2(4): 293-303. |
| 10 | MARCELLA Bini, FRANCESCO Monteforte, IRENE Quinzeni, et al. Hybrid compounds for improving drugs solubility: Synthesis, physico-chemical and pharmaceutical characterization of Nimesulide-LDH[J]. Journal of Solid State Chemistry, 2019, 272: 131-137. |
| 11 | GIULIA Balducci, LAURA Bravo Diaz, GREGORY Duncan H. Recent progress in the synthesis of nanostructured magnesium hydroxide[J]. CrystEngComm, 2017, 19(41): 6067-6084. |
| 12 | 申红艳, 刘有智. 纳米氢氧化镁的制备及其原位改性[J]. 化工进展, 2017, 36(1): 294-298. |
| SHEN Hongyan, LIU Youzhi. Preparation and in situ modification of magnesium hydroxide nanoparticles[J]. Chemical Industry and Engineering Progress, 2017, 36(1): 294-298. | |
| 13 | RAJABIMASHHADI Zahra, NAGHIZADEH Rahim, ZOLRIASATEIN Ashkan, et al. Novel synthesis of nano Mg(OH)2 by means of hydrothermal method with different surfactants[J]. Nanomaterials, 2023, 13(3): 454. |
| 14 | 武俊, 钱永芳, 吕丽华, 等. 静电纺PVP/Mg(OH)2阻燃纳米纤维材料的制备及性能[J]. 上海纺织科技, 2022, 50(7): 23-26, 52. |
| WU Jun, QIAN Yongfang, Lihua LYU, et al. Preparation and properties of electrospun PVP/Mg(OH)2 flame retardant nanofiber materials[J]. Shanghai Textile Science & Technology, 2022, 50(7): 23-26, 52. | |
| 15 | SONG Xingfu, SUN Shuying, ZHANG Dengke, et al. Synthesis and characterization of magnesium hydroxide by batch reaction crystallization[J]. Frontiers of Chemical Science and Engineering, 2011, 5(4): 416-421. |
| 16 | WANG Wei, QIAO Xueliang, CHEN Jianguo, et al. Facile synthesis of magnesium oxide nanoplates via chemical precipitation[J]. Materials Letters, 2007, 61(14/15): 3218-3220. |
| 17 | HENRIST C, MATHIEU J P, VOGELS C, et al. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution[J]. Journal of Crystal Growth, 2003, 249(1/2): 321-330. |
| 18 | 申红艳, 刘有智, 马鹏程, 等. 不同制备方法对纳米氢氧化镁性能的影响[J]. 化工进展, 2016, 35(4): 1149-1153. |
| SHEN Hongyan, LIU Youzhi, MA Pengcheng, et al. Effect of different preparation methods on the properties of magnesium hydroxide nanoparticles[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1149-1153. | |
| 19 | CHEN Dehong, ZHU Lunyu, ZHANG Huaiping, et al. Magnesium hydroxide nanoparticles with controlled morphologies via wet coprecipitation[J]. Materials Chemistry and Physics, 2008, 109(2/3): 224-229. |
| 20 | GOVINDARAJU K, ANAND K V, ANBARASU S, et al. Seaweed (Turbinaria ornata)-assisted green synthesis of magnesium hydroxide [Mg(OH)2] nanomaterials and their anti-mycobacterial activity[J]. Materials Chemistry and Physics, 2020, 239: 122007. |
| 21 | PILARSKA Agnieszka, WYSOKOWSKI Marcin, MARKIEWICZ Ewa, et al. Synthesis of magnesium hydroxide and its calcinates by a precipitation method with the use of magnesium sulfate and poly(ethylene glycols)[J]. Powder Technology, 2013, 235: 148-157. |
| 22 | ZHENG Jun, ZHOU Wei. Solution-phase synthesis of magnesium hydroxide nanotubes[J]. Materials Letters, 2014, 127: 17-19. |
| 23 | YOUSEFI Sadegh, GHASEMI Behrooz. Mg(OH)2 nanostructures using impure brine: Optimization of synthesis parameters by Taguchi robust design and study of optical properties[J]. Research on Chemical Intermediates, 2021, 47(5): 2029-2047. |
| 24 | SIERRA-FERNANDEZ A, Gomez-Villalba LS, MILOSEVIC O, et al. Synthesis and morpho-structural characterization of nanostructured magnesium hydroxide obtained by a hydrothermal method[J]. Ceramics International, 2014, 40(8): 12285-12292. |
| 25 | 任鹏飞, 陈建铭, 宋云华, 等. 水热合成制备超细氢氧化镁阻燃剂[J]. 化工进展, 2005, 24(2): 186-189. |
| REN Pengfei, CHEN Jianming, SONG Yunhua, et al. Study on the preparation of ultrafine magnesium hydroxide flame retardant in different hydrothermal treatment methods[J]. Chemical Industry and Engineering Progress, 2005, 24(2): 186-189. | |
| 26 | LI Y, SUI M, DING Y, et al. Preparation of Mg(OH)2 nanorods[J]. Advanced Materials, 2000, 12(11): 818-821. |
| 27 | FAN Weiliu, SUN Sixiu, YOU Liping, et al. Solvothermal synthesis of Mg(OH)2 nanotubes using Mg10(OH)18Cl2·5H2O nanowires as precursors[J]. J Mater Chem, 2003, 13(12): 3062-3065. |
| 28 | CHEN Yongbin, ZHOU Tao, FANG Huaxiong, et al. A novel preparation of nano-sized hexagonal Mg(OH)2 [J]. Procedia Engineering, 2015, 102: 388-394. |
| 29 | JIN Dalai, GU Xiaoyun, YU Xiaojing, et al. Hydrothermal synthesis and characterization of hexagonal Mg(OH)2 nano-flake as a flame retardant[J]. Materials Chemistry and Physics, 2008, 112(3): 962-965. |
| 30 | ZONG Luyao, LI Liyan, ZHANG Jiyi, et al. Synthesis of high dispersion and uniform nano-sized flame retardant-used hexagonal Mg(OH)2 [J]. Journal of Cluster Science, 2016, 27(6): 1831-1841. |
| 31 | QIAN Binbin, LIU Huiling, MA Bing, et al. Bulk trash to nano treasure: Synthesis of two-dimensional brucite nanosheet from high-magnesium nickel slag[J]. Journal of Cleaner Production, 2022, 333: 130196. |
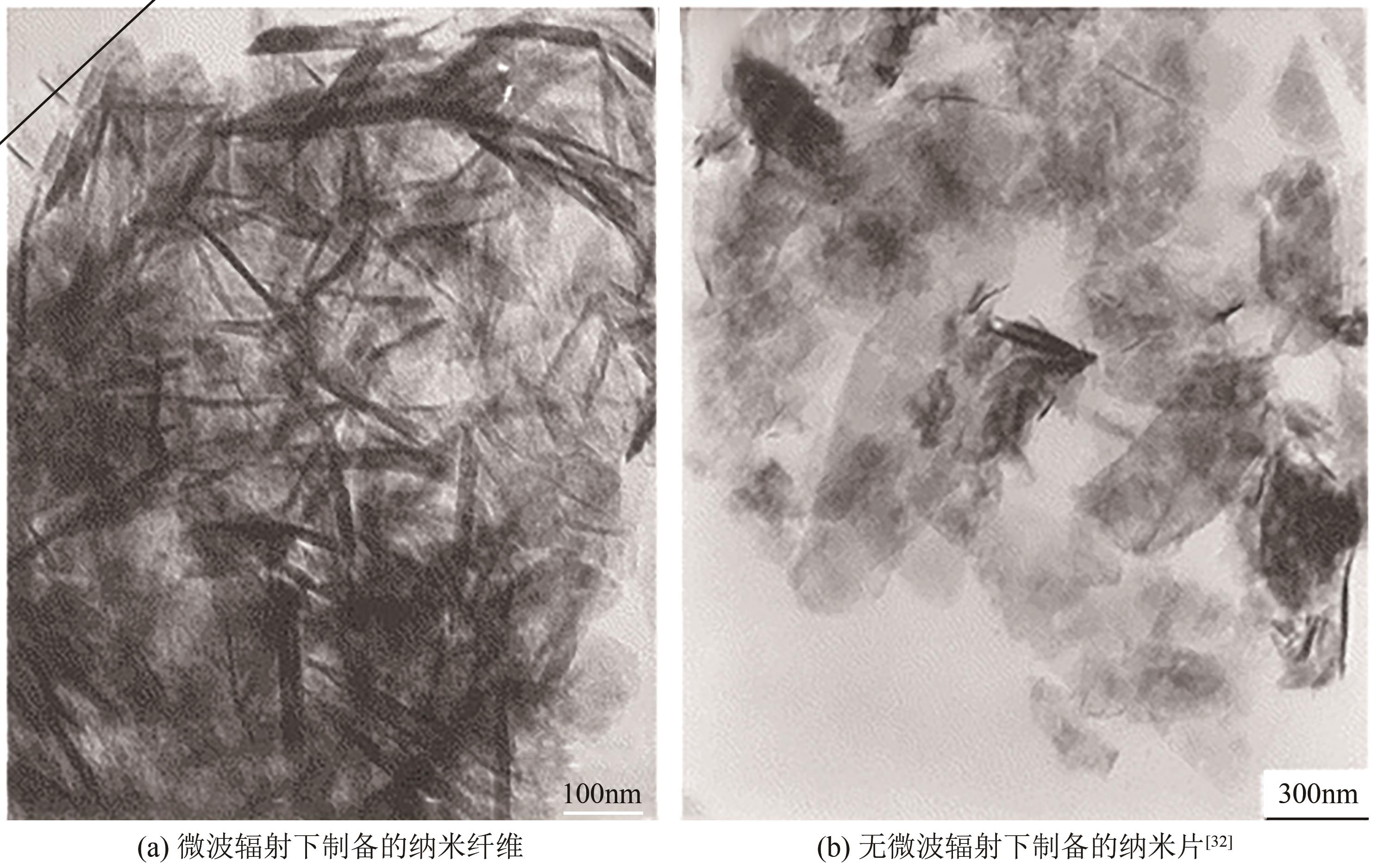
| 32 | WU Huaqiang, SHAO Mingwang, GU Jiashan, et al. Microwave-assisted synthesis of fibre-like Mg(OH)2 nanoparticles in aqueous solution at room temperature[J]. Materials Letters, 2004, 58(16): 2166-2169. |
| 33 | Faten AL-HAZMI, UMAR Ahmad, DAR G N, et al. Microwave assisted rapid growth of Mg(OH)2 nanosheet networks for ethanol chemical sensor application[J]. Journal of Alloys and Compounds, 2012, 519: 4-8. |
| 34 | HATTORI Yoshiaki, MUKASA Shinobu, TOYOTA Hiromichi, et al. Continuous synthesis of magnesium-hydroxide, zinc-oxide, and silver nanoparticles by microwave plasma in water[J]. Materials Chemistry and Physics, 2011, 131(1/2): 425-430. |
| 35 | AL-GAASHANI R, RADIMAN S, AL-DOURI Y, et al. Investigation of the optical properties of Mg(OH)2 and MgO nanostructures obtained by microwave-assisted methods[J]. Journal of Alloys and Compounds, 2012, 521: 71-76. |
| 36 | BEALL Gary W, DURAIA El-Shazly M, Farid EL-TANTAWY, et al. Rapid fabrication of nanostructured magnesium hydroxide and hydromagnesite via microwave-assisted technique[J]. Powder Technology, 2013, 234: 26-31. |
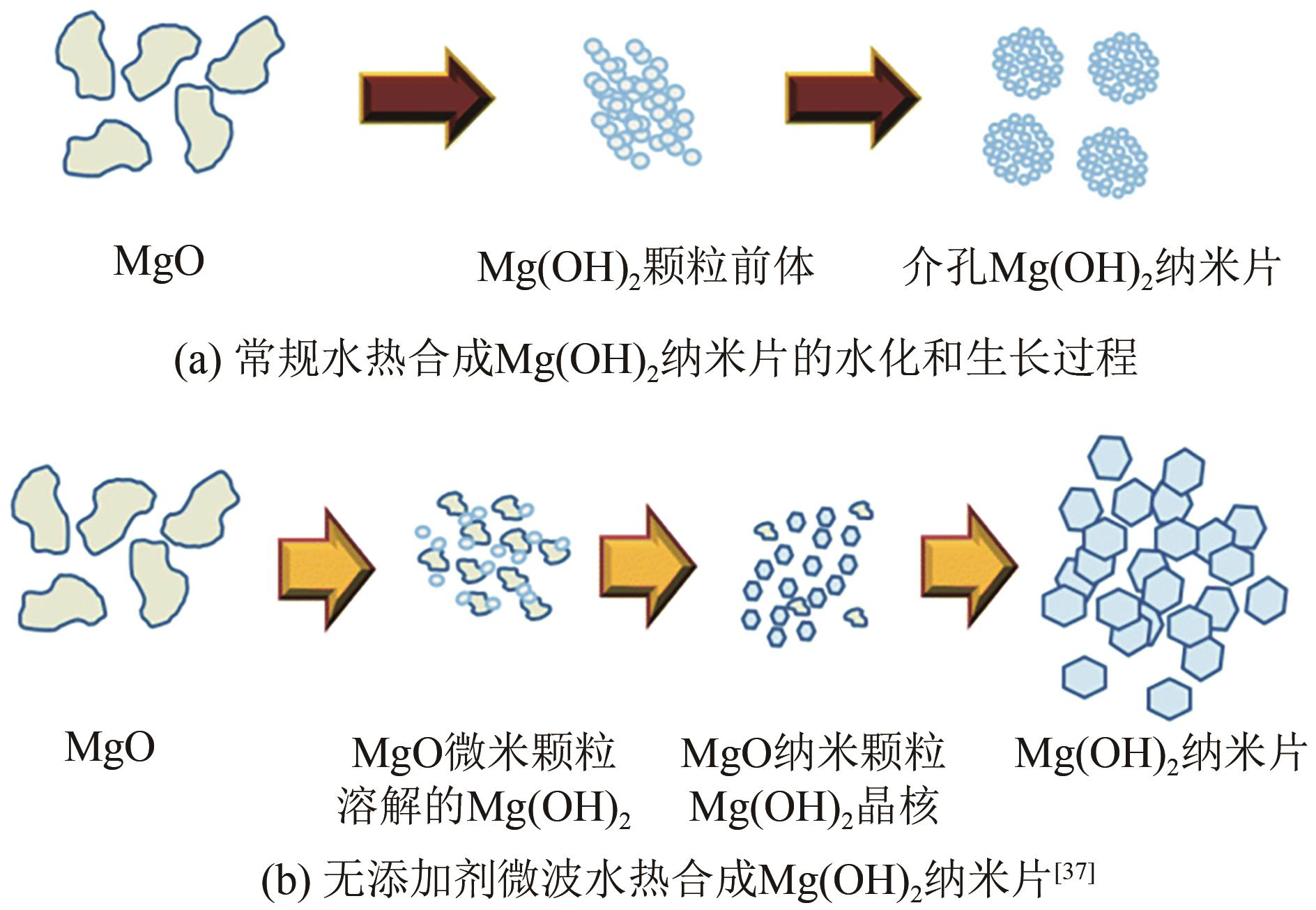
| 37 | HANLON James M, LAURA Bravo Diaz, GIULIA Balducci, et al. Rapid surfactant-free synthesis of Mg(OH)2 nanoplates and pseudomorphic dehydration to MgO[J]. CrystEngComm, 2015, 17(30): 5672-5679. |
| 38 | YU Jimmy C, XU Anwu, ZHANG Lizhi, et al. Synthesis and characterization of porous magnesium hydroxide and oxide nanoplates[J]. The Journal of Physical Chemistry B, 2004, 108(1): 64-70. |
| 39 | OLGA Baidukova, SKORB Ekaterina V. Ultrasound-assisted synthesis of magnesium hydroxide nanoparticles from magnesium[J]. Ultrasonics-Sonochemistry, 2016, 31: 423-428. |
| 40 | WU Haihong, GAO Chunjuan, MA Laibo, et al. Controllable microemulsion method for the synthesis of Mg(OH)2/PS core-shell structures[J]. Micro & Nano Letters, 2021, 16(8): 413-418. |
| 41 | LIN Shengnan, ZHANG Tingan, YANG Han. Preparation and mechanism analysis of nano-Mg(OH)2 by electroconversion of MgCl2 [J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2023, 31(7): 667-674. |
| 42 | SANTHOSH Balanand, KUMAR Muthusundar, MATHEWS Jeen Maria, et al. A facile hydrous mechano-synthesis of magnesium hydroxide [Hy-Mg(OH)2] nano fillers for flame-retardant polyester composites[J]. Chemical Engineering Journal Advances, 2023, 14: 100466. |
| 43 | GUI H, ZHANG X, LIU Y, et al. Effect of dispersion of nano-magnesium hydroxide on the flammability of flame retardant ternary composites[J]. Composites Science and Technology, 2007, 67(6): 974-980. |
| 44 | HAN Kyaw Thet, LHOSUPASIRIRAT Siraprapa, SRIKHIRIN Pongsid, et al. Development of flame retardant stearic acid doped graphite powder and magnesium hydroxide nanoparticles, material for thermal energy storage applications[J]. Journal of Physics: Conference Series, 2022, 2175(1): 012043. |
| 45 | JURAN Noh, INSUNG Kang, JAEHYUK Choi, et al. Surface modification of magnesium hydroxide nanoparticles with hexylphosphoric acid to improve thermal stabilities of polyethylene composites[J]. Polymer Bulletin, 2016, 73(10): 2855-2866. |
| 46 | FENG Xi, WANG Zhengyu, MA Hongli, et al. Development of bio-based magnesium phosphate flame retardant for simultaneously improved flame retardancy, smoke suppression and mechanical properties of HDPE[J]. Journal of Applied Polymer Science, 2023, 140(23): e53927. |
| 47 | YAO Meng, WU Hongjuan, LIU Hao, et al. In-situ growth of boron nitride for the effect of layer-by-layer assembly modified magnesium hydroxide on flame retardancy, smoke suppression, toxicity and char formation in EVA[J]. Polymer Degradation and Stability, 2021, 183: 109417. |
| 48 | WANG Sen, LIANG Shitong, WANG Kesong, et al. Enhanced flame retardancy, smoke suppression, and acid resistance of polypropylene/magnesium hydroxide composite by expandable graphite and microencapsulated red phosphorus[J]. Journal of Vinyl and Additive Technology, 2023, 29(2): 395-409. |
| 49 | JIAO Wanli, ZHONG Shaofeng, YU Xueman. Preparation and properties of superhydrophobic and flame-retardant cotton fabric[J]. Chinese Journal of Applied Chemistry, 2020, 37: 301-306. |
| 50 | 李志斌, 唐辉, 罗大伟, 等. 废弃PET化学回收及制备不饱和聚酯树脂的研究进展[J]. 化工进展, 2022, 41(6): 3279-3292. |
| LI Zhibin, TANG Hui, LUO Dawei, et al. Progress in chemical recycling of waste PET and preparation of unsaturated polyester resins[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3279-3292. | |
| 51 | WANG Ying, LIU Yanjing, LI Xiyue, et al. Preparation of nano-Mg(OH)2 and its flame retardant and antibacterial modification on polyethylene terephthalate fabrics[J]. Polymers, 2022, 15(1): 7. |
| 52 | LI Na, LI Zhi, LIU Zhiqi, et al. Magnesium hydroxide micro-whiskers as super-reinforcer to improve fire retardancy and mechanical property of epoxy resin[J]. Polymer Composites, 2022, 43(4): 1996-2009. |
| 53 | ZHAO Jiangqi, ZHANG Ximu, TU Rui, et al. Mechanically robust, flame-retardant and anti-bacterial nanocomposite films comprised of cellulose nanofibrils and magnesium hydroxide nanoplatelets in a regenerated cellulose matrix[J]. Cellulose, 2014, 21(3): 1859-1872. |
| 54 | WANG Qilei. Thermo-oxidative ageing and stability studies of different magnesium hydroxide/glass fiber/nitrile butadiene rubber nano-composites[J]. Proceedings of the Institution of Mechanical Engineers, Part L. Journal of Materials: Design and Applications, 2016, 230(1): 175-181. |
| 55 | STARK Nicole M, WHITE Robert H, MUELLER Scott A, et al. Evaluation of various fire retardants for use in wood flour-polyethylene composites[J]. Polymer Degradation and Stability, 2010, 95(9): 1903-1910. |
| 56 | Bi S, Song X. Esterified galactomannan mixed with Mg(OH)2 and Sb2O3 for making reinforced flame-retardant paper[J]. Chemistry and Industry of Forest Products, 2022, 42(3): 97-104. |
| 57 | LI Xiujuan, GUO Ruisong, QIAN Xiaodong. Preparation and absorption carbon monoxide properties of a novel flame retardants based fire-fighting foam[J]. Frontiers in Materials, 2021, 8: 646509. |
| 58 | LAZAR Simone T, KOLIBABA Thomas J, GRUNLAN Jaime C. Flame-retardant surface treatments[J]. Nature Reviews Materials, 2020, 5(4): 259-275. |
| 59 | QIU Xiaoqing, LI Zhiwei, LI Xiaohong, et al. Flame retardant coatings prepared using layer by layer assembly: A review[J]. Chemical Engineering Journal, 2018, 334: 108-122. |
| 60 | LIU Bowen, ZHAO Haibo, WANG Yuzhong. Advanced flame-retardant methods for polymeric materials[J]. Advanced Materials, 2022, 34(46): 2107905. |
| [1] | 慕铭, 赵伟伟, 陈光孟, 刘小青. 基于激光诱导石墨烯的应变传感器研究进展[J]. 化工进展, 2024, 43(9): 4970-4979. |
| [2] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [3] | 任国瑜, 妥云, 郑文杰, 谯泽庭, 任壮壮, 赵娅莉, 尚军飞, 陈晓东, 高祥虎. 超疏水纳米涂层技术研究进展及应用[J]. 化工进展, 2024, 43(8): 4450-4463. |
| [4] | 孙忻茹, 张秋怡, 卓建坤, 杨润, 姚强. CaCl2复合热化学储热材料的研究进展[J]. 化工进展, 2024, 43(8): 4506-4515. |
| [5] | 石佳博, 张宇轩, 陈雪峰, 谭蕉君. 单宁酸-纳米协同改性胶原纤维多孔材料的制备及其油水分离性能[J]. 化工进展, 2024, 43(8): 4624-4629. |
| [6] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [7] | 王丽娜, 武金升. 共价有机框架材料的合成与应用研究进展[J]. 化工进展, 2024, 43(7): 3834-3856. |
| [8] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| [9] | 赵伟刚, 张倩倩, 蓝钰玲, 闫雯, 周晓剑, 范毜仔, 杜官本. 真空绝热板芯材的研究进展与展望[J]. 化工进展, 2024, 43(7): 3910-3922. |
| [10] | 江慧珍, 罗凯, 王艳, 费华, 吴登科, 叶卓铖, 曹雄金. 废弃生物质复合相变材料的构建与应用[J]. 化工进展, 2024, 43(7): 3934-3945. |
| [11] | 杜倩, 侯明, 高冀芸, 杨黎, 鲁元佳, 郭胜惠. f-Ti3C2T x /ZIF-8异质结构增强NO2气体传感器的敏感性能[J]. 化工进展, 2024, 43(7): 3946-3954. |
| [12] | 张世蕊, 范朕连, 宋慧平, 张丽娜, 高宏宇, 程淑艳, 程芳琴. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| [13] | 刘梦凡, 王华伟, 王亚楠, 张艳茹, 蒋旭彤, 孙英杰. Bio-FeMnCeO x 活化PMS降解四环素效能与机制[J]. 化工进展, 2024, 43(6): 3492-3502. |
| [14] | 杨磊, 邱广薇, 李思言, 葛宏程, 孙园园, 王菲, 范晓光. 基于温度和葡萄糖双重响应性共聚物微囊的胰岛素控释载体[J]. 化工进展, 2024, 43(6): 3277-3284. |
| [15] | 卢欣欣, 蔡东仁, 詹国武. 基于固体前体构建集成催化剂及CO2加氢研究进展[J]. 化工进展, 2024, 43(5): 2786-2802. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||