化工进展 ›› 2023, Vol. 42 ›› Issue (11): 5929-5942.DOI: 10.16085/j.issn.1000-6613.2022-2314
• 资源与环境化工 • 上一篇
生物电芬顿系统在废水处理中的研究进展
武诗宇( ), 杜志平(
), 杜志平( ), 申婧, 李剑锋, 程芳琴(
), 申婧, 李剑锋, 程芳琴( ), 赵华章
), 赵华章
- 山西大学资源与环境工程研究所,山西省黄河实验室,山西 太原 030006
-
收稿日期:2022-12-14修回日期:2023-01-25出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:杜志平,程芳琴 -
作者简介:武诗宇(1994—),男,博士研究生,研究方向为生物电化学水处理技术。E-mail:wushiyu126@126.com。 -
基金资助:国家自然科学基金(22272098);山西省重点研发计划(202102090301028);中央引导地方科技发展专项资金项目(YDZX20191400002539);山西省高等学校科技创新项目
Treatment of wastewater by bio-electro-Fenton system: a review
WU Shiyu( ), DU Zhiping(
), DU Zhiping( ), SHEN Jing, LI Jianfeng, CHENG Fangqin(
), SHEN Jing, LI Jianfeng, CHENG Fangqin( ), ZHAO Huazhang
), ZHAO Huazhang
- Shanxi Laboratory for Yellow River, Institute of Resources and Environmental Engineering, Shanxi University, Taiyuan 030006, Shanxi, China
-
Received:2022-12-14Revised:2023-01-25Online:2023-11-20Published:2023-12-15 -
Contact:DU Zhiping, CHENG Fangqin
摘要:
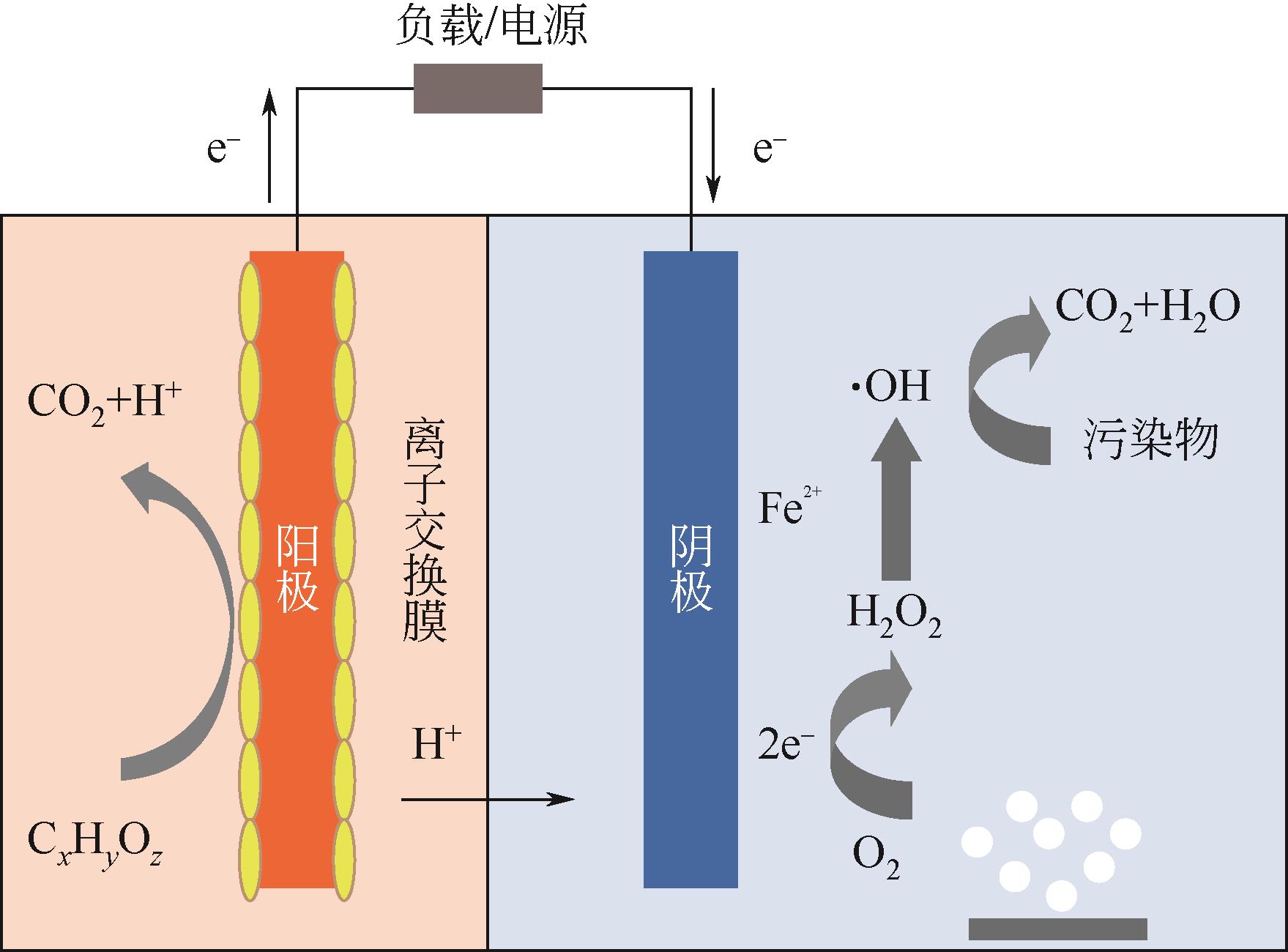
随着工业化进程加快,废水的类型和排放量日益增加,对自然环境和人类社会造成了严重危害,因此亟需开发经济高效的废水处理技术。生物电芬顿(BEF)系统是将生物电化学系统与芬顿氧化工艺耦合的水处理技术,其因高效、绿色、节能的特点而受到广泛关注。本文综述了BEF系统的基本原理,讨论了阳极和阴极性能的主要影响因素(微生物胞外电子传递过程、阳极材料、阴极材料、芬顿反应催化剂和系统运行条件),总结了BEF系统在各类废水(染料废水、制药废水、垃圾渗滤液、煤化工废水、养猪废水等)处理中的应用,最后指出BEF系统当前面临的挑战及未来的研究方向,包括开发高效的电极材料和催化剂、耦合其他水处理工艺、降低膜材料成本和开展中试研究等,旨在为BEF系统的进一步发展提供参考。
中图分类号:
引用本文
武诗宇, 杜志平, 申婧, 李剑锋, 程芳琴, 赵华章. 生物电芬顿系统在废水处理中的研究进展[J]. 化工进展, 2023, 42(11): 5929-5942.
WU Shiyu, DU Zhiping, SHEN Jing, LI Jianfeng, CHENG Fangqin, ZHAO Huazhang. Treatment of wastewater by bio-electro-Fenton system: a review[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5929-5942.
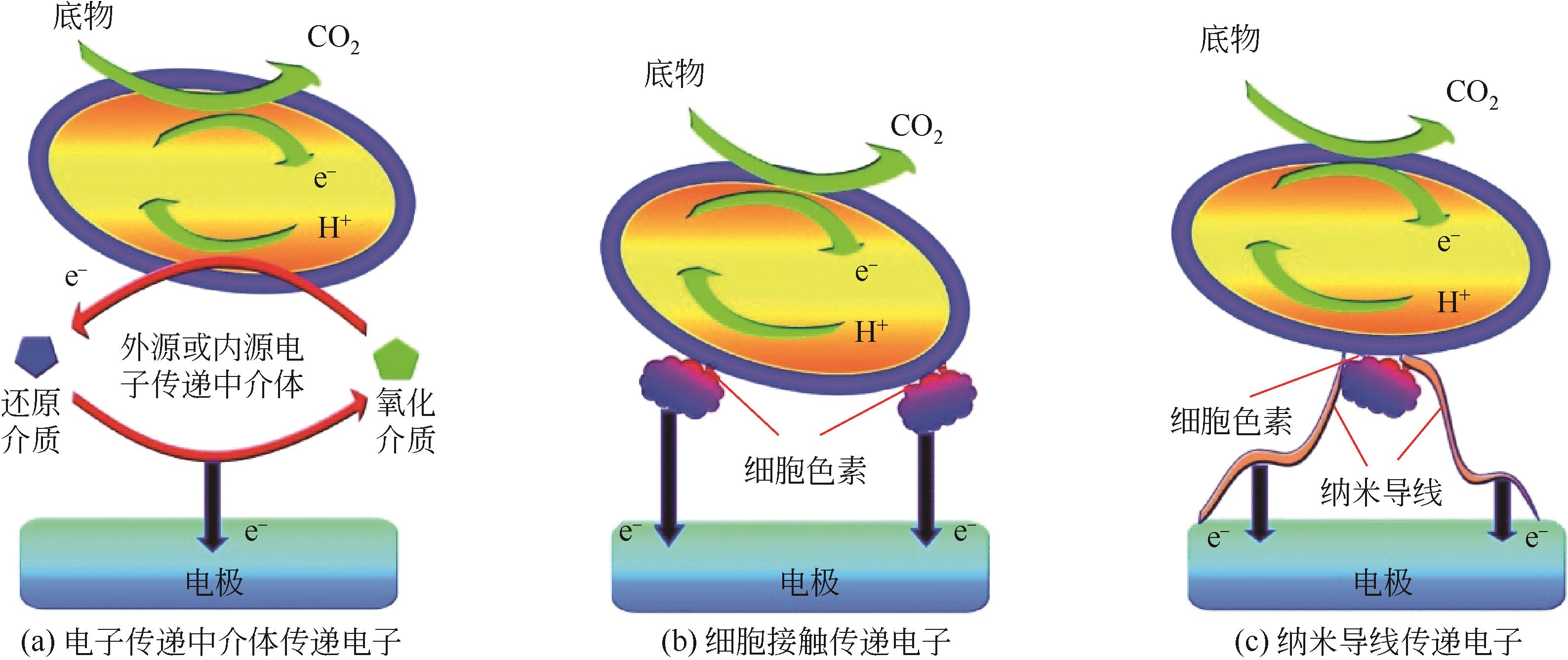
| 微生物 | 门 | 种 | 电子传递机质 | 参考文献 |
|---|---|---|---|---|
| 细菌 | 变形菌门 | 沼泽红假单胞菌 | 细胞接触传递 | [ |
| 嗜酸性氧化亚铁硫杆菌 | 细胞接触传递 | [ | ||
| 大肠埃希菌 | 电子传递中介体传递 | [ | ||
| 铜绿假单胞菌 | 电子传递中介体传递 | [ | ||
| 运动发酵单胞菌 | 细胞接触传递、电子传递中介体传递 | [ | ||
| 希瓦氏菌 | 细胞接触传递、纳米导线传递、电子传递中介体传递 | [ | ||
| 硫还原地杆菌 | 细胞接触传递、纳米导线传递 | [ | ||
| 厚壁菌门 | 丁酸梭菌 | 细胞接触传递 | [ | |
| 真菌 | 子囊菌门 | 异常汉逊酵母 | 细胞接触传递 | [ |
| 酿酒酵母菌 | 细胞接触传递、电子传递中介体传递 | [ | ||
| 古菌 | 广古菌门 | 产甲烷菌 | 细胞接触传递、纳米导线传递 | [ |
表1 常见产电微生物类群及其电子传递机制
| 微生物 | 门 | 种 | 电子传递机质 | 参考文献 |
|---|---|---|---|---|
| 细菌 | 变形菌门 | 沼泽红假单胞菌 | 细胞接触传递 | [ |
| 嗜酸性氧化亚铁硫杆菌 | 细胞接触传递 | [ | ||
| 大肠埃希菌 | 电子传递中介体传递 | [ | ||
| 铜绿假单胞菌 | 电子传递中介体传递 | [ | ||
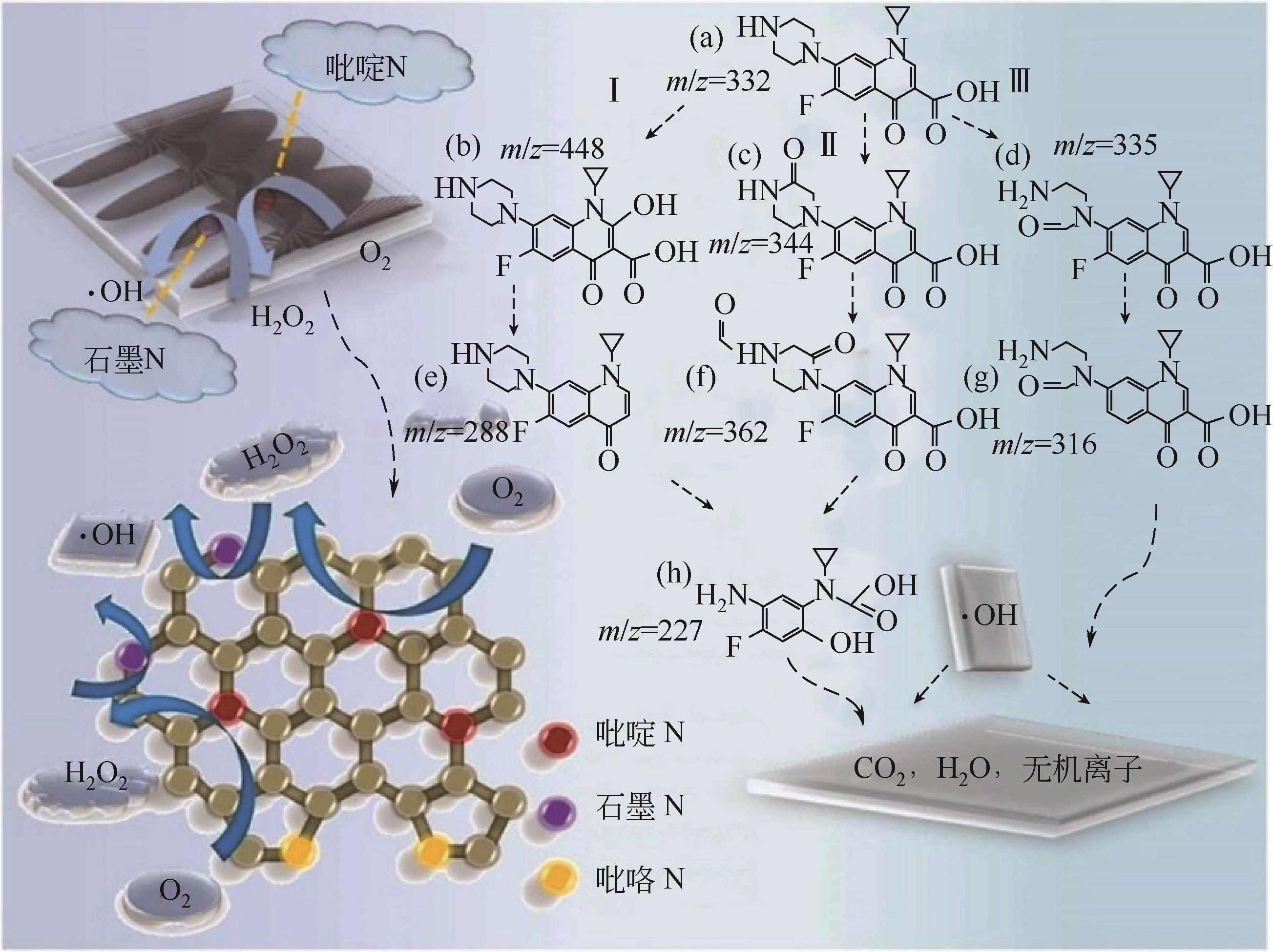
| 运动发酵单胞菌 | 细胞接触传递、电子传递中介体传递 | [ | ||
| 希瓦氏菌 | 细胞接触传递、纳米导线传递、电子传递中介体传递 | [ | ||
| 硫还原地杆菌 | 细胞接触传递、纳米导线传递 | [ | ||
| 厚壁菌门 | 丁酸梭菌 | 细胞接触传递 | [ | |
| 真菌 | 子囊菌门 | 异常汉逊酵母 | 细胞接触传递 | [ |
| 酿酒酵母菌 | 细胞接触传递、电子传递中介体传递 | [ | ||
| 古菌 | 广古菌门 | 产甲烷菌 | 细胞接触传递、纳米导线传递 | [ |
| 废水类型 | 阳极 | 阴极 | 催化剂 | 运行条件 | 污染物浓度 | 去除率 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| 染料废水 | |||||||
| 橙黄Ⅱ | 碳毡 | PPy/AQDS/碳毡 | γ-FeOOH | pH=7,外部电阻1000Ω,曝气量100mL/min,γ-FeOOH 1g/L | 0.2mmol/L | 100% | [ |
| 橙黄Ⅱ | 碳毡 | 碳纳米管/ γ-FeOOH | pH=7,外部电阻1000Ω | 0.1mmol/L | 100% | [ | |
| 甲基橙 | 碳纸+ 颗粒活性炭 | 负载Pt碳纸+ 颗粒活性炭 | FeSO4·7H2O | pH=3,外部电阻1000Ω,曝气量150mL/min,FeSO4·7H2O 0.05mol/L | 300mg/L | 86.39% | [ |
| 甲基橙 | 石墨纤维刷 | Fe2O3/活性碳毡 | pH=3,外部电阻100Ω,曝气量750mL/min | 5mg/L | 86.7% | [ | |
| 罗丹明B | 活性碳毡 | Fe@Fe2O3/ 活性碳毡 | pH=3,外部电阻120Ω,曝气量300mL/min | 10mg/L | 95.0% | [ | |
| 橙G | 碳刷 | 石墨板 | Fe2+ | pH=2,外部电阻10Ω,曝气量8mL/min,Fe2+ 0.01mol/L | 400mg/L | 99.6% | [ |
| 酸性橙7 | 碳布 | 碳毡 | FeVO4 | pH=3,外部电阻1000Ω,FeVO4 12.5g/L | 50mg/L | 89% | [ |
酸性绿50 结晶紫 | 石墨棒 | 石墨棒 | 铁藻酸盐微珠 | pH=2,外部电阻1000Ω,曝气量2L/min,铁藻酸盐微珠10g(铁浓度150mg/L) | 10mg/L | 94% 83% | [ |
| 亚甲基蓝 | 碳刷 | 石墨板 | Fe2+ | pH=3,外部电阻5Ω,曝气量10mL/min,Fe2+ 2mmol/L | 50mg/L | 100% | [ |
| 亚甲基蓝 | 碳刷 | 石墨板 | Fe2+ | pH=2,外加电压0.4V,曝气量350mL/min,Fe2+ 0.2mmol/L | 20mg/L | 99% | [ |
| 橙G | 100% | ||||||
| 甲苯胺蓝 | 98% | ||||||
| 麦尔多拉蓝 | 99% | ||||||
| 罗丹明B | 100% | ||||||
| 罗丹明6G | 100% | ||||||
| 雷马素蓝RR | 葡萄糖氧化酶 固定化碳毡 | 碳毡 | FeSO4·7H2O | pH=3.5,外部电阻10Ω,FeSO4·7H2O 1.5g/L | 50mg/L | COD 34% | [ |
| 含PPCPs废水 | |||||||
| 对硝基苯酚 | 碳毡 | 碳毡 | 废铁屑 | pH=3,外部电阻1000Ω,废铁屑10g | 1mmol/L | 100% | [ |
| 对硝基苯酚 | 碳毡 | 碳毡 | 褐铁矿 | pH=2,外部电阻300Ω,曝气量100mL/min,褐铁矿2.24g/L | 0.25mmol/L | 96% | [ |
磺胺甲𫫇唑 诺氟沙星 | 碳毡 | γ-FeOOH石墨烯聚丙烯酰胺碳化气凝胶 | 自然pH,外部电阻1000Ω | 0.1mmol/L | 97.4% 96.1% | [ | |
| 土霉素 | 碳毡 | FeCo/氧化 石墨烯/碳毡 | pH=7.2,外部电阻1000Ω,曝气量50mL/min | 20mg/L | 80.34% | [ | |
| 四环素 | 碳毡 | 碳毡 | Fe2+ | pH=3,外部电阻250Ω,溶解氧≥8.4mg/L,Fe2+ 1mg/L | 20mg/L | 100% | [ |
17β-雌二醇 17α-乙炔基雌二醇 | 碳毡+ 颗粒石墨 | Fe@Fe2O3/非催化碳毡 | pH=3,外部电阻1000Ω,曝气量100mL/min | 20μg/L 20μg/L | 81% 56% | [ | |
| 双酚A | 石墨棒 | 石墨棒+ 石墨颗粒 | FeSO4·7H2O | pH=3,外部电阻10Ω, FeSO4·7H2O 1.25mmol/L | 1mg/L | 75% | [ |
| 雌酮 | 100% | ||||||
| 磺胺二甲基嘧啶 | 97% | ||||||
| 三氯卡班 | 98% | ||||||
| 扑热息痛 | 石墨毡 | 石墨板 | FeSO4·7H2O | pH=2,外部电阻20Ω, FeSO4·7H2O 5mg/L | 10mg/L | 70% | [ |
| 含PPCPs废水 | |||||||
| 酮洛芬 | 碳刷 | 石墨板 | FeSO4·7H2O | pH=2,外加电压0.5V,曝气量8mL/min,FeSO4·7H2O 5mmol/L | 40μg/L | 59% | [ |
| 双氯芬酸 | 87% | ||||||
| 布洛芬 | 80% | ||||||
| 萘普生 | 75% | ||||||
| 美托洛尔 | 碳刷 | 石墨板 | FeSO4·7H2O | pH=3,外加电压 0.2V,曝气量2mL/min,FeSO4·7H2O 0.2mmol/L | 0.5mg/L | 70.1% | [ |
| 垃圾渗滤液 | |||||||
| 垃圾渗滤液 | 碳布 | nZVI@MAC/不锈钢网 | pH=7.57,外部电阻1000Ω | COD 3520mg/L | 83.8% | [ | |
| 垃圾渗滤液 | 碳毡 | 磁黄铁矿负载石墨片 | pH=2.7,外部电阻500Ω,曝气量8mL/min | COD 1022mg/L | 78% | [ | |
| 垃圾渗滤液 | 碳毡 | 碳毡 | FeSO4·7H2O | pH=7.6,FeSO4·7H2O 60mg/L | COD (2152±624)mg/L | 49.3% | [ |
| 其他类型废水 | |||||||
| 煤气化废水 | 碳毡 | FeVO4/碳毡 | pH=7,外部电阻500Ω,曝气量100mL/min | COD 346mg/L | 83.7% | [ | |
| 苯胺废水 | 碳刷 | 石墨板 | FeSO4 | pH=3,外加电压0.5V,曝气量16mL/min,FeSO4 10mmol/L | (4460±52)mg/L | 97.1% | [ |
| 养猪废水 | 石墨棒+ 石墨颗粒 | Fe@Fe2O3/非催化碳毡 | pH=3,外部电阻100Ω,曝气量300mL/min | COD 1652mg/L | 62.2% | [ | |
| 中药废水 | 石墨板 | Fe@Fe2O3/石墨 | pH=3,外部电阻100Ω,曝气量300mL/min | COD (6183±309)mg/L | 84.02% | [ | |
| 含油废水 | 石墨毡 | γ-FeOOH/石墨毡 | pH=3,外部电阻500Ω,曝气量200mL/min,温度35℃ | 油浓度154mg/L | 95.3% | [ | |
表2 BEF系统对各类废水处理性能
| 废水类型 | 阳极 | 阴极 | 催化剂 | 运行条件 | 污染物浓度 | 去除率 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| 染料废水 | |||||||
| 橙黄Ⅱ | 碳毡 | PPy/AQDS/碳毡 | γ-FeOOH | pH=7,外部电阻1000Ω,曝气量100mL/min,γ-FeOOH 1g/L | 0.2mmol/L | 100% | [ |
| 橙黄Ⅱ | 碳毡 | 碳纳米管/ γ-FeOOH | pH=7,外部电阻1000Ω | 0.1mmol/L | 100% | [ | |
| 甲基橙 | 碳纸+ 颗粒活性炭 | 负载Pt碳纸+ 颗粒活性炭 | FeSO4·7H2O | pH=3,外部电阻1000Ω,曝气量150mL/min,FeSO4·7H2O 0.05mol/L | 300mg/L | 86.39% | [ |
| 甲基橙 | 石墨纤维刷 | Fe2O3/活性碳毡 | pH=3,外部电阻100Ω,曝气量750mL/min | 5mg/L | 86.7% | [ | |
| 罗丹明B | 活性碳毡 | Fe@Fe2O3/ 活性碳毡 | pH=3,外部电阻120Ω,曝气量300mL/min | 10mg/L | 95.0% | [ | |
| 橙G | 碳刷 | 石墨板 | Fe2+ | pH=2,外部电阻10Ω,曝气量8mL/min,Fe2+ 0.01mol/L | 400mg/L | 99.6% | [ |
| 酸性橙7 | 碳布 | 碳毡 | FeVO4 | pH=3,外部电阻1000Ω,FeVO4 12.5g/L | 50mg/L | 89% | [ |
酸性绿50 结晶紫 | 石墨棒 | 石墨棒 | 铁藻酸盐微珠 | pH=2,外部电阻1000Ω,曝气量2L/min,铁藻酸盐微珠10g(铁浓度150mg/L) | 10mg/L | 94% 83% | [ |
| 亚甲基蓝 | 碳刷 | 石墨板 | Fe2+ | pH=3,外部电阻5Ω,曝气量10mL/min,Fe2+ 2mmol/L | 50mg/L | 100% | [ |
| 亚甲基蓝 | 碳刷 | 石墨板 | Fe2+ | pH=2,外加电压0.4V,曝气量350mL/min,Fe2+ 0.2mmol/L | 20mg/L | 99% | [ |
| 橙G | 100% | ||||||
| 甲苯胺蓝 | 98% | ||||||
| 麦尔多拉蓝 | 99% | ||||||
| 罗丹明B | 100% | ||||||
| 罗丹明6G | 100% | ||||||
| 雷马素蓝RR | 葡萄糖氧化酶 固定化碳毡 | 碳毡 | FeSO4·7H2O | pH=3.5,外部电阻10Ω,FeSO4·7H2O 1.5g/L | 50mg/L | COD 34% | [ |
| 含PPCPs废水 | |||||||
| 对硝基苯酚 | 碳毡 | 碳毡 | 废铁屑 | pH=3,外部电阻1000Ω,废铁屑10g | 1mmol/L | 100% | [ |
| 对硝基苯酚 | 碳毡 | 碳毡 | 褐铁矿 | pH=2,外部电阻300Ω,曝气量100mL/min,褐铁矿2.24g/L | 0.25mmol/L | 96% | [ |
磺胺甲𫫇唑 诺氟沙星 | 碳毡 | γ-FeOOH石墨烯聚丙烯酰胺碳化气凝胶 | 自然pH,外部电阻1000Ω | 0.1mmol/L | 97.4% 96.1% | [ | |
| 土霉素 | 碳毡 | FeCo/氧化 石墨烯/碳毡 | pH=7.2,外部电阻1000Ω,曝气量50mL/min | 20mg/L | 80.34% | [ | |
| 四环素 | 碳毡 | 碳毡 | Fe2+ | pH=3,外部电阻250Ω,溶解氧≥8.4mg/L,Fe2+ 1mg/L | 20mg/L | 100% | [ |
17β-雌二醇 17α-乙炔基雌二醇 | 碳毡+ 颗粒石墨 | Fe@Fe2O3/非催化碳毡 | pH=3,外部电阻1000Ω,曝气量100mL/min | 20μg/L 20μg/L | 81% 56% | [ | |
| 双酚A | 石墨棒 | 石墨棒+ 石墨颗粒 | FeSO4·7H2O | pH=3,外部电阻10Ω, FeSO4·7H2O 1.25mmol/L | 1mg/L | 75% | [ |
| 雌酮 | 100% | ||||||
| 磺胺二甲基嘧啶 | 97% | ||||||
| 三氯卡班 | 98% | ||||||
| 扑热息痛 | 石墨毡 | 石墨板 | FeSO4·7H2O | pH=2,外部电阻20Ω, FeSO4·7H2O 5mg/L | 10mg/L | 70% | [ |
| 含PPCPs废水 | |||||||
| 酮洛芬 | 碳刷 | 石墨板 | FeSO4·7H2O | pH=2,外加电压0.5V,曝气量8mL/min,FeSO4·7H2O 5mmol/L | 40μg/L | 59% | [ |
| 双氯芬酸 | 87% | ||||||
| 布洛芬 | 80% | ||||||
| 萘普生 | 75% | ||||||
| 美托洛尔 | 碳刷 | 石墨板 | FeSO4·7H2O | pH=3,外加电压 0.2V,曝气量2mL/min,FeSO4·7H2O 0.2mmol/L | 0.5mg/L | 70.1% | [ |
| 垃圾渗滤液 | |||||||
| 垃圾渗滤液 | 碳布 | nZVI@MAC/不锈钢网 | pH=7.57,外部电阻1000Ω | COD 3520mg/L | 83.8% | [ | |
| 垃圾渗滤液 | 碳毡 | 磁黄铁矿负载石墨片 | pH=2.7,外部电阻500Ω,曝气量8mL/min | COD 1022mg/L | 78% | [ | |
| 垃圾渗滤液 | 碳毡 | 碳毡 | FeSO4·7H2O | pH=7.6,FeSO4·7H2O 60mg/L | COD (2152±624)mg/L | 49.3% | [ |
| 其他类型废水 | |||||||
| 煤气化废水 | 碳毡 | FeVO4/碳毡 | pH=7,外部电阻500Ω,曝气量100mL/min | COD 346mg/L | 83.7% | [ | |
| 苯胺废水 | 碳刷 | 石墨板 | FeSO4 | pH=3,外加电压0.5V,曝气量16mL/min,FeSO4 10mmol/L | (4460±52)mg/L | 97.1% | [ |
| 养猪废水 | 石墨棒+ 石墨颗粒 | Fe@Fe2O3/非催化碳毡 | pH=3,外部电阻100Ω,曝气量300mL/min | COD 1652mg/L | 62.2% | [ | |
| 中药废水 | 石墨板 | Fe@Fe2O3/石墨 | pH=3,外部电阻100Ω,曝气量300mL/min | COD (6183±309)mg/L | 84.02% | [ | |
| 含油废水 | 石墨毡 | γ-FeOOH/石墨毡 | pH=3,外部电阻500Ω,曝气量200mL/min,温度35℃ | 油浓度154mg/L | 95.3% | [ | |
| 54 | LI Xiaohu, JIN Xiangdan, ZHAO Nannan, et al. Efficient treatment of aniline containing wastewater in bipolar membrane microbial electrolysis cell-Fenton system[J]. Water Research, 2017, 119: 67-72. |
| 55 | ZHANG Lijuan, YIN Xuejiao, LI Sam Fong Yau. Bio-electrochemical degradation of paracetamol in a microbial fuel cell-Fenton system[J]. Chemical Engineering Journal, 2015, 276: 185-192. |
| 56 | ROZENDAL René A, LEONE Emilie, Jürg KELLER, et al. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system[J]. Electrochemistry Communications, 2009, 11(9): 1752-1755. |
| 57 | SOLTANI Fatemeh, NAVIDJOUY Nahid, KHORSANDI Hassan, et al. A novel bio-electro-Fenton system with dual application for the catalytic degradation of tetracycline antibiotic in wastewater and bioelectricity generation[J]. RSC Advances, 2021, 11(44): 27160-27173. |
| 58 | FENG Chunhua, LI Fangbai, Hongjian MAI, et al. Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment[J]. Environmental Science & Technology, 2010, 44(5): 1875-1880. |
| 59 | LI Xiaohu, JIN Xiangdan, ZHAO Nannan, et al. Novel bio-electro-Fenton technology for azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell[J]. Bioresource Technology, 2017, 228: 322-329. |
| 60 | LUO Yong, ZHANG Renduo, LIU Guangli, et al. Simultaneous degradation of refractory contaminants in both the anode and cathode chambers of the microbial fuel cell[J]. Bioresource Technology, 2011, 102(4): 3827-3832. |
| 61 | DE DIOS María Ángeles Fernández, DEL CAMPO Araceli González, FRANCISCO Jesús Fernández, et al. Bacterial-fungal interactions enhance power generation in microbial fuel cells and drive dye decolourisation by an ex situ and in situ electro-Fenton process[J]. Bioresource Technology, 2013, 148: 39-46. |
| 62 | ZHANG Yifeng, WANG Yong, ANGELIDAKI Irini. Alternate switching between microbial fuel cell and microbial electrolysis cell operation as a new method to control H2O2 level in Bioelectro-Fenton system[J]. Journal of Power Sources, 2015, 291: 108-116. |
| 63 | ZOU Rusen, ANGELIDAKI Irini, JIN Biao, et al. Feasibility and applicability of the scaling-up of bio-electro-Fenton system for textile wastewater treatment[J]. Environment International, 2020, 134: 105352. |
| 64 | KAHOUSH May, BEHARY Nemeshwaree, GUAN Jinping, et al. Genipin-mediated immobilization of glucose oxidase enzyme on carbon felt for use as heterogeneous catalyst in sustainable wastewater treatment[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105633. |
| 65 | WANG Yuezhu, ZHANG Hanmin, FENG Yujie, et al. Bio-electron-Fenton (BEF) process driven by sediment microbial fuel cells (SMFCs) for antibiotics desorption and degradation[J]. Biosensors and Bioelectronics, 2019, 136: 8-15. |
| 66 | ZHENG Linshan, LIN Xiaoqiu, LIU Yuanfeng, et al. Synergistically enhanced oxygen reduction reaction and oxytetracycline mineralization by FeCoO/GO modified cathode in microbial fuel cell[J]. Science of the Total Environment, 2022, 808: 151873. |
| 67 | LONG Sha, ZHAO Lin, CHEN Jinchen, et al. Tetracycline inhibition and transformation in microbial fuel cell systems: Performance, transformation intermediates, and microbial community structure[J]. Bioresource Technology, 2021, 322: 124534. |
| 68 | XU Nan, ZENG Yaqiong, LI Jie, et al. Removal of 17 β-estrodial in a bio-electro-Fenton system: Contribution of oxidation and generation of hydroxyl radicals with the Fenton reaction and carbon felt cathode[J]. RSC Advances, 2015, 5(70): 56832-56840. |
| 69 | WANG Yinhan, FENG Cuijie, LI Yan, et al. Enhancement of emerging contaminants removal using Fenton reaction driven by H2O2-producing microbial fuel cells[J]. Chemical Engineering Journal, 2017, 307: 679-686. |
| 70 | NADAIS Helena, LI Xiaohu, ALVES Nadine, et al. Bio-electro-Fenton process for the degradation of non-Steroidal anti-Inflammatory Drugs in wastewater[J]. Chemical Engineering Journal, 2018, 338: 401-410. |
| 71 | YANG Xiaoyong, ZOU Rusen, TANG Kai, et al. Degradation of metoprolol from wastewater in a bio-electro-Fenton system[J]. Science of the Total Environment, 2021, 771: 145385. |
| 72 | LI Yan, LU Anhuai, DING Hongrui, et al. Microbial fuel cells using natural pyrrhotite as the cathodic heterogeneous Fenton catalyst towards the degradation of biorefractory organics in landfill leachate[J]. Electrochemistry Communications, 2010, 12(7): 944-947. |
| 73 | HASSAN Muhammad, POUS Narcis, XIE Bing, et al. Influence of iron species on integrated microbial fuel cell and electro-Fenton process treating landfill leachate[J]. Chemical Engineering Journal, 2017, 328: 57-65. |
| 74 | XU Nan, ZHOU Shungui, YUAN Yong, et al. Coupling of anodic biooxidation and cathodic bioelectro-Fenton for enhanced swine wastewater treatment[J]. Bioresource Technology, 2011, 102(17): 7777-7783. |
| 75 | BIRJANDI Noushin, YOUNESI Habibollah, GHOREYSHI Ali Asghar, et al. Electricity generation through degradation of organic matters in medicinal herbs wastewater using bio-electro-Fenton system[J]. Journal of Environmental Management, 2016, 180: 390-400. |
| 76 | 韩英杰, 杜英才, 张吉强, 等. 生物电Fenton系统对餐饮含油废水处理研究[J]. 工业水处理, 2017, 37(10): 78-81. |
| HAN Yingjie, DU Yingcai, ZHANG Jiqiang, et al. Research on the treatment of catering oil-bearing wastewater by the bioelectro-Fenton system[J]. Industrial Water Treatment, 2017, 37(10): 78-81. | |
| 1 | LI Shengnan, HUA Tao, LI Fengxiang, et al. Bio-electro-Fenton systems for sustainable wastewater treatment: Mechanisms, novel configurations, recent advances, LCA and challenges. An updated review[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(8): 2083-2097. |
| 2 | LI Xiaohu, CHEN Si, ANGELIDAKI Irini, et al. Bio-electro-Fenton processes for wastewater treatment: Advances and prospects[J]. Chemical Engineering Journal, 2018, 354: 492-506. |
| 3 | POTTER M C. Electrical effects accompanying the decomposition of organic compounds[J]. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character, 1911, 84(571): 260-276. |
| 4 | Schröder UWE. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency[J]. Physical Chemistry Chemical Physics, 2007, 9(21): 2619-2629. |
| 5 | ZHU Xiuping, NI Jinren. Simultaneous processes of electricity generation and p-nitrophenol degradation in a microbial fuel cell[J]. Electrochemistry Communications, 2009, 11(2): 274-277. |
| 6 | WANG Weiye, ZHAO Qingliang, DING Jing, et al. Development of an MFC-powered BEF system with novel Fe-Mn-Mg/CF composite cathode to degrade refractory pollutants[J]. Journal of Cleaner Production, 2021, 326: 129348. |
| 7 | SATHE S M, CHAKRABORTY Indrajit, DUBEY B K, et al. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges[J]. Environmental Research, 2022, 204: 112135. |
| 8 | 马晨, 周顺桂, 庄莉, 等. 微生物胞外呼吸电子传递机制研究进展[J]. 生态学报, 2011, 31(7): 2008-2018. |
| MA Chen, ZHOU Shungui, ZHUANG Li, et al. Electron transfer mechanism of extracellular respiration: A review[J]. Acta Ecologica Sinica, 2011, 31(7): 2008-2018. | |
| 9 | HASSAN Muhammad, Hugo OLVERA-VARGAS, ZHU Xiuping, et al. Microbial electro-Fenton: An emerging and energy-efficient platform for environmental remediation[J]. Journal of Power Sources, 2019, 424: 220-244. |
| 10 | ZHAO Cuie, GAI Panpan, SONG Rongbin, et al. Nanostructured material-based biofuel cells: Recent advances and future prospects[J]. Chemical Society Reviews, 2017, 46(5): 1545-1564. |
| 11 | JIANG Yuanyuan, NI Pengjuan, CHEN Chuanxia, et al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Advanced Energy Materials, 2018, 8(31): 1801909. |
| 12 | BOSE A, GARDEL E J, VIDOUDEZ C, et al. Electron uptake by iron-oxidizing phototrophic bacteria[J]. Nature Communications, 2014, 5: 3391. |
| 13 | HE Ziming, LIU Jing, QIAO Yan, et al. Architecture engineering of hierarchically porous chitosan/vacuum-stripped graphene scaffold as bioanode for high performance microbial fuel cell[J]. Nano Letters, 2012, 12(9): 4738-4741. |
| 14 | Yarzábal ANDRÉS, Brasseur GAËL, JEANINE Ratouchniak, et al. The high-molecular-weight cytochrome c Cyc2 of Acidithiobacillus ferrooxidans is an outer membrane protein[J]. Journal of Bacteriology, 2002, 184(1): 313-317. |
| 15 | FENG Jiao, QIAN Ying, WANG Zhen, et al. Enhancing the performance of Escherichia coli-inoculated microbial fuel cells by introduction of the phenazine-1-carboxylic acid pathway[J]. Journal of Biotechnology, 2018, 275: 1-6. |
| 16 | GENG Boyu, CAO Lianying, LI Feng, et al. Potential of Zymomonas mobilis as an electricity producer in ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 36. |
| 17 | ZHAO Cuie, WANG Ying, SHI Fengjian, et al. High biocurrent generation in Shewanella-inoculated microbial fuel cells using ionic liquid functionalized graphenenanosheets as an anode[J]. Chemical Communications, 2013, 49(59): 6668-6670. |
| 18 | POORNA Subramanian, SAHAND Pirbadian, EL-NAGGAR Mohamed Y, et al. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(14): E3246-E3255. |
| 19 | YANG Yun, DING Yuanzhao, HU Yidan, et al. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway[J]. ACS Synthetic Biology, 2015, 4(7): 815-823. |
| 20 | GEMMA Reguera, NEVIN Kelly P, NICOLL Julie S, et al. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells[J]. Applied and Environmental Microbiology, 2006, 72(11): 7345-7348. |
| 21 | ZACHAROFF Lori, CHAN Chi Ho, BOND Daniel R. Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens [J]. Bioelectrochemistry, 2016, 107: 7-13. |
| 22 | 代凤, 刘建, 孙霞, 等. 不同阳极电势驯化对于丁酸梭菌葡萄糖代谢影响研究[J]. 四川大学学报(自然科学版), 2017, 54(2): 399-404. |
| DAI Feng, LIU Jian, SUN Xia, et al. The glucose metabolism variation of clostridium butyricum during domestication with different anode potentials[J]. Journal of Sichuan University (Natural Science Edition), 2017, 54(2): 399-404. | |
| 77 | RAMOS M D N, SANTANA C S, VELLOSO C C V, et al. A review on the treatment of textile industry effluents through Fenton processes[J]. Process Safety and Environmental Protection, 2021, 155: 366-386. |
| 78 | WU Chuanwei, CHEN Weiming, GU Zhepei, et al. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment[J]. Science of the Total Environment, 2021, 762: 143131. |
| 23 | PRASAD D, ARUN S, MURUGESAN M, et al. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell[J]. Biosensors and Bioelectronics, 2007, 22(11): 2604-2610. |
| 24 | WALKER Alyssa L, WALKER Charles W. Biological fuel cell and an application as a reserve power source[J]. Journal of Power Sources, 2006, 160(1): 123-129. |
| 25 | MARDIANA Ummy, INNOCENT Christophe, JARRAR Haytem, et al. Electropolymerized neutral red as redox mediator for yeast fuel cell[J]. International Journal of Electrochemical Science, 2015, 10(11): 8886-8898. |
| 26 | NAROA Uria, ISABEL Ferrera, JORDI Mas. Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms[J]. BMC Microbiology, 2017, 17(1): 1-12. |
| 27 | LI Ming, ZHOU Minghua, TIAN Xiaoyu, et al. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity[J]. Biotechnology Advances, 2018, 36(4): 1316-1327. |
| 28 | LIU Jing, QIAO Yan, LU Zhisong, et al. Enhance electron transfer and performance of microbial fuel cells by perforating the cell membrane[J]. Electrochemistry Communications, 2012, 15(1): 50-53. |
| 29 | 刘向, 张君奇, 张保财, 等. 强化产电微生物与电极间电子传递速率的研究进展[J]. 生物工程学报, 2021, 37(2): 361-377. |
| LIU Xiang, ZHANG Junqi, ZHANG Baocai, et al. Progress in enhancing electron transfer rate between exoelectrogenic microorganisms and electrode interface[J]. Chinese Journal of Biotechnology, 2021, 37(2): 361-377. | |
| 30 | LEANG Ching, MALVANKAR Nikhil S, FRANKS Ashley E, et al. Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production[J]. Energy & Environmental Science, 2013, 6(6): 1901-1908. |
| 31 | LI Feng, LI Yuanxiu, CAO Yingxiu, et al. Modular engineering to increase intracellular NAD(H/+) promotes rate of extracellular electron transfer of Shewanella oneidensis [J]. Nature Communications, 2018, 9: 3637. |
| 32 | LIU Ting, YU Yangyang, DENG Xiaopeng, et al. Enhanced Shewanella biofilm promotes bioelectricity generation[J]. Biotechnology and Bioengineering, 2015, 112(10): 2051-2059. |
| 33 | SONAWANE Jayesh M, ABHISHEK Yadav, GHOSH Prakash C, et al. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells[J]. Biosensors and Bioelectronics, 2017, 90: 558-576. |
| 34 | KARTHIKEYAN Rengasamy, WANG Bin, XUAN Jin, et al. Interfacial electron transfer and bioelectrocatalysis of carbonized plant material as effective anode of microbial fuel cell[J]. Electrochimica Acta, 2015, 157: 314-323. |
| 35 | REN Hao, Soonjae PYO, LEE Jae-Ik, et al. A high power density miniaturized microbial fuel cell having carbon nanotube anodes[J]. Journal of Power Sources, 2015, 273: 823-830. |
| 36 | HINDATU Y, ANNUAR M S M, GUMEL A M. Mini-review: Anode modification for improved performance of microbial fuel cell[J]. Renewable and Sustainable Energy Reviews, 2017, 73: 236-248. |
| 37 | TANG Xinhua, GUO Kun, LI Haoran, et al. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes[J]. Bioresource Technology, 2011, 102(3): 3558-3560. |
| 38 | 王维大, 李浩然, 冯雅丽, 等. 微生物燃料电池的研究应用进展[J]. 化工进展, 2014, 33(5): 1067-1076. |
| WANG Weida, LI Haoran, FENG Yali, et al. Research and application advances in microbial fuel cell[J]. Chemical Industry and Engineering Progress, 2014, 33(5): 1067-1076. | |
| 39 | 赵娟, 牛艳艳, 袁磊, 等. 微生物燃料电池阳极修饰研究进展[J]. 煤炭与化工, 2018, 41(10): 133-135, 141. |
| ZHAO Juan, NIU Yanyan, YUAN Lei, et al. Study progress of anode modification in microbial fuel cell[J]. Coal and Chemical Industry, 2018, 41(10): 133-135, 141. | |
| 40 | HUANG Tao, LIU Longfei, TAO Junjun, et al. Microbial fuel cells coupling with the three-dimensional electro-Fenton technique enhances the degradation of methyl orange in the wastewater[J]. Environmental Science and Pollution Research, 2018, 25(18): 17989-18000. |
| 41 | FENG Chunhua, LI Fangbai, LIU Haiyang, et al. A dual-chamber microbial fuel cell with conductive film-modified anode and cathode and its application for the neutral electro-Fenton process[J]. Electrochimica Acta, 2010, 55(6): 2048-2054. |
| 42 | CHEN Ruyan, ZHANG Jing, ZHANG Keyi, et al. In-situ degradation of organic pollutants by bioelectrical-Fenton reaction with a metal-free polyaniline-derived nitrogen-doped carbon nanofibre electrode[J]. Journal of Alloys and Compounds, 2022, 901: 163710. |
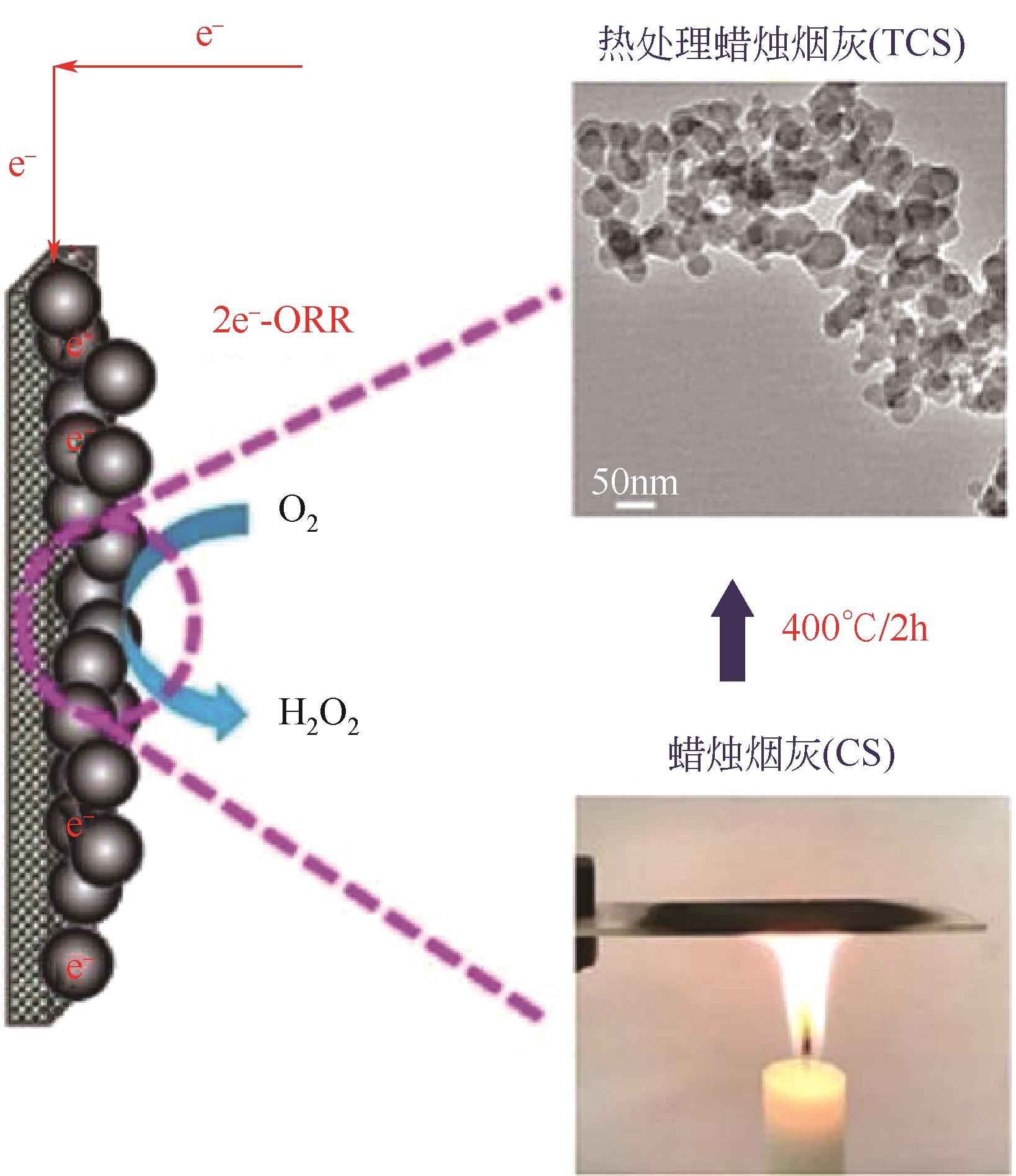
| 43 | REN Yueping, YAN Yating, WANG Yue, et al. Thermally treated candle soot as a novel catalyst for hydrogen peroxide in situ production enhancement in the bio-electro-Fenton system[J]. Chemosphere, 2021, 262: 127839. |
| 44 | CHEN Jiayi, LI Nan, ZHAO Lin. Three-dimensional electrode microbial fuel cell for hydrogen peroxide synthesis coupled to wastewater treatment[J]. Journal of Power Sources, 2014, 254: 316-322. |
| 45 | TAO Huchun, WEI Xueyan, ZHANG Lijuan, et al. Degradation of p-nitrophenol in a BES-Fenton system based on limonite[J]. Journal of Hazardous Materials, 2013, 254/255: 236-241. |
| 46 | ZHUANG Li, ZHOU Shungui, YUAN Yong, et al. A novel bioelectro-Fenton system for coupling anodic COD removal with cathodic dye degradation[J]. Chemical Engineering Journal, 2010, 163(1/2): 160-163. |
| 47 | WANG Xiangqin, LIU Chuanping, YUAN Yong, et al. Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral pH conditions[J]. Journal of Hazardous Materials, 2014, 275: 200-209. |
| 48 | LI Shengnan, HUA Tao, YUAN Chung-Shin, et al. Degradation pathways, microbial community and electricity properties analysis of antibiotic sulfamethoxazole by bio-electro-Fenton system[J]. Bioresource Technology, 2020, 298: 122501. |
| 49 | LING Ting, HUANG Bin, ZHAO Mingxing, et al. Repeated oxidative degradation of methyl orange through bio-electro-Fenton in bioelectrochemical system (BES)[J]. Bioresource Technology, 2016, 203: 89-95. |
| 50 | WANG Dongliang, HOU Huijie, HU Jingping, et al. A bio-electro-Fenton system with a facile anti-biofouling air cathode for efficient degradation of landfill leachate[J]. Chemosphere, 2019, 215: 173-181. |
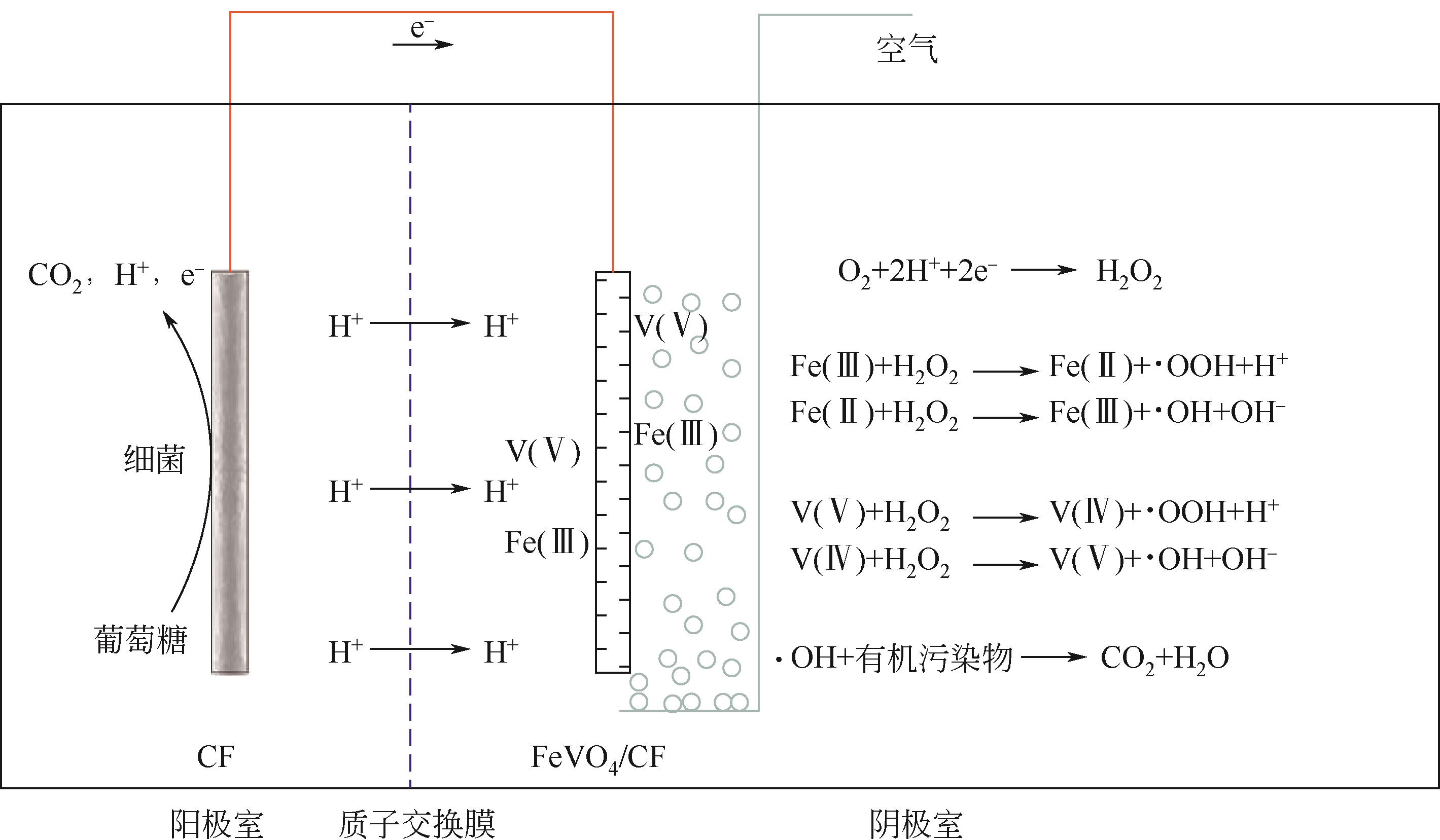
| 51 | XU Peng, XU Hao, SHI Zhou. A novel bio-electro-Fenton process with FeVO4/CF cathode on advanced treatment of coal gasification wastewater[J]. Separation and Purification Technology, 2018, 194: 457-461. |
| 52 | LI Biao, YAN Zhiying, LIU Xiaona, et al. Enhanced Bio-electro-Fenton degradation of phenolic compounds based on a novel Fe-Mn/Graphite felt composite cathode[J]. Chemosphere, 2019, 234: 260-268. |
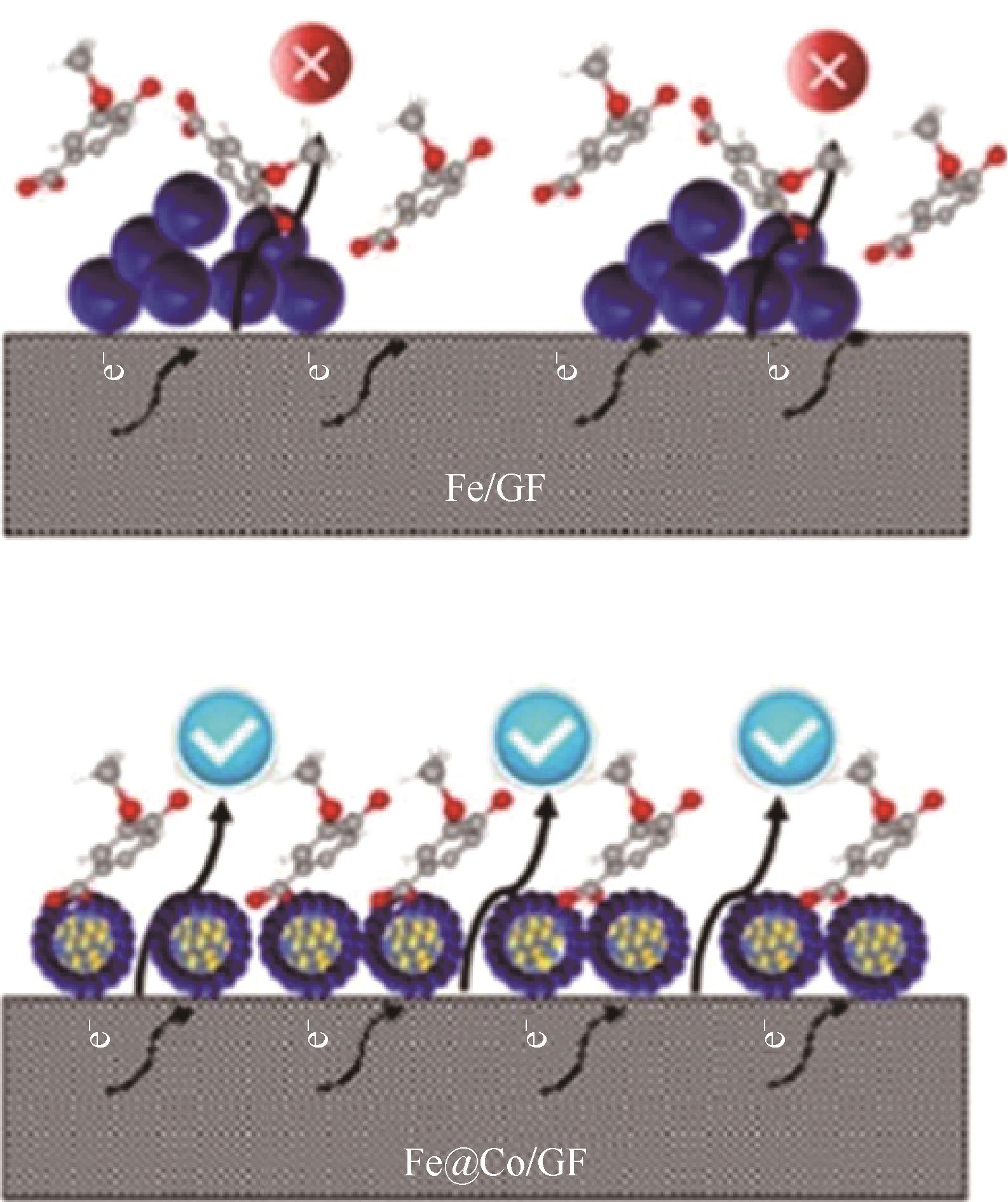
| 53 | LI Biao, SUN Jiadong, TANG Chen, et al. A novel core-shell Fe@Co nanoparticles uniformly modified graphite felt cathode (Fe@Co/GF) for efficient bio-electro-Fenton degradation of phenolic compounds[J]. Science of the Total Environment, 2021, 760: 143415. |
| [1] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [2] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [3] | 杨子育, 朱玲, 王文龙, 于超凡, 桑义敏. 阴燃法处理含油污泥的研究及应用进展[J]. 化工进展, 2023, 42(7): 3760-3769. |
| [4] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [5] | 朱紫旋, 陈俊江, 张星星, 李祥, 刘文如, 吴鹏. 基于短程反硝化厌氧氨氧化新型污水生物脱氮工艺的研究进展[J]. 化工进展, 2023, 42(4): 2091-2100. |
| [6] | 苏景振, 詹健. 生物炭对水环境中微塑料的去除研究进展[J]. 化工进展, 2023, 42(10): 5445-5458. |
| [7] | 应璐瑶, 王荣昌. 菌藻共生系统削减抗生素类污染物的去除途径及胁迫响应[J]. 化工进展, 2023, 42(1): 469-479. |
| [8] | 张丽珠, 王欢, 李琼, 杨东杰. 木质素衍生吸附材料及其在废水处理中的应用研究进展[J]. 化工进展, 2022, 41(7): 3731-3744. |
| [9] | 郭之晗, 徐云翔, 李天皓, 黄子川, 刘文如, 沈耀良. 好氧颗粒污泥长期稳定运行研究进展[J]. 化工进展, 2022, 41(5): 2686-2697. |
| [10] | 李海涛, 汪东. 精对苯二甲酸生产废水处理与CO2协同利用技术的实践与展望[J]. 化工进展, 2022, 41(3): 1132-1135. |
| [11] | 陈诗雨, 许志成, 杨婧, 徐浩, 延卫. 微生物燃料电池在废水处理中的研究进展[J]. 化工进展, 2022, 41(2): 951-963. |
| [12] | 张莉红, 李杰, 王亚娥, 谢慧娜, 赵炜, 李婧. Feammox: 一种新型自养生物脱氮技术[J]. 化工进展, 2022, 41(1): 391-399. |
| [13] | 潘柔杏, 于庆君, 唐晓龙, 易红宏, 高凤雨, 赵顺征, 周远松, 刘媛媛. 被动NOx吸附剂在柴油车冷启动排放控制中的研究进展[J]. 化工进展, 2022, 41(1): 400-417. |
| [14] | 代校军, 成艳, 王晓晗, 黄文斌, 魏强, 周亚松. 小粒径SAPO-11分子筛合成的研究进展[J]. 化工进展, 2021, 40(S1): 191-203. |
| [15] | 李东梅, 吴丹萍, 吴敏, 潘波. 污水处理厂运行工况对温室气体排放的影响[J]. 化工进展, 2021, 40(12): 6897-6906. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||