化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5486-5497.DOI: 10.16085/j.issn.1000-6613.2023-1639
• 工业催化 • 上一篇
碳基单原子催化剂在电催化二氧化碳还原中的研究进展
- 中海油化工与新材料科学研究院(北京)有限公司,北京 102209
-
收稿日期:2023-09-15修回日期:2024-02-02出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:李永恒 -
作者简介:李永恒(1989—),男,博士,工程师,研究方向为碳资源化学利用。E-mail:liyh90@cnooc.com.cn。 -
基金资助:中国海洋石油集团有限公司科技项目(KJGG-2022-12-CCUS-030401)
Research progress of carbon based single atom catalysts for electrocatalytic reduction of carbon dioxide
LI Yongheng( ), WANG Wenbo, XIN Jing, WU Chongchong, SU Mengjun, YANG Guoming
), WANG Wenbo, XIN Jing, WU Chongchong, SU Mengjun, YANG Guoming
- Institute of Chemicals & Advanced Materials (Beijing), China National Offshore Oil Corporation, Beijing 102209, China
-
Received:2023-09-15Revised:2024-02-02Online:2024-10-15Published:2024-10-29 -
Contact:LI Yongheng
摘要:
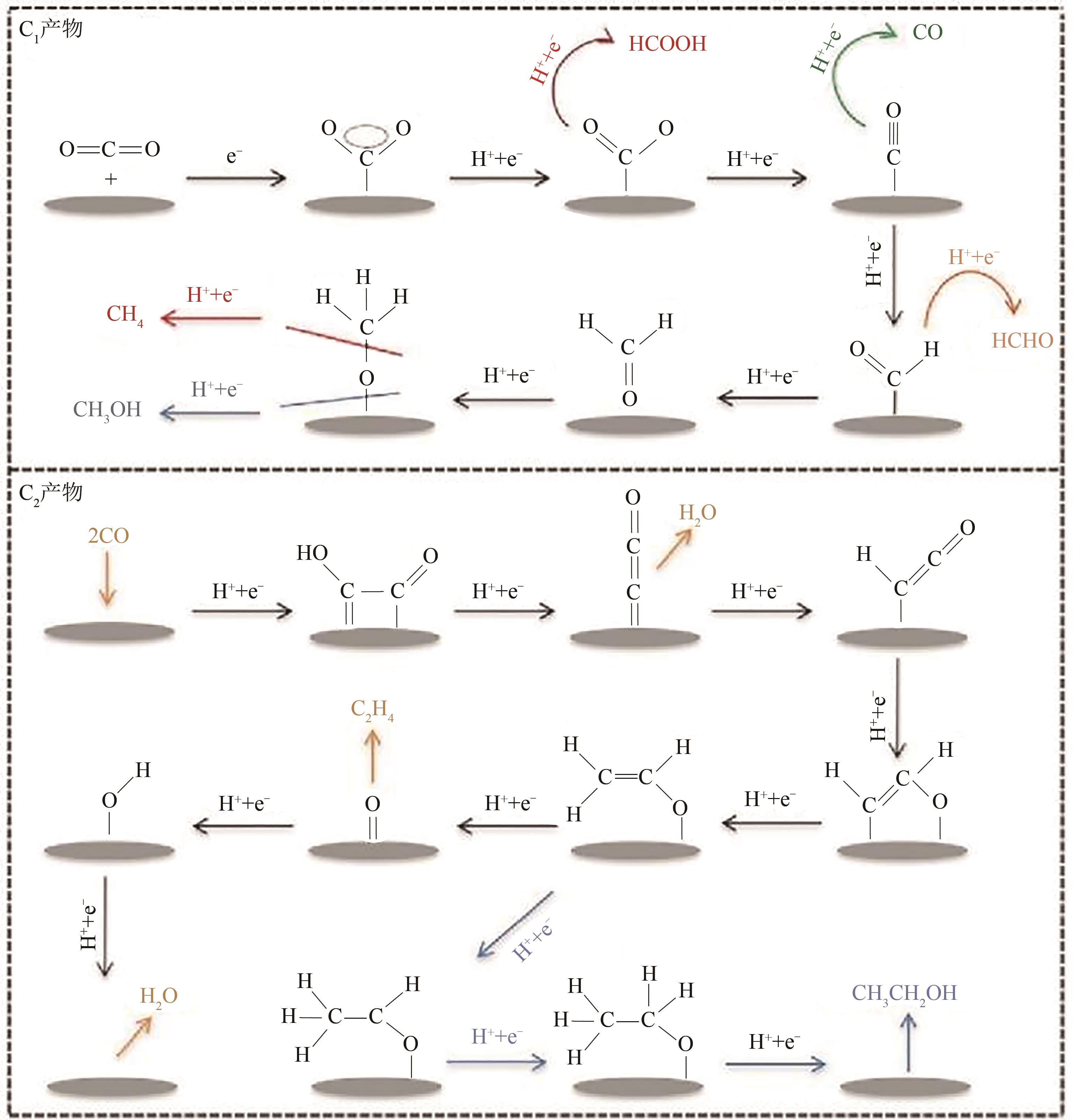
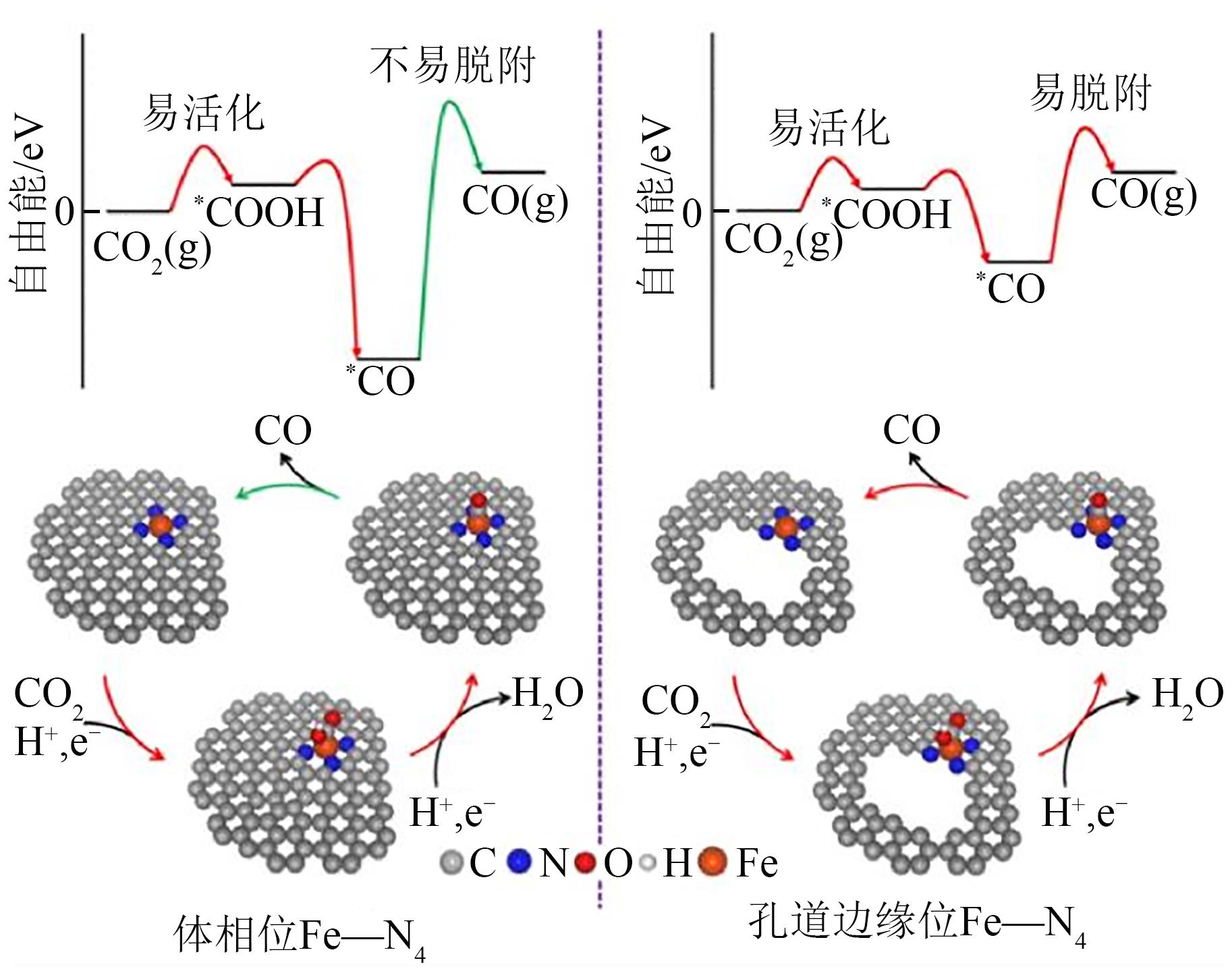
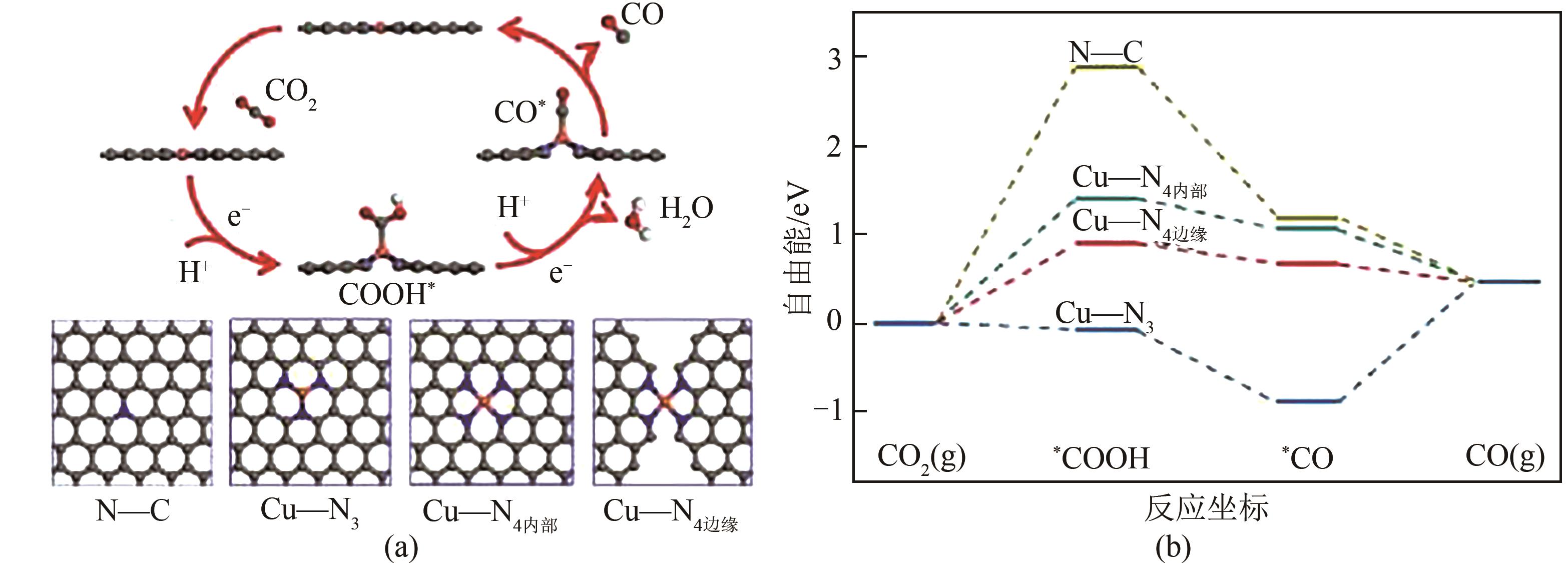
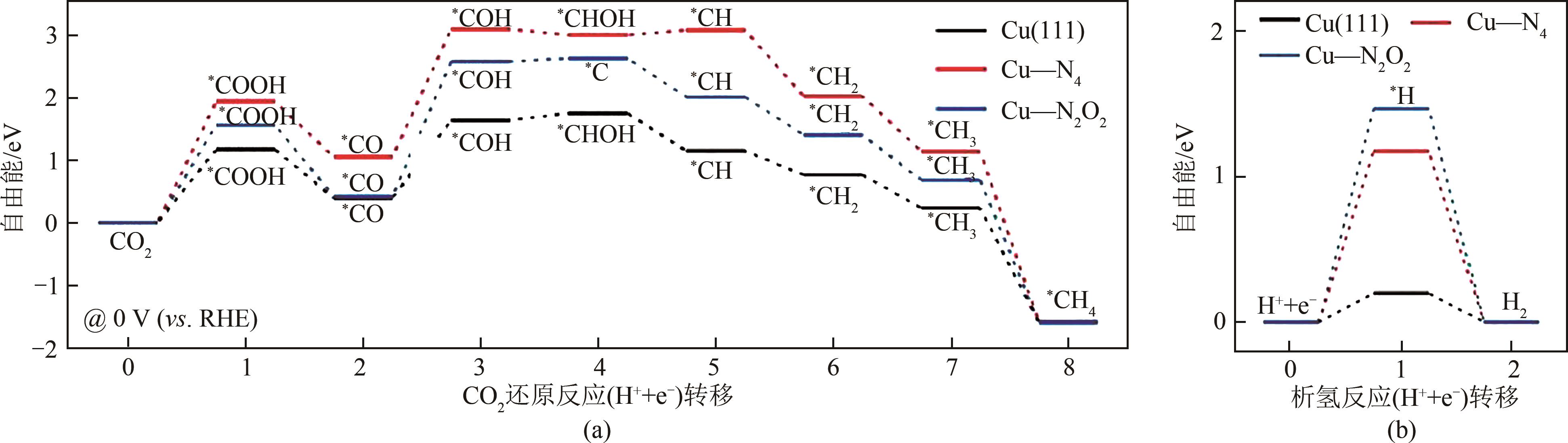
电催化CO2还原技术是一种极富潜力的CO2利用手段,开发具有高附加值产品选择性的电催化剂对该技术至关重要。近年来,碳基单原子材料作为一种原子利用率高、化学结构可调的新型催化剂在电催化CO2还原反应中表现出极高的研究价值。本文介绍了电催化CO2还原为不同产物的反应路径,总结了不同金属种类包括Fe、Cu、Ni、Co等的碳基单原子催化剂在电催化CO2还原反应中的催化性能,分别阐述了单原子配位环境调节、电子结构调节、活性位点数量调控等调节策略对催化剂性能和反应过程等的影响。最后针对电催化CO2还原反应碳基单原子催化剂的设计合成,从如何提高生成多碳产物的选择性以及鉴别反应过程中活性位点的真实状态等方面进行了展望。
中图分类号:
引用本文
李永恒, 王文波, 辛靖, 吴冲冲, 苏梦军, 杨国明. 碳基单原子催化剂在电催化二氧化碳还原中的研究进展[J]. 化工进展, 2024, 43(10): 5486-5497.
LI Yongheng, WANG Wenbo, XIN Jing, WU Chongchong, SU Mengjun, YANG Guoming. Research progress of carbon based single atom catalysts for electrocatalytic reduction of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5486-5497.
| 反应路径 | 反应平衡电位(vs. RHE)/V | 产物 |
|---|---|---|
| -0.1 | 一氧化碳(CO) | |
| +0.03 | 甲醇(CH3OH) | |
| -0.12 | 甲酸(HCOOH) | |
| +0.17 | 甲烷(CH4) | |
| +0.08 | 乙烯(C2H4) | |
| +0.11 | 乙酸(CH3COOH) | |
| +0.06 | 乙醛(CH3CHO) | |
| +0.09 | 乙醇(C2H5OH) | |
| +0.14 | 乙烷(C2H6) | |
| +0.1 | 丙醇(C3H7OH) |
表1 不同反应路径对应的反应平衡电位和产物(pH=7,电解液浓度1mol/L,25℃,0.1MPa)[6,11-13]
| 反应路径 | 反应平衡电位(vs. RHE)/V | 产物 |
|---|---|---|
| -0.1 | 一氧化碳(CO) | |
| +0.03 | 甲醇(CH3OH) | |
| -0.12 | 甲酸(HCOOH) | |
| +0.17 | 甲烷(CH4) | |
| +0.08 | 乙烯(C2H4) | |
| +0.11 | 乙酸(CH3COOH) | |
| +0.06 | 乙醛(CH3CHO) | |
| +0.09 | 乙醇(C2H5OH) | |
| +0.14 | 乙烷(C2H6) | |
| +0.1 | 丙醇(C3H7OH) |
| 催化剂 | 电解液 | 产物 | 电压(vs. RHE) /V | 法拉第效率 /% | 最大电流密度 /mA∙cm-2 | 稳定性 /h | 转化频率 /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Fe-SA/ZIF | 0.1mol/L KHCO3 | CO | -1.0~-0.4 | 98 | 18 | 40 | — | [ |
| FeN4Cl/NC | 0.5mol/L KHCO3 | CO | -0.7~-0.3 | 90.5 | 40 | 18 | 1566 | [ |
| Fe-N/P-C | 0.5mol/L KHCO3 | CO | -0.7~-0.4(>90%) | 98 | 20 | 24 | 508.8 | [ |
| FeN4/石墨烯边缘 | 0.1mol/L KHCO3 | CO | -0.6~-0.4 | 94 | — | 9 | 1630 | [ |
| Fe2—N-C | 0.5mol/L KHCO3 | CO | -0.9~-0.5 | 80 | 45 | 20 | 26,637 | [ |
| Fe-n-f-CNTs(碳纳米管) | 0.5mol/L KHCO3 | CH3CH2OH | -1.2~-0.6 | 45 | 60 | — | — | [ |
| Cu SAs/NC | 0.1mol/L KHCO3 | CO | -0.9~-0.5(>70%) | 92 | 8.9 | 30 | — | [ |
| Cu—N4—C/1100 | 0.1mol/L KHCO3 | CO | -1.1~-0.6(>90%) | 98 | 14 | 40 | 1012 | [ |
| 2Bn-Cu@UiO-67 | 1.0mol/L KOH | CH4 | -1.6~-1.1(>70%) | 81 | 420 | — | 58680 | [ |
| Cu-C(石墨炔) | 0.1mol/L KHCO3 | CH4 | — | 66 | 40 | 10 | 2311 | [ |
| CuSAs/TCNFs (贯通孔碳纳米纤维) | 0.1mol/L KHCO3 | CH3OH | -0.9~-0.4 | 44 | 93 | 50 | — | [ |
| Cu3(2,3,7,8,12, B-六羟基三环喹唑啉)2 | 0.1mol/L KHCO3 | CH3OH | -0.8~-0.2 | 53.6 | 45 | — | — | [ |
| CuNi-DSA(双单原子)/ CNFs(碳纳米纤维) | 0.1mol/L KHCO3 | CO | -1.18~-0.78 | 99.60 | 42 | 25 | 2870 | [ |
| Cu/C | 0.1mol/L KHCO3 | CH3CH2OH | -1.2~-0.4 | 91 | 25 | 16 | — | [ |
| Cu-SA/NPC(氮磷共掺杂碳) | 0.1mol/L KHCO3 | CH3COCH3 | -0.96~-0.16 | 36.7 | 15 | — | — | [ |
| Ni-SAs-NC | 0.5mol/L KHCO3 | CO | -1.0~-0.6(>90%) | 98 | 31 | 30 | — | [ |
| MeNiPc(甲酯化酞菁镍)/ (石墨烯) | 0.5mol/L KHCO3 | CO | -0.93~-0.58(>90%) | 99.40 | 28 | 12 | 8310 | [ |
| Ni-N-MEGO | 0.5mol/L KHCO3 | CO | -0.7~-0.55(>90%) | 92.10 | 28.6 | 21 | 864 | [ |
| Ni-SAs@BNC | 0.5mol/L KHCO3 | CO | -1.2~-0.6(>97%) | 99 | 67.91 | 40 | — | [ |
| SACs Co—N2C3 | 0.1mol/L KHCO3 | CO | -1.1~-0.4(>90%) | 92 | 8.3 | 40 | 1451 | [ |
| CoSA/HCNFs (非自支撑碳纳米纤维) | 0.1mol/L KHCO3 | CO | -0.9~-0.4(>90%) | 97 | 67 | 50 | — | [ |
| Co-HNC(空心氮掺杂碳) | 0.1mol/L KHCO3 | 合成气 | -1.0~-0.7 | 100 | — | 24 | — | [ |
| CoPc(酞菁钴)/石墨烯 | 0.5mol/L KHCO3 | CO | -1.6~-0.6 | 85.40 | — | 30 | — | [ |
| CoPc/MWCNT(多壁碳纳米管) | 0.5mol/L K2HPO4 | CH3OH | — | 65 | 100 | 8 | — | [ |
| CoPor-N3/CNTs | 0.1mol/L KHCO3 | CO | -0.7~-0.35(>46%) | 96 | 20.3 | 24 | 550 | [ |
| COF@CoPor | 0.5mol/L KHCO3 | CO | -1.0~-0.5(>84.2%) | 73.8 | 12.5 | 10 | 4578 | [ |
| SACs Co-ZIF | 0.5mol/L KHCO3 | CO | -0.7~-0.5(>45%) | 60 | 12 | 10 | — | [ |
| 2D-Co-COF500 | 0.5mol/L KHCO3 | CO | -1.0~-0.5(>80.2%) | 96.50 | 17.9 | 15 | 336 | [ |
| CoN4/石墨烯 | 0.1mol/L KHCO3 | CO | -0.96~-0.26 | 95 | 19 | 15 | — | [ |
| Co—N5/HNPCSs (空心氮掺杂多孔碳球) | 0.2mol/L NaHCO3 | CO | -0.88~-0.57(>90%) | 99 | 17.5 | 10 | 480.2 | [ |
| Sb SA/NC | 0.5mol/L KHCO3 | HCOOH | -1.0~-0.7 | 94 | 25 | 10 | — | [ |
| Pd2 DAC(双原子催化剂) | 0.5mol/L KHCO3 | CO | -0.9~-0.7 | 98.20 | 38 | 12 | — | [ |
| Bi SA | 0.5mol/L KHCO3 | CO | -1.2~-0.8 | >90 | 29.3 | 40 | 9504 | [ |
| Bi SAs/NC | 0.1mol/L NaHCO3 | CO | -0.625~-0.375 | 97 | — | — | 10118 | [ |
| Cd-NC SACs | 0.5mol/L KHCO3 | CO | -0.90~-0.65 | 91.40 | 17.5 | 10 | — | [ |
| Ga—N3S-PC | 0.5mol/L KHCO3 | CO | -0.30~-0.20 | 92 | 175 | 24 | — | [ |
| SnPc(酞菁锡)/CNT-OH | 1mol/L KOH | HCOOH | -1.2~-0.7 | 89.40 | 135 | 9 | — | [ |
| ZnN4S1/P-HC(空心碳) | 0.1mol/L KHCO3 | CO | -0.9~-0.4 | 约100 | 35 | 30 | 1241 | [ |
| InCe/CN | 0.1mol/L KHCO3 | HCOOH | -1.4~-1.2 | 77 | — | 6 | — | [ |
表2 不同碳基单原子催化剂的电催化CO2还原反应表现
| 催化剂 | 电解液 | 产物 | 电压(vs. RHE) /V | 法拉第效率 /% | 最大电流密度 /mA∙cm-2 | 稳定性 /h | 转化频率 /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Fe-SA/ZIF | 0.1mol/L KHCO3 | CO | -1.0~-0.4 | 98 | 18 | 40 | — | [ |
| FeN4Cl/NC | 0.5mol/L KHCO3 | CO | -0.7~-0.3 | 90.5 | 40 | 18 | 1566 | [ |
| Fe-N/P-C | 0.5mol/L KHCO3 | CO | -0.7~-0.4(>90%) | 98 | 20 | 24 | 508.8 | [ |
| FeN4/石墨烯边缘 | 0.1mol/L KHCO3 | CO | -0.6~-0.4 | 94 | — | 9 | 1630 | [ |
| Fe2—N-C | 0.5mol/L KHCO3 | CO | -0.9~-0.5 | 80 | 45 | 20 | 26,637 | [ |
| Fe-n-f-CNTs(碳纳米管) | 0.5mol/L KHCO3 | CH3CH2OH | -1.2~-0.6 | 45 | 60 | — | — | [ |
| Cu SAs/NC | 0.1mol/L KHCO3 | CO | -0.9~-0.5(>70%) | 92 | 8.9 | 30 | — | [ |
| Cu—N4—C/1100 | 0.1mol/L KHCO3 | CO | -1.1~-0.6(>90%) | 98 | 14 | 40 | 1012 | [ |
| 2Bn-Cu@UiO-67 | 1.0mol/L KOH | CH4 | -1.6~-1.1(>70%) | 81 | 420 | — | 58680 | [ |
| Cu-C(石墨炔) | 0.1mol/L KHCO3 | CH4 | — | 66 | 40 | 10 | 2311 | [ |
| CuSAs/TCNFs (贯通孔碳纳米纤维) | 0.1mol/L KHCO3 | CH3OH | -0.9~-0.4 | 44 | 93 | 50 | — | [ |
| Cu3(2,3,7,8,12, B-六羟基三环喹唑啉)2 | 0.1mol/L KHCO3 | CH3OH | -0.8~-0.2 | 53.6 | 45 | — | — | [ |
| CuNi-DSA(双单原子)/ CNFs(碳纳米纤维) | 0.1mol/L KHCO3 | CO | -1.18~-0.78 | 99.60 | 42 | 25 | 2870 | [ |
| Cu/C | 0.1mol/L KHCO3 | CH3CH2OH | -1.2~-0.4 | 91 | 25 | 16 | — | [ |
| Cu-SA/NPC(氮磷共掺杂碳) | 0.1mol/L KHCO3 | CH3COCH3 | -0.96~-0.16 | 36.7 | 15 | — | — | [ |
| Ni-SAs-NC | 0.5mol/L KHCO3 | CO | -1.0~-0.6(>90%) | 98 | 31 | 30 | — | [ |
| MeNiPc(甲酯化酞菁镍)/ (石墨烯) | 0.5mol/L KHCO3 | CO | -0.93~-0.58(>90%) | 99.40 | 28 | 12 | 8310 | [ |
| Ni-N-MEGO | 0.5mol/L KHCO3 | CO | -0.7~-0.55(>90%) | 92.10 | 28.6 | 21 | 864 | [ |
| Ni-SAs@BNC | 0.5mol/L KHCO3 | CO | -1.2~-0.6(>97%) | 99 | 67.91 | 40 | — | [ |
| SACs Co—N2C3 | 0.1mol/L KHCO3 | CO | -1.1~-0.4(>90%) | 92 | 8.3 | 40 | 1451 | [ |
| CoSA/HCNFs (非自支撑碳纳米纤维) | 0.1mol/L KHCO3 | CO | -0.9~-0.4(>90%) | 97 | 67 | 50 | — | [ |
| Co-HNC(空心氮掺杂碳) | 0.1mol/L KHCO3 | 合成气 | -1.0~-0.7 | 100 | — | 24 | — | [ |
| CoPc(酞菁钴)/石墨烯 | 0.5mol/L KHCO3 | CO | -1.6~-0.6 | 85.40 | — | 30 | — | [ |
| CoPc/MWCNT(多壁碳纳米管) | 0.5mol/L K2HPO4 | CH3OH | — | 65 | 100 | 8 | — | [ |
| CoPor-N3/CNTs | 0.1mol/L KHCO3 | CO | -0.7~-0.35(>46%) | 96 | 20.3 | 24 | 550 | [ |
| COF@CoPor | 0.5mol/L KHCO3 | CO | -1.0~-0.5(>84.2%) | 73.8 | 12.5 | 10 | 4578 | [ |
| SACs Co-ZIF | 0.5mol/L KHCO3 | CO | -0.7~-0.5(>45%) | 60 | 12 | 10 | — | [ |
| 2D-Co-COF500 | 0.5mol/L KHCO3 | CO | -1.0~-0.5(>80.2%) | 96.50 | 17.9 | 15 | 336 | [ |
| CoN4/石墨烯 | 0.1mol/L KHCO3 | CO | -0.96~-0.26 | 95 | 19 | 15 | — | [ |
| Co—N5/HNPCSs (空心氮掺杂多孔碳球) | 0.2mol/L NaHCO3 | CO | -0.88~-0.57(>90%) | 99 | 17.5 | 10 | 480.2 | [ |
| Sb SA/NC | 0.5mol/L KHCO3 | HCOOH | -1.0~-0.7 | 94 | 25 | 10 | — | [ |
| Pd2 DAC(双原子催化剂) | 0.5mol/L KHCO3 | CO | -0.9~-0.7 | 98.20 | 38 | 12 | — | [ |
| Bi SA | 0.5mol/L KHCO3 | CO | -1.2~-0.8 | >90 | 29.3 | 40 | 9504 | [ |
| Bi SAs/NC | 0.1mol/L NaHCO3 | CO | -0.625~-0.375 | 97 | — | — | 10118 | [ |
| Cd-NC SACs | 0.5mol/L KHCO3 | CO | -0.90~-0.65 | 91.40 | 17.5 | 10 | — | [ |
| Ga—N3S-PC | 0.5mol/L KHCO3 | CO | -0.30~-0.20 | 92 | 175 | 24 | — | [ |
| SnPc(酞菁锡)/CNT-OH | 1mol/L KOH | HCOOH | -1.2~-0.7 | 89.40 | 135 | 9 | — | [ |
| ZnN4S1/P-HC(空心碳) | 0.1mol/L KHCO3 | CO | -0.9~-0.4 | 约100 | 35 | 30 | 1241 | [ |
| InCe/CN | 0.1mol/L KHCO3 | HCOOH | -1.4~-1.2 | 77 | — | 6 | — | [ |
| 1 | LI Minhan, WANG Haifeng, LUO Wei, et al. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction[J]. Advanced Materials, 2020, 32(34): e2001848. |
| 2 | LI Xiaoxin, CHAI Guoliang, XU Xiao, et al. Electrocatalytic reduction of CO2 to CO over iron phthalocyanine-modified graphene nanocomposites[J]. Carbon, 2020, 167: 658-667. |
| 3 | 赵锦波, 卞凤鸣. CO2化学转化基础与应用研究进展[J]. 化工进展, 2022, 41(S1): 524-535. |
| ZHAO Jinbo, BIAN Fengming. Progress on basis and application of CO2 chemical conversion technologies[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 524-535. | |
| 4 | ZHANG Zedong, ZHU Jiexin, CHEN Shenghua, et al. Liquid fluxional Ga single atom catalysts for efficient electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2023, 62(3): e202215136. |
| 5 | 华亚妮, 冯少广, 党欣悦, 等. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240. |
| HUA Yani, FENG Shaoguang, DANG Xinyue, et al. Research progress of CO2 electrocatalytic reduction to syngas[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1224-1240. | |
| 6 | ZHANG Shilei, YUE Pengtao, ZHOU Yue, et al. Ni single atoms embedded in graphene nanoribbon sieves for high-performance CO2 reduction to CO[J]. Small, 2023, 19(43): e2303016. |
| 7 | JIANG Liyun, YANG Qingqing, XIA Zhaoming, et al. Recent progress of theoretical studies on electro- and photo-chemical conversion of CO2 with single-atom catalysts[J]. RSC Advances, 2023, 13(9): 5833-5850. |
| 8 | KOOLEN Cedric David, LUO Wen, Andreas ZÜTTEL. From single crystal to single atom catalysts: Structural factors influencing the performance of metal catalysts for CO2 electroreduction[J]. ACS Catalysis, 2023, 13(2): 948-973. |
| 9 | JIA Chen, DASTAFKAN Kamran, ZHAO Chuan. Key factors for designing single-atom metal-nitrogen-carbon catalysts for electrochemical CO2 reduction[J]. Current Opinion in Electrochemistry, 2022, 31: 100854. |
| 10 | ZHU Mengnan, JIANG Haoqing, ZHANG Bowen, et al. Nanosecond laser confined bismuth moiety with tunable structures on graphene for carbon dioxide reduction[J]. ACS Nano, 2023, 17(9): 8705-8716. |
| 11 | SHAO Wei, ZHANG Xiaodong. Atomic-level engineering of two-dimensional electrocatalysts for CO2 reduction[J]. Nanoscale, 2021, 13(15): 7081-7095. |
| 12 | FU Zhanzhao, WU Mingliang, ZHOU Yipeng, et al. Support-based modulation strategies in single-atom catalysts for electrochemical CO2 reduction: Graphene and conjugated macrocyclic complexes[J]. Journal of Materials Chemistry A, 2022, 10(11): 5699-5716. |
| 13 | WANG Maohuai, KONG Lingyan, LU Xiaoqing, et al. First-row transition metal embedded pyrazine-based graphynes as high-performance single atom catalysts for the CO2 reduction reaction[J]. Journal of Materials Chemistry A, 2022, 10(16): 9048-9058. |
| 14 | CHOI Hyeonuk, LEE Dong-Kyu, HAN Mi-Kyung, et al. Review—Non-noble metal-based single-atom catalysts for efficient electrochemical CO2 reduction reaction[J]. Journal of the Electrochemical Society, 2020, 167(16): 164503. |
| 15 | JIANG Zhuoli, WANG Tao, PEI Jiajing, et al. Discovery of main group single Sb–N4 active sites for CO2 electroreduction to formate with high efficiency[J]. Energy & Environmental Science, 2020, 13(9): 2856-2863. |
| 16 | ZHANG Erhuan, WANG Tao, YU Ke, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction[J]. Journal of the American Chemical Society, 2019, 141(42): 16569-16573. |
| 17 | 张少阳, 商阳阳, 赵瑞花, 等. 电催化还原二氧化碳制一氧化碳催化剂研究进展[J]. 化工进展, 2022, 41(4): 1848-1857. |
| ZHANG Shaoyang, SHANG Yangyang, ZHAO Ruihua, et al. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1848-1857. | |
| 18 | JEONG Gyoung Hwa, TAN Ying Chuan, SONG Jun Tae, et al. Synthetic multiscale design of nanostructured Ni single atom catalyst for superior CO2 electroreduction[J]. Chemical Engineering Journal, 2021, 426: 131063. |
| 19 | WANG Minjie, WANG Li, LI Qingbin, et al. Regulating the coordination geometry and oxidation state of single-atom Fe sites for enhanced oxygen reduction electrocatalysis[J]. Small, 2023, 19(24): e2300373. |
| 20 | ZHANG Zhengping, SUN Junting, WANG Feng, et al. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework[J]. Angewandte Chemie International Edition, 2018, 130(29): 9176-9181. |
| 21 | LIU Jing, JIAO Menggai, MEI Bingbao, et al. Carbon-supported divacancy-anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2019, 131(4): 1175-1179. |
| 22 | LAI Weihong, ZHANG Binwei, HU Zhenpeng, et al. The quasi-Pt-allotrope catalyst: Hollow PtCo@single-atom Pt1 on nitrogen-doped carbon toward superior oxygen reduction[J]. Advanced Functional Materials, 2019, 29(13): 1807340. |
| 23 | ZHANG Ningqiang, ZHANG Xinxin, KANG Yikun, et al. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(24): 13388-13393. |
| 24 | WANG Shuguang, ZHOU Peng, ZHOU Lei, et al. A unique gas-migration, trapping, and emitting strategy for high-loading single atomic Cd sites for carbon dioxide electroreduction[J]. Nano Letters, 2021, 21(10): 4262-4269. |
| 25 | ZHAI Lipeng, YANG Shuai, LU Chenbao, et al. CoN5 sites constructed by anchoring Co porphyrins on vinylene-linked covalent organic frameworks for electroreduction of carbon dioxide[J]. Small, 2022, 18(32): e2200736. |
| 26 | YANG Xiao, CHENG Jun, YANG Xian, et al. Single Ni active sites with a nitrogen and phosphorus dual coordination for an efficient CO2 reduction[J]. Nanoscale, 2022, 14(18): 6846-6853. |
| 27 | MEDFORD Andrew J, VOJVODIC Aleksandra, HUMMELSHØJ Jens S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. Journal of Catalysis, 2015, 328: 36-42. |
| 28 | WU Qing, WU Chongchong. Mechanism insights on single-atom catalysts for CO2 conversion[J]. Journal of Materials Chemistry A, 2023, 11(10): 4876-4906. |
| 29 | KORTLEVER Ruud, SHEN Jing, SCHOUTEN Klaas Jan P, et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide[J]. The Journal of Physical Chemistry Letters, 2015, 6(20): 4073-4082. |
| 30 | TANG Tianmi, WANG Zhenlu, GUAN Jingqi. Optimizing the electrocatalytic selectivity of carbon dioxide reduction reaction by regulating the electronic structure of single-atom M-N-C materials[J]. Advanced Functional Materials, 2022, 32(19): 2111504. |
| 31 | LI Meiping, Qing LYU, SI Wenyan, et al. Sp-hybridized nitrogen as new anchoring sites of iron single atoms to boost the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2022, 61(38): e202208238. |
| 32 | HU Riming, LI Yongcheng, WANG Fuhe, et al. Rational prediction of multifunctional bilayer single atom catalysts for the hydrogen evolution, oxygen evolution and oxygen reduction reactions[J]. Nanoscale, 2020, 12(39): 20413-20424. |
| 33 | HU Chun, SONG Erhong, WANG Maoyu, et al. Partial-single-atom, partial-nanoparticle composites enhance water dissociation for hydrogen evolution[J]. Advanced Science, 2021, 8(2): 2001881. |
| 34 | YU Ke, SUN Kaian, CHEONG Weng-Chon Max), et al. Oxalate-assisted synthesis of hollow carbon nanocage with Fe single atoms for electrochemical CO2 reduction[J]. Small, 2023, 19(39): 2302611. |
| 35 | CHENG Huiyuan, WU Xuemei, LI Xiangcun, et al. Zeolitic imidazole framework-derived FeN5-doped carbon as superior CO2 electrocatalysts[J]. Journal of Catalysis, 2021, 395: 63-69. |
| 36 | XIE Yuxin, LIU Nian, LI Xin, et al. The influence of single-atom Fe2+/3+N4 spin state on the electroreduction of CO2 to CO/HCOOH by analyzing proton/electron transfer mechanisms and free energy evolutions[J]. The Journal of Physical Chemistry C, 2021, 125(39): 21460-21470. |
| 37 | LI Ke, ZHANG Shengbo, ZHANG Xiuli, et al. Atomic tuning of single-atom Fe-N-C catalysts with phosphorus for robust electrochemical CO2 reduction[J]. Nano Letters, 2022, 22(4): 1557-1565. |
| 38 | PAN Fuping, LI Boyang, SARNELLO Erik, et al. Pore-edge tailoring of single-atom iron-nitrogen sites on graphene for enhanced CO2 reduction[J]. ACS Catalysis, 2020, 10(19): 10803-10811. |
| 39 | NI Wenpeng, LIU Zhixiao, ZHANG Yan, et al. Electroreduction of carbon dioxide driven by the intrinsic defects in the carbon plane of a single Fe-N4 site[J]. Advanced Materials, 2021, 33(1): 2003238. |
| 40 | WANG Ying, PARK Byoung Joon, PAIDI Vinod K, et al. Precisely constructing orbital coupling-modulated dual-atom Fe pair sites for synergistic CO2 electroreduction[J]. ACS Energy Letters, 2022, 7(2): 640-649. |
| 41 | LAKSHMANAN Keseven, HUANG Wei-Hsiang, CHALA Soressa Abera, et al. Highly active oxygen coordinated configuration of Fe single-atom catalyst toward electrochemical reduction of CO2 into multi-carbon products[J]. Advanced Functional Materials, 2022, 32(24): 2109310. |
| 42 | ZHAO Kun, NIE Xiaowa, WANG Haozhi, et al. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon[J]. Nature Communications, 2020, 11(1): 2455. |
| 43 | CHU Senlin, KANG Changwoo, PARK Woonghyeon, et al. Single atom and defect engineering of CuO for efficient electrochemical reduction of CO2 to C2H4 [J]. SmartMat, 2022, 3(1): 194-205. |
| 44 | XU Haiping, REBOLLAR Dominic, HE Haiying, et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper[J]. Nature Energy, 2020, 5: 623-632. |
| 45 | CHENG Huiyuan, WU Xuemei, LI Xiangcun, et al. Construction of atomically dispersed Cu-N4 sites via engineered coordination environment for high-efficient CO2 electroreduction[J]. Chemical Engineering Journal, 2021, 407: 126842. |
| 46 | CAI Yanming, FU Jiaju, ZHOU Yang, et al. Insights on forming N,O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane[J]. Nature Communications, 2021, 12(1): 586. |
| 47 | CHENG Yi, ZHAO Shiyong, LI Haobo, et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2 [J]. Applied Catalysis B: Environmental, 2019, 243: 294-303. |
| 48 | AN Beibei, ZHOU Jingsheng, ZHU Zhiyong, et al. Uncovering the coordination effect on the Ni single-atom catalysts for CO2 reduction including vacancy defect and non-vacancy defect structures[J]. Fuel, 2022, 310: 122472. |
| 49 | GU Xiaokang, JIAO Yuying, WEI Bo, et al. Boron bridged NiN4B2C x single-atom catalyst for superior electrochemical CO2 reduction[J]. Materials Today, 2022, 54: 63-71. |
| 50 | CHENG Huiyuan, FAN Zihao, WU Xuemei, et al. Coordination engineering of the hybrid Co-C and Co-N active sites for efficient catalyzing CO2 electroreduction[J]. Journal of Catalysis, 2022, 405: 634-640. |
| 51 | MIAO Qiyang, LU Chengbao, XU Qing, et al. CoN2O2 sites in carbon nanosheets by template-pyrolysis of COFs for CO2RR[J]. Chemical Engineering Journal, 2022, 450: 138427. |
| 52 | HU Chenghong, ZHANG Yue, HU Anqian, et al. Near- and long-range electronic modulation of single metal sites to boost CO2 electrocatalytic reduction[J]. Advanced Materials, 2023, 35(19): e2209298. |
| 53 | DENG Yachen, ZHAO Jian, WANG Shifu, et al. Operando spectroscopic analysis of axial oxygen-coordinated single-Sn-atom sites for electrochemical CO2 reduction[J]. Journal of the American Chemical Society, 2023, 145(13): 7242-7251. |
| 54 | YANG Fangqi, MAO Xinyu, MA Mingfeng, et al. Scalable strategy to fabricate single Cu atoms coordinated carbons for efficient electroreduction of CO2 to CO[J]. Carbon, 2020, 168: 528-535. |
| 55 | CHEN Shenghua, LI Wenhao, JIANG Wenjun, et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis[J]. Angewandte Chemie International Edition, 2022, 61(4): e202114450. |
| 56 | SHI Guodong, XIE Yunlong, DU Lili, et al. Constructing Cu-C bonds in a graphdiyne-regulated Cu single-atom electrocatalyst for CO2 reduction to CH4 [J]. Angewandte Chemie International Edition, 2022, 61(23): e202203569. |
| 57 | YANG Hengpan, WU Yu, LI Guodong, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. Journal of the American Chemical Society, 2019, 141(32): 12717-12723. |
| 58 | LIU Jingjuan, YANG Dan, ZHOU Yi, et al. Tricycloquinazoline-based 2D conductive metal-organic frameworks as promising electrocatalysts for CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(26): 14473-14479. |
| 59 | HAO Jican, ZHUANG Zechao, HAO Jiace, et al. Interatomic electronegativity offset dictates selectivity when catalyzing the CO2 reduction reaction[J]. Advanced Energy Materials, 2022, 12(26): 2200579. |
| 60 | WANG Weijuan, CAO Changsheng, WANG Kaiwen, et al. Boosting CO2 electroreduction to CO with abundant nickel single atom active sites[J]. Inorganic Chemistry Frontiers, 2021, 8(10): 2542-2548. |
| 61 | HU Meng ke, ZHOU Shenghua, MA Dong dong, et al. New insight into heterointerfacial effect for heterogenized metallomacrocycle catalysts in executing electrocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental, 2022, 310: 121324. |
| 62 | YANG Hengpan, LIN Qing, WU Yu, et al. Highly efficient utilization of single atoms via constructing 3D and free-standing electrodes for CO2 reduction with ultrahigh current density[J]. Nano Energy, 2020, 70: 104454. |
| 63 | SONG Xiaokai, ZHANG Hao, YANG Yuqi, et al. Bifunctional nitrogen and cobalt codoped hollow carbon for electrochemical syngas production[J]. Advanced Science, 2018, 5(7): 1800177. |
| 64 | REN Xinyi, LIU Song, LI Huicong, et al. Electron-withdrawing functional ligand promotes CO2 reduction catalysis in single atom catalyst[J]. Science China Chemistry, 2020, 63(12): 1727-1733. |
| 65 | REN Xinyi, ZHAO Jian, LI Xuning, et al. In-situ spectroscopic probe of the intrinsic structure feature of single-atom center in electrochemical CO/CO2 reduction to methanol[J]. Nature Communications, 2023, 14(1): 3401. |
| 66 | WANG Tingxia, GUO Lulu, PEI Hao, et al. Electron-rich pincer ligand-coupled cobalt porphyrin polymer with single-atom sites for efficient (photo)electrocatalytic CO2 reduction at ultralow overpotential[J]. Small, 2021, 17(45): e2102957. |
| 67 | CHEN Zhangsen, ZHANG Gaixia, HU Qingmin, et al. The deep understanding into the promoted carbon dioxide electroreduction of ZIF-8-derived single‐atom catalysts by the simple grinding process[J]. Small Structures, 2022, 3(7): 2200031. |
| 68 | ZHANG Huinian, WANG Huiqi, JIA Suping, et al. CoN4 active sites in a graphene matrix for the highly efficient electrocatalysis of CO2 reduction[J]. New Carbon Materials, 2022, 37(4): 734-742. |
| 69 | PAN Yuan, LIN Rui, CHEN Yinjuan, et al. Design of single-atom Co-N5 catalytic site: A robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability[J]. Journal of the American Chemical Society, 2018, 140(12): 4218-4221. |
| 70 | LIANG Zhong, SONG Lianpeng, SUN Mingzi, et al. Atomically dispersed indium and cerium sites for selectively electroreduction of CO2 to formate[J]. Nano Research, 2023, 17(6): 8757-8764. |
| [1] | 王正峰, 谢雨杭, 李伟科, 范永春, 康钟尹, 付乾. 多孔炭修饰的吸附催化一体化电极高效电解碳酸氢盐[J]. 化工进展, 2024, 43(9): 4892-4899. |
| [2] | 刘振涛, 梅金林, 王春雅, 段爱军, 巩雁军, 徐春明, 王喜龙. 一步法加氢制生物航煤催化剂研究进展[J]. 化工进展, 2024, 43(9): 4909-4924. |
| [3] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| [4] | 修浩然, 王云刚, 白彦渊, 刘涛, 张兴邦, 张益嘉. H2O2低温催化氧化法脱硫脱硝中试实验特性[J]. 化工进展, 2024, 43(9): 4941-4950. |
| [5] | 付维, 宁淑英, 蔡晨, 陈佳音, 周皞, 苏亚欣. Cu改性MIL-100(Fe)催化剂的SCR-C3H6脱硝特性[J]. 化工进展, 2024, 43(9): 4951-4960. |
| [6] | 梁宏成, 赵冬妮, 权银, 李敬妮, 胡欣怡. SEI膜形貌与结构对锂离子电池性能的影响[J]. 化工进展, 2024, 43(9): 5049-5062. |
| [7] | 吴剑扬, 王汝娜, 陈耀, 申兰耀, 于永利, 蒋宁, 邱景义, 周恒辉. 锂离子电池高镍正极材料前体的制备工艺[J]. 化工进展, 2024, 43(9): 5079-5085. |
| [8] | 李美萱, 成建凤, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王俊莲, 王春霞, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 高电压镍锰酸锂正极材料的合成与电化学机理[J]. 化工进展, 2024, 43(9): 5086-5094. |
| [9] | 屈芸, 成丽媛, 代国亮, 王刚, 郭羽晴, 孙洁. PAN/MXene同轴纤维电极的制备及性能[J]. 化工进展, 2024, 43(9): 5113-5122. |
| [10] | 马红周, 党煜博, 王耀宁, 曾劲阳, 赵小军, 史建伟. 废锌基脱硫剂与铜锌基催化剂协同真空碳热提取锌[J]. 化工进展, 2024, 43(9): 5275-5281. |
| [11] | 胡婷霞, 赵立欣, 姚宗路, 霍丽丽, 贾吉秀, 谢腾. 双金属催化剂在生物质焦油催化蒸汽重整领域的研究进展[J]. 化工进展, 2024, 43(8): 4354-4365. |
| [12] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [13] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [14] | 张子杭, 王树荣. 生物质热解转化与产物低碳利用研究进展[J]. 化工进展, 2024, 43(7): 3692-3708. |
| [15] | 龚德成, 沈倩, 朱贤青, 黄云, 夏奡, 张敬苗, 朱恂, 廖强. 微藻超临界水气化制取富氢合成气的研究进展[J]. 化工进展, 2024, 43(7): 3709-3728. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||