化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4694-4703.DOI: 10.16085/j.issn.1000-6613.2023-1118
• 资源与环境化工 • 上一篇
脱硫石膏转化α-半水石膏的特征及机理:实验与模拟
- 东南大学能源与环境学院,江苏 南京 210096
-
收稿日期:2023-07-05修回日期:2023-11-01出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:仲兆平 -
作者简介:怀立业(1998—),男,硕士研究生,研究方向为固体废弃物处理。E-mail:220210595@seu.edu.cn。 -
基金资助:清洁高效燃煤发电与污染控制国家重点实验室开放基金(D20227K076)
Characteristics and mechanism of desulfurization gypsum to α-hemihydrate gypsum: Experiments and simulations
HUAI Liye( ), ZHONG Zhaoping(
), ZHONG Zhaoping( ), YANG Yuxuan
), YANG Yuxuan
- School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
-
Received:2023-07-05Revised:2023-11-01Online:2024-08-15Published:2024-09-02 -
Contact:ZHONG Zhaoping
摘要:
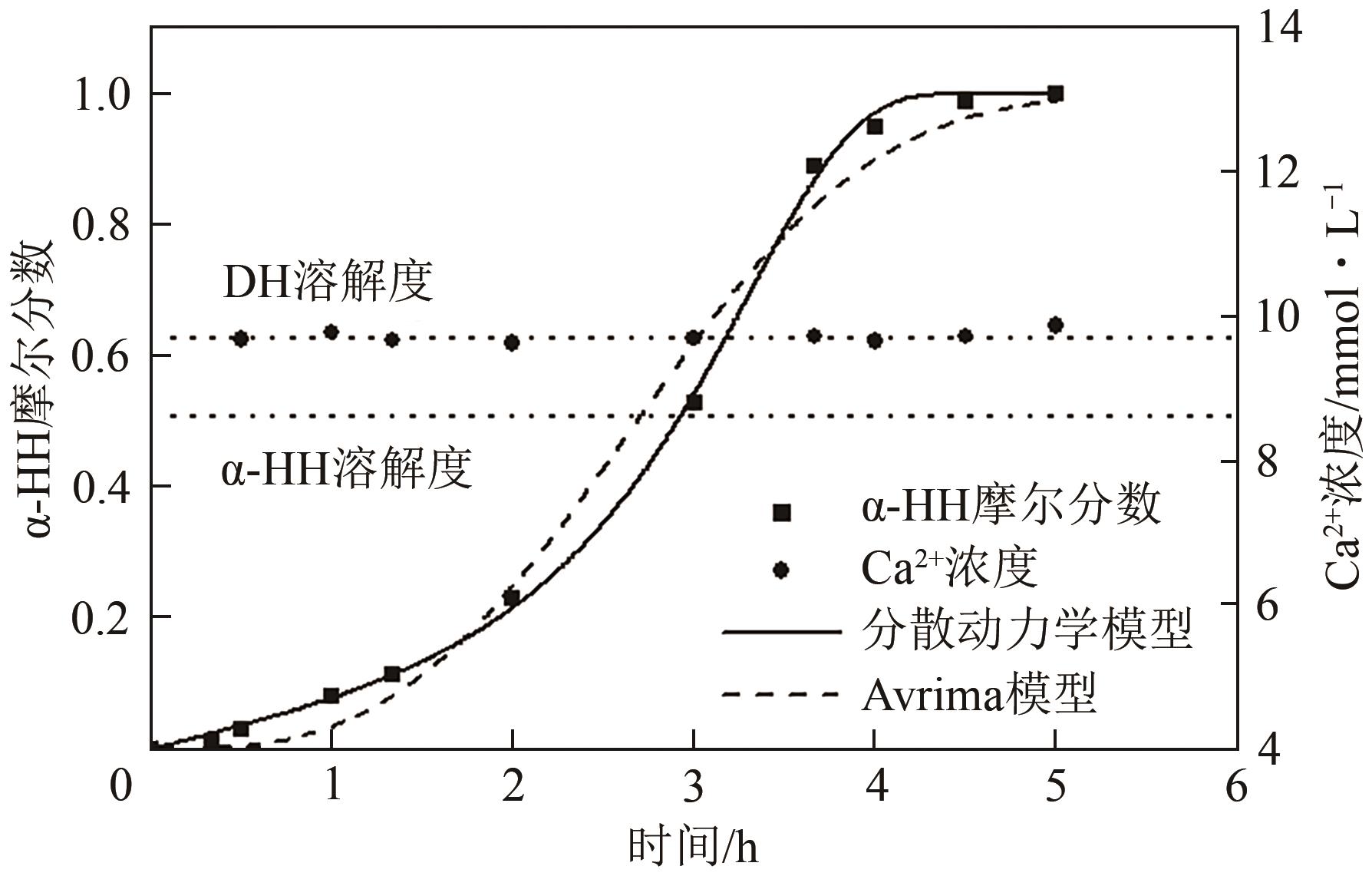
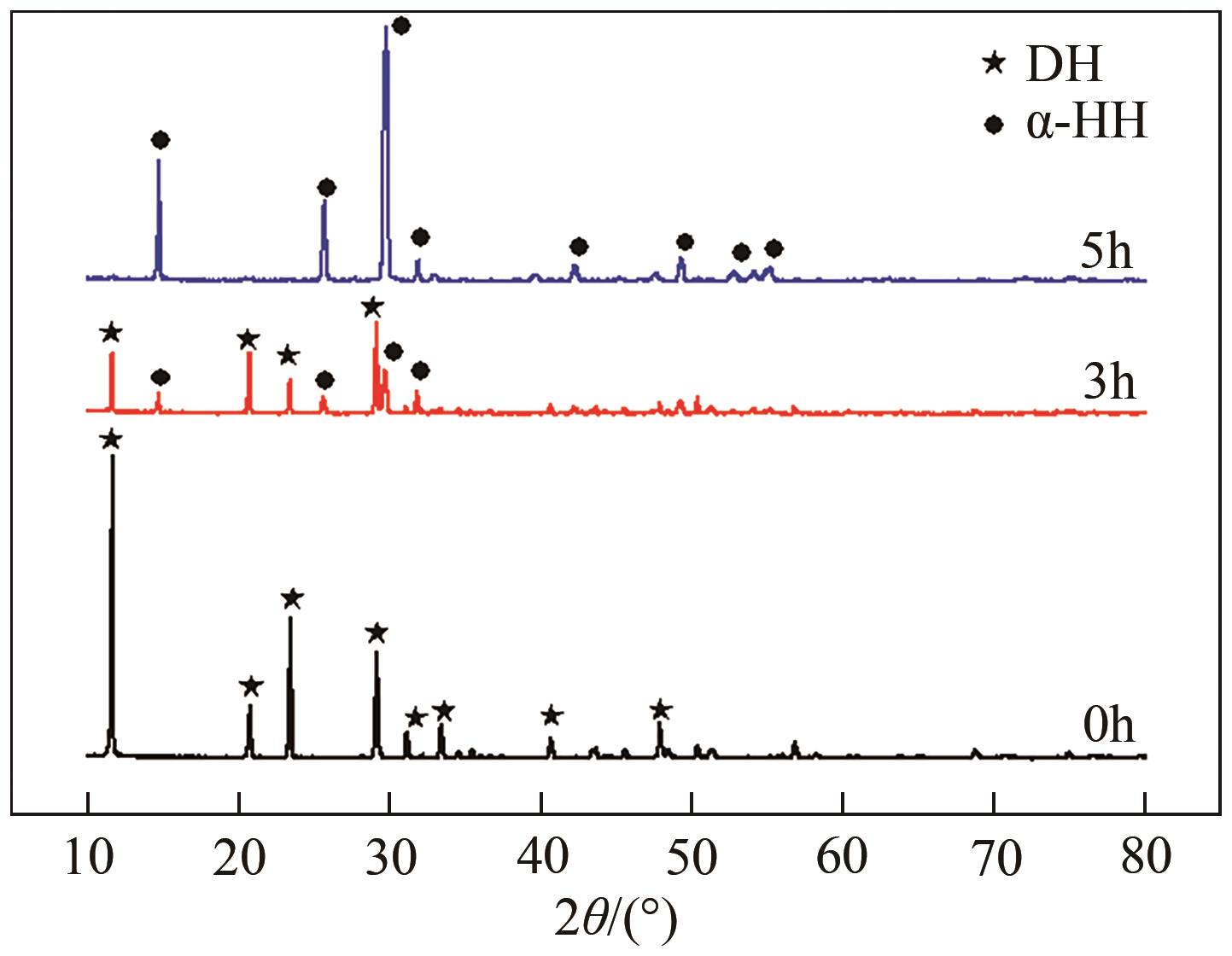
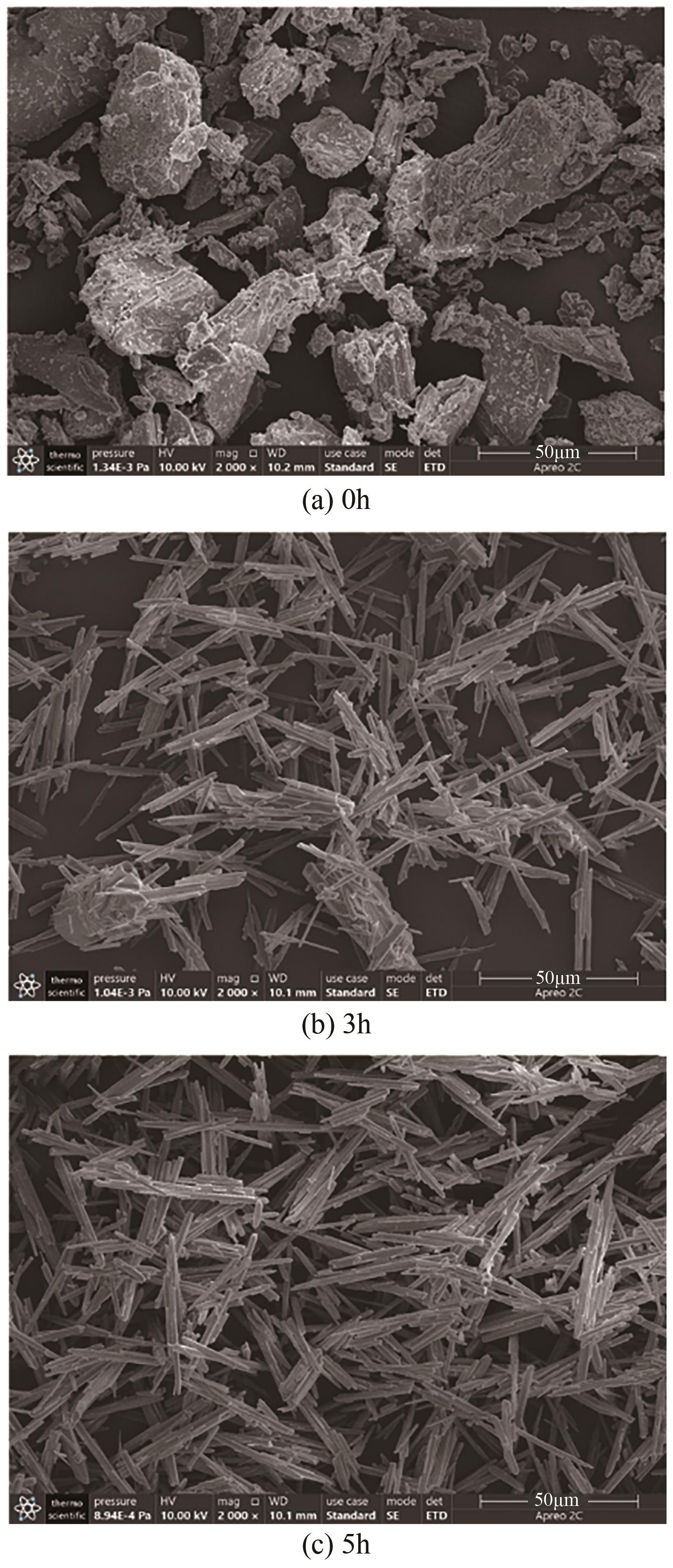
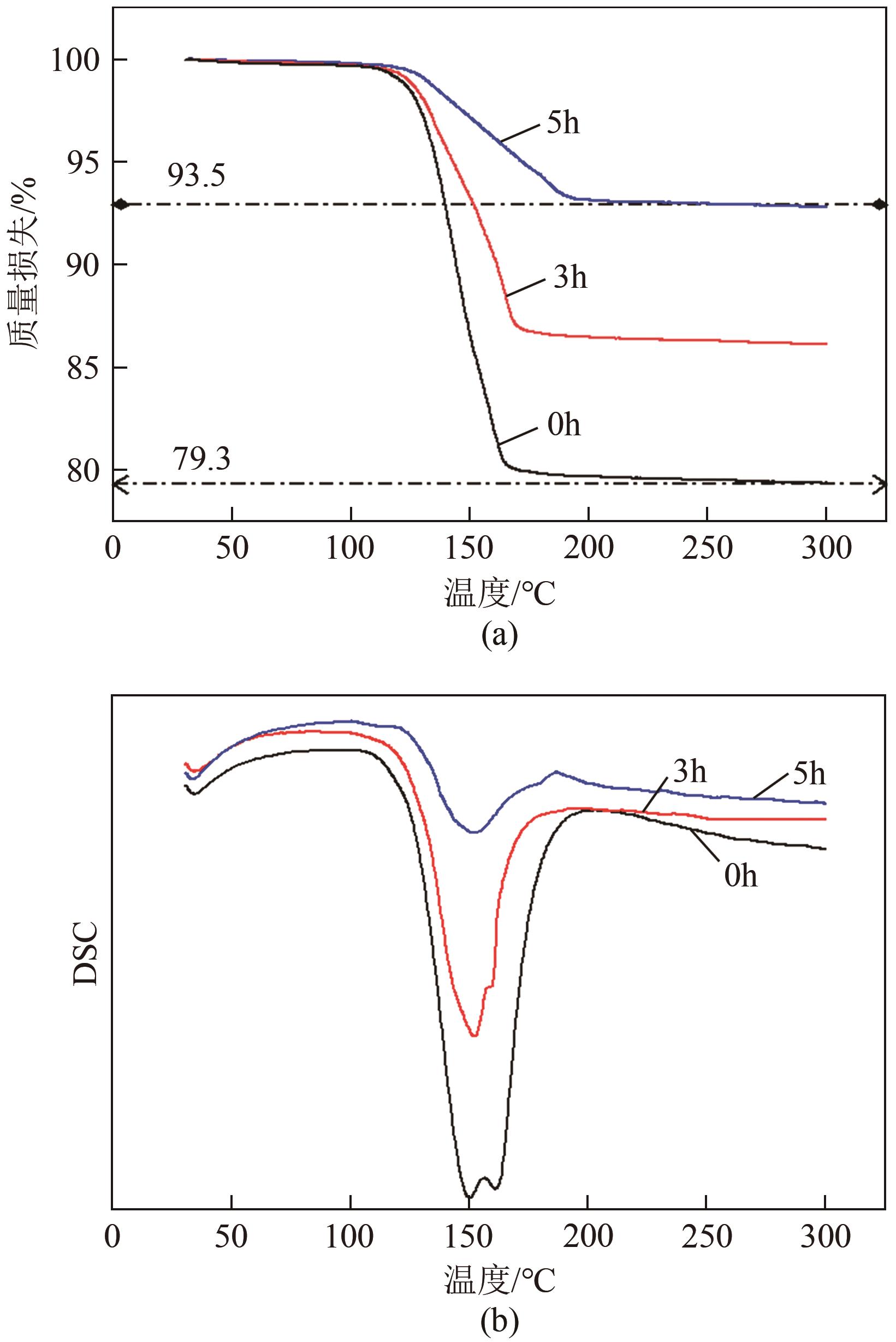
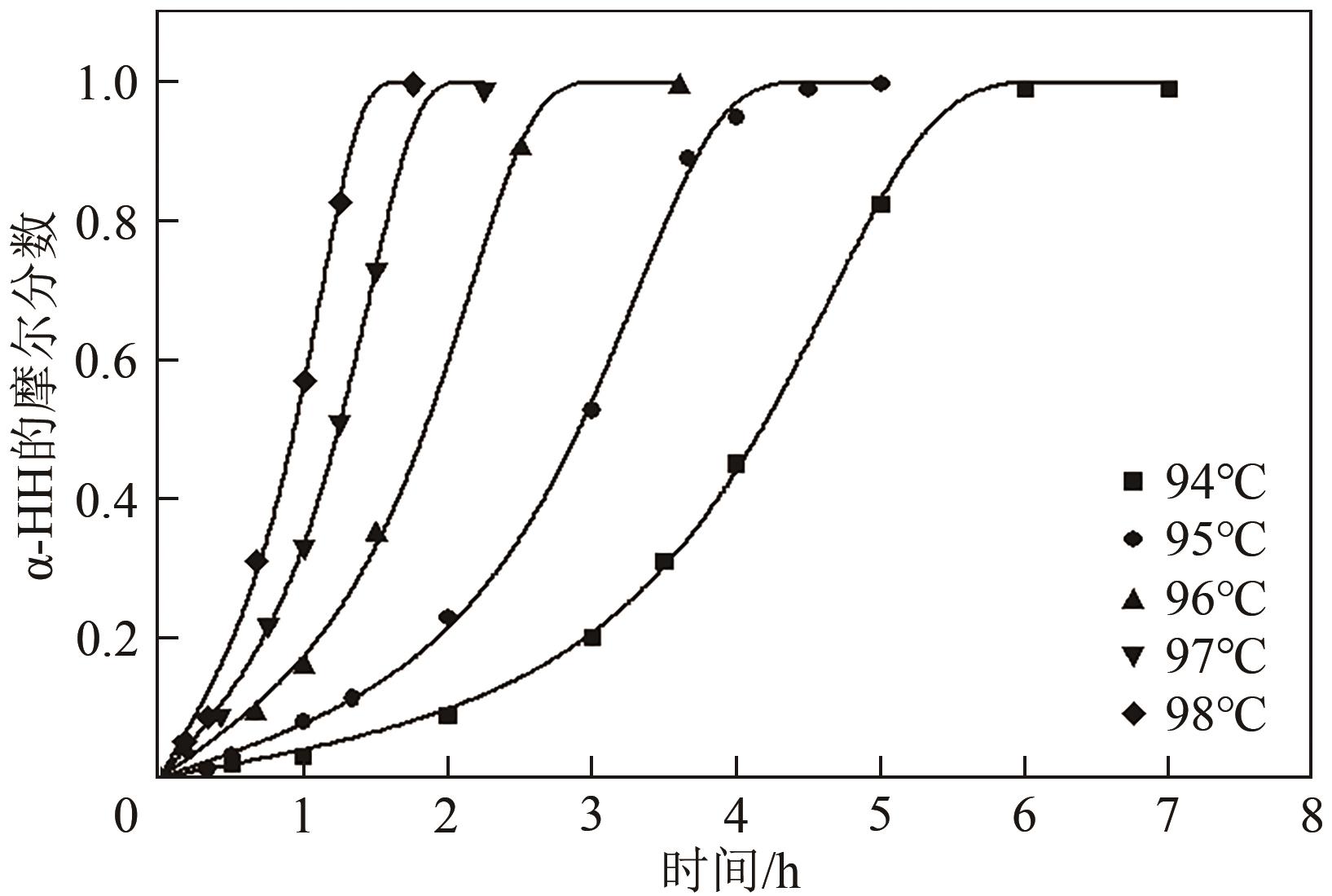
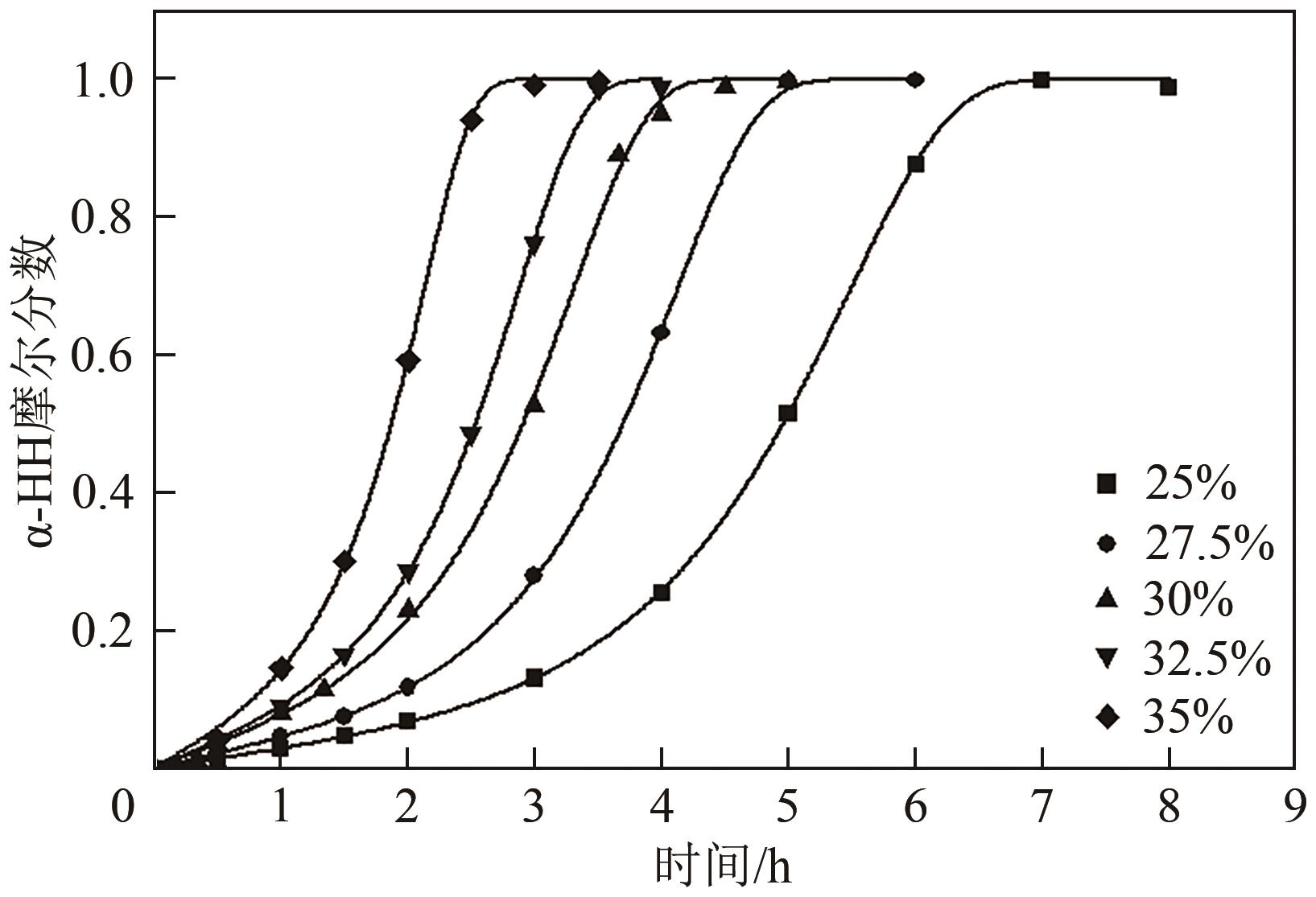
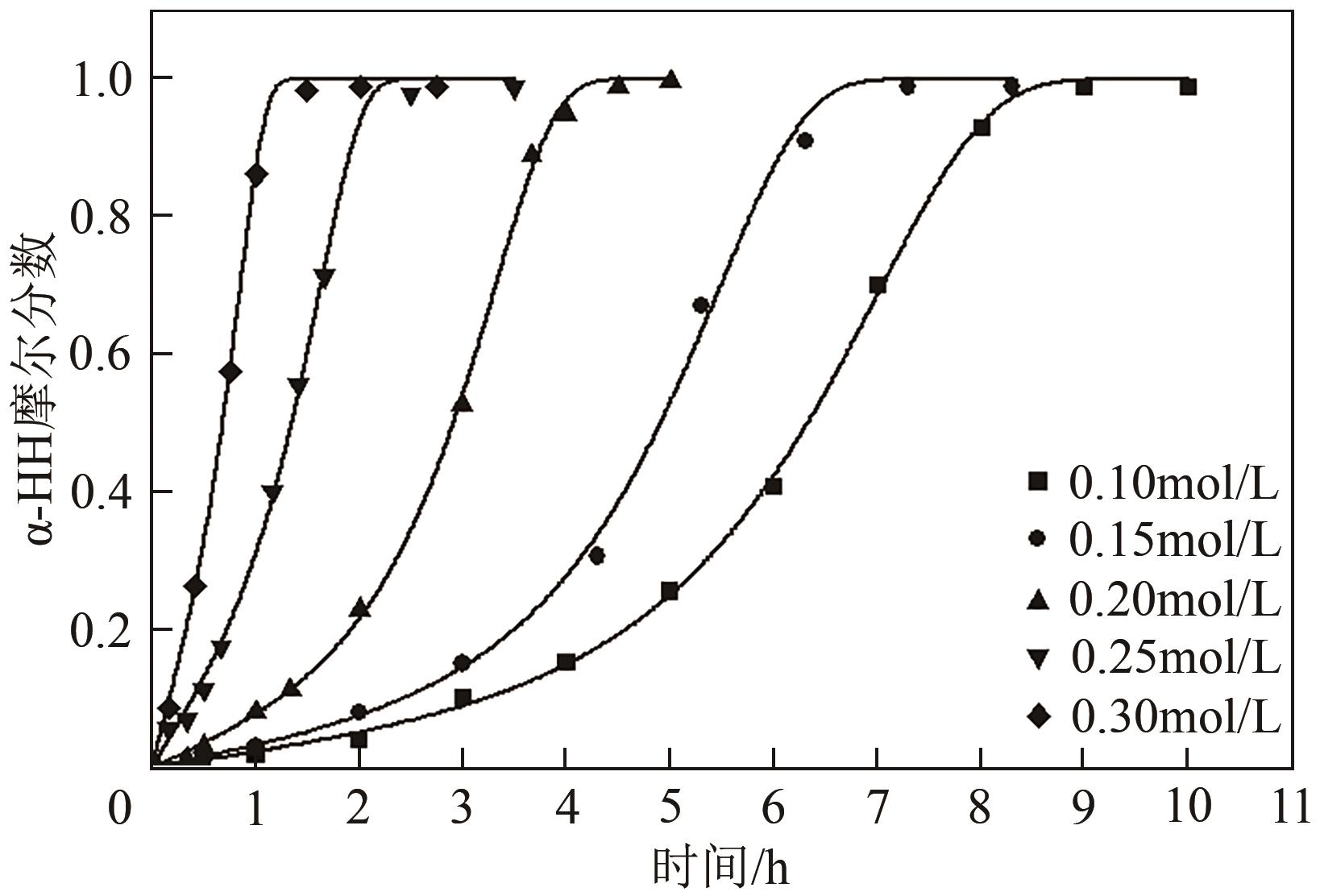
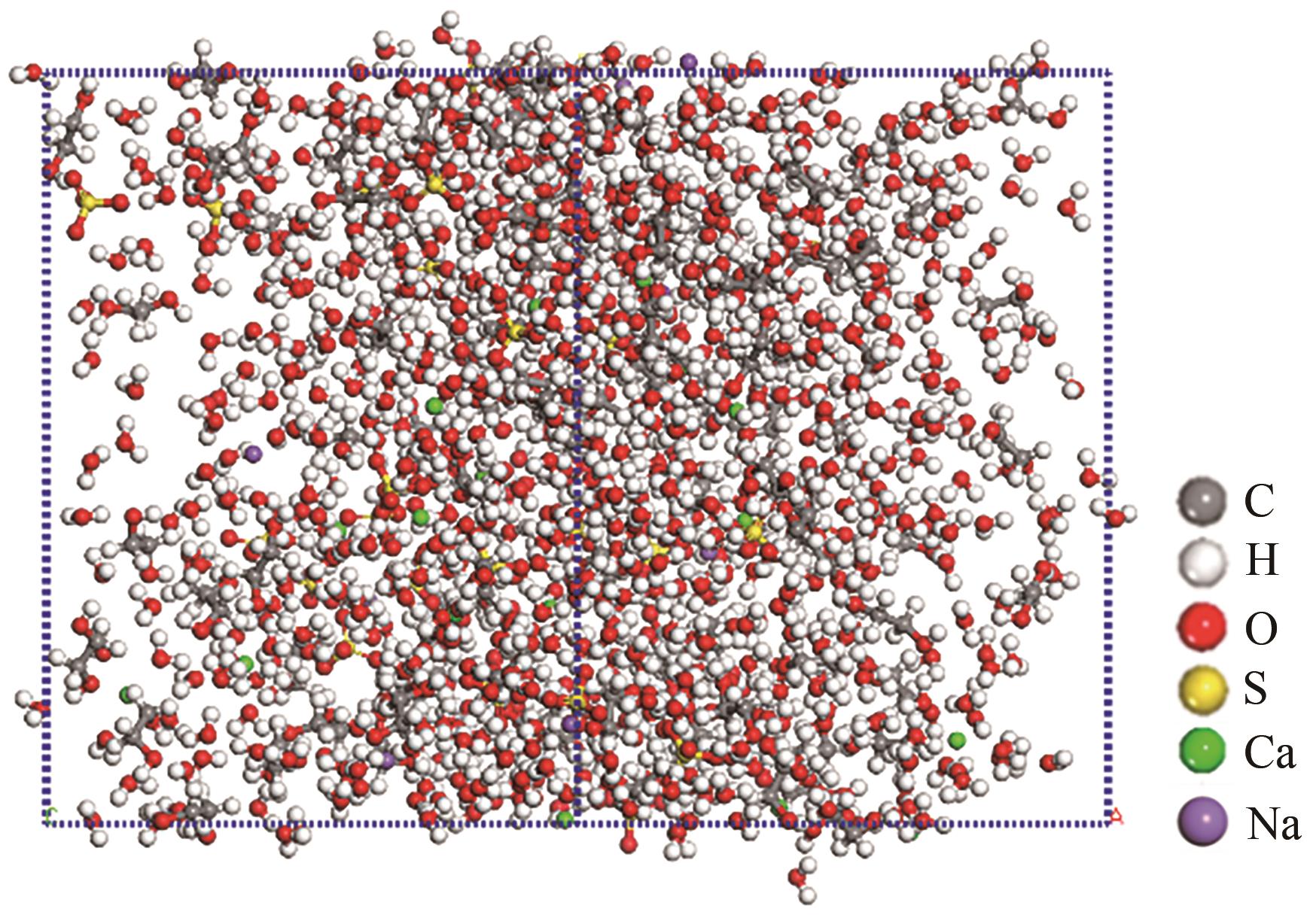
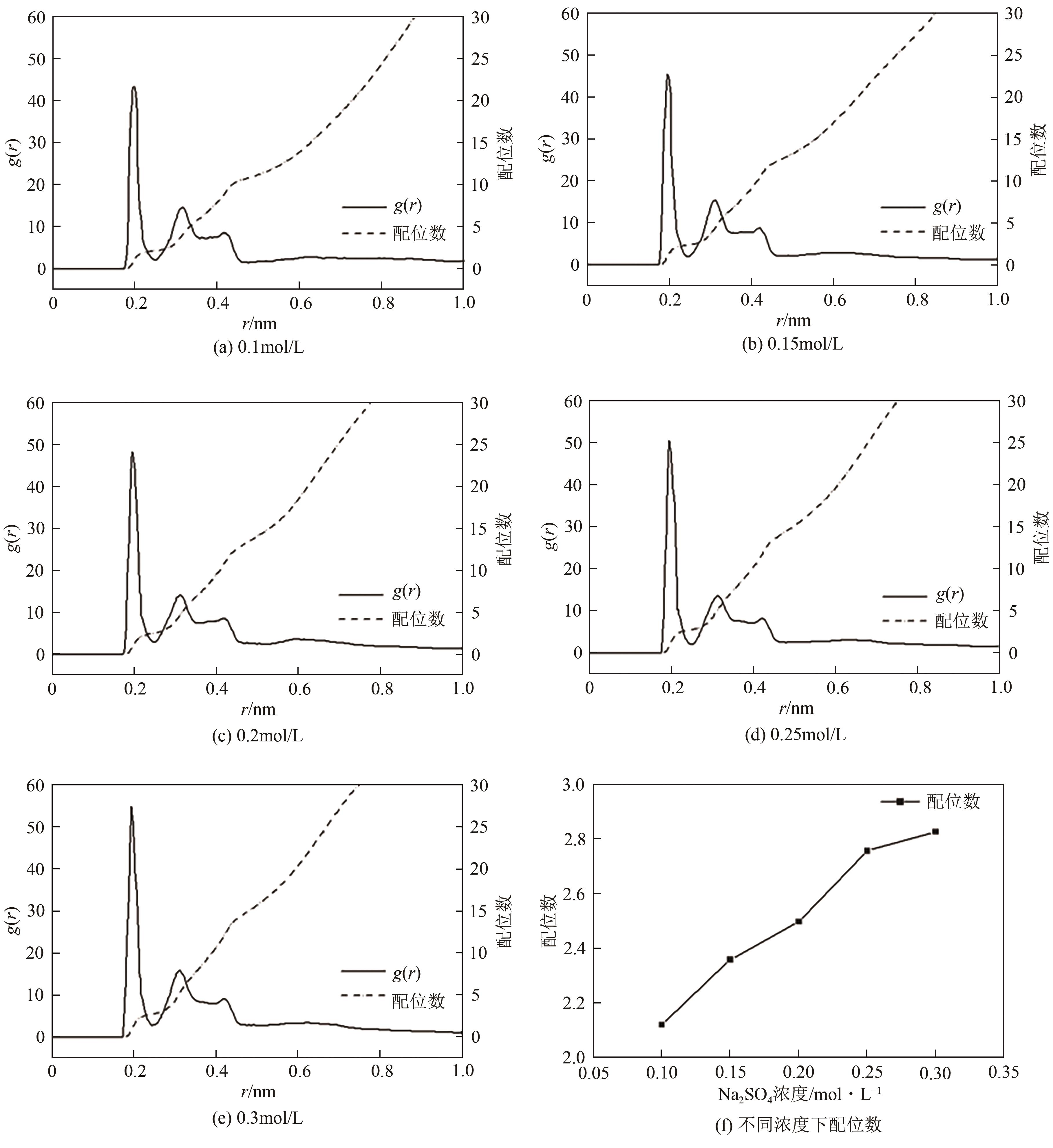
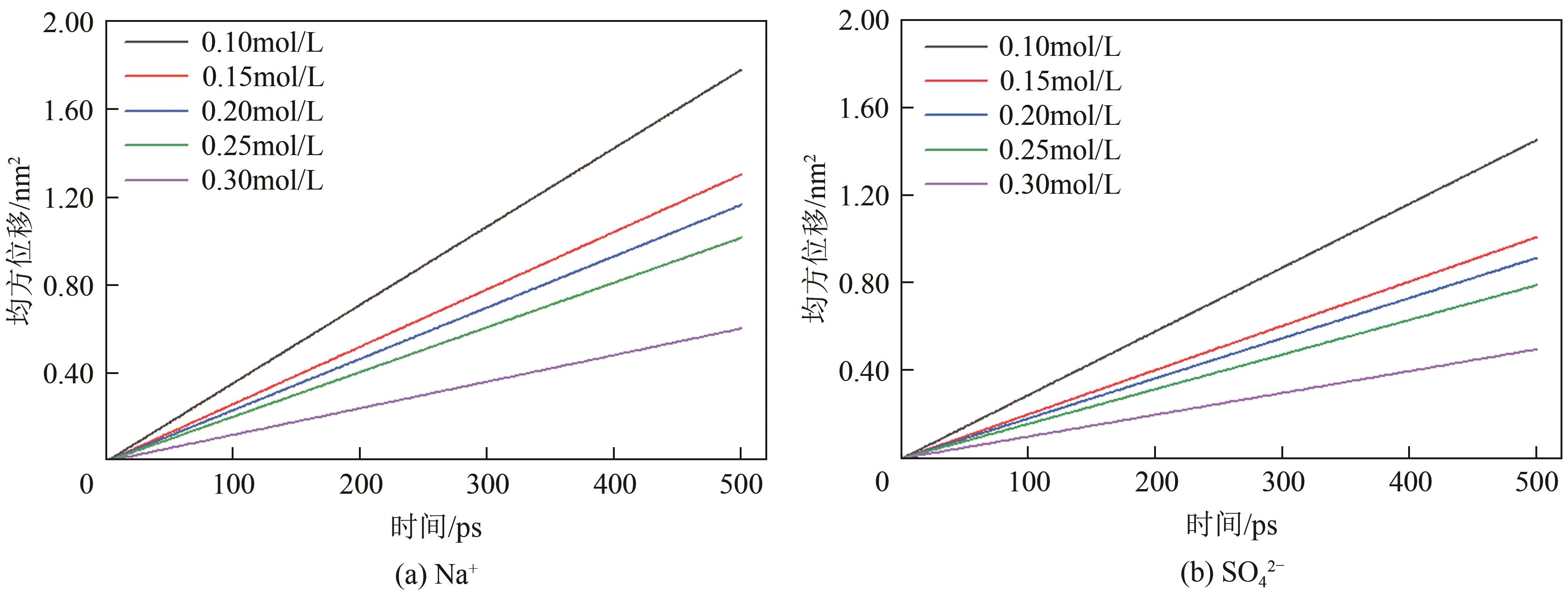
脱硫石膏的高效利用可以有效缓解一系列环境问题,变废为宝。本文利用二水石膏在含有微量无水硫酸钠的乙二醇水溶液中制备α-半水石膏,探究温度(94~98℃)、乙二醇体积分数(25%~35%)、硫酸钠浓度(0.1~0.3mol/L)对二水石膏向α-半水石膏脱水转化过程的α-半水石膏摩尔分数和动力学参数变化。研究发现,在乙二醇水溶液中,二水石膏向α-半水石膏脱水转化过程符合分散动力学模型。随着温度和乙二醇浓度的增加,动力学参数α基本保持不变,而参数β显著增加,导致活化熵∆S*增大,进而导致能垒Ea降低,促进了二水石膏向α-半水石膏脱水转化。微量无水硫酸钠的添加,显著缩短了α-半水石膏的成核诱导时间。通过分子动力学模拟,增加硫酸钠的浓度,Na+与SO42-的配位数增加,扩散系数绝对差值∆D降低,从而导致配位能力增加,解耦能力降低。本研究对高效利用脱硫石膏、掌握其向α-半水石膏的转化特征具有重要意义。
中图分类号:
引用本文
怀立业, 仲兆平, 杨宇轩. 脱硫石膏转化α-半水石膏的特征及机理:实验与模拟[J]. 化工进展, 2024, 43(8): 4694-4703.
HUAI Liye, ZHONG Zhaoping, YANG Yuxuan. Characteristics and mechanism of desulfurization gypsum to α-hemihydrate gypsum: Experiments and simulations[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4694-4703.
| 温度/℃ | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 94 | 0.2821 | 0.1393 | 0.9995 | 2.01 | 0.23 | 5.93 | 1.61×10-5 | 1.83×10-5 | 0.853 | 1.10 |
| 95 | 0.2992 | 0.2021 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.58×10-5 | 1.76×10-5 | 0.854 | 1.13 |
| 96 | 0.3177 | 0.2779 | 0.9978 | 0.63 | 0.39 | 2.94 | 1.55×10-5 | 1.7×10-5 | 0.855 | 1.15 |
| 97 | 0.2392 | 0.3783 | 0.9995 | 0.53 | 0.60 | 2.01 | 1.53×10-5 | 1.63×10-5 | 0.855 | 1.17 |
| 98 | 0.2572 | 0.6314 | 0.9998 | 0.41 | 0.75 | 1.61 | 1.50×10-5 | 1.57×10-5 | 0.856 | 1.20 |
表1 不同温度下的动力学参数
| 温度/℃ | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 94 | 0.2821 | 0.1393 | 0.9995 | 2.01 | 0.23 | 5.93 | 1.61×10-5 | 1.83×10-5 | 0.853 | 1.10 |
| 95 | 0.2992 | 0.2021 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.58×10-5 | 1.76×10-5 | 0.854 | 1.13 |
| 96 | 0.3177 | 0.2779 | 0.9978 | 0.63 | 0.39 | 2.94 | 1.55×10-5 | 1.7×10-5 | 0.855 | 1.15 |
| 97 | 0.2392 | 0.3783 | 0.9995 | 0.53 | 0.60 | 2.01 | 1.53×10-5 | 1.63×10-5 | 0.855 | 1.17 |
| 98 | 0.2572 | 0.6314 | 0.9998 | 0.41 | 0.75 | 1.61 | 1.50×10-5 | 1.57×10-5 | 0.856 | 1.20 |
| 乙二醇体积分数/% | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 25.0 | 0.2522 | 0.1093 | 0.9997 | 2.59 | 0.21 | 6.85 | 1.55×10-5 | 1.7×10-5 | 0.872 | 1.09 |
| 27.5 | 0.2365 | 0.1810 | 0.9998 | 1.80 | 0.26 | 5.21 | 1.55×10-5 | 1.7×10-5 | 0.866 | 1.11 |
| 30.0 | 0.2992 | 0.2421 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.55×10-5 | 1.7×10-5 | 0.854 | 1.13 |
| 32.5 | 0.2532 | 0.3234 | 0.9996 | 1.06 | 0.34 | 3.74 | 1.55×10-5 | 1.7×10-5 | 0.839 | 1.16 |
| 35.0 | 0.2965 | 0.5798 | 0.9993 | 0.77 | 0.44 | 2.78 | 1.55×10-5 | 1.7×10-5 | 0.823 | 1.19 |
表2 不同乙二醇浓度下的动力学参数
| 乙二醇体积分数/% | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 25.0 | 0.2522 | 0.1093 | 0.9997 | 2.59 | 0.21 | 6.85 | 1.55×10-5 | 1.7×10-5 | 0.872 | 1.09 |
| 27.5 | 0.2365 | 0.1810 | 0.9998 | 1.80 | 0.26 | 5.21 | 1.55×10-5 | 1.7×10-5 | 0.866 | 1.11 |
| 30.0 | 0.2992 | 0.2421 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.55×10-5 | 1.7×10-5 | 0.854 | 1.13 |
| 32.5 | 0.2532 | 0.3234 | 0.9996 | 1.06 | 0.34 | 3.74 | 1.55×10-5 | 1.7×10-5 | 0.839 | 1.16 |
| 35.0 | 0.2965 | 0.5798 | 0.9993 | 0.77 | 0.44 | 2.78 | 1.55×10-5 | 1.7×10-5 | 0.823 | 1.19 |
| Na2SO4浓度/mol·L-1 | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.3364 | 0.0644 | 0.9994 | 3.19 | 0.16 | 8.76 | 1.55×10-5 | 1.7×10-5 | 0.889 | 1.06 |
| 0.15 | 0.3132 | 0.1031 | 0.9976 | 2.40 | 0.20 | 6.87 | 1.55×10-5 | 1.7×10-5 | 0.871 | 1.09 |
| 0.20 | 0.2992 | 0.2421 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.55×10-5 | 1.7×10-5 | 0.854 | 1.13 |
| 0.25 | 0.2760 | 0.6871 | 0.9988 | 0.39 | 0.47 | 2.29 | 1.55×10-5 | 1.7×10-5 | 0.834 | 1.22 |
| 0.30 | 0.2967 | 2.0467 | 0.9995 | 0.17 | 0.83 | 1.26 | 1.55×10-5 | 1.7×10-5 | 0.751 | 1.37 |
表3 不同Na2SO4浓度下的动力学参数
| Na2SO4浓度/mol·L-1 | α/h | β/h-2 | R2 | tind/h | v/h-1 | ttot/h | Ksp,DH | Ksp,HH | aw | SHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.3364 | 0.0644 | 0.9994 | 3.19 | 0.16 | 8.76 | 1.55×10-5 | 1.7×10-5 | 0.889 | 1.06 |
| 0.15 | 0.3132 | 0.1031 | 0.9976 | 2.40 | 0.20 | 6.87 | 1.55×10-5 | 1.7×10-5 | 0.871 | 1.09 |
| 0.20 | 0.2992 | 0.2421 | 0.9992 | 1.21 | 0.29 | 4.35 | 1.55×10-5 | 1.7×10-5 | 0.854 | 1.13 |
| 0.25 | 0.2760 | 0.6871 | 0.9988 | 0.39 | 0.47 | 2.29 | 1.55×10-5 | 1.7×10-5 | 0.834 | 1.22 |
| 0.30 | 0.2967 | 2.0467 | 0.9995 | 0.17 | 0.83 | 1.26 | 1.55×10-5 | 1.7×10-5 | 0.751 | 1.37 |
| Na2SO4浓度 /mol·L-1 | D(Na+) /10-5cm2·s-1 | D(SO42-) /10-5cm2·s-1 | ∆D/10-5cm2·s-1 |
|---|---|---|---|
| 0.1 | 5.95 | 5.22 | 1.1 |
| 0.15 | 4.36 | 3.37 | 0.98 |
| 0.2 | 3.9 | 3.05 | 0.82 |
| 0.25 | 3.39 | 2.64 | 0.76 |
| 0.3 | 2.02 | 1.66 | 0.36 |
表4 不同浓度下Na+与SO42-的D及∆D
| Na2SO4浓度 /mol·L-1 | D(Na+) /10-5cm2·s-1 | D(SO42-) /10-5cm2·s-1 | ∆D/10-5cm2·s-1 |
|---|---|---|---|
| 0.1 | 5.95 | 5.22 | 1.1 |
| 0.15 | 4.36 | 3.37 | 0.98 |
| 0.2 | 3.9 | 3.05 | 0.82 |
| 0.25 | 3.39 | 2.64 | 0.76 |
| 0.3 | 2.02 | 1.66 | 0.36 |
| 1 | JIANG Guangming, WANG Hao, CHEN Qiaoshan, et al. Preparation of alpha-calcium sulfate hemihydrate from FGD gypsum in chloride-free Ca(NO3)2 solution under mild conditions[J]. Fuel, 2016, 174: 235-241. |
| 2 | 中华人民共和国生态环境部. 2020年全国大、中城市固体废物污染环境防治年报[EB/OL]. (2020-12-28) [2023-06-25]. . |
| Ministry of Ecology and Environment of the People's Republic of China. Annual report on the prevention and control of environmental pollution by solid waste in large and medium cities in 2020 [EB/OL]. (2020-12-28) [2023-06-25]. . | |
| 3 | 王文飚, 许月阳, 薛建明, 等. 燃煤电厂脱硫技术研究进展及建议[J]. 电力科技与环保, 2020, 36(3): 1-5. |
| WANG Wenbiao, XU Yueyang, XUE Jianming, et al. The research progress and suggestions of desulfurization technology in coal-fired power plants[J]. Electric Power Technology and Environmental Protection, 2020, 36(3): 1-5. | |
| 4 | ZHU Zhencai, XU Lei, CHEN Guo'an. Effect of different whiskers on the physical and tribological properties of non-metallic friction materials[J]. Materials & Design, 2011, 32(1): 54-61. |
| 5 | ZHAO Wenpeng, GAO Chuanhui, SANG Hongfei, et al. Calcium sulfate hemihydrate whisker reinforced polyvinyl alcohol with improved shape memory effect[J]. RSC Advances, 2016, 6(58): 52982-52986. |
| 6 | JIANG Guangming, CHEN Qiaoshan, JIA Caiyun, et al. Controlled synthesis of monodisperse α-calcium sulfate hemihydrate nanoellipsoids with a porous structure[J]. Physical Chemistry Chemical Physics, 2015, 17(17): 11509-11515. |
| 7 | 郝卓, 赵建华, 石秀芹, 等. 脱硫石膏液相法生产高强度α-石膏的工艺研究[J]. 电力科技与环保, 2011, 27(3): 33-34. |
| HAO Zhuo, ZHAO Jianhua, SHI Xiuqin, et al. Research on parameters of liquid phase process in FGD gypsum utilization[J]. Electric Power Technology and Environmental Protection, 2011, 27(3): 33-34. | |
| 8 | 张小军, 许旭斌, 冯斌, 等. 630MW机组石膏高含水率原因分析及处理[J]. 电力科技与环保, 2022, 38(3): 238-244. |
| ZHANG Xiaojun, XU Xubin, FENG Bin, et al. Analysis and treatment of high water content of gypsum in a 630MW unit[J]. Electric Power Technology and Environmental Protection, 2022, 38(3): 238-244. | |
| 9 | LI Xianbo, ZHANG Qin, SHEN Zhihui, et al. L-aspartic acid: A crystal modifier for preparation of hemihydrate from phosphogypsum in CaCl2 solution[J]. Journal of Crystal Growth, 2019, 511: 48-55. |
| 10 | GUAN Qingjun, SUN Wei, HU Yuehua, et al. A facile method of transforming FGD gypsum to α-CaSO4·0.5H2O whiskers with cetyltrimethylammonium bromide (CTAB) and KCl in glycerol-water solution[J]. Scientific Reports, 2017, 7: 7085. |
| 11 | GUAN Baohong, YANG Li, FU Hailu, et al. Alpha-calcium sulfate hemihydrate preparation from FGD gypsum in recycling mixed salt solutions[J]. Chemical Engineering Journal, 2011, 174(1): 296-303. |
| 12 | FU Hailuo, HUANG Jianshi, SHEN Luming, et al. Sodium cation-mediated crystallization of α-hemihydrate whiskers from gypsum in ethylene glycol-water solutions[J]. Crystal Growth & Design, 2018, 18(11): 6694-6701. |
| 13 | GUAN Baohong, YANG Liuchun, WU Zhongbiao, et al. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions[J]. Fuel, 2009, 88(7): 1286-1293. |
| 14 | LI Zhibao, DEMOPOULOS George P. Model-based construction of calcium sulfate phase-transition diagrams in the HCl-CaCl2-H2O system between 0 and 100℃[J]. Industrial & Engineering Chemistry Research, 2006, 45(13): 4517-4524. |
| 15 | FU Hailu, JIANG Guangming, WANG Hao, et al. Solution-mediated transformation kinetics of calcium sulfate dihydrate to α-calcium sulfate hemihydrate in CaCl2 solutions at elevated temperature[J]. Industrial & Engineering Chemistry Research, 2013, 52(48): 17134-17139. |
| 16 | FELDMANN Thomas, DEMOPOULOS George P. Influence of impurities on crystallization kinetics of calcium sulfate dihydrate and hemihydrate in strong HCl-CaCl2 solutions[J]. Industrial & Engineering Chemistry Research, 2013, 52(19): 6540-6549. |
| 17 | JIANG Guangming, FU Hailu, SAVINO Keith, et al. Nonlattice cation-SO4 2– ion pairs in calcium sulfate hemihydrate nucleation[J]. Crystal Growth & Design, 2013, 13(11): 5128-5134. |
| 18 | WANG Bingqi, YANG Lin, CAO Jianxin. The influence of impurities on the dehydration and conversion process of calcium sulfate dihydrate to α-calcium sulfate hemihydrate in the two-step wet-process phosphoric acid production[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(43): 14365-14374. |
| 19 | MAO Xiulong, SONG Xingfu, LU Guimin, et al. Control of crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions by additives: Experimental and molecular dynamics simulation studies[J]. Industrial and Engineering Chemistry Research, 2015, 54(17): 4781-4787. |
| 20 | 孔令云, 全秀洁, 李朝波, 等. 乳化剂在集料化学成分表面吸附行为的分子模拟与试验论证[J]. 化工进展, 2020, 39(8): 3196-3204. |
| KONG Lingyun, QUAN Xiujie, LI Chaobo, et al. Molecular simulation and experimental demonstration of adsorption behavior of emulsifier on surface of chemical composition of aggregate[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3196-3204. | |
| 21 | GUAN Baohong, JIANG Guangming, FU Hailu, et al. Thermodynamic preparation window of alpha calcium sulfate hemihydrate from calcium sulfate dihydrate in non-electrolyte glycerol-water solution under mild conditions[J]. Industrial and Engineering Chemistry Research, 2011, 50(23): 13561-13567. |
| 22 | AVRAMI Melvin. Kinetics of phase change. I. General theory[J]. The Journal of Chemical Physics, 1939, 7(12): 1103-1112. |
| 23 | SKRDLA Peter J, ROBERTSON Rebecca T. Semiempirical equations for modeling solid-state kinetics based on a Maxwell-Boltzmann distribution of activation energies: Applications to a polymorphic transformation under crystallization slurry conditions and to the thermal decomposition of AgMnO4 crystals[J]. The Journal of Physical Chemistry B, 2005, 109(21): 10611-10619. |
| 24 | JIANG Guangming, FU Wenyang, WANG Yuzheng, et al. Calcium sulfate hemihydrate nanowires: One robust material in separation of water from water-in-oil emulsion[J]. Environmental Science & Technology, 2017, 51(18): 10519-10525. |
| 25 | WANG Bingqi, YANG Lin, LUO Tong, et al. Study on the kinetics of hydration transformation from hemihydrate phosphogypsum to dihydrate phosphogypsum in simulated wet process phosphoric acid[J]. ACS Omega, 2021, 6(11): 7342-7350. |
| 26 | COLLINS Kim D. Ion hydration: Implications for cellular function, polyelectrolytes, and protein crystallization[J]. Biophysical Chemistry, 2006, 119(3): 271-281. |
| [1] | 何海霞, 万亚萌, 李帆帆, 牛心雨, 张静雯, 李涛, 任保增. 盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺[J]. 化工进展, 2024, 43(8): 4230-4245. |
| [2] | 向瑞, 艾波, 吴高胜, 李瑜哲, 宗睿, 许保云, 杜丽君. 锂电添加剂FEC-VC二元体系固液平衡数据的测定及回归[J]. 化工进展, 2024, 43(8): 4246-4252. |
| [3] | 谢娟, 贺文, 赵勖丞, 李帅辉, 卢真真, 丁哲宇. 分子动力学模拟在沥青体系中的应用研究进展[J]. 化工进展, 2024, 43(8): 4432-4449. |
| [4] | 丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612. |
| [5] | 冼学权, 杜芳黎, 刘忠林, 刘婉玉, 黎演明, 龙思宇, 黄华林. 利用PEG/Na2CO3双水相乳液法制备碳酸钙微球及其形成机理[J]. 化工进展, 2024, 43(6): 3221-3231. |
| [6] | 蒋晨光, 张胜振, 张翠清, 郭屹, 孙永伟. 基于DFSS方法优化52#费托蜡的制备工艺[J]. 化工进展, 2024, 43(4): 1742-1753. |
| [7] | 周逸寰, 解强, 周红阳, 梁鼎成, 刘金昌. 基于分子模拟的多孔炭材料结构模型构建方法研究进展[J]. 化工进展, 2024, 43(3): 1535-1551. |
| [8] | 董晓涵, 田月, 苏毅. 含钛高炉渣制备复合吸附剂及其铬吸附性能[J]. 化工进展, 2024, 43(3): 1552-1564. |
| [9] | 张梁, 马骥, 贺高红, 姜晓滨, 肖武. 膜调控的头孢呋辛钠溶析-冷却耦合结晶成核介稳区测定及分析[J]. 化工进展, 2024, 43(1): 260-268. |
| [10] | 王一棪, 王达锐, 沈震浩, 何俊琳, 孙洪敏, 杨为民. 全结晶MCM-22分子筛催化剂的制备及其催化性能[J]. 化工进展, 2024, 43(1): 285-291. |
| [11] | 冯瑶光, 陈奎, 赵佳伟, 王娜, 王霆, 黄欣, 周丽娜, 郝红勋. 溶液结晶过程强化[J]. 化工进展, 2024, 43(1): 87-99. |
| [12] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [13] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [14] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [15] | 王达锐, 孙洪敏, 薛明伟, 王一棪, 刘威, 杨为民. 微波法高效合成全结晶ZSM-5分子筛催化剂及其催化性能[J]. 化工进展, 2023, 42(7): 3582-3588. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||