化工进展 ›› 2024, Vol. 43 ›› Issue (6): 3310-3321.DOI: 10.16085/j.issn.1000-6613.2023-0725
• 资源与环境化工 • 上一篇
耦合生物质氧化转化的CO2电化学还原
闫哲1( ), 刘畅2(
), 刘畅2( ), 王丰旭1, 周宏旺1, 刘樨2, 赵雪冰2(
), 王丰旭1, 周宏旺1, 刘樨2, 赵雪冰2( )
)
- 1.江苏华电句容发电有限公司,江苏 镇江 212413
2.清华大学化学工程系应用化学研究所,北京 100084
-
收稿日期:2023-05-04修回日期:2023-08-07出版日期:2024-06-15发布日期:2024-07-02 -
通讯作者:赵雪冰 -
作者简介:闫哲(1987—),男,高级工程师,研究方向为热力系统建模和故障诊断。E-mail:yz_jrhd@126.com
刘畅(1993—),男,博士后,研究方向为污染物的电化学降解与CO2的电化学转化。E-mail: 467673846@qq.com。 -
基金资助:国家重点研发计划(2022YFC2105900);清华大学-江苏华电句容发电有限公司二氧化碳转化合作项目(20212002252)
Electrochemical reduction of CO2 coupled with oxidative conversion of biomass
YAN Zhe1( ), LIU Chang2(
), LIU Chang2( ), WANG Fengxu1, ZHOU Hongwang1, LIU Xi2, ZHAO Xuebing2(
), WANG Fengxu1, ZHOU Hongwang1, LIU Xi2, ZHAO Xuebing2( )
)
- 1.Jiangsu Huadian Jurong Power Station Co. , Ltd. , Zhenjiang 212413, Jiangsu, China
2.Institute of Applied Chemistry, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2023-05-04Revised:2023-08-07Online:2024-06-15Published:2024-07-02 -
Contact:ZHAO Xuebing
摘要:
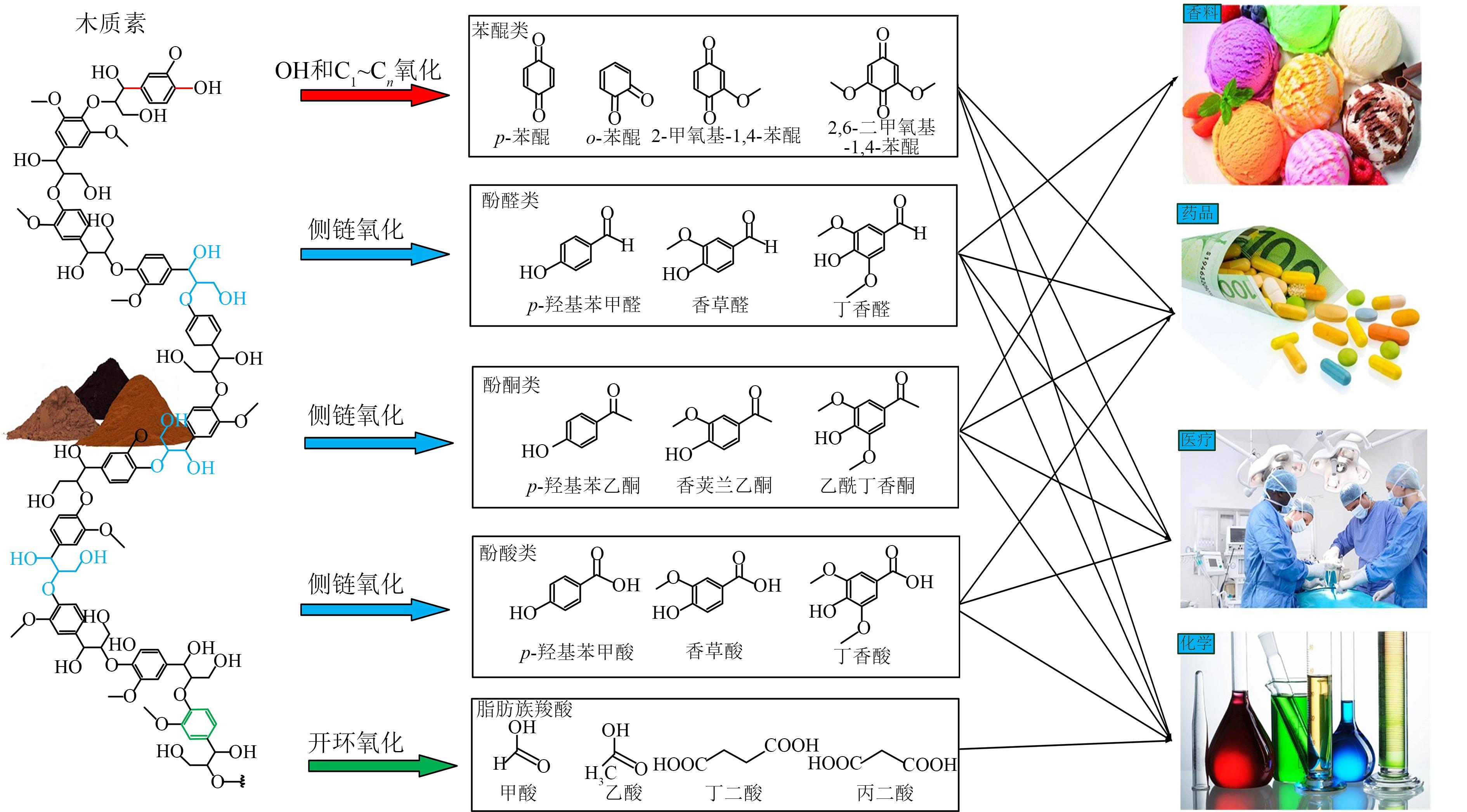
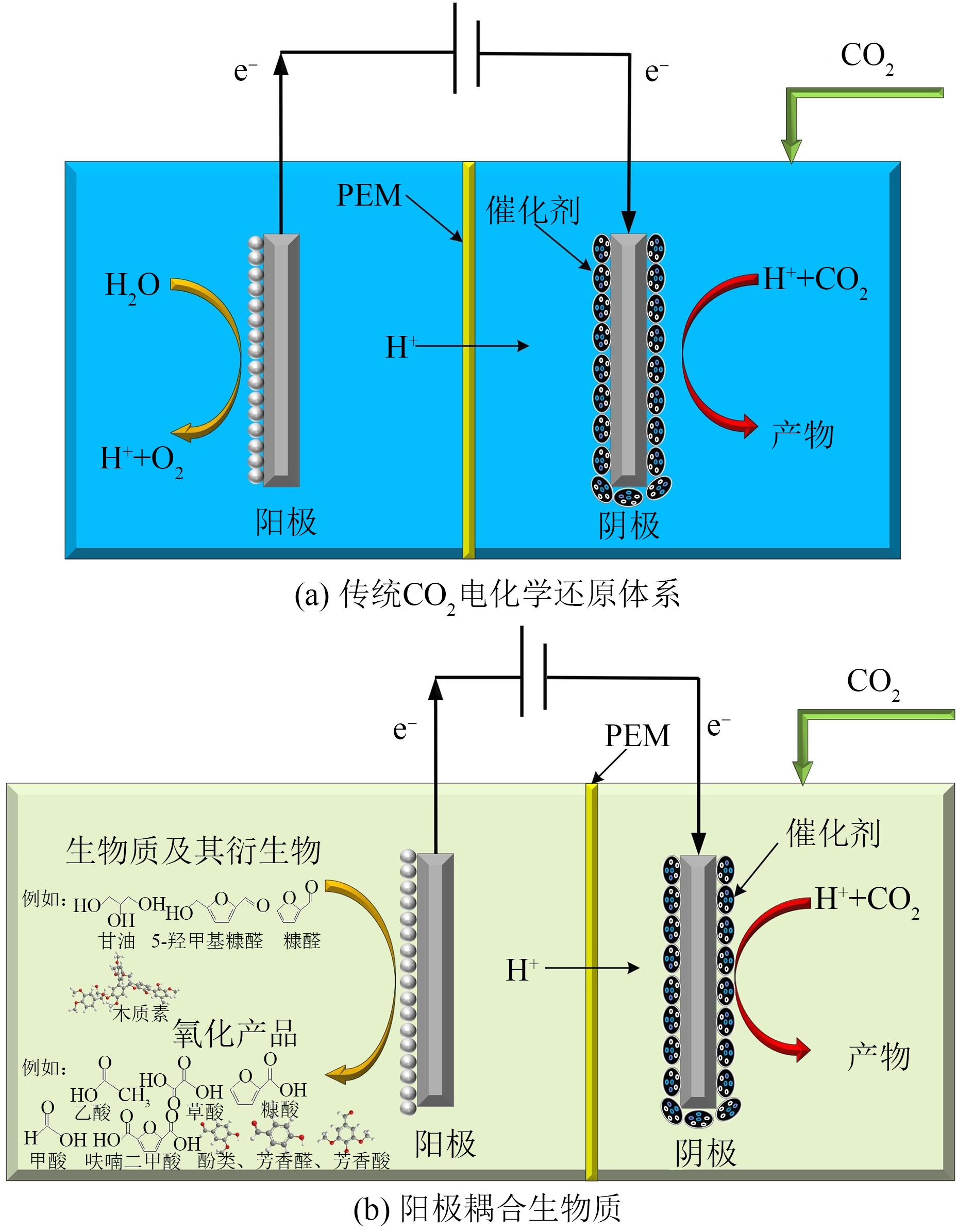
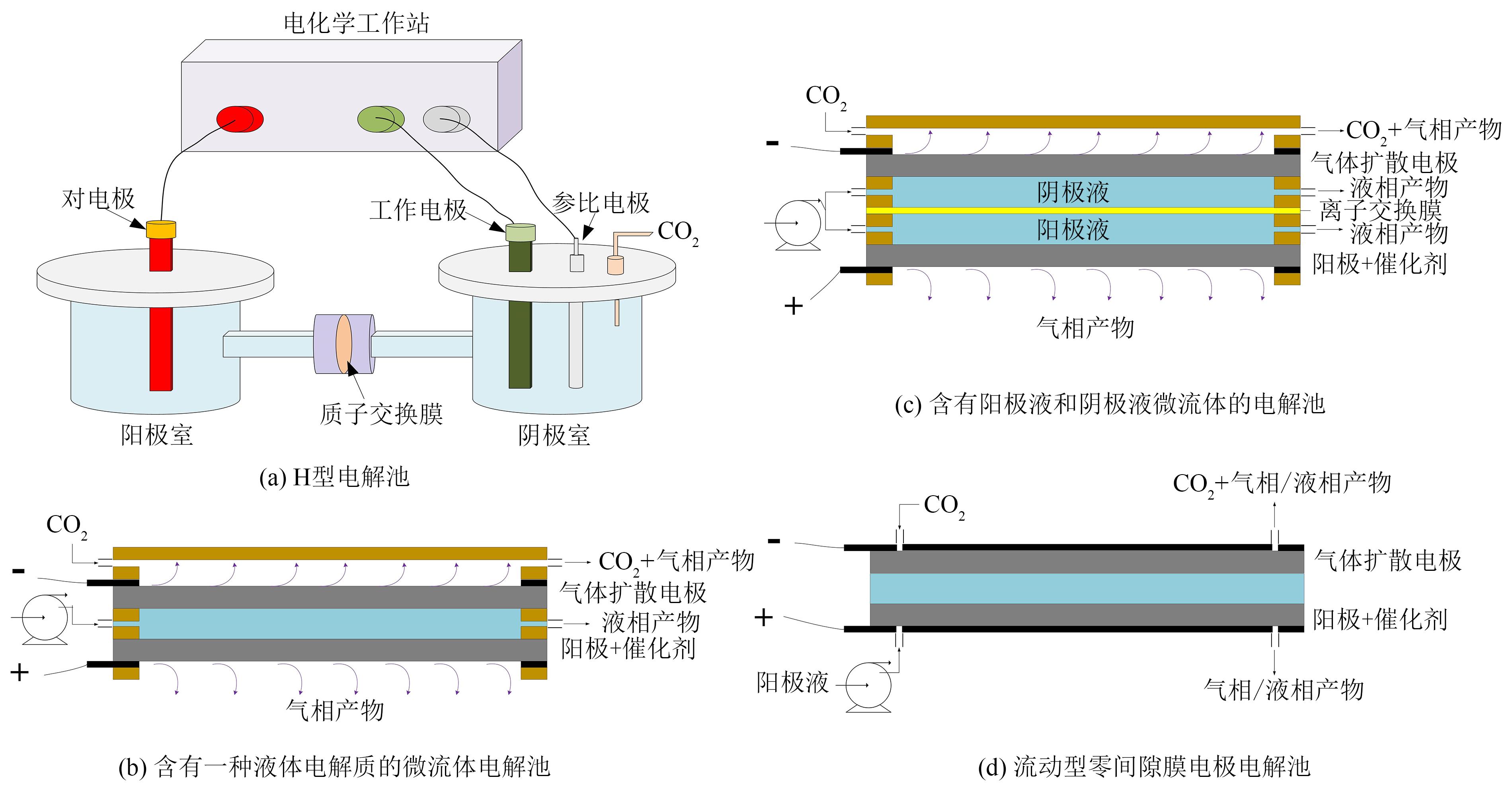
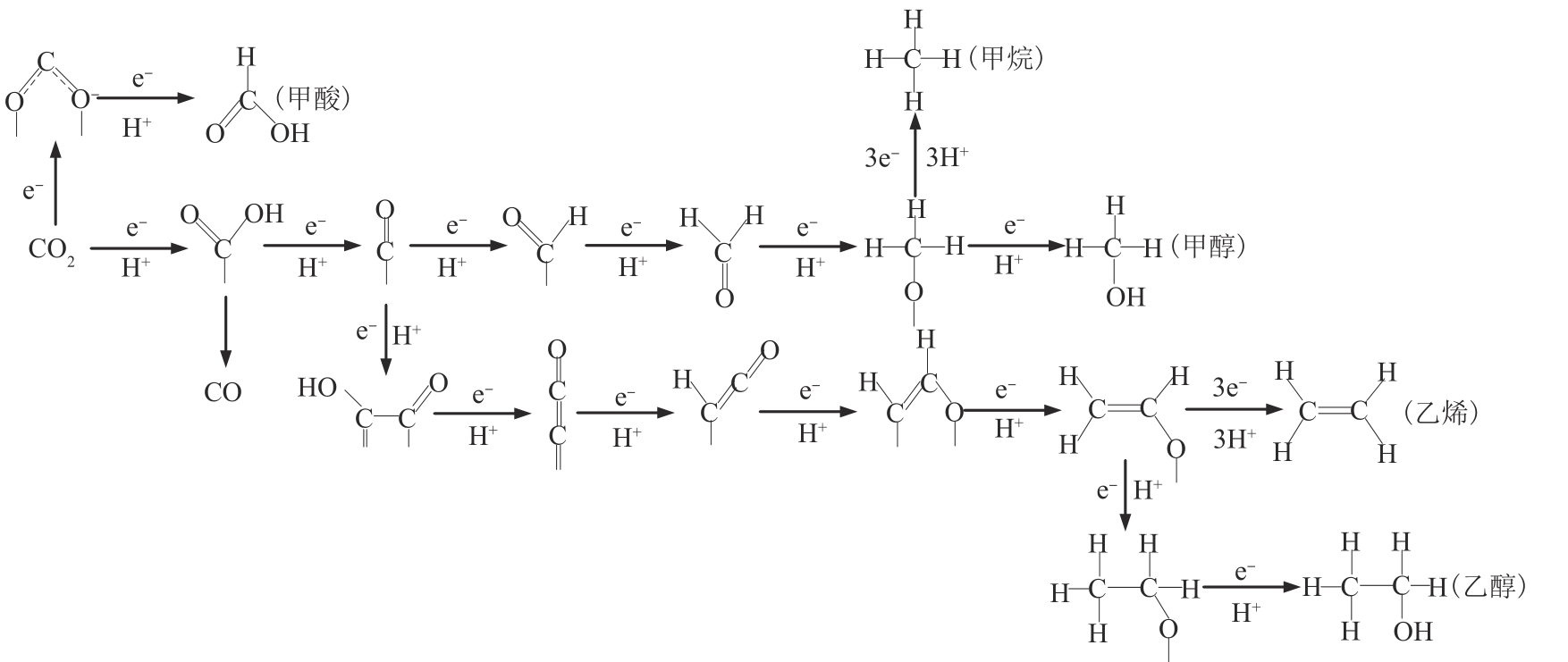
化石能源的大规模使用使得大气中CO2的含量不断增加。发展生物质能源等可再生能源,结合CO2的转化利用对降低化石能源的依赖和减少碳排放具有重要的意义。利用可再生电能驱动CO2的电化学还原生成燃料和化学品是CO2转化利用的一个有效途径。本文对CO2电化学转化以及耦合生物质氧化转化新路线的主要研究进展进行了综述。重点介绍了CO2电化学还原的催化剂、反应系统、反应机制等方面的最新进展,以及阳极耦合生物质及其衍生醇类、醛类等氧化转化的新技术。可再生电能驱动的生物质氧化转化与CO2电还原相耦合不仅可以获得高值化学品,而且可以显著减少CO2的净排放。因此,本文可为生物质和CO2转化提供新的思路。
中图分类号:
引用本文
闫哲, 刘畅, 王丰旭, 周宏旺, 刘樨, 赵雪冰. 耦合生物质氧化转化的CO2电化学还原[J]. 化工进展, 2024, 43(6): 3310-3321.
YAN Zhe, LIU Chang, WANG Fengxu, ZHOU Hongwang, LIU Xi, ZHAO Xuebing. Electrochemical reduction of CO2 coupled with oxidative conversion of biomass[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3310-3321.
| 还原反应方程式 | 标准电势/V |
|---|---|
| CO2+e- | -1.90 |
| CO2+2H++2e- | -0.53 |
| CO2+H2O+2e- | -1.35 |
| CO2+2H++2e- | -0.61 |
| CO2+H2O+2e- | -1.49 |
| CO2+4H++4e- | -0.48 |
| CO2+3H2O+4e- | -1.31 |
| CO2+6H++6e- | -0.38 |
| CO2+5H2O+6e- | -1.23 |
| CO2+8H++8e- | -0.24 |
| CO2+6H2O+8e- | -1.07 |
| CO2+4H++4e- | -2.00 |
| CO2+2H2O+4e- | -1.04 |
| 2CO2+12H++12e- | -0.34 |
| 2CO2+8H2O+12e- | -1.17 |
| 2CO2+14H++14e- | -0.27 |
| 2CO2+2H++2e- | -0.91 |
| 2CO2+2e- | -1.00 |
| 2CO2+12H++12e- | -0.33 |
| 2CO2+9H2O+12e- | -1.16 |
| 3CO2+18H++18e- | -0.32 |
| 2H++2e- | -0.42 |
表1 电化学还原CO2的化学方程式及标准电势[18, 41]
| 还原反应方程式 | 标准电势/V |
|---|---|
| CO2+e- | -1.90 |
| CO2+2H++2e- | -0.53 |
| CO2+H2O+2e- | -1.35 |
| CO2+2H++2e- | -0.61 |
| CO2+H2O+2e- | -1.49 |
| CO2+4H++4e- | -0.48 |
| CO2+3H2O+4e- | -1.31 |
| CO2+6H++6e- | -0.38 |
| CO2+5H2O+6e- | -1.23 |
| CO2+8H++8e- | -0.24 |
| CO2+6H2O+8e- | -1.07 |
| CO2+4H++4e- | -2.00 |
| CO2+2H2O+4e- | -1.04 |
| 2CO2+12H++12e- | -0.34 |
| 2CO2+8H2O+12e- | -1.17 |
| 2CO2+14H++14e- | -0.27 |
| 2CO2+2H++2e- | -0.91 |
| 2CO2+2e- | -1.00 |
| 2CO2+12H++12e- | -0.33 |
| 2CO2+9H2O+12e- | -1.16 |
| 3CO2+18H++18e- | -0.32 |
| 2H++2e- | -0.42 |
| 产物 | 催化剂 | 法拉第效率/% | 过电势/V | 电流密度/mA·cm-2 | 电解液 |
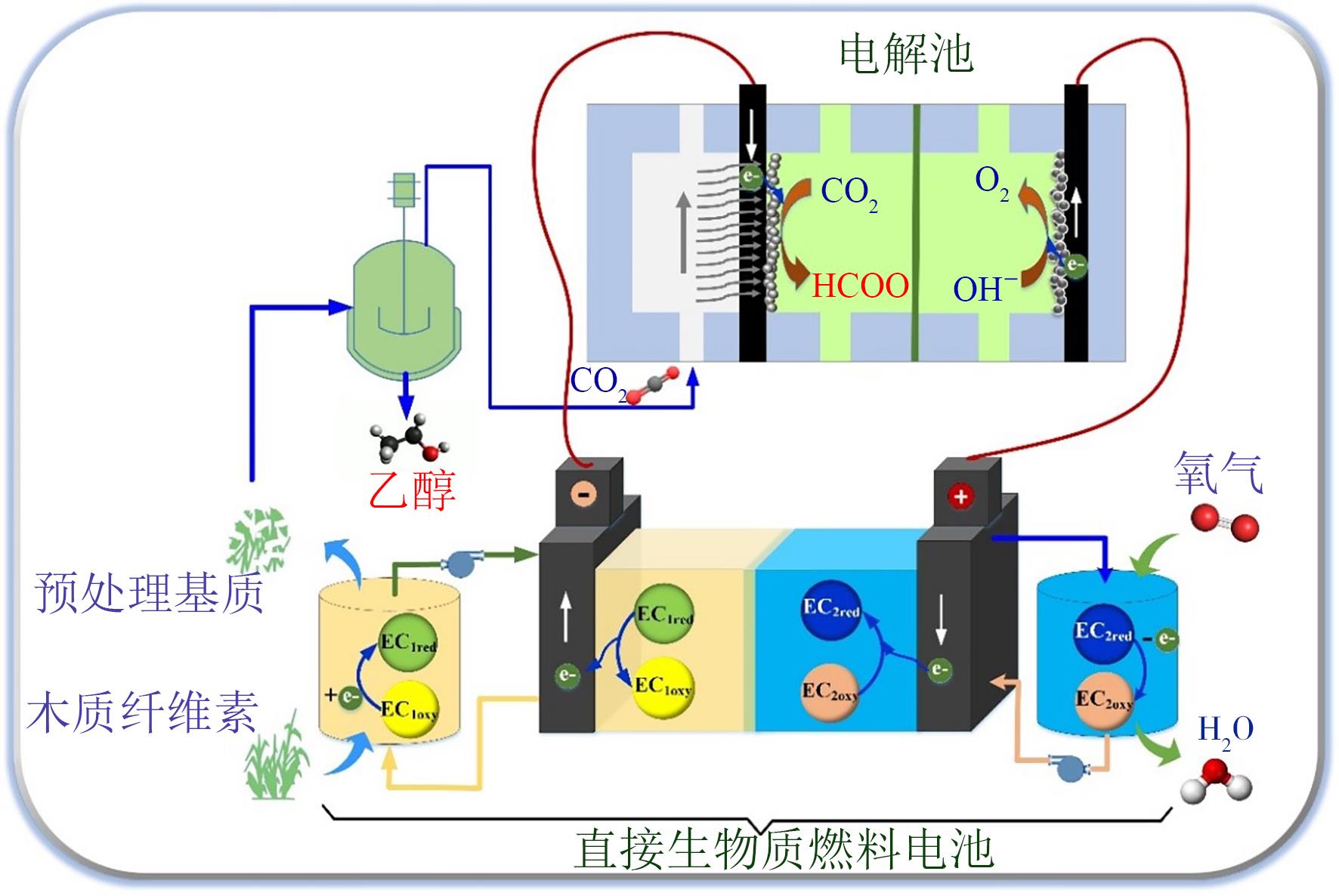
|---|---|---|---|---|---|
| HCOOH | Pb | 97.4 | -1.19 | 5.0 | 0.1mol/L KHCO3 |
| Sn | 88.4 | -1.04 | 5.0 | 0.1mol/L KHCO3 | |
| Pd/C | 99 | -0.15 | 2.4~7.0 | 2.8mol/L KHCO3 | |
| Pd70Pt30/C | 90 | -0.36 | 4.0~7.5 | 0.2mol/L PO43– | |
| CO | Au | 87.1 | -0.64 | 5 | 0.1mol/L KHCO3 |
| Au NPs | 97 | -0.58 | 3.49 ± 0.61 | 0.1mol/L KHCO3 | |
| OD-Au | >96 | -0.25 | 2~4 | 0.5mol/L NaHCO3 | |
| Ag | 94 | -0.99 | 约5 | 0.1mol/L KHCO3 | |
| CH4 | 铜箔 | 40.4 | -1.34 | 约7 | 0.1mol/L KHCO3 |
| Cu(210) | 64 | -1.29 | 5 | 0.1mol/L KHCO3 | |
| C2H4 | 铜箔 | 26.0 | -1.13 | 1~2 | 0.1mol/L KHCO3 |
| Cu | 60 | -0.98 | 约15 | 0.1mol/L KHCO3 | |
| 卤化铜 | 60.5~79.5 | -2.11 | 46.1~39.2 | 3mol/L KBr | |
| 石墨/碳 NPs/Cu/PTFE | 70 | -0.63 | 75~100 | 7mol/L KOH | |
| CH3OH | Cu2O | 38 | -0.43 | 1~2 | 0.5mol/L KHCO3 |
| Mo | 84 | -0.33 | 0.12 | 0.2mol/L Na2SO4 | |
| C2H5OH | 铜箔 | 9.8 | -1.14 | 约0.6 | 0.1mol/L KHCO3 |
| Cu2O | 9~16 | -1.08 | 30~35 | 0.1mol/L KHCO3 | |
| CuO | 36.1 | — | 约11.7 | 0.2mol/L KI | |
| Cu/CNS | 63 | -1.29 | 2 | 0.1mol/L KHCO3 |
表2 CO2电化学还原合成不同产物的常用催化剂[58]
| 产物 | 催化剂 | 法拉第效率/% | 过电势/V | 电流密度/mA·cm-2 | 电解液 |
|---|---|---|---|---|---|
| HCOOH | Pb | 97.4 | -1.19 | 5.0 | 0.1mol/L KHCO3 |
| Sn | 88.4 | -1.04 | 5.0 | 0.1mol/L KHCO3 | |
| Pd/C | 99 | -0.15 | 2.4~7.0 | 2.8mol/L KHCO3 | |
| Pd70Pt30/C | 90 | -0.36 | 4.0~7.5 | 0.2mol/L PO43– | |
| CO | Au | 87.1 | -0.64 | 5 | 0.1mol/L KHCO3 |
| Au NPs | 97 | -0.58 | 3.49 ± 0.61 | 0.1mol/L KHCO3 | |
| OD-Au | >96 | -0.25 | 2~4 | 0.5mol/L NaHCO3 | |
| Ag | 94 | -0.99 | 约5 | 0.1mol/L KHCO3 | |
| CH4 | 铜箔 | 40.4 | -1.34 | 约7 | 0.1mol/L KHCO3 |
| Cu(210) | 64 | -1.29 | 5 | 0.1mol/L KHCO3 | |
| C2H4 | 铜箔 | 26.0 | -1.13 | 1~2 | 0.1mol/L KHCO3 |
| Cu | 60 | -0.98 | 约15 | 0.1mol/L KHCO3 | |
| 卤化铜 | 60.5~79.5 | -2.11 | 46.1~39.2 | 3mol/L KBr | |
| 石墨/碳 NPs/Cu/PTFE | 70 | -0.63 | 75~100 | 7mol/L KOH | |
| CH3OH | Cu2O | 38 | -0.43 | 1~2 | 0.5mol/L KHCO3 |
| Mo | 84 | -0.33 | 0.12 | 0.2mol/L Na2SO4 | |
| C2H5OH | 铜箔 | 9.8 | -1.14 | 约0.6 | 0.1mol/L KHCO3 |
| Cu2O | 9~16 | -1.08 | 30~35 | 0.1mol/L KHCO3 | |
| CuO | 36.1 | — | 约11.7 | 0.2mol/L KI | |
| Cu/CNS | 63 | -1.29 | 2 | 0.1mol/L KHCO3 |
| 阴极CO2电化学还原反应 | 电流密度 /mA·cm-2 | 阳极反应 | 电压 /V | |||
|---|---|---|---|---|---|---|
| 催化剂 | 生成产物 | 法拉第效率/% | 催化剂 | 反应底物 | ||
| Ag GDL | CO | 100.9 | 2.29 | Pt | 甘油 | 0.9 |
| Ag GDL | CO | 98.85 | 4.55 | Pt | 葡萄糖 | 1.2 |
| Sn GDL | HCOOH | 85.5 | 82.52 | Pt | 甘油 | 2.3 |
| Sn GDL | HCOOH | 86.39 | 52.46 | Pt | 葡萄糖 | 1.4 |
表3 耦合不同阳极底物氧化的CO2电化学还原效果比较[71-72]
| 阴极CO2电化学还原反应 | 电流密度 /mA·cm-2 | 阳极反应 | 电压 /V | |||
|---|---|---|---|---|---|---|
| 催化剂 | 生成产物 | 法拉第效率/% | 催化剂 | 反应底物 | ||
| Ag GDL | CO | 100.9 | 2.29 | Pt | 甘油 | 0.9 |
| Ag GDL | CO | 98.85 | 4.55 | Pt | 葡萄糖 | 1.2 |
| Sn GDL | HCOOH | 85.5 | 82.52 | Pt | 甘油 | 2.3 |
| Sn GDL | HCOOH | 86.39 | 52.46 | Pt | 葡萄糖 | 1.4 |
| 1 | FIGUEROA José D, FOUT Timothy, PLASYNSKI Sean, et al. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program[J]. International Journal of Greenhouse Gas Control, 2008, 2(1): 9-20. |
| 2 | YANG Yuechao, MU Tiancheng. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): Pathway, mechanism, catalysts and coupling reactions[J]. Green Chemistry, 2021, 23(12): 4228-4254. |
| 3 | WANG Jiu, SHIRVANI Hamed, ZHAO Heng, et al. Lignocellulosic biomass valorization via bio-photo/electro hybrid catalytic systems[J]. Biotechnology Advances, 2023, 66: 108157. |
| 4 | DAHMEN Nicolaus, LEWANDOWSKI Iris, ZIBEK Susanne, et al. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives[J]. GCB Bioenergy, 2019, 11(1): 107-117. |
| 5 | Bill Vaneck Bot, Jean Gaston Tamba, Olivier Thierry Sosso. Assessment of biomass briquette energy potential from agricultural residues in Cameroon[J]. Biomass Conversion and Biorefinery, 2022: 1-13. |
| 6 | CHEN Yu, MU Tiancheng. Application of deep eutectic solvents in biomass pretreatment and conversion[J]. Green Energy & Environment, 2019, 4(2): 95-115. |
| 7 | ZHOU Ziyuan, LIU Dehua, ZHAO Xuebing. Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose[J]. Renewable and Sustainable Energy Reviews, 2021, 146: 111169. |
| 8 | JAYARAMAN Theerthagiri, KARUPPASAMY K, JUHYEON Park, et al. Electrochemical conversion of biomass-derived aldehydes into fine chemicals and hydrogen: A review[J]. Environmental Chemistry Letters, 2023, 21(3): 1555-1583. |
| 9 | SHARMA Bhawna, LARROCHE Christian, DUSSAP Claude-Gilles. Comprehensive assessment of 2G bioethanol production[J]. Bioresource Technology, 2020, 313: 123630. |
| 10 | GAO Daihong, OUYANG Denghao, ZHAO Xuebing. Electro-oxidative depolymerization of lignin for production of value-added chemicals[J]. Green Chemistry, 2022, 24(22): 8585-8605. |
| 11 | OUYANG Denghao, WANG Fangqian, GAO Daihong, et al. Light-driven lignocellulosic biomass conversion for production of energy and chemicals[J]. iScience, 2022, 25(10): 105221. |
| 12 | Sajid Muhammad, ZHAO Xuebing, LIU Dehua. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes[J]. Green Chemistry, 2018, 20(24): 5427-5453. |
| 13 | TAPIA John Frederick D, LEE Jui-Yuan, Raymond E H OOI, et al. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems[J]. Sustainable Production and Consumption, 2018, 13: 1-15. |
| 14 | VARGHESE Anish Mathai, KARANIKOLOS Georgios N. CO2 capture adsorbents functionalized by amine-bearing polymers: A review[J]. International Journal of Greenhouse Gas Control, 2020, 96: 103005. |
| 15 | OSMAN Ahmed I, MAHMOUD Hefny, ABDEL MAKSOUD M I A, et al. Recent advances in carbon capture storage and utilisation technologies: A review[J]. Environmental Chemistry Letters, 2021, 19(2): 797-849. |
| 16 | ZHANG Zhien, PAN Shuyuan, LI Hao, et al. Recent advances in carbon dioxide utilization[J]. Renewable and Sustainable Energy Reviews, 2020, 125: 109799. |
| 17 | AGHAIE Mahsa, REZAEI Nima, ZENDEHBOUDI Sohrab. A systematic review on CO2 capture with ionic liquids: Current status and future prospects[J]. Renewable and Sustainable Energy Reviews, 2018, 96: 502-525. |
| 18 | WANG Genxiang, CHEN Junxiang, DING Yichun, et al. Electrocatalysis for CO2 conversion: From fundamentals to value-added products[J]. Chemical Society Reviews, 2021, 50(8): 4993-5061. |
| 19 | XIE Huan, WANG Tanyuan, LIANG Jiashun, et al. Cu-based nanocatalysts for electrochemical reduction of CO2 [J]. Nano Today, 2018, 21: 41-54. |
| 20 | LI Chen, WANG Yuwei, XIAO Nan, et al. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction[J]. Carbon, 2019, 151: 46-52. |
| 21 | 崔凯. 中国生物质产业地图[M]. 北京: 中国轻工业出版社, 2007. |
| CUI Kai. Industrial map of China’s biomass[M]. Beijing: China Light Industry Press, 2007. | |
| 22 | 李思敏. 木质素及其衍生物定向转化制备液体燃料的反应机理和产物调控研究[D]. 杭州: 浙江大学, 2021. |
| LI Simin. Study on reaction mechanism and product regulation of liquid fuel prepared by directional conversion of lignin and its derivatives[D].Hangzhou: Zhejiang University, 2021. | |
| 23 | 李庆林, 宋涛, 杨勇. 生物质炭基材料在有机催化转化中的应用[J]. 化工进展, 2021, 40(4): 1966-1982. |
| LI Qinglin, SONG Tao, YANG Yong. Biomass-derived carbon materials for organic transformations[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1966-1982. | |
| 24 | YOO Chang Geun, MENG Xianzhi, PU Yunqiao, et al. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review[J]. Bioresource Technology, 2020, 301: 122784. |
| 25 | 庄雨婷, 王建华, 向智艳, 等. 半纤维素及其衍生物转化为γ-戊内酯及其动力学研究进展[J]. 化工进展, 2022, 41(7): 3519-3533. |
| ZHUANG Yuting, WANG Jianhua, XIANG Zhiyan, et al. Research progress in preparation and kinetics of γ-valerolactone synthesis from hemicellulose and its derivatives[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3519-3533. | |
| 26 | LIU Chao, WU Shiliang, ZHANG Huiyan, et al. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review[J]. Fuel Processing Technology, 2019, 191: 181-201. |
| 27 | ZHOU Ziyuan, OUYANG Denghao, LIU Dehua, et al. Oxidative pretreatment of lignocellulosic biomass for enzymatic hydrolysis: Progress and challenges[J]. Bioresource Technology, 2023, 367: 128208. |
| 28 | NWOSU Ugochukwu, WANG Aiguo, PALMA Bruna, et al. Selective biomass photoreforming for valuable chemicals and fuels: A critical review[J]. Renewable and Sustainable Energy Reviews, 2021, 148: 111266. |
| 29 | PAYORMHORM Jiraporn, CHUANGCHOTE Surawut, KIATKITTIPONG Kunlanan, et al. Xylitol and gluconic acid productions via photocatalytic-glucose conversion using TiO2 fabricated by surfactant-assisted techniques: Effects of structural and textural properties[J]. Materials Chemistry and Physics, 2017, 196: 29-36. |
| 30 | KOBAYAKAWA Koichi, SATO Yuichi, NAKAMURA Shigeo, et al. Photodecomposition of kraft lignin catalyzed by titanium dioxide[J]. Bulletin of the Chemical Society of Japan, 1989, 62(11): 3433-3436. |
| 31 | FAN Hongxian, LI Gang, YANG Fang, et al. Photodegradation of cellulose under UV light catalysed by TiO2 [J]. Journal of Chemical Technology & Biotechnology, 2011, 86(8): 1107-1112. |
| 32 | MA Ben, WANG Yingyong, GUO Xiaoning, et al. Photocatalytic synthesis of 2,5-diformylfuran from 5-hydroxymethyfurfural or fructose over bimetallic Au-Ru nanoparticles supported on reduced graphene oxides[J]. Applied Catalysis A: General, 2018, 552: 70-76. |
| 33 | ZHOU Baowen, SONG Jinliang, ZHANG Zhanrong, et al. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2 [J]. Green Chemistry, 2017, 19(4): 1075-1081. |
| 34 | Dinh CAO-THANG, THOMAS Burdyny, GOLAM Kibria MD, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface[J]. Science, 2018, 360(6390): 783-787. |
| 35 | CHEN Chunlin, WANG Lingchen, ZHU Bin, et al. 2,5-Furandicarboxylic acid production via catalytic oxidation of 5-hydroxymethylfurfural: Catalysts, processes and reaction mechanism[J]. Journal of Energy Chemistry, 2021, 54: 528-554. |
| 36 | QIANG Ye, OUYANG Denghao, YOU Li, et al. Liquid flow fuel cell with an electrodeposition-modified nickel foam anode for efficient oxidation of 5-hydroxymethylfurfural to produce 2,5-furandicarboxylic acid with co-generation of electricity[J]. Chemical Engineering Journal, 2023, 469: 143832. |
| 37 | 刘南, 祁峰, 李力, 等. 纤维素降解辅助蛋白及其作用机理研究进展[J]. 化工进展, 2018, 37(3): 1118-1129. |
| LIU Nan, QI Feng, LI Li, et al. Auxiliary proteins for boosting enzymatic hydrolysis of cellulose and the action mechanisms[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1118-1129. | |
| 38 | ZHOU M, FAKAYODE O A, REN M, et al. Laccase-catalyzed lignin depolymerization in deep eutectic solvents: Challenges and prospects[J]. Bioresources and Bioprocessing, 2023, 10(1): 1-22. |
| 39 | QIN Yezhi, LI Yanmei, ZONG Minhua, et al. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents[J]. Green Chemistry, 2015, 17(7): 3718-3722. |
| 40 | ZHANG Xueying, ZONG Minhua, LI Ning. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid[J]. Green Chemistry, 2017, 19(19): 4544-4551. |
| 41 | 张轩, 黄耀桢, 邵秀丽, 等. 结构化铜基催化剂电化学还原CO2为多碳产物研究进展[J]. 化工进展, 2021, 40(7): 3736-3746. |
| ZHANG Xuan, HUANG Yaozhen, SHAO Xiuli, et al. Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3736-3746. | |
| 42 | LIANG Shuyu, ALTAF Naveed, HUANG Liang, et al. Electrolytic cell design for electrochemical CO2 reduction[J]. Journal of CO2 Utilization, 2020, 35: 90-105. |
| 43 | KORTLEVER Ruud, PETERS Ines, KOPER Sander, et al. Electrochemical CO2 reduction to formic acid at low overpotential and with high faradaic efficiency on carbon-supported bimetallic Pd–Pt nanoparticles[J]. ACS Catalysis, 2015, 5(7): 3916-3923. |
| 44 | DONG Wenfei, ZHANG Nan, LI Sanxiu, et al. A Mn single atom catalyst with Mn-N2O2 sites integrated into carbon nanosheets for efficient electrocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2022, 10(20): 10892-10901. |
| 45 | NIU Zhuangzhuang, CHI Liping, LIU Ren, et al. Rigorous assessment of CO2 electroreduction products in a flow cell[J]. Energy & Environmental Science, 2021, 14(8): 4169-4176. |
| 46 | ALBO Jonathan, BEOBIDE Garikoitz, Pedro CASTAÑO, et al. Methanol electrosynthesis from CO2 at Cu2O/ZnO prompted by pyridine-based aqueous solutions[J]. Journal of CO2 Utilization, 2017, 18: 164-172. |
| 47 | LEE Jonghyeok, Jinkyu LIM, Chi-Woo ROH, et al. Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes[J]. Journal of CO2 Utilization, 2019, 31: 244-250. |
| 48 | YU Libo, WANG Jingjing, HU Xun, et al. A nanocatalyst network for electrochemical reduction of CO2 over microchanneled solid oxide electrolysis cells[J]. Electrochemistry Communications, 2018, 86: 72-75. |
| 49 | 葛睿, 胡旭, 董灵玉, 等. 电化学耦合阴极二氧化碳还原与阳极氧化合成[J]. 化工进展, 2021, 40(9): 5132-5144. |
| GE Rui, HU Xu, DONG Lingyu, et al. Electrochemical coupling between cathodic carbon dioxide reduction and anodic oxidation synthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5132-5144. | |
| 50 | VASS Á, ENDRŐDI B, JANÁKY C. Coupling electrochemical carbon dioxide conversion with value-added anode processes: An emerging paradigm[J]. Current Opinion in Electrochemistry, 2021, 25: 100621. |
| 51 | WU Zhizheng, GAO Feiyue, GAO Minrui. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction[J]. Energy & Environmental Science, 2021, 14(3): 1121-1139. |
| 52 | GUO Weiwei, LIU Shoujie, TAN Xingxing, et al. Highly efficient CO2 electroreduction to methanol through atomically dispersed Sn coupled with defective CuO catalysts[J]. Angewandte Chemie International Edition, 2021, 60(40): 21979-21987. |
| 53 | WANG Guozhi, MA Yangbo, WANG Juan, et al. Metal functionalization of two-dimensional nanomaterials for electrochemical carbon dioxide reduction[J]. Nanoscale, 2023, 15(14): 6456-6475. |
| 54 | ITO Yoshikazu, KUKUNURI Suresh, JEONG Samuel, et al. Phase-dependent electrochemical CO2 reduction ability of NiSn alloys for formate generation[J]. ACS Applied Energy Materials, 2021, 4(7): 7122-7128. |
| 55 | LI Chengbo, JI Yuan, WANG Youpeng, et al. Applications of metal-organic frameworks and their derivatives in electrochemical CO2 reduction[J]. Nano-Micro Letters, 2023, 15(1): 113. |
| 56 | PERRY Samuel C, LEUNG Pui-ki, WANG Ling, et al. Developments on carbon dioxide reduction: Their promise, achievements, and challenges[J]. Current Opinion in Electrochemistry, 2020, 20: 88-98. |
| 57 | WANG Jiajun, LI Xiaopeng, CUI Bingfeng, et al. A review of non-noble metal-based electrocatalysts for CO2 electroreduction[J]. Rare Metals, 2021, 40(11): 3019-3037. |
| 58 | BIRDJA Yuvraj Y, Elena PÉREZ-GALLENT, FIGUEIREDO Marta C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nature Energy, 2019, 4(9): 732-745. |
| 59 | Chatterjee Sudipta, Dutta Indranil, Lum Yanwei, et al. Enabling storage and utilization of low-carbon electricity: Power to formic acid[J]. Energy & Environmental Science, 2021, 14(3): 1194-1246. |
| 60 | FAN Lei, XIA Chuan, ZHU Peng, et al. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor[J]. Nature Communications, 2020, 11: 3633. |
| 61 | WEN Guobin, LEE Dong Un, REN Bohua, et al. Carbon dioxide electroreduction: Orbital interactions in Bi-Sn bimetallic electrocatalysts for highly selective electrochemical CO2 reduction toward formate production[J]. Advanced Energy Materials, 2018, 8(31): 1870138. |
| 62 | KUMAR Amit, Prosenjit DAW, MILSTEIN David. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics[J]. Chemical Reviews, 2022, 122(1): 385-441. |
| 63 | LIANG Zhifu, WANG Jianghao, TANG Pengyi, et al. Molecular engineering to introduce carbonyl between nickel salophen active sites to enhance electrochemical CO2 reduction to methanol[J]. Applied Catalysis B: Environmental, 2022, 314: 121451. |
| 64 | WANG Liwen, XU Yida, CHEN Teng, et al. Ternary heterostructural CoO/CN/Ni catalyst for promoted CO2 electroreduction to methanol[J]. Journal of Catalysis, 2021, 393: 83-91. |
| 65 | SUN S N, DONG L Z, LI J R, et al. Redox-active crystalline coordination catalyst for hybrid electrocatalytic methanol oxidation and CO2 reduction[J]. Angewandte Chemie International Edition, 2022, 61: 1-8. |
| 66 | LI Rui, XIANG Kun, PENG Zhikun, et al. Recent advances on electrolysis for simultaneous generation of valuable chemicals at both anode and cathode[J]. Advanced Energy Materials, 2021, 11(46): 2102292. |
| 67 | ZHAO Xin, DU Lijie, YOU Bo, et al. Integrated design for electrocatalytic carbon dioxide reduction[J]. Catalysis Science & Technology, 2020, 10(9): 2711-2720. |
| 68 | YOU Bo, LIU Xuan, LIU Xin, et al. Efficient H2 evolution coupled with oxidative refining of alcohols via a hierarchically porous nickel bifunctional electrocatalyst[J]. ACS Catalysis, 2017, 7(7): 4564-4570. |
| 69 | HAO Shuai, YANG Libin, LIU Danni, et al. Integrating natural biomass electro-oxidation and hydrogen evolution: Using a porous Fe-doped CoP nanosheet array as a bifunctional catalyst[J]. Chemical Communications, 2017, 53(42): 5710-5713. |
| 70 | WEI Xinfa, WANG Shun, HUA Zile, et al. Metal-organic framework nanosheet electrocatalysts for efficient H2 production from methanol solution: Methanol-assisted water splitting or methanol reforming?[J]. ACS Applied Materials & Interfaces, 2018, 10(30): 25422-25428. |
| 71 | VERMA Sumit, LU Shawn, KENIS Paul J A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption[J]. Nature Energy, 2019, 4(6): 466-474. |
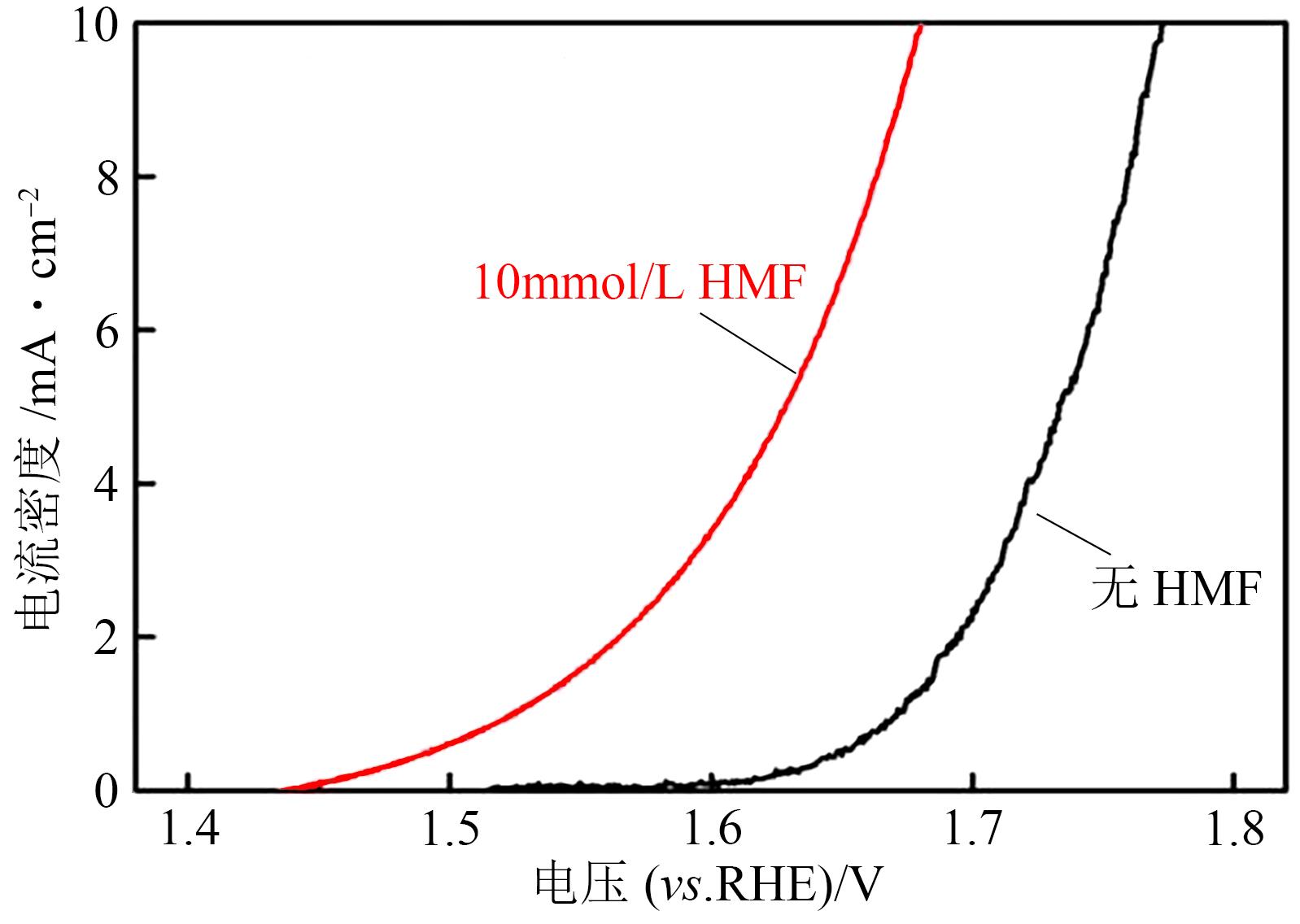
| 72 | BI Jiahui, ZHU Qinggong, GUO Weiwei, et al. Simultaneous CO2 reduction and 5-hydroxymethylfurfural oxidation to value-added products by electrocatalysis[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(24): 8043-8050. |
| 73 | BAI Xinyu, HOU Qidong, QIAN Hengli, et al. Selective oxidation of glucose to gluconic acid and glucaric acid with chlorin e6 modified carbon nitride as metal-free photocatalyst[J]. Applied Catalysis B: Environmental, 2022, 303: 120895. |
| 74 | LIU Yunpeng, ZOU Ren, QIN Binhao, et al. Energy-efficient monosaccharides electrooxidation coupled with green hydrogen production by bifunctional Co9S8/Ni3S2 electrode[J]. Chemical Engineering Journal, 2022, 446: 136950. |
| 75 | LI Tengfei, CAO Yang, HE Jingfu, et al. Electrolytic CO2 reduction in tandem with oxidative organic chemistry[J]. ACS Central Science, 2017, 3(7): 778-783. |
| 76 | WANG Ying, GONELL Sergio, MATHIYAZHAGAN Ulaganatha Raja, et al. Simultaneous electrosynthesis of syngas and an aldehyde from CO2 and an alcohol by molecular electrocatalysis[J]. ACS Applied Energy Materials, 2019, 2(1): 97-101. |
| 77 | WEINGARTEN Ronen, Alexandra RODRIGUEZ-BEUERMAN, CAO Fei, et al. Selective conversion of cellulose to hydroxymethylfurfural in polar aprotic solvents[J]. ChemCatChem, 2014, 6(8): 2229-2234. |
| 78 | CHOI Seungwoo, BALAMURUGAN Mani, LEE Kang-Gyu, et al. Mechanistic investigation of biomass oxidation using nickel oxide nanoparticles in a CO2-saturated electrolyte for paired electrolysis[J]. The Journal of Physical Chemistry Letters, 2020, 11(8): 2941-2948. |
| 79 | YOU Bo, JIANG Nan, LIU Xuan, et al. Simultaneous H2 generation and biomass upgrading in water by an efficient noble-metal-free bifunctional electrocatalyst[J]. Angewandte Chemie International Edition, 2016, 55(34): 9913-9917. |
| 80 | Hyeonmyeong OH, CHOI Yuri, SHIN Changhwan, et al. Phosphomolybdic acid as a catalyst for oxidative valorization of biomass and its application as an alternative electron source[J]. ACS Catalysis, 2020, 10(3): 2060-2068. |
| 81 | OUYANG Denghao, HAN Yazhu, WANG Fangqiang, et al. All-iron ions mediated electron transfer for biomass pretreatment coupling with direct generation of electricity from lignocellulose[J]. Bioresource Technology, 2022, 344: 126189. |
| 82 | DING Yi, DU Bo, ZHAO Xuebing, et al. Phosphomolybdic acid and ferric iron as efficient electron mediators for coupling biomass pretreatment to produce bioethanol and electricity generation from wheat straw[J]. Bioresource Technology, 2017, 228: 279-289. |
| 83 | WANG Fangqian, OUYANG Denghao, LI Bo, et al. Promoting transfer of endogenous electrons well increases the carbon and energy efficiency of lignocellulosic biomass conversion to fuels and chemicals[J]. Energy Conversion and Management, 2022, 258: 115552. |
| [1] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [2] | 周爱国, 郑家乐, 杨川箬, 杨小艺, 赵俊德, 李兴春. 直接空气二氧化碳捕集技术工业化进展[J]. 化工进展, 2024, 43(6): 2928-2939. |
| [3] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [4] | 张真, 张凡, 云祉婷. 绿氢在石化和化工行业的减碳经济性分析[J]. 化工进展, 2024, 43(6): 3021-3028. |
| [5] | 何世坤, 张文豪, 冯君锋, 潘晖. 负载金属型固体酸催化木质纤维生物质定向转化为乙酰丙酸甲酯[J]. 化工进展, 2024, 43(6): 3042-3050. |
| [6] | 陈富强, 仲兆平, 戚仁志. 铜基催化剂电还原二氧化碳为甲酸研究进展[J]. 化工进展, 2024, 43(6): 3051-3060. |
| [7] | 曾壮, 李柯志, 苑志伟, 杜金涛, 李卓师, 王悦. CO/CO2 加氢制低碳醇改性费托合成催化剂研究进展[J]. 化工进展, 2024, 43(6): 3061-3079. |
| [8] | 龚雪梅, 蒋军, 王超, 梅长彤. 纳米纤维素疏水改性及其功能化应用研究进展[J]. 化工进展, 2024, 43(6): 3187-3198. |
| [9] | 冯勇强, 王洁茹, 王超娴, 李芳, 苏婉婷, 孙宇, 赵彬然. γ-Al2O3 负载的Ni、Fe、Cu对介质阻挡放电等离子体转化CO2/CH4的影响[J]. 化工进展, 2024, 43(5): 2705-2713. |
| [10] | 解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722. |
| [11] | 周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738. |
| [12] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| [13] | 黄澎, 邹颖, 王宝焕, 王逍妍, 赵勇, 梁鑫, 胡迪. 二氧化碳电催化还原反应制合成气催化剂研究进展[J]. 化工进展, 2024, 43(5): 2760-2775. |
| [14] | 卢欣欣, 蔡东仁, 詹国武. 基于固体前体构建集成催化剂及CO2加氢研究进展[J]. 化工进展, 2024, 43(5): 2786-2802. |
| [15] | 武西宁, 张宁, 秦佳敏, 徐龙, 魏朝阳, 马晓迅. 低冷量下强化CO2吸收的甲醇基纳米流体性能[J]. 化工进展, 2024, 43(5): 2811-2822. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||