化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6215-6232.DOI: 10.16085/j.issn.1000-6613.2023-1763
• 材料科学与技术 • 上一篇
基于煤层气甲烷富集的固体多孔材料研究进展
程春晖1( ), 明淑君2, 庞磊3, 田士东4, 李克伦4, 李涛1(
), 明淑君2, 庞磊3, 田士东4, 李克伦4, 李涛1( )
)
- 1.华中科技大学化学与化工学院,能量转换与存储材料化学教育部重点实验室,材料化学与服役失效湖北省 重点实验室,湖北 武汉 430074
2.黄冈师范学院化学化工学院,湖北 黄冈 438000
3.东风商用车有限公司 技术中心,湖北 武汉 430056
4.陕西煤业化工技术研究院有限责任公司,陕西 西安 710100
-
收稿日期:2023-10-09修回日期:2023-12-18出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:李涛 -
作者简介:程春晖(1995—),男,博士研究生,研究方向为固体多孔吸附剂材料的合成与改性。E-mail:chunhuicheng@hust.edu.cn。 -
基金资助:国家重点研发计划(2020YFB1711200)
Developments in solid porous materials for methane enrichment in coalbed gas
CHENG Chunhui1( ), MING Shujun2, PANG Lei3, TIAN Shidong4, LI Kelun4, LI Tao1(
), MING Shujun2, PANG Lei3, TIAN Shidong4, LI Kelun4, LI Tao1( )
)
- 1.School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, MOE Key Laboratory of Material Chemistry for Energy Conversion and Storage, Hubei Key Laboratory of Material Chemistry and Service Failure, Wuhan 430074, Hubei, China
2.School of Chemistry and Chemical Engineering, Huanggang Normal University, Huanggang 438000, Hubei, China
3.Technical Center, Dongfeng Commercial Vehicle Co. , Ltd. , Wuhan 430056, Hubei, China
Shaanxi Coal Chemical Industry Technology Research Institute Co. , Ltd. , Xi’an 710100, Shaanxi, China
-
Received:2023-10-09Revised:2023-12-18Online:2024-11-15Published:2024-12-07 -
Contact:LI Tao
摘要:
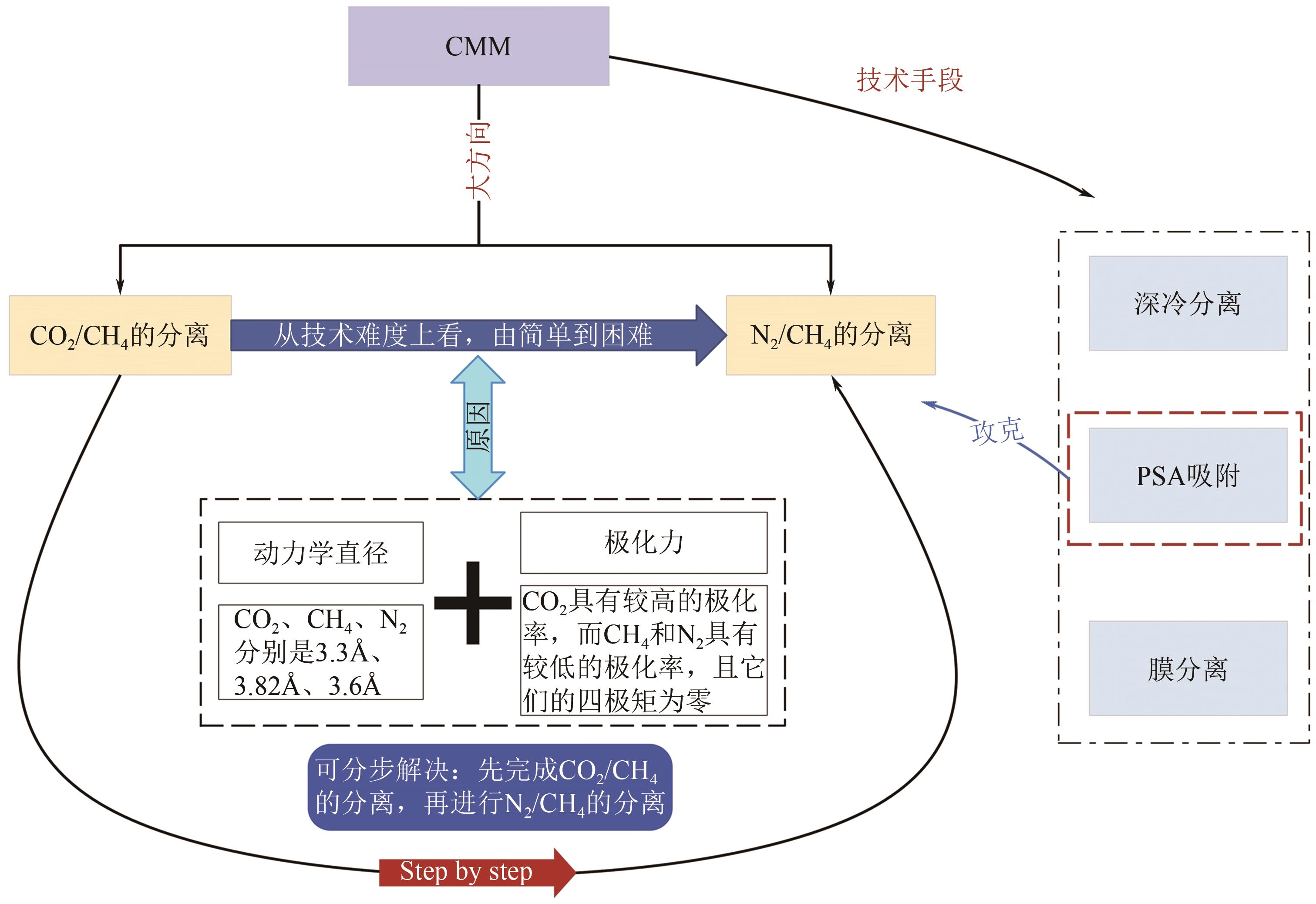
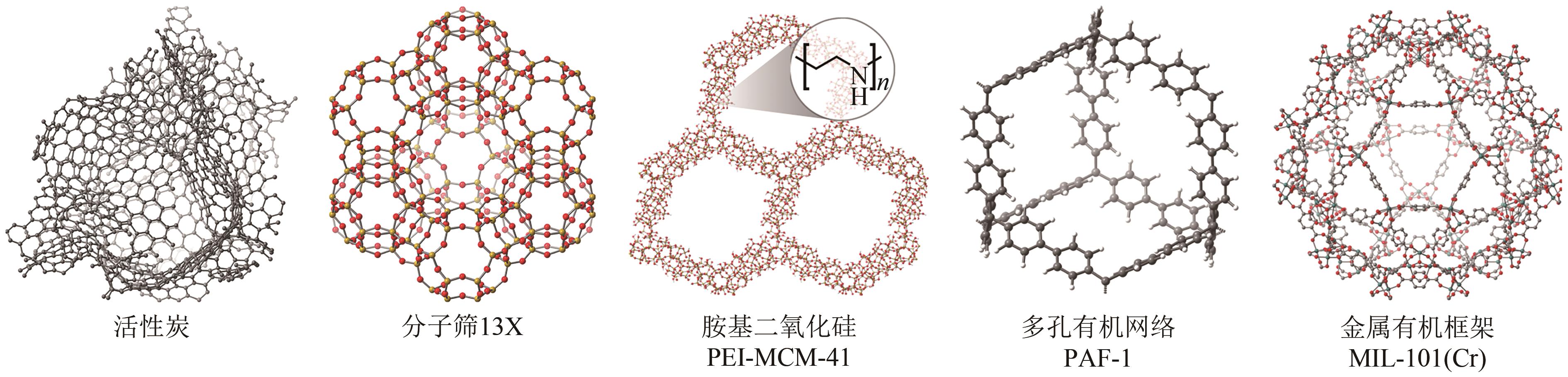
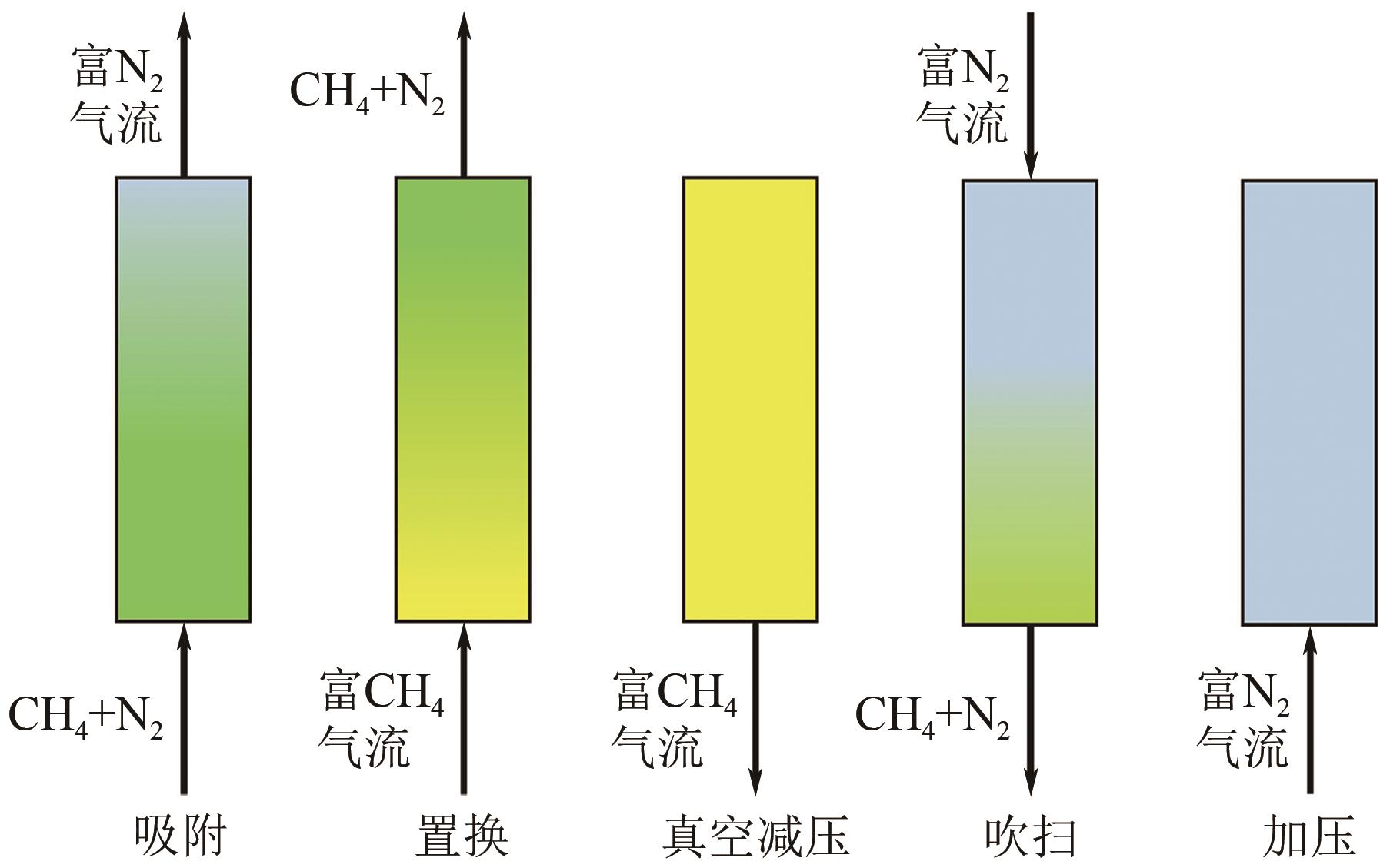
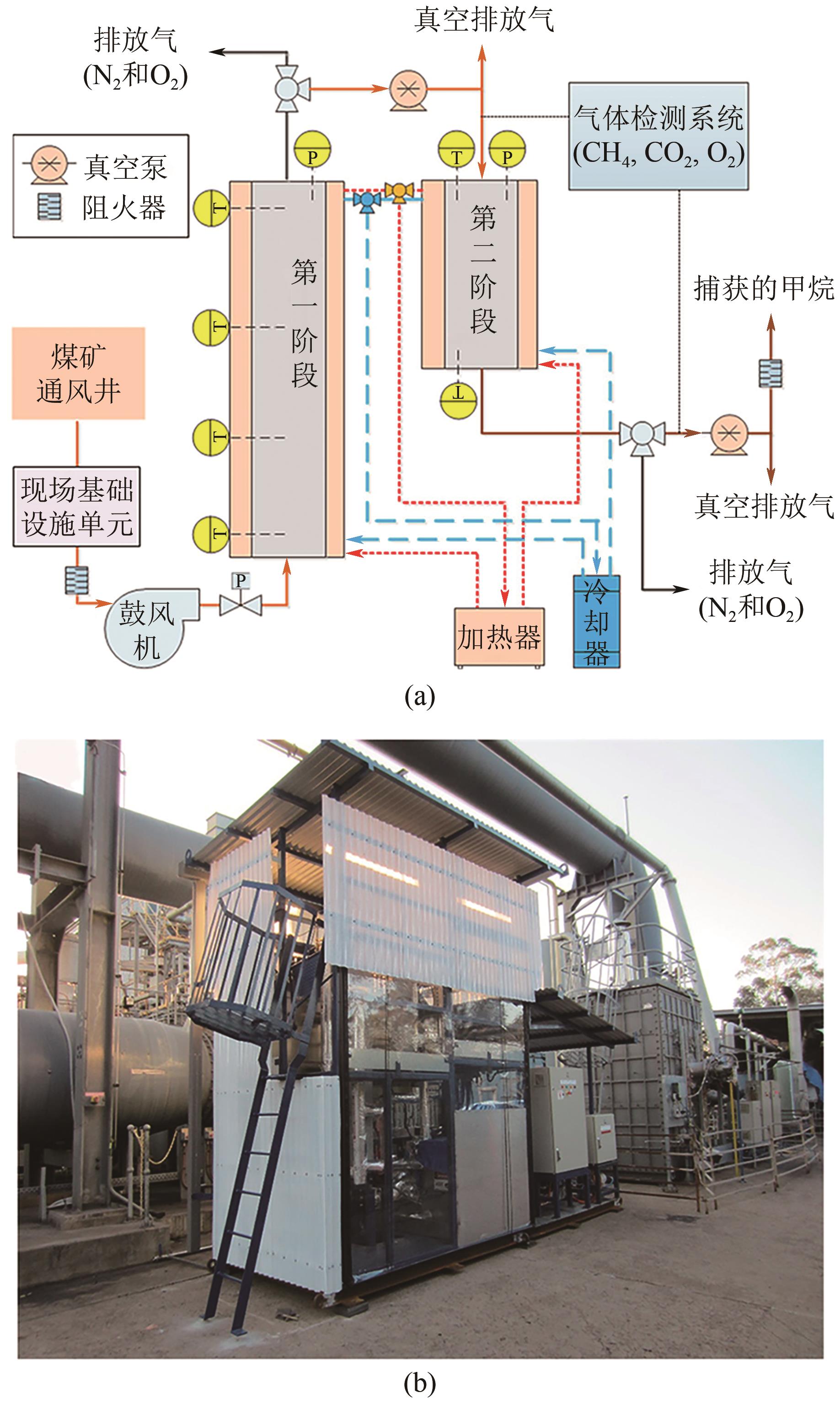
煤层气(CMM)中的甲烷不仅是一种重要的绿色可持续的低碳清洁能源,亦是一种令人担忧的爆炸和温室气体污染物。因此寻找一种稳定且高效的甲烷富集技术,对于增加目前煤炭开采业的安全系数及克服常规天然气短缺问题具有重要意义。然而煤层气中存在大量杂质已成为其工业化大规模利用的关键技术障碍,尤其是对二氧化碳和氮气污染物的高效分离。本文综述了在煤层气甲烷富集真空变压吸附工艺(VPSA)过程中的两个主要方向:二氧化碳捕集(CO2/CH4分离)和天然气净化(CH4/N2分离),其中制备高效分离甲烷气体混合物的固体吸附剂是VPSA技术的核心突破点。进而重点分析和比较了碳基吸附材料、沸石分子筛和金属有机骨架这三种典型的固体吸附剂在上述两个方向上分离或吸附甲烷的差异性与相似性,其中在吸附剂表面耦合不同功能的官能团或在纳米尺度上微调其孔隙结构将是不断提高煤层气甲烷富集吸附分离效率的有效途径。此外还讨论了固体多孔材料未来发展面临的挑战和发展方向,希望在帮助研究人员了解CMM甲烷富集吸附剂的技术前提下,理解并设计出新型吸附剂以期满足工业上多重苛刻的甲烷分离要求。
中图分类号:
引用本文
程春晖, 明淑君, 庞磊, 田士东, 李克伦, 李涛. 基于煤层气甲烷富集的固体多孔材料研究进展[J]. 化工进展, 2024, 43(11): 6215-6232.
CHENG Chunhui, MING Shujun, PANG Lei, TIAN Shidong, LI Kelun, LI Tao. Developments in solid porous materials for methane enrichment in coalbed gas[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6215-6232.
| 技术 | 变压吸附分离 | 低温深冷分离 | 膜分离 | 水合物基分离 |
|---|---|---|---|---|
| 机理 | 吸附选择性 | 沸点不同 | 渗透率差异 | 甲烷水合物结晶 |
| 相变 | 无 | 有 | 无 | 有 |
| 压力 | PSA需要高压;VPSA仅需常压 | 高压 | 高压 | 高压 |
| 优点 | 高安全性、长寿命、高操作灵活性、低能耗 | 富集性能好 | 操作灵活性高、能耗低 | 富集性能好 |
| 缺点 | 一次操作的富集性能有限 | 能耗高、工艺复杂、爆炸风险高 | 选择性低、膜寿命短 | 能耗高、水合物形成速率低、 爆炸风险大 |
| 发展现状 | 商业化应用 | 局部试验阶段 | 实验室开发阶段 | 实验室开发阶段 |
| 潜在问题 | 低产出投入比 | 高成本和爆炸风险 | 放大问题、膜耐久性 | 放大问题、技术不成熟 |
表1 煤层气甲烷富集不同工艺的对比[6]
| 技术 | 变压吸附分离 | 低温深冷分离 | 膜分离 | 水合物基分离 |
|---|---|---|---|---|
| 机理 | 吸附选择性 | 沸点不同 | 渗透率差异 | 甲烷水合物结晶 |
| 相变 | 无 | 有 | 无 | 有 |
| 压力 | PSA需要高压;VPSA仅需常压 | 高压 | 高压 | 高压 |
| 优点 | 高安全性、长寿命、高操作灵活性、低能耗 | 富集性能好 | 操作灵活性高、能耗低 | 富集性能好 |
| 缺点 | 一次操作的富集性能有限 | 能耗高、工艺复杂、爆炸风险高 | 选择性低、膜寿命短 | 能耗高、水合物形成速率低、 爆炸风险大 |
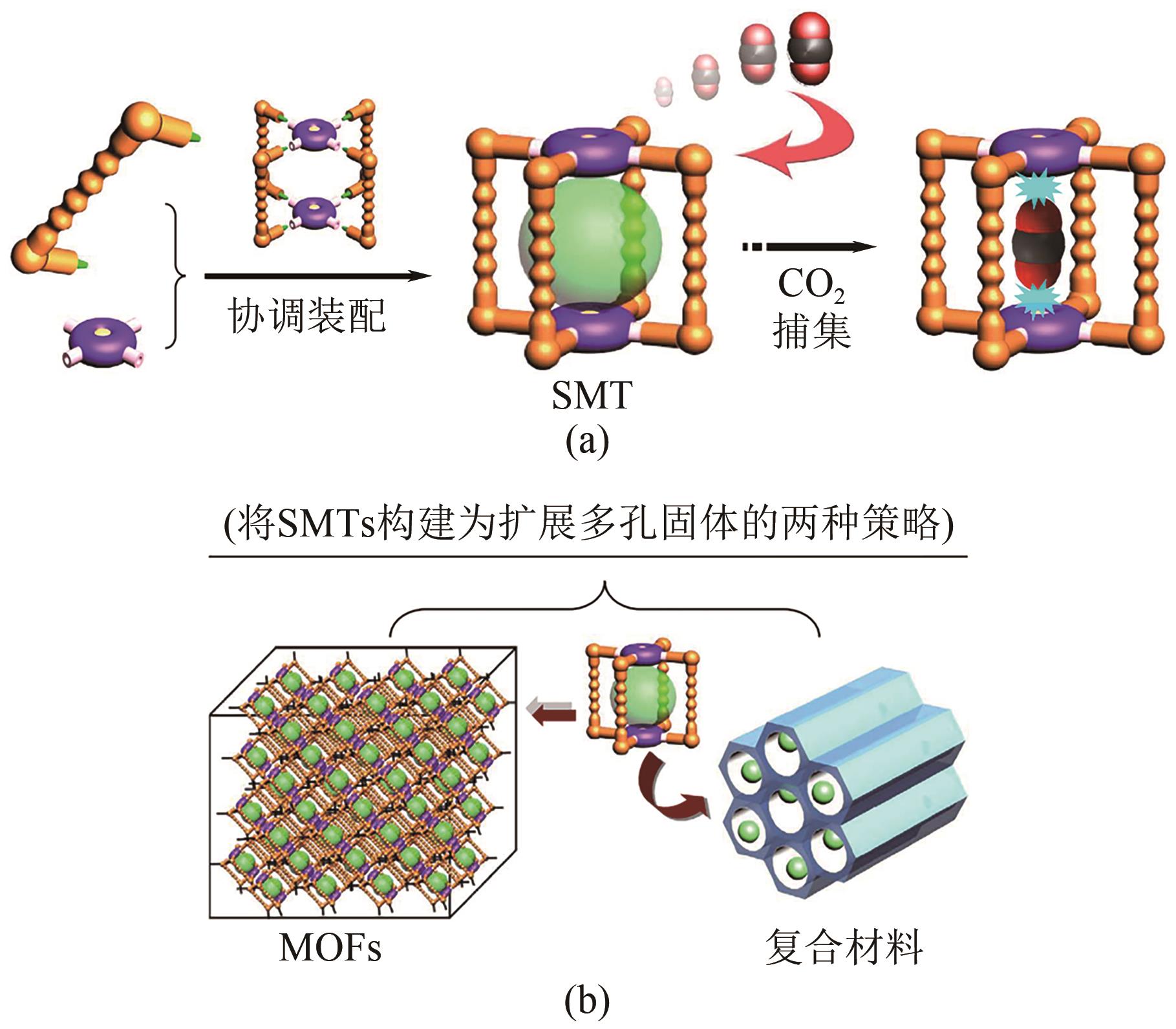
| 发展现状 | 商业化应用 | 局部试验阶段 | 实验室开发阶段 | 实验室开发阶段 |
| 潜在问题 | 低产出投入比 | 高成本和爆炸风险 | 放大问题、膜耐久性 | 放大问题、技术不成熟 |
| 吸附剂类型 | 优点 | 缺点 |
|---|---|---|
| 活性炭 | 成本低廉、比表面积大、易于修饰 | 孔隙分布杂乱、选择性低、机械稳定性差 |
| 沸石分子筛 | 良好的热和机械稳定性、高的表面积 | 再生能耗高、对水敏感 |
| 有机聚合物 | 可定制化孔隙结构和化学性质 | 热和机械稳定性差、合成复杂、再生性弱 |
| 金属有机框架 | 极高的表面积、可定制化孔隙结构和化学性质 | 制造成本高、对水敏感、热和机械稳定性差 |
| 二氧化硅 | 良好的热稳定性和机械强度 | 吸附量和选择性低 |
| 碳纳米管 | 高表面积、可调节的化学性质和孔结构 | 制造成本高、分散性和加工性能需优化 |
表2 不同吸附剂类型的优缺点[22-23]
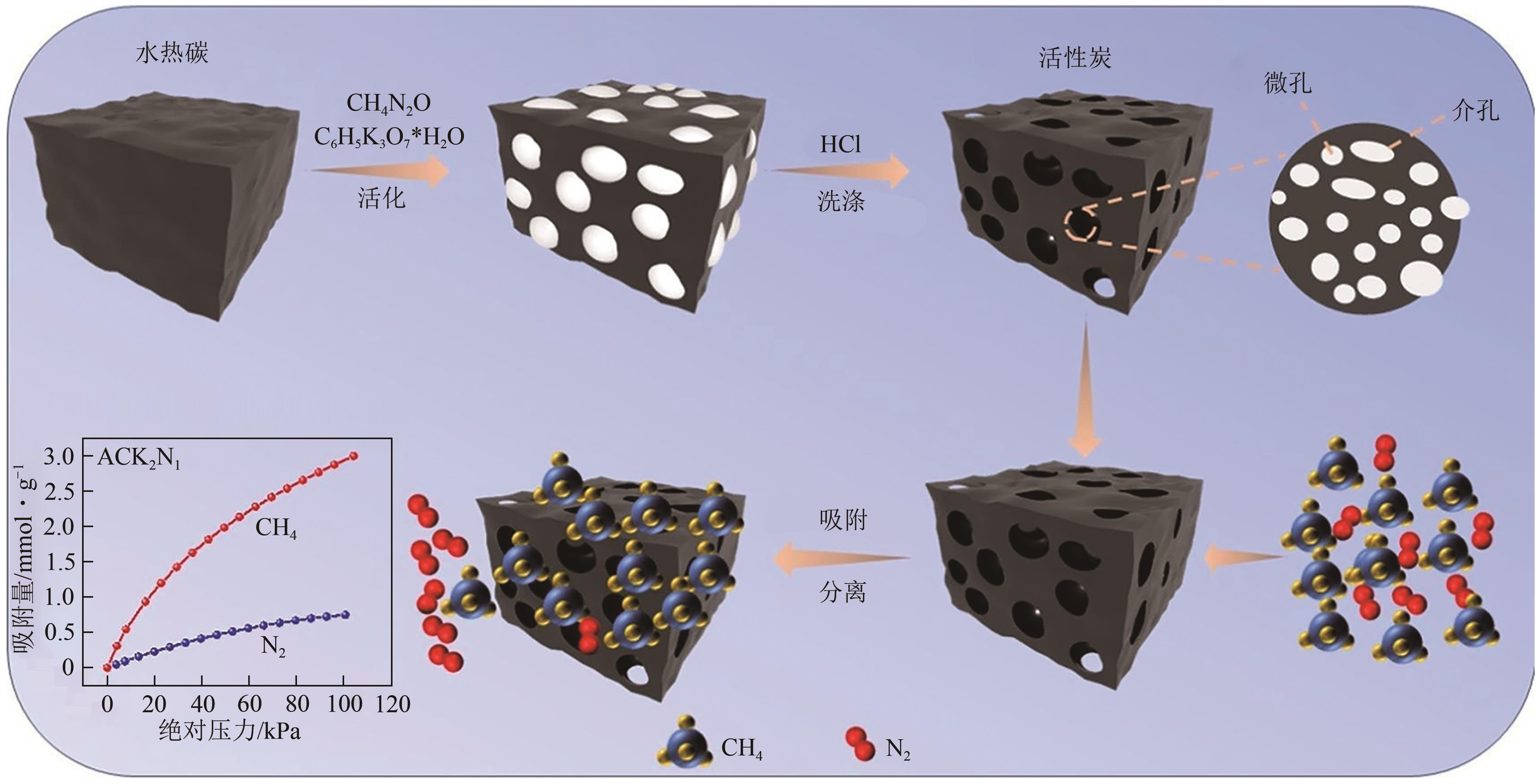
| 吸附剂类型 | 优点 | 缺点 |
|---|---|---|
| 活性炭 | 成本低廉、比表面积大、易于修饰 | 孔隙分布杂乱、选择性低、机械稳定性差 |
| 沸石分子筛 | 良好的热和机械稳定性、高的表面积 | 再生能耗高、对水敏感 |
| 有机聚合物 | 可定制化孔隙结构和化学性质 | 热和机械稳定性差、合成复杂、再生性弱 |
| 金属有机框架 | 极高的表面积、可定制化孔隙结构和化学性质 | 制造成本高、对水敏感、热和机械稳定性差 |
| 二氧化硅 | 良好的热稳定性和机械强度 | 吸附量和选择性低 |
| 碳纳米管 | 高表面积、可调节的化学性质和孔结构 | 制造成本高、分散性和加工性能需优化 |
| 1 | 中华人民共和国自然资源部. 中国矿产资源报告-2022[M]. 北京: 地质出版社, 2022. |
| Ministry of Natural Resources of the People’s Republic of China. China mineral resources-2022[M]. Beijing: Geological Publishing House, 2022. | |
| 2 | LU Yiyu, ZHANG Huidong, ZHOU Zhe, et al. Current status and effective suggestions for efficient exploitation of coalbed methane in China: A review[J]. Energy & Fuels, 2021, 35(11): 9102-9123. |
| 3 | MILLER Scot M, MICHALAK Anna M, DETMERS Robert G, et al. China’s coal mine methane regulations have not curbed growing emissions[J]. Nature Communications, 2019, 10: 303. |
| 4 | NANDANWAR Sachin U, CORBIN David R, SHIFLETT Mark B. A review of porous adsorbents for the separation of nitrogen from natural gas[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13355-13369. |
| 5 | YANG Zhuxian, HUSSAIN Mian Zahid, Pablo MARÍN, et al. Enrichment of low concentration methane: An overview of ventilation air methane[J]. Journal of Materials Chemistry A, 2022, 10(12): 6397-6413. |
| 6 | WANG Xinxin, ZHOU Fubao, LING Yihan, et al. Overview and outlook on utilization technologies of low-concentration coal mine methane[J]. Energy & Fuels, 2021, 35(19): 15398-15423. |
| 7 | SU Shi, BEATH Andrew, GUO Hua, et al. An assessment of mine methane mitigation and utilisation technologies[J]. Progress in Energy and Combustion Science, 2005, 31(2): 123-170. |
| 8 | HOLMES R I. Mitigating ventilation air methane cost-effectively from a colliery in Australia[J]. Journal of Applied Engineering Sciences, 2016, 6(1): 41-50. |
| 9 | LI Xiyue, GE Binbin, YAN Jin, et al. Review on hydrate-based CH4 separation from low-concentration coalbed methane in China[J]. Energy & Fuels, 2021, 35(10): 8494-8509. |
| 10 | QUARANTA Isabella C C, PINHEIRO Larissa S, GONÇALVES Daniel V, et al. Multiscale design of a pressure swing adsorption process for natural gas purification[J]. Adsorption, 2021, 27(7): 1055-1066. |
| 11 | WIHEEB A D, HELWANI Z, KIM J, et al. Pressure swing adsorption technologies for carbon dioxide capture[J]. Separation & Purification Reviews, 2016, 45(2): 108-121. |
| 12 | 李云赫, 闵秀博, 余忆玄, 等. 甲烷与氮气吸附分离研究进展[J]. 石油学报(石油加工), 2022, 38(6): 1520-1530. |
| LI Yunhe, MIN Xiubo, YU Yixuan, et al. Research progress in adsorption separation of methane and nitrogen[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2022, 38(6): 1520-1530. | |
| 13 | David URSUEGUÍA, Eva DÍAZ, Salvador ORDÓÑEZ. Metal-organic frameworks (MOFs) as methane adsorbents: From storage to diluted coal mining streams concentration[J]. Science of the Total Environment, 2021, 790: 148211. |
| 14 | PALMER J C, LLOBET A, S-H YEON, et al. Modeling the structural evolution of carbide-derived carbons using quenched molecular dynamics[J]. Carbon, 2010, 48(4): 1116-1123. |
| 15 | KHORAMZADEH Elham, MOFARAHI Masoud, LEE Changha. Equilibrium adsorption study of CO2 and N2 on synthesized zeolites 13X, 4A, 5A, and beta[J]. Journal of Chemical & Engineering Data, 2019, 64(12): 5648-5664. |
| 16 | UGLIENGO P, SODUPE M, MUSSO F, et al. Realistic models of hydroxylated amorphous silica surfaces and MCM-41 mesoporous material simulated by large-scale periodic B3LYP calculations[J]. Advanced Materials, 2008, 20(23): 4579-4583. |
| 17 | THOMAS Jens M H, TREWIN Abbie. Amorphous PAF-1: Guiding the rational design of ultraporous materials[J]. The Journal of Physical Chemistry C, 2014, 118(34): 19712-19722. |
| 18 | FÉREY G, MELLOT-DRAZNIEKS C, SERRE C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309(5743): 2040-2042. |
| 19 | SIEGELMAN Rebecca L, KIM Eugene J, LONG Jeffrey R. Porous materials for carbon dioxide separations[J]. Nature Materials, 2021, 20(8): 1060-1072. |
| 20 | BOER Dina G, LANGERAK Jort, PESCARMONA Paolo P. Zeolites as selective adsorbents for CO2 separation[J]. ACS Applied Energy Materials, 2023, 6(5): 2634-2656. |
| 21 | HEDAYATI Ali, DELICA Beñat Anda, Susana PEREZ-GIL, et al. Evaluation of high-performance adsorbents for separation of CO2 from industrial effluent gases[J]. Greenhouse Gases: Science and Technology, 2023, 13(2): 216-226. |
| 22 | SHEN Jiaxuan, WANG Xiaodong, CHEN Yani. Adsorbents for adsorption separation of CO2 and CH4: A literature review[J]. The Canadian Journal of Chemical Engineering, 2023, 101(12): 7115-7133. |
| 23 | LIU Rushuai, SHI Xiaodong, WANG Chengtong, et al. Advances in post-combustion CO2 capture by physical adsorption: From materials innovation to separation practice[J]. ChemSusChem, 2021, 14(6): 1428-1471. |
| 24 | ZHOU Yan, FU Qiang, SHEN Yuanhui, et al. Upgrade of low-concentration oxygen-bearing coal bed methane by a vacuum pressure swing adsorption process: Performance study and safety analysis[J]. Energy & Fuels, 2016, 30(2): 1496-1509. |
| 25 | WANG Xinxin, WANG Zujing, WEI Kangwei, et al. Kinetic-separation vacuum swing adsorption for safe and efficient enrichment of low concentration coal mine gas[J]. Separation and Purification Technology, 2022, 299: 121683. |
| 26 | QIAN Zhiling, YANG Ying, LI Ping, et al. An improved vacuum pressure swing adsorption process with the simulated moving bed operation mode for CH4/N2 separation to produce high-purity methane[J]. Chemical Engineering Journal, 2021, 419: 129657. |
| 27 | OLAJOSSY Andrzej. Effective recovery of methane from coal mine methane gas by vacuum pressure swing adsorption: A pilot scale case study[J]. Chemical Engineering and Science, 2013, 1(4): 46-54. |
| 28 | HU Guoping, ZHAO Qinghu, TAO Lefu, et al. Enrichment of low grade CH4 from N2/CH4 mixtures using vacuum swing adsorption with activated carbon[J]. Chemical Engineering Science, 2021, 229: 116152. |
| 29 | Jun-Seok BAE, SU Shi, YU Xinxiang, et al. Site trials of ventilation air methane enrichment with two-stage vacuum, temperature, and vacuum swing adsorption[J]. Industrial & Engineering Chemistry Research, 2020, 59(35): 15732-15741. |
| 30 | MADEJSKI P, KAROLINA Chmiel, NAVANEETHAN Subramanian, et al. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies[J]. Energies, 2022, 15(3): 887. |
| 31 | ZENG Hongxue, QU Xinghong, XU Dong, et al. Porous adsorption materials for carbon dioxide capture in industrial flue gas[J]. Frontiers in Chemistry, 2022, 10: 939701. |
| 32 | YUSOF S M, TEH L P. Bifunctional materials for CO2 adsorption: Short review[J]. Journal of Chemical Engineering and Industrial Biotechnology, 2021, 7(2): 15-19. |
| 33 | 佟思琦, 建伟伟, 海秋岩, 等. 多孔固体材料吸附CO2的研究进展[J]. 辽宁石油化工大学学报, 2022, 42(2): 30-37. |
| TONG Siqi, JIAN Weiwei, Qiuyan HAI, et al. Research progress of porous solid materials for CO2 adsorption and removal[J]. Journal of Liaoning Petrochemical University, 2022, 42(2): 30-37. | |
| 34 | SHI Shuo, LIU Yangxian. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption[J]. Fuel, 2021, 306: 121762. |
| 35 | Dariusz WAWRZYŃCZAK, Izabela MAJCHRZAK-KUCĘBA, PEVIDA Covadonga, et al. The carbon chain in carbon dioxide industrial utilization technologies: A case study[M]. Boca Raton: CRC Press, 2022. |
| 36 | Joanna SREŃSCEK-NAZZAL, Karolina KIEŁBASA. Advances in modification of commercial activated carbon for enhancement of CO2 capture[J]. Applied Surface Science, 2019, 494: 137-151. |
| 37 | WEI Jianwen, LIN Zhifeng, HE Zeyu, et al. Bagasse activated carbon with TETA/TEPA modification and adsorption properties of CO2 [J]. Water, Air, & Soil Pollution, 2017, 228(4): 128. |
| 38 | LI Yao, LIU Nan, ZHANG Tao, et al. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation[J]. Energy, 2020, 211: 118561. |
| 39 | LI Yi, LI Lin, YU Jihong. Applications of zeolites in sustainable chemistry[J]. Chem, 2017, 3(6): 928-949. |
| 40 | MAGHFIRAH A, ILMI M M, FAJAR A T N, et al. A review on the green synthesis of hierarchically porous zeolite[J]. Materials Today Chemistry, 2020, 17: 100348. |
| 41 | LIU Qingling, MACE Amber, BACSIK Zoltan, et al. NaKA sorbents with high CO2-over-N2 selectivity and high capacity to adsorb CO2 [J]. Chemical Communications, 2010, 46(25): 4502-4504. |
| 42 | ZHOU Yu, ZHANG Jianlin, WANG Lei, et al. Self-assembled iron-containing mordenite monolith for carbon dioxide sieving[J]. Science, 2021, 373(6552): 315-320. |
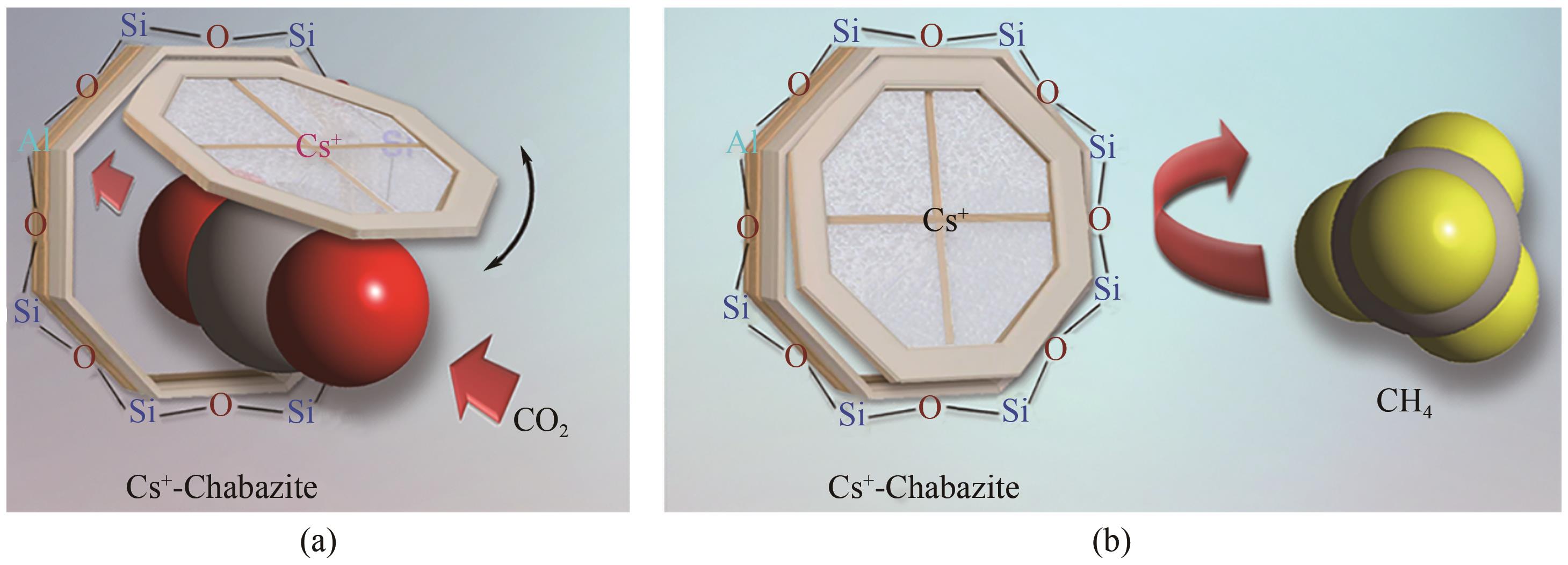
| 43 | SHANG Jin, LI Gang, SINGH Ranjeet, et al. Discriminative separation of gases by a “molecular trapdoor” mechanism in chabazite zeolites[J]. Journal of the American Chemical Society, 2012, 134(46): 19246-19253. |
| 44 | 刘佳奇, 尚华, 唐轩, 等. 分子筛基CH4-N2分离材料的研究进展[J]. 化工进展, 2019, 38(1): 449-456. |
| LIU Jiaqi, SHANG Hua, TANG Xuan, et al. Zeolite based materials for CH4-N2 separation[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 449-456. | |
| 45 | KIM Chaehoon, CHO Hae Sung, CHANG Shuai, et al. An ethylenediamine-grafted Y zeolite: A highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation[J]. Energy & Environmental Science, 2016, 9(5): 1803-1811. |
| 46 | LI Hailian, EDDAOUDI Mohamed, O’KEEFFE M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402(6759): 276-279. |
| 47 | BOYD Peter G, CHIDAMBARAM Arunraj, Enrique GARCÍA-DÍEZ, et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture[J]. Nature, 2019, 576(7786): 253-256. |
| 48 | USMAN Muhammad, IQBAL Naseem, NOOR Tayyaba, et al. Advanced strategies in metal-organic frameworks for CO2 capture and separation[J]. The Chemical Record, 2022, 22(7): e202100230. |
| 49 | LI Jianrong, YU Jiamei, LU Weigang, et al. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption[J]. Nature Communications, 2013, 4: 1538. |
| 50 | LI Bin, CHEN Banglin. A flexible metal-organic framework with double interpenetration for highly selective CO2 capture at room temperature[J]. Science China Chemistry, 2016, 59(8): 965-969. |
| 51 | BRITT David, FURUKAWA Hiroyasu, WANG Bo, et al. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(49): 20637-20640. |
| 52 | LIN Jianbin, NGUYEN Tai T T, RAMANATHAN Vaidhyanathan, et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture[J]. Science, 2021, 374(6574): 1464-1469. |
| 53 | HAMEDI Homa, KARIMI Iftekhar A, GUNDERSEN Truls. Optimal cryogenic processes for nitrogen rejection from natural gas[J]. Computers & Chemical Engineering, 2018, 112: 101-111. |
| 54 | HAMEDI Homa. An innovative integrated process for helium and NGL recovery and nitrogen removal[J]. Cryogenics, 2021, 113: 103224. |
| 55 | LEE Yongseok, Youngsub LIM, LEE Won Bo. Integrated process design and optimization of nitrogen recovery in natural gas processing[J]. Industrial & Engineering Chemistry Research, 2019, 58(4): 1658-1674. |
| 56 | KENNEDY Dean A, MUJCIN Maja, TRUDEAU Emily, et al. Pure and binary adsorption equilibria of methane and nitrogen on activated carbons, desiccants, and zeolites at different pressures[J]. Journal of Chemical & Engineering Data, 2016, 61(9): 3163-3176 |
| 57 | TADDEI Marco, PETIT Camille. Engineering metal-organic frameworks for adsorption-based gas separations: From process to atomic scale[J]. Molecular Systems Design & Engineering, 2021, 6(11): 841-875. |
| 58 | KENNEDY D A, MUJČIN M, ABOU-ZEID C, et al. Cation exchange modification of clinoptilolite-thermodynamic effects on adsorption separations of carbon dioxide, methane, and nitrogen[J]. Microporous and Mesoporous Materials, 2019, 274: 327-341. |
| 59 | TAGLIABUE Marco, FARRUSSENG David, VALENCIA Susana, et al. Natural gas treating by selective adsorption: Material science and chemical engineering interplay[J]. Chemical Engineering Journal, 2009, 155(3): 553-566. |
| 60 | PAN Hongyan, ZHAO Jingyun, LIN Qian, et al. Preparation and characterization of activated carbons from bamboo sawdust and its application for CH4 selectivity adsorption from a CH4/N2 system[J]. Energy & Fuels, 2016, 30(12): 10730-10738. |
| 61 | PAN Hongyan, YI Yun, LIN Qian, et al. Effect of surface chemistry and textural properties of activated carbons for CH4 selective adsorption through low-concentration coal bed methane[J]. Journal of Chemical & Engineering Data, 2016, 61(6): 2120-2127. |
| 62 | Arash ARAMI-NIYA, RUFFORD Thomas E, ZHU Zhonghua. Activated carbon monoliths with hierarchical pore structure from tar pitch and coal powder for the adsorption of CO2, CH4 and N2 [J]. Carbon, 2016, 103: 115-124. |
| 63 | YUAN Desheng, ZHENG Yuannan, LI Qingzhao, et al. Effects of pore structure of prepared coal-based activated carbons on CH4 enrichment from low concentration gas by IAST method[J]. Powder Technology, 2018, 333: 377-384. |
| 64 | QU Donglei, YANG Ying, LU Kai, et al. Microstructure effect of carbon materials on the low-concentration methane adsorption separation from its mixture with nitrogen[J]. Adsorption, 2018, 24(4): 357-369. |
| 65 | LI Ziyi, LIU Yingshu, ZHANG Chuanzhao, et al. Methane recovery from coal bed gas using modified activated carbons: A combined method for assessing the role of functional groups[J]. Energy & Fuels, 2015, 29(10): 6858-6865. |
| 66 | YAO Kexin, CHEN Yanli, LU Yue, et al. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading[J]. Carbon, 2017, 122: 258-265. |
| 67 | ZHANG Li, DONG Yonggang, ZHANG Dan, et al. Facile preparation of nitrogen-doped microporous carbon from potassium citrate/urea for effective CH4 separation and uptake[J]. Fuel, 2023, 351: 128915. |
| 68 | DUSSELIER Michiel, DAVIS Mark E. Small-pore zeolites: Synthesis and catalysis[J]. Chemical Reviews, 2018, 118(11): 5265-5329. |
| 69 | REINOSO Deborath, ADROVER María, PEDERNERA Marisa. Green synthesis of nanocrystalline faujasite zeolite[J]. Ultrasonics Sonochemistry, 2018, 42: 303-309. |
| 70 | KNYAZEVA E E, IVANOVA I I. Synthesis of nanoscale zeolites[J]. Petroleum Chemistry, 2019, 59(3): 262-274. |
| 71 | TANG Xuan, LIU Jiaqi, SHANG Hua, et al. Gas diffusion and adsorption capacity enhancement via ultrasonic pretreatment for hydrothermal synthesis of K-KFI zeolite with nano/micro-scale crystals[J]. Microporous and Mesoporous Materials, 2020, 297: 110036. |
| 72 | YANG Jiangfeng, LIU Jiaqi, LIU Puxu, et al. K-chabazite zeolite nanocrystal aggregates for highly efficient methane separation[J]. Angewandte Chemie International Edition, 2022, 61(8): e202116850. |
| 73 | YANG Jiangfeng, TANG Xuan, LIU Jiaqi, et al. Down-sizing the crystal size of ZK-5 zeolite for its enhanced CH4 adsorption and CH4/N2 separation performances[J]. Chemical Engineering Journal, 2021, 406: 126599. |
| 74 | KENNEDY D A, TEZEL F H. Cation exchange modification of clinoptilolite—Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide[J]. Microporous and Mesoporous Materials, 2018, 262: 235-250. |
| 75 | WU Yaqi, YUAN Danhua, ZENG Shu, et al. Significant enhancement in CH4/N2 separation with amine-modified zeolite Y[J]. Fuel, 2021, 301: 121077. |
| 76 | KENCANA Kevin S, MIN Jung Gi, Christian KEMP K, et al. Nanocrystalline Ag-ZK-5 zeolite for selective CH4/N2 separation[J]. Separation and Purification Technology, 2022, 282: 120027. |
| 77 | SHANG Jin, LI Gang, GU Qinfen, et al. Temperature controlled invertible selectivity for adsorption of N2 and CH4 by molecular trapdoor chabazites[J]. Chemical Communications, 2014, 50(35): 4544-4546. |
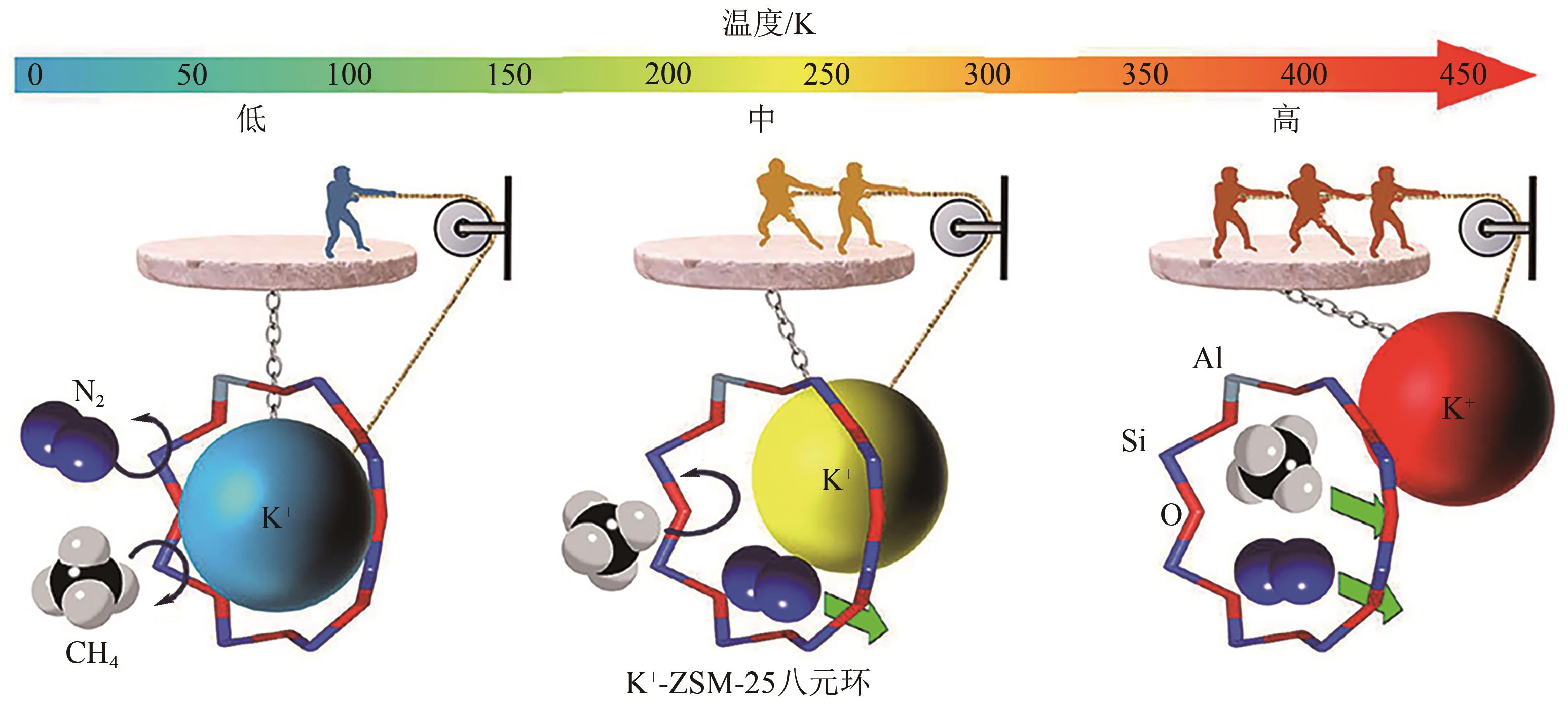
| 78 | ZHAO Jianhua, MOUSAVI Seyed Hesam, XIAO Gongkui, et al. Nitrogen rejection from methane via a “trapdoor” K-ZSM-25 zeolite[J]. Journal of the American Chemical Society, 2021, 143(37): 15195-15204. |
| 79 | YILMAZ Gamze, Shing Bo PEH, ZHAO Dan, et al. Atomic-and molecular-level design of functional metal-organic frameworks (MOFs) and derivatives for energy and environmental applications[J]. Advanced Science, 2019, 6(21): 1901129. |
| 80 | ZHOU Sheng, SHEKHAH Osama, Adrian RAMÍREZ, et al. Asymmetric pore windows in MOF membranes for natural gas valorization[J]. Nature, 2022, 606(7915): 706-712. |
| 81 | Labeeb ALI, MAHMOUD Eyas. Recent advances in the design of metal-organic frameworks for methane storage and delivery[J]. Journal of Porous Materials, 2021, 28(1): 213-230. |
| 82 | NIU Zheng, CUI Xili, PHAM Tony, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 83 | YOON Ji Woong, CHANG Hyunju, LEE Seung-Joon, et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites[J]. Nature Materials, 2017, 16(5): 526-531. |
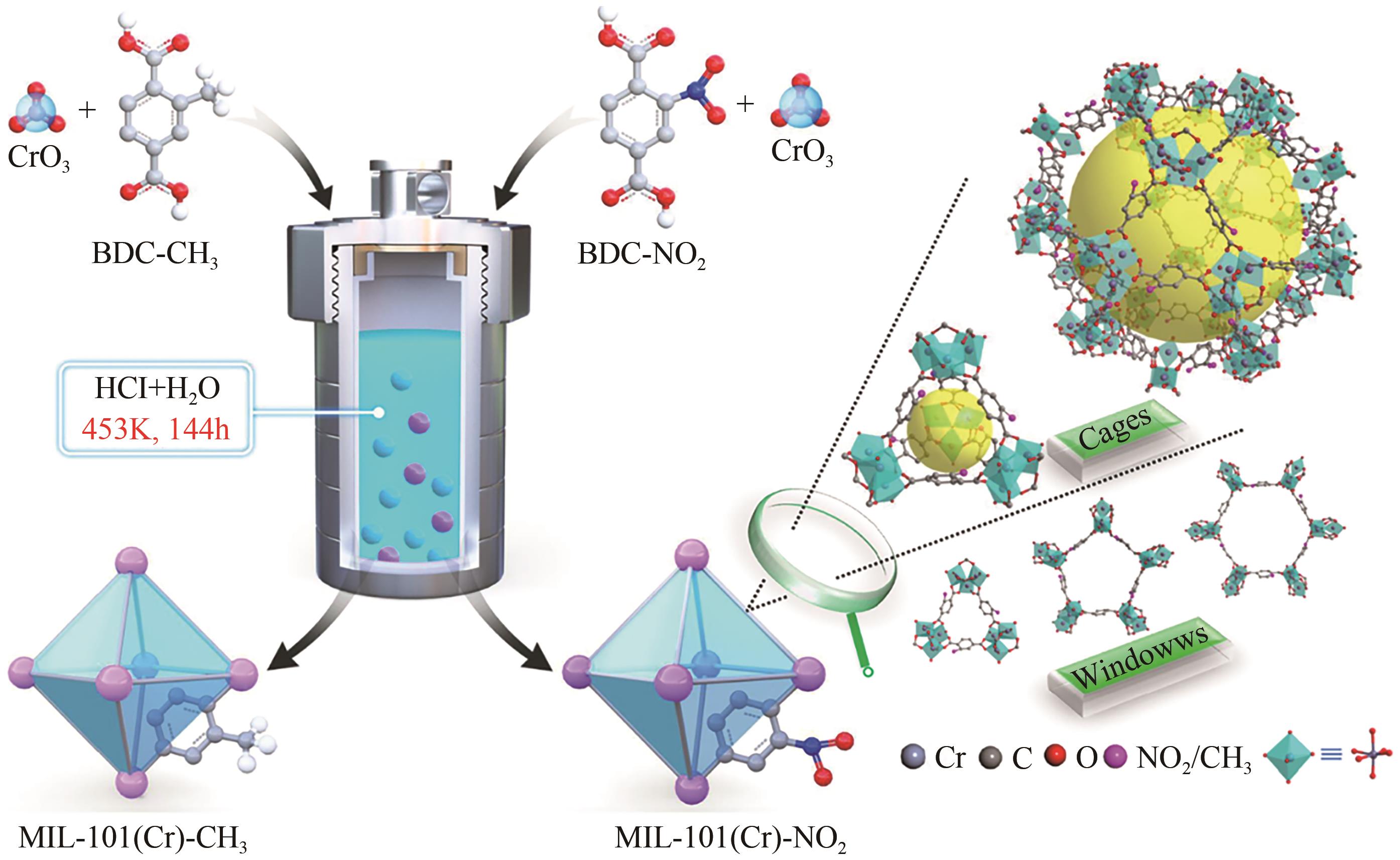
| 84 | ZHANG Feifei, SHANG Hua, WANG Li, et al. Substituent-induced electron-transfer strategy for selective adsorption of N2 in MIL-101(Cr)-X metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2022, 14(1): 2146-2154. |
| 85 | JARAMILLO David E, REED Douglas A, JIANG Henry Z H, et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites[J]. Nature Materials, 2020, 19(5): 517-521. |
| [1] | 于梦洁, 吴语童, 罗发祥, 豆义波. 低浓度二氧化碳还原光催化剂结构设计的研究进展[J]. 化工进展, 2024, 43(S1): 335-350. |
| [2] | 王博葳, 郑明朕, 王乐萌, 付东, 王珊, 朱生俊, 赵昆, 张盼. Na2SO4电解制备NaOH捕集CO2[J]. 化工进展, 2024, 43(S1): 604-614. |
| [3] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [4] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [5] | 武西宁, 张宁, 秦佳敏, 徐龙, 魏朝阳, 马晓迅. 低冷量下强化CO2吸收的甲醇基纳米流体性能[J]. 化工进展, 2024, 43(5): 2811-2822. |
| [6] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| [7] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [8] | 孙伟吉, 刘浪, 方治余, 朱梦博, 解耿, 何伟, 高宇恒. 改性镁渣的湿法碳酸化工艺[J]. 化工进展, 2024, 43(4): 2161-2173. |
| [9] | 徐泽文, 王明, 王强, 侯影飞. 胺基材料在二氧化碳分离膜领域研究进展[J]. 化工进展, 2024, 43(3): 1374-1386. |
| [10] | 张瑞凯, 张会书, 郑龙云, 曾爱武. CO2吸收过程中气相分压对Rayleigh对流传质特性的影响[J]. 化工进展, 2024, 43(2): 913-924. |
| [11] | 韦诗慧, 郑璇, 王燕, 汪洋, 纪龙, 晏水平. 富CO2氨水溶液的生物质灰矿化解吸特性[J]. 化工进展, 2024, 43(10): 5950-5957. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [14] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [15] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||