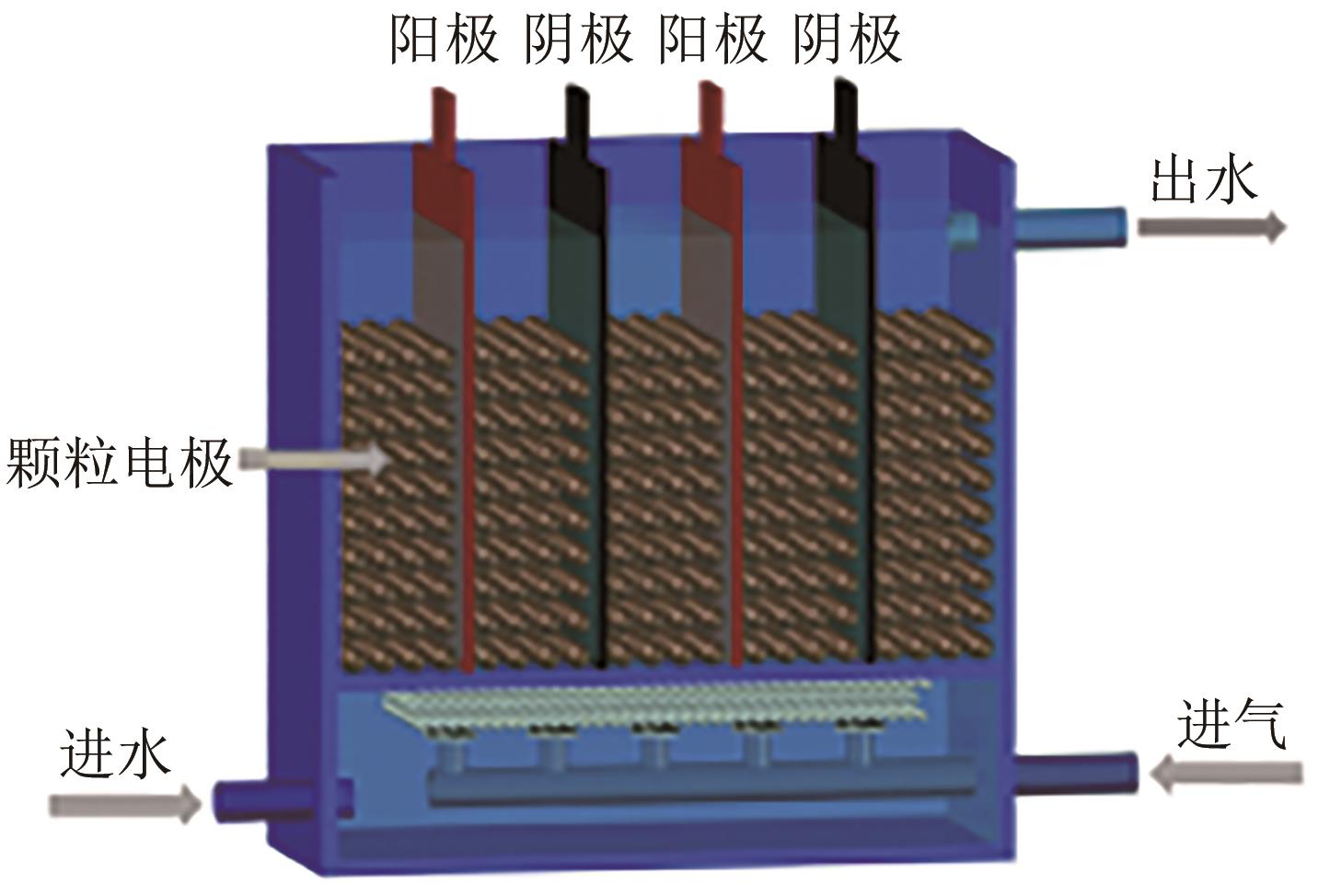
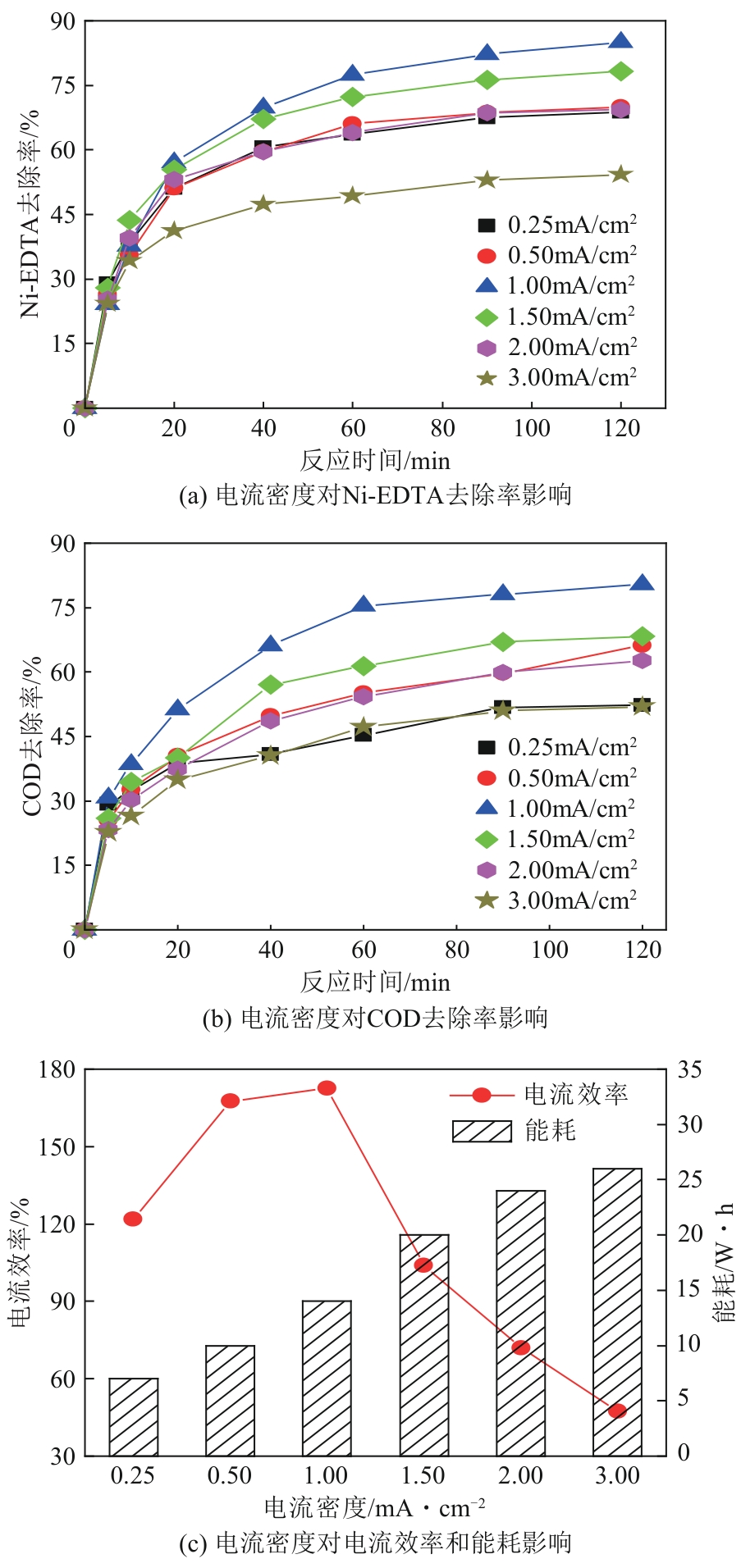
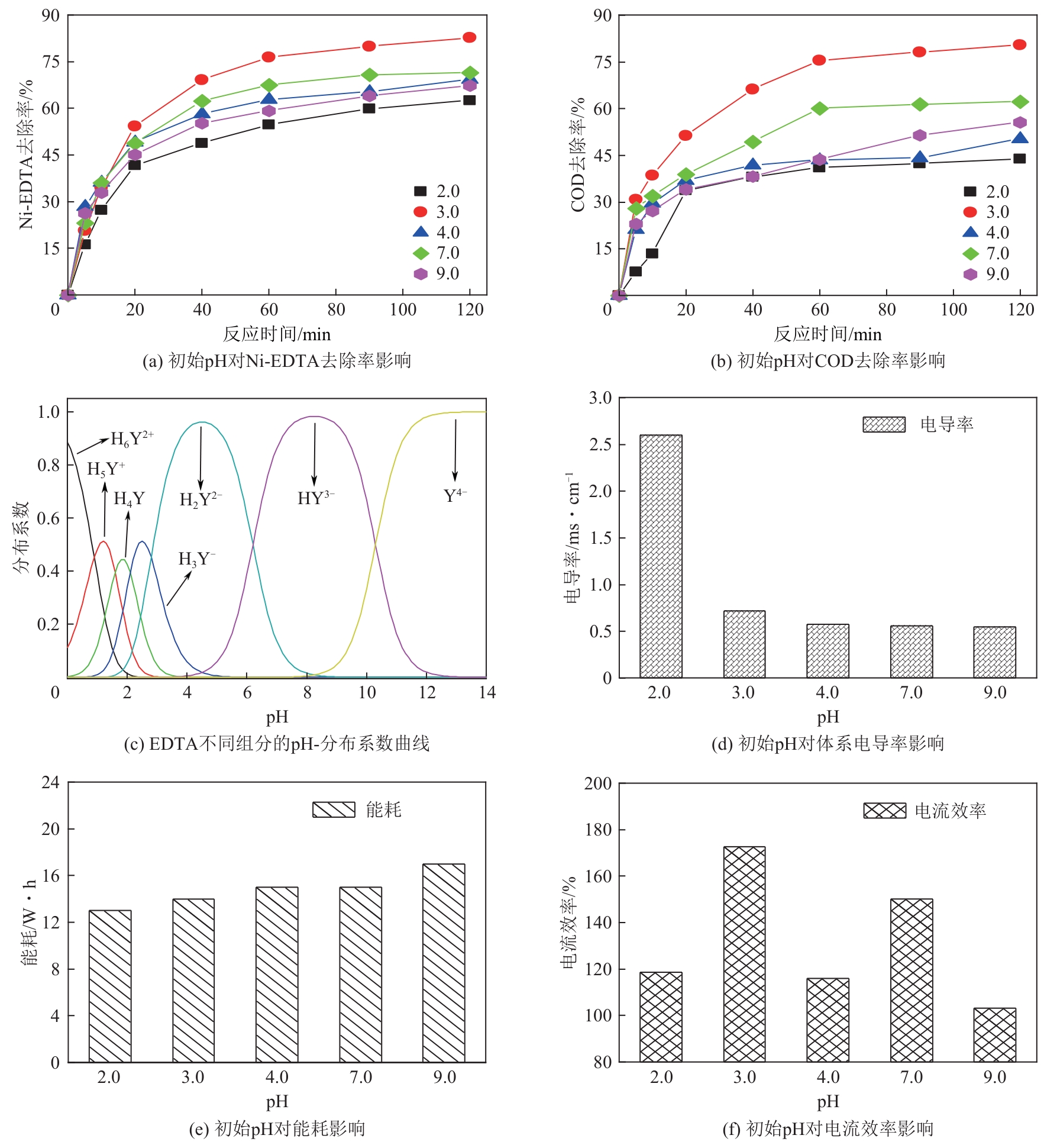
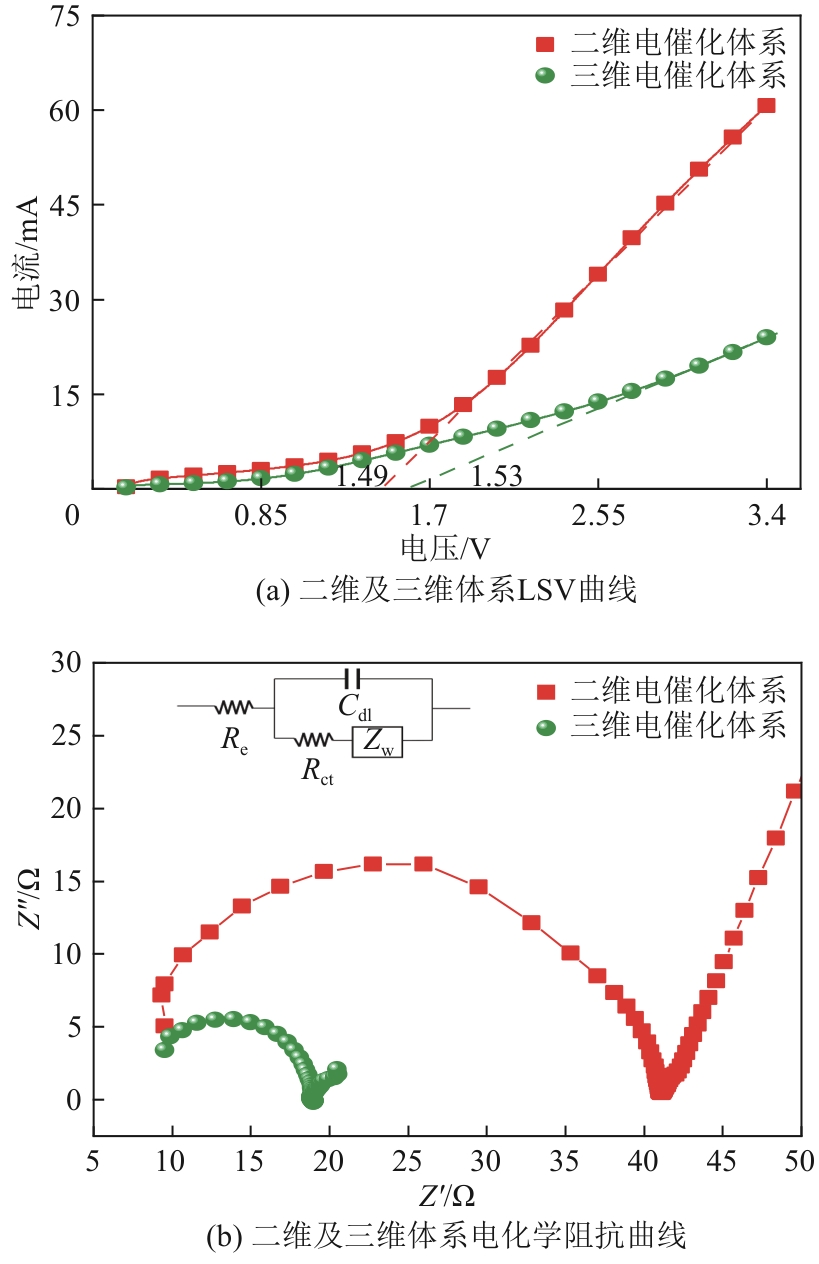
| 1 |
ZHUANG Ping, MCBRIDE Murray B, XIA Hanping, et al. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China[J]. Science of the Total Environment, 2009, 407(5): 1551-1561.
|
| 2 |
DONG Xiaoqing, LI Chaolin, LI Ji, et al. Application of a system dynamics approach for assessment of the impact of regulations on cleaner production in the electroplating industry in China[J]. Journal of Cleaner Production, 2012, 20(1): 72-81.
|
| 3 |
Olcay TÜNAY, KABDASLI Isik, Rüya TASLI. Pretreatment of complexed metal wastewaters[J]. Water Science and Technology, 1994, 29(9): 265-274.
|
| 4 |
关智杰. 臭氧催化氧化-重金属捕集深度处理化学镀镍废水的研究[D]. 广州: 广东工业大学, 2019.
|
|
GUAN Zhijie. Study on advanced treatment of electroless nickel plating wastewater by ozone catalytic oxidation-heavy metal chelation[D]. Guangzhou: Guangdong University of Technology, 2019.
|
| 5 |
戎关镛. 关于金属-EDTA络合物的条件稳定常数[J]. 冶金分析, 1990, 10(3): 59-61.
|
|
RONG Guanyong. Conditional stability constants of metal-EDTA complexes [J]. Metallurgical Analysis, 1990, 10(3): 59-61.
|
| 6 |
HE Hongping, WU Deli, ZHAO Linghui, et al. Sequestration of chelated copper by structural Fe(Ⅱ): Reductive decomplexation and transformation of CuII-EDTA[J]. Journal of Hazardous Materials, 2016, 309: 116-125.
|
| 7 |
贾鹏, 俞马宏. SBR间歇活性污泥法处理络合铜废水试验研究[J]. 水处理技术, 2012, 38(S1): 76-79.
|
|
JIA Peng, YU Mahong. Study on the treatment of complexation of copper by active sludge on SBR[J]. Technology of Water Treatment, 2012, 38(S1): 76-79.
|
| 8 |
YANG Xin, WANG Jinnan, CHENG Cheng. Preparation of new spongy adsorbent for removal of EDTA-Cu(Ⅱ) and EDTA-Ni(Ⅱ) from water [J]. Chinese Chemical Letters, 2013, 24(5): 383-385.
|
| 9 |
MOHSEN-NIA M, MONTAZERI P, MODARRESS H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes[J]. Desalination, 2007, 217(1/2/3): 276-281.
|
| 10 |
ZHAO Wei, LIU Zicheng, YUAN Yuan, et al. Insight into Cu(Ⅱ) adsorption on polyamine resin in the presence of HEDP by tracking the evolution of amino groups and Cu(Ⅱ)-HEDP complexes[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5256-5263.
|
| 11 |
OZTURK Dilara, YILMAZ Alper Erdem. Treatment of slaughterhouse wastewater with the electrochemical oxidation process: Role of operating parameters on treatment efficiency and energy consumption[J]. Journal of Water Process Engineering, 2019, 31: 100834.
|
| 12 |
MARTÍNEZ-HUITLE Carlos Alberto, PANIZZA Marco. Electrochemical oxidation of organic pollutants for wastewater treatment[J]. Current Opinion in Electrochemistry, 2018, 11: 62-71.
|
| 13 |
符远航, 刘安迪, 黄纬斌, 等. 负载多壁碳纳米管的多孔Ti/SnO2-Sb-Ni电极电催化氧化双酚A[J]. 环境科学, 2022, 43(5): 2640-2649.
|
|
FU Yuanhang, LIU Andi, HUANG Weibin, et al. Electrocatalytic oxidation of bisphenol A by porous Ti/SnO2-Sb-Ni electrode loaded with multi-wall carbon nanotubes[J]. Environmental Science, 2022, 43(5): 2640-2649.
|
| 14 |
石秋俊, 刘安迪, 唐柏彬, 等. Ni掺杂Sb-SnO2瓷环粒子电极电催化氧化磺胺嘧啶[J]. 环境科学, 2020, 41(4): 1725-1733.
|
|
SHI Qiujun, LIU Andi, TANG Bobin, et al. Electrocatalytic oxidation of sulfadiazine with Ni-doped Sb-SnO2 ceramic ring particle electrode[J]. Environmental Science, 2020, 41(4): 1725-1733.
|
| 15 |
HU Xinxin, YANG Shuai, YOU Xinyu, et al. Electrocatalytic decomplexation of Cu-EDTA and simultaneous recovery of Cu with Ni/GO-PAC particle electrode[J]. Chemical Engineering Journal, 2022, 428: 131468.
|
| 16 |
唐益洲. 三维电催化氧化处理柠檬酸络合镍废水的研究[D]. 长沙: 湖南师范大学, 2017.
|
|
TANG Yizhou. Study on the advanced treatment of nickel citrate by three-dimensional electrocatalytic oxidation[D]. Changsha: Hunan Normal University, 2017.
|
| 17 |
杨舒敏. 三维电催化生物滤池深度处理含抗生素畜禽养殖废水效能及机制研究[D]. 济南: 济南大学, 2021.
|
|
YANG Shumin. Study on the efficiency and mechanism of advanced treatment of antibiotic-containing livestock wastewater by three-dimensional electrocatalytic biological filter[D]. Jinan: University of Jinan, 2021.
|
| 18 |
DURANTE Christian, CUSCOV Marco, ISSE Abdirisak Ahmed, et al. Advanced oxidation processes coupled with electro-coagulation for the exhaustive abatement of Cr-EDTA[J]. Water Research, 2011, 45(5): 2122-2130.
|
| 19 |
YANG Xiupei, ZOU Ruyi, HUO Feng, et al. Preparation and characterization of Ti/SnO2-Sb2O3-Nb2O5/PbO2 thin film as electrode material for the degradation of phenol[J]. Journal of Hazardous Materials, 2009, 164(1): 367-373.
|
| 20 |
ZHOU Minghua, WANG Wei, CHI Meiling. Enhancement on the simultaneous removal of nitrate and organic pollutants from groundwater by a three-dimensional bio-electrochemical reactor[J]. Bioresource Technology, 2009, 100(20): 4662-4668.
|
| 21 |
DUAN Tigang, WEN Qing, CHEN Ye, et al. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets[J]. Journal of Hazardous Materials, 2014, 280: 304-314.
|
| 22 |
THOMAS R A, LAWLOR K, BAILEY M, et al. Biodegradation of metal-EDTA complexes by an enriched microbial population[J]. Applied and Environmental Microbiology, 1998, 64(4): 1319-1322.
|
| 23 |
ZHAO Xu, GUO Libao, HU Chengzhi, et al. Simultaneous destruction of Nickel(Ⅱ)-EDTA with TiO2/Ti film anode and electrodeposition of nickel ions on the cathode[J]. Applied Catalysis B: Environmental, 2014, 144: 478-485.
|
| 24 |
卫昶, 陈霞玲, 周伟舫. Pb及Pb-7w/OSb合金在氧析出电位区生长的阳极膜[J]. 化学学报, 1992(5): 467-472.
|
|
WEI Chang, CHEN Xialing, ZHOU Weifang. Anodic films grown at oxygen evolved potential range on Pb and Pb-7w/OSb alloy [J]. Acta Chimica Sinica, 1992, 50(5): 467-472.
|
| 25 |
NI Congcong, WANG Jingru, GUAN Yuxin, et al. Self-powered peroxi-coagulation for the efficient removal of p-arsanilic acid: pH-dependent shift in the contributions of peroxidation and electrocoagulation[J]. Chemical Engineering Journal, 2020, 391: 123495.
|
| 26 |
ZHU Xiuping, NI Jinren, XING Xuan, et al. Synergies between electrochemical oxidation and activated carbon adsorption in three-dimensional boron-doped diamond anode system[J]. Electrochimica Acta, 2011, 56(3): 1270-1274.
|
| 27 |
孟昭瑞, 高宁. 有机化合物的紫外光谱分析[J]. 西部资源, 2017(6): 179-182.
|
|
MENG Zhaorui, GAO Ning. Ultraviolet spectral analysis of organic compounds [J]. Western Resources, 2017(6): 179-182.
|
| 28 |
刘泽坤. UV/H2O2工艺处理Ni-EDTA废水效能研究[D]. 哈尔滨: 哈尔滨工业大学, 2018.
|
|
LIU Zekun. Treatment of Ni-EDTA containing wastewater by UV/H2O2 process[D]. Harbin: Harbin Institute of Technology, 2018.
|
 ), 冯岩1(
), 冯岩1( ), 卢玉玉1, 贾新强3, 邱立平1,2
), 卢玉玉1, 贾新强3, 邱立平1,2
 ), FENG Yan1(
), FENG Yan1( ), LU Yuyu1, JIA Xinqiang3, QIU Liping1,2
), LU Yuyu1, JIA Xinqiang3, QIU Liping1,2