化工进展 ›› 2022, Vol. 41 ›› Issue (11): 5858-5869.DOI: 10.16085/j.issn.1000-6613.2022-0197
微波协同氧化锆@碳纳米管强化果糖制5-羟甲基糠醛
慕诗芸1( ), 刘凯1, 吕孝琦1, 矫义来3(
), 刘凯1, 吕孝琦1, 矫义来3( ), 李鑫钢1, 李洪1, 范晓雷4, 高鑫1,2(
), 李鑫钢1, 李洪1, 范晓雷4, 高鑫1,2( )
)
- 1.天津大学化工学院,精馏技术国家工程研究中心,天津 300350
2.物质绿色创造与制造海河实验室,天津 300192
3.中国科学院金属研究所,辽宁 沈阳 110016
4.曼彻斯特大学工程学院化学工程系,英国 曼彻斯特 M13 9PL
-
收稿日期:2022-02-06修回日期:2022-04-23出版日期:2022-11-25发布日期:2022-11-28 -
通讯作者:矫义来,高鑫 -
作者简介:慕诗芸(1997—),女,硕士研究生,研究方向为微波强化反应过程。E-mail:mushiyun1@163.com。 -
基金资助:欧盟“地平线2020”研究与创新基金(872102);物质绿色创造与制造海河实验室项目
Conversion of fructose to 5-hydroxymethylfurfural catalyzed by microwave-assisted zirconia@carbon nanotubes
MU Shiyun1( ), LIU Kai1, LYU Xiaoqi1, JIAO Yilai3(
), LIU Kai1, LYU Xiaoqi1, JIAO Yilai3( ), LI Xingang1, LI Hong1, FAN Xiaolei4, GAO Xin1,2(
), LI Xingang1, LI Hong1, FAN Xiaolei4, GAO Xin1,2( )
)
- 1.National Engineering Research Center of Distillation Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
2.Haihe Laboratory of Sustainable Chemical Transformations, Tianjin 300192, China
3.Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, Liaoning, China
4.Department of Chemical Engineering, School of Engineering, The University of Manchester, Manchester M13 9PL, United Kingdom
-
Received:2022-02-06Revised:2022-04-23Online:2022-11-25Published:2022-11-28 -
Contact:JIAO Yilai, GAO Xin
摘要:
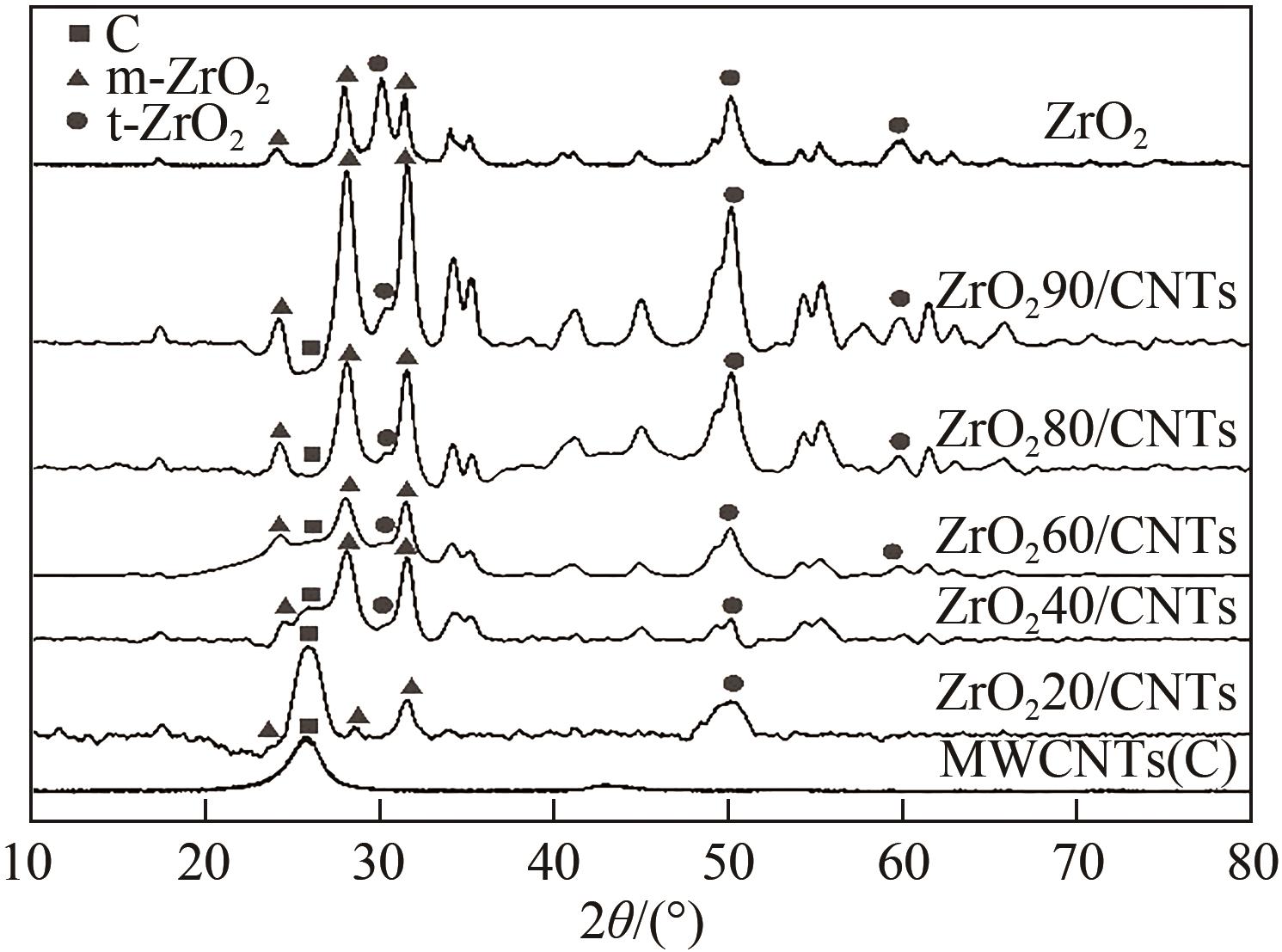
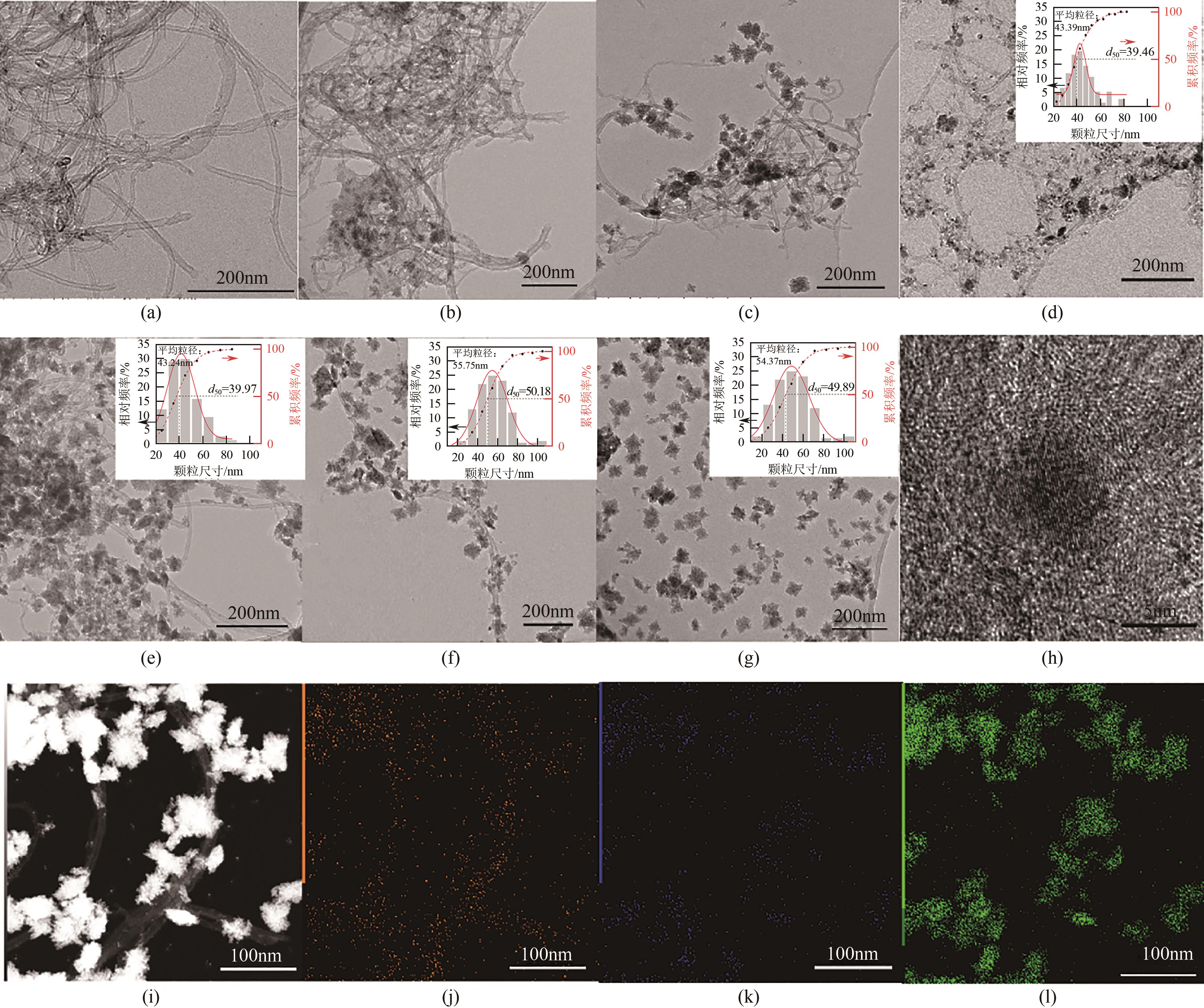
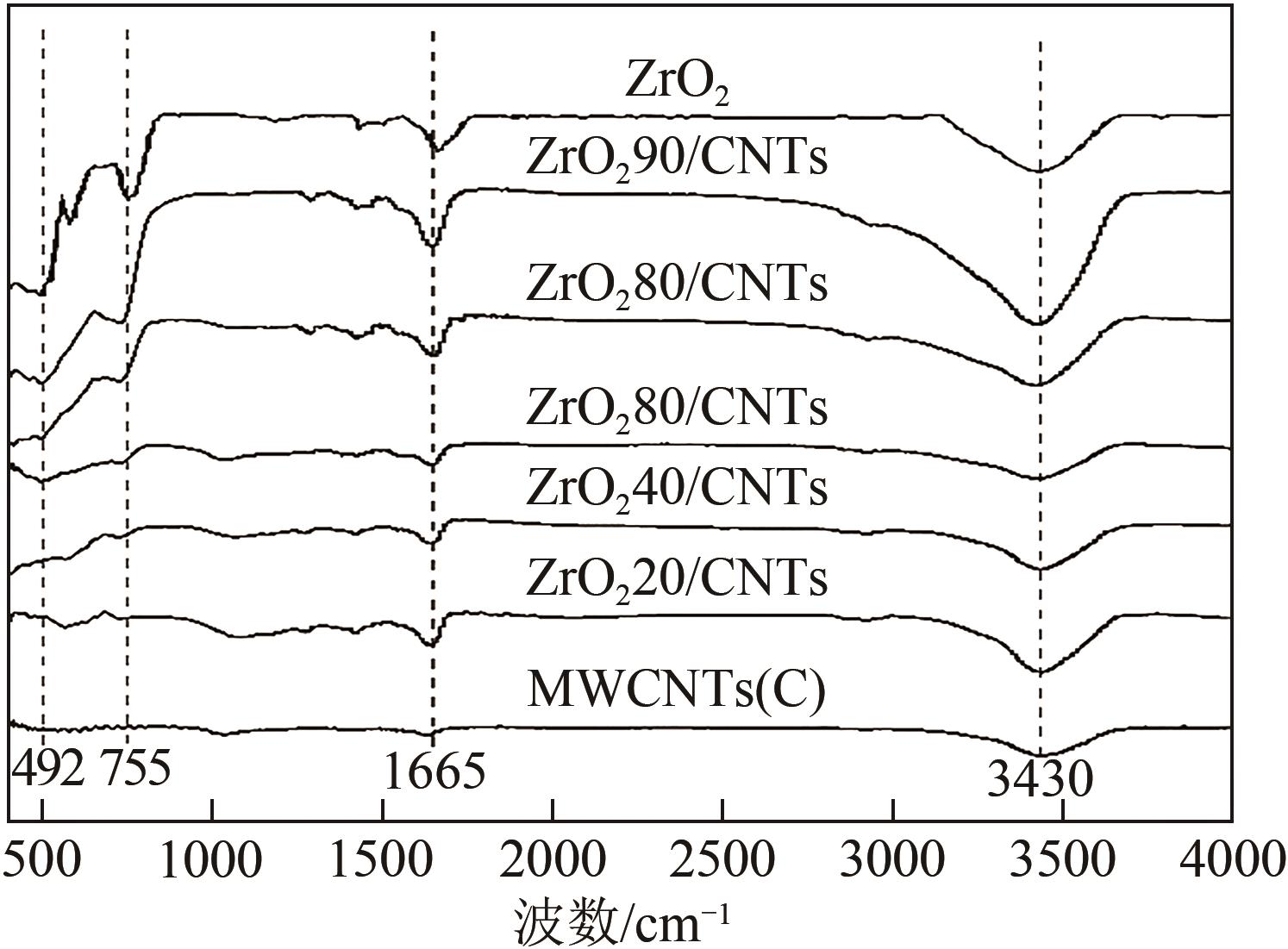
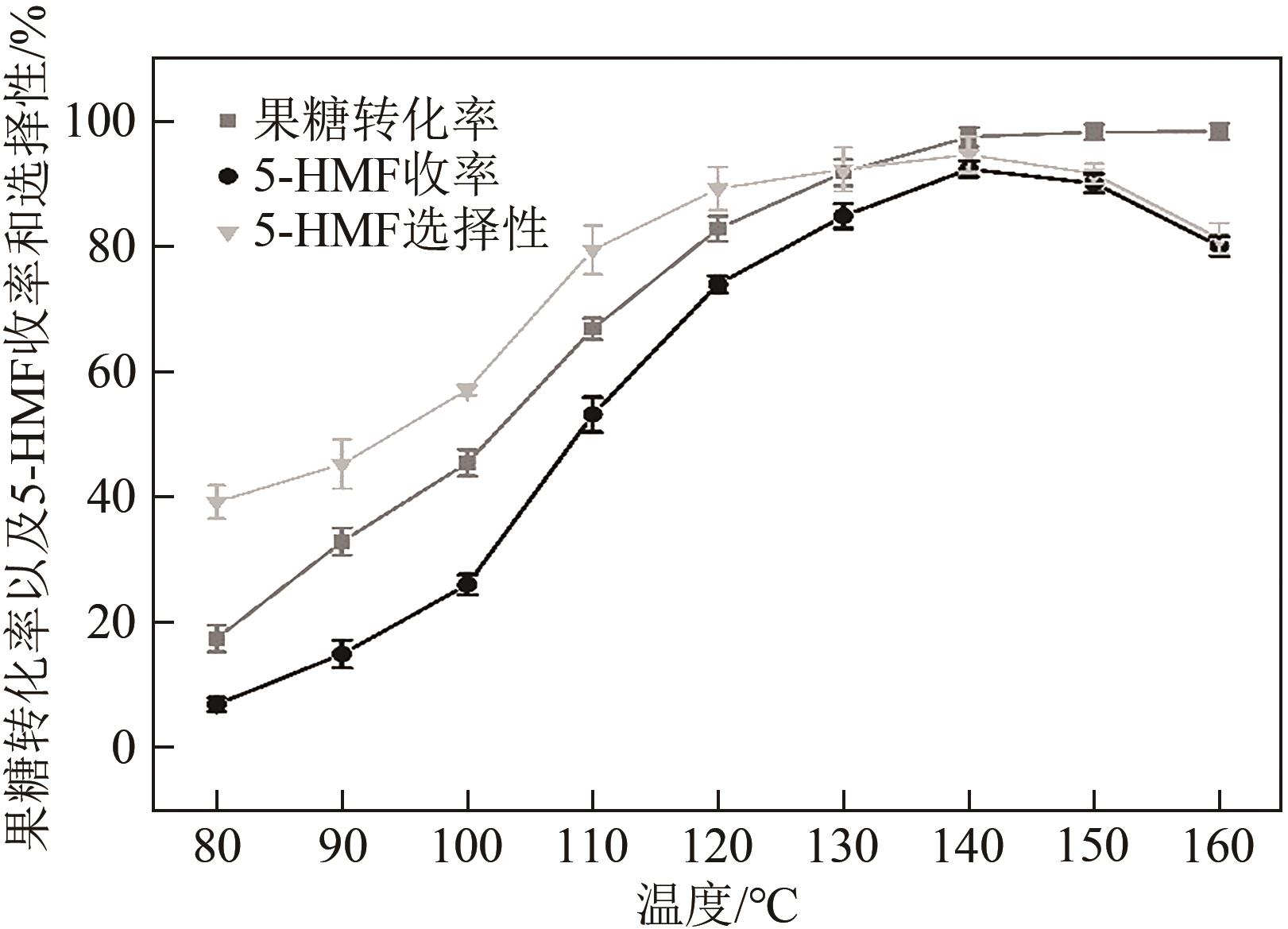
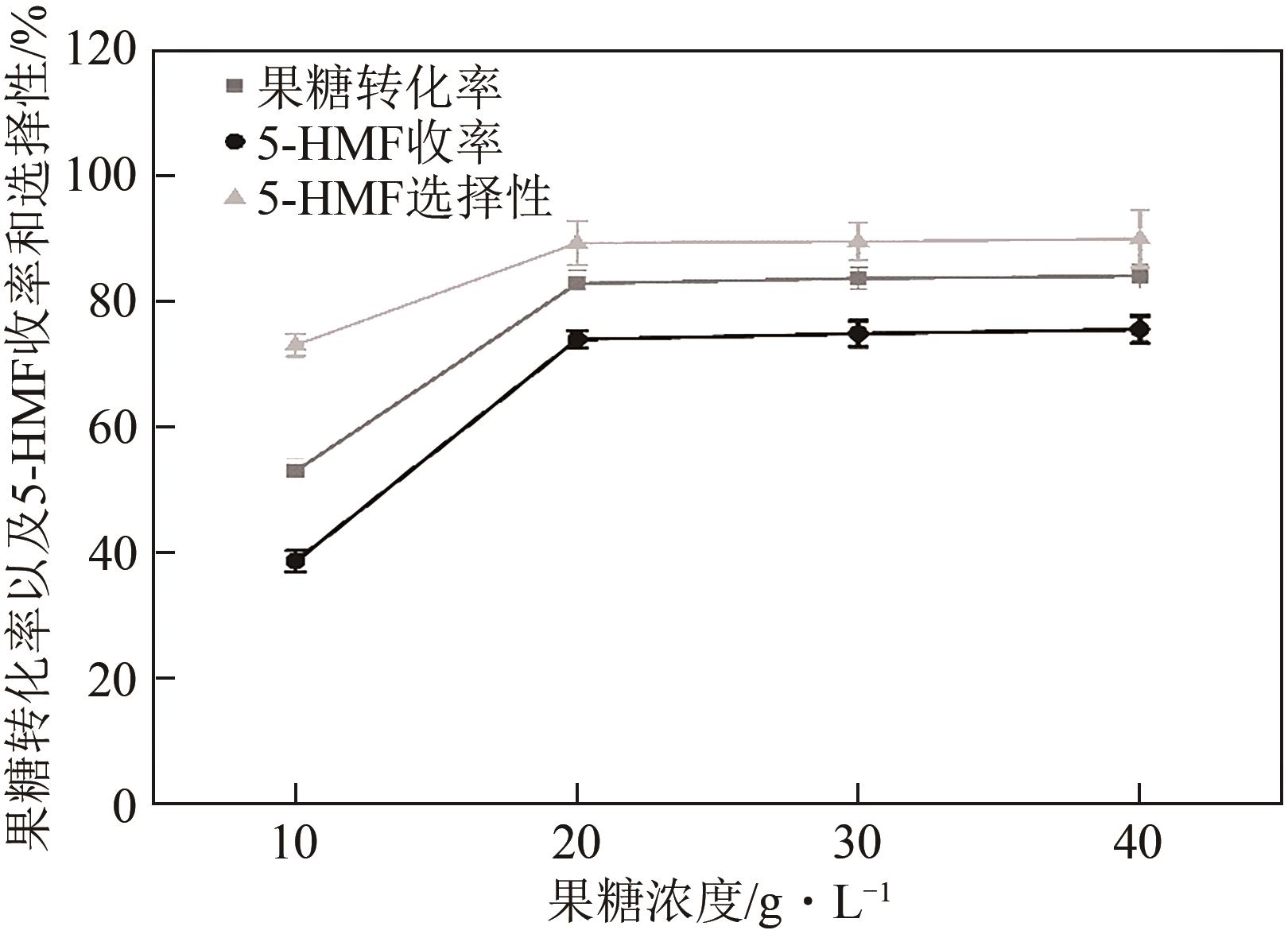
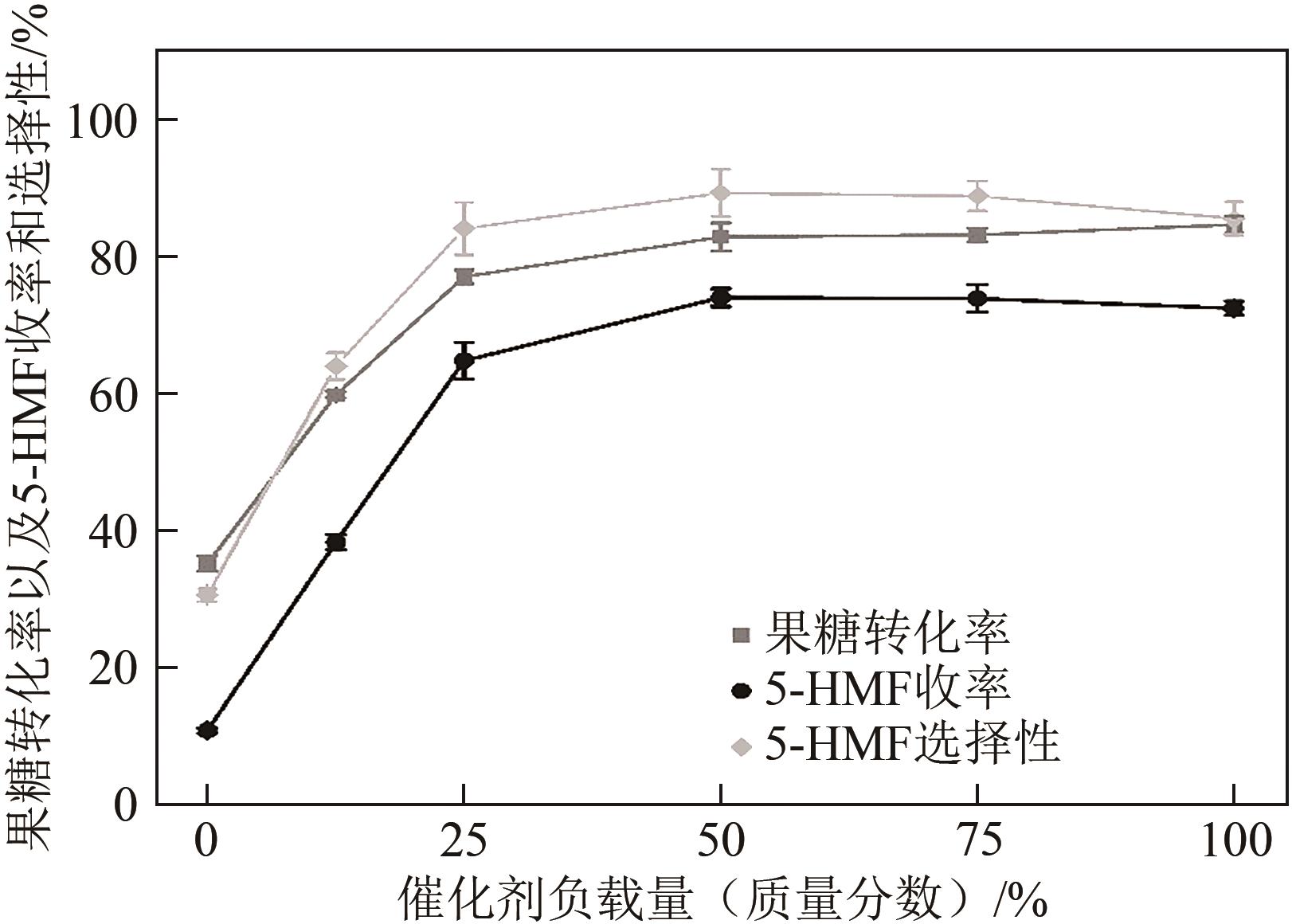
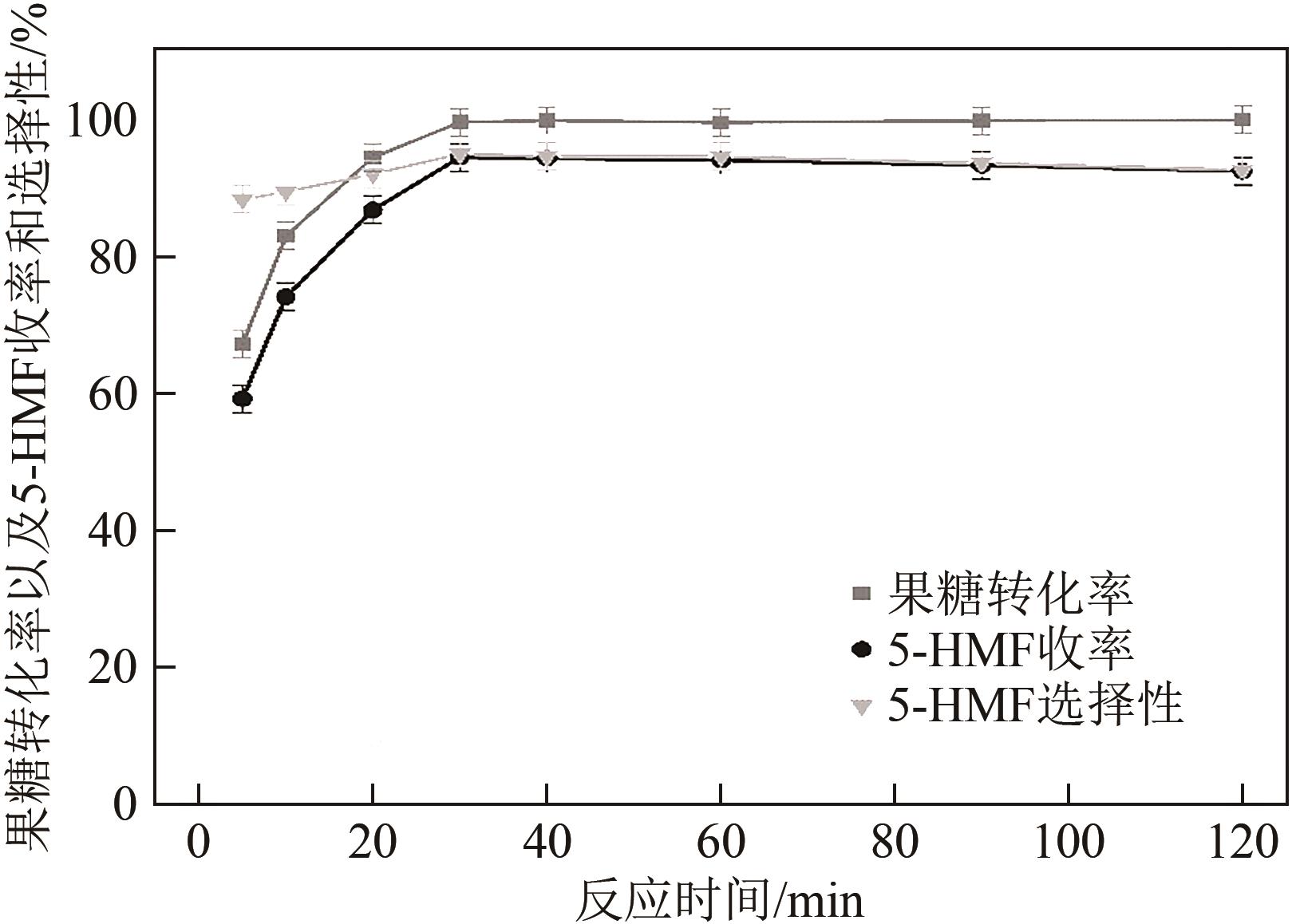
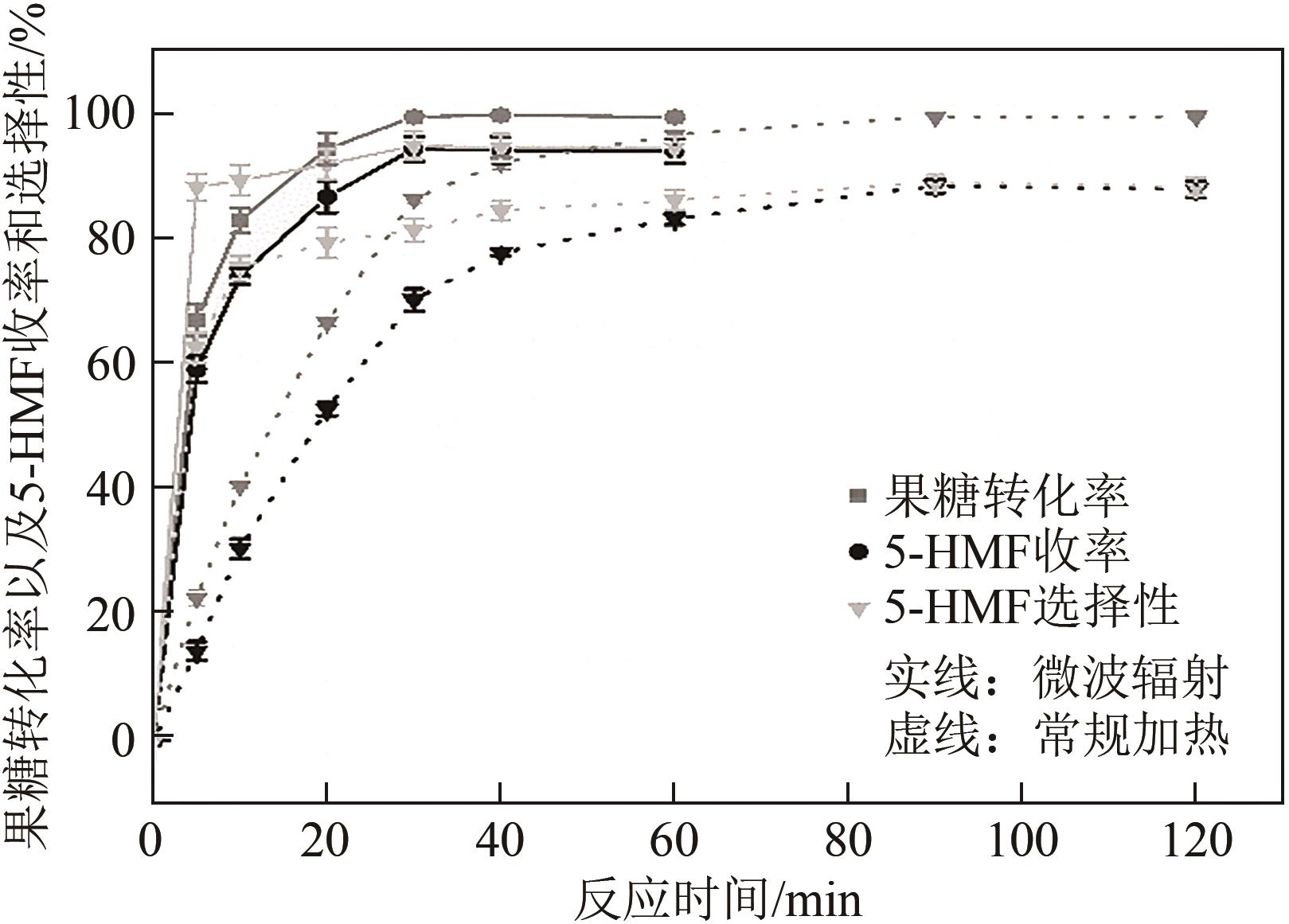
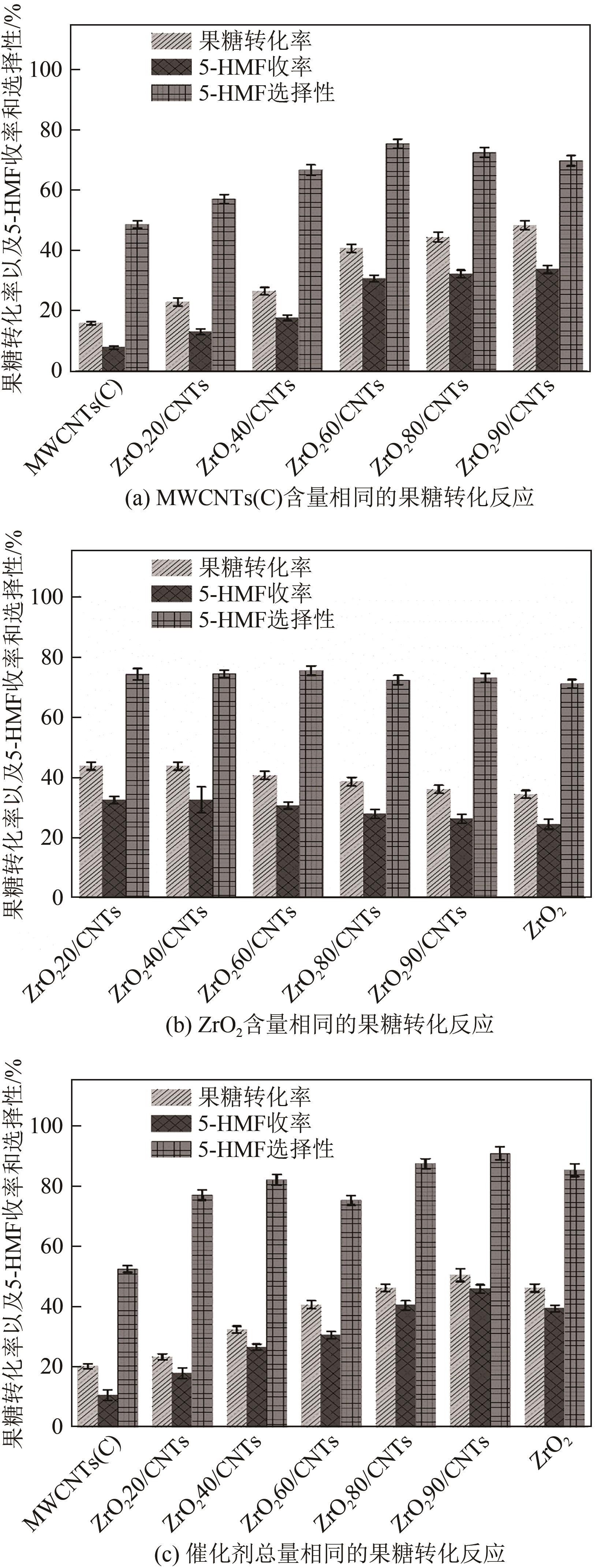
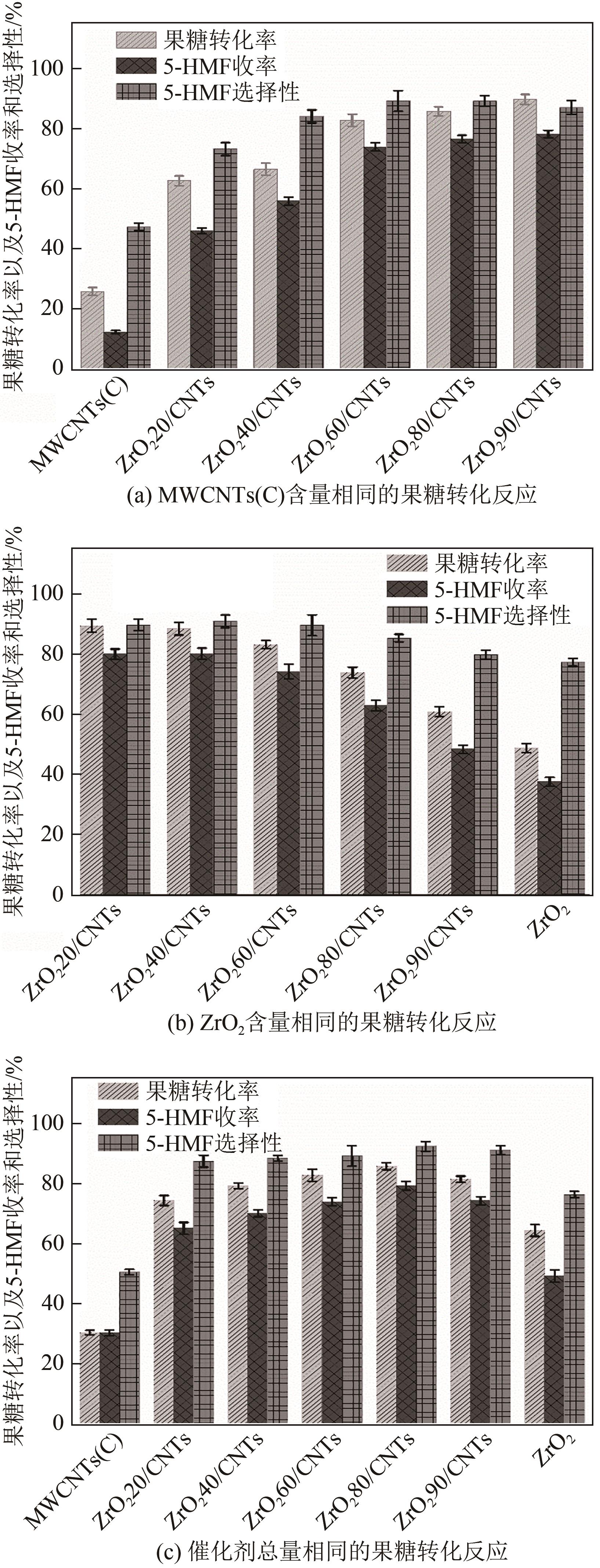
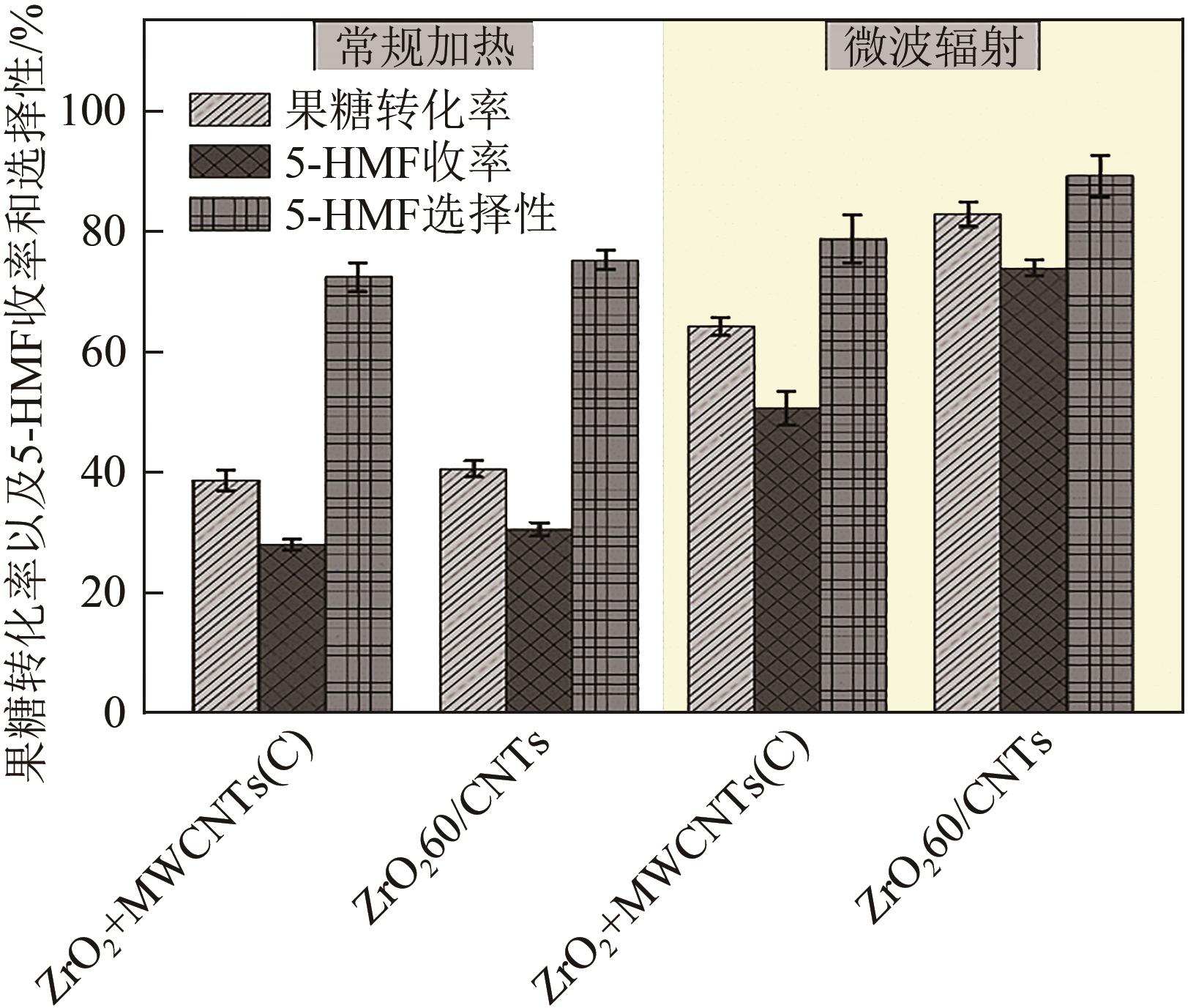
糖类催化转化是生产生物质基燃料和高附加值化学品的重要途径,而微波能量的使用可使这一过程更具商业可行性。本文探究了微波辐射下微波响应型催化剂碳纳米管负载氧化锆[ZrO2/MWCNTs(C)]催化的果糖高效分解制5-羟甲基糠醛(5-HMF)过程。首先,采用水热法制备了性能优异的氧化锆@碳纳米管催化剂,并对其进行表征;进一步考察了催化剂用量、果糖浓度、反应温度和反应时间对反应产物5-HMF收率的影响,并通过调节各组分在反应过程中的实际含量,探究微波强化的作用机理。研究结果表明在相对温和的条件下(120℃、常压),微波辐射下的5-HMF收率(约74%)远高于常规加热条件下的5-HMF收率(约31%);采用最佳ZrO260/CNTs用量(ZrO2质量分数约为60%),微波场中,140℃常压条件下反应10min,可以实现约98%的果糖转化率和92%的5-HMF收率。通过探究载体吸波性能与活性位点催化性能之间的耦合匹配关系,揭示了微波协同催化过程强化机理归因于具有强吸波性能碳质载体的选择性加热和活性位点ZrO2之间的协同耦合作用。
中图分类号:
引用本文
慕诗芸, 刘凯, 吕孝琦, 矫义来, 李鑫钢, 李洪, 范晓雷, 高鑫. 微波协同氧化锆@碳纳米管强化果糖制5-羟甲基糠醛[J]. 化工进展, 2022, 41(11): 5858-5869.
MU Shiyun, LIU Kai, LYU Xiaoqi, JIAO Yilai, LI Xingang, LI Hong, FAN Xiaolei, GAO Xin. Conversion of fructose to 5-hydroxymethylfurfural catalyzed by microwave-assisted zirconia@carbon nanotubes[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5858-5869.
| 样品 | ε′ | ε″ | tanδ |
|---|---|---|---|
| MWCNTs(C) | 847.09 | 927.74 | 1.0952 |
| ZrO220/CNTs | 44.72 | 132.46 | 2.9620 |
| ZrO240/CNTs | 38.31 | 73.14 | 1.9092 |
| ZrO260/CNTs | 22.67 | 36.13 | 1.5937 |
| ZrO280/CNTs | 21.61 | 15.81 | 0.7316 |
| ZrO290/CNTs | 7.08 | 1.05 | 0.1483 |
| ZrO2 | 2.61 | 0.11 | 0.0421 |
表1 不同ZrO2负载量复合材料的介电性能参数
| 样品 | ε′ | ε″ | tanδ |
|---|---|---|---|
| MWCNTs(C) | 847.09 | 927.74 | 1.0952 |
| ZrO220/CNTs | 44.72 | 132.46 | 2.9620 |
| ZrO240/CNTs | 38.31 | 73.14 | 1.9092 |
| ZrO260/CNTs | 22.67 | 36.13 | 1.5937 |
| ZrO280/CNTs | 21.61 | 15.81 | 0.7316 |
| ZrO290/CNTs | 7.08 | 1.05 | 0.1483 |
| ZrO2 | 2.61 | 0.11 | 0.0421 |
| 催化剂样品 | Zr测量值 | Zr理论值 | ZrO2实际负载量 |
|---|---|---|---|
| ZrO220CNTs | 11.74 | 14.81 | 15.87 |
| ZrO240/CNTs | 27.96 | 29.61 | 37.78 |
| ZrO260/CNTs | 42.79 | 44.42 | 57.82 |
| ZrO280/CNTs | 57.31 | 59.23 | 77.45 |
| ZrO290/CNTs | 64.56 | 66.63 | 87.24 |
| ZrO2 | 74.15 | 74.03 | 100.00 |
表2 ZrO2/MWCNTs(C)催化剂的Zr质量分数 (%)
| 催化剂样品 | Zr测量值 | Zr理论值 | ZrO2实际负载量 |
|---|---|---|---|
| ZrO220CNTs | 11.74 | 14.81 | 15.87 |
| ZrO240/CNTs | 27.96 | 29.61 | 37.78 |
| ZrO260/CNTs | 42.79 | 44.42 | 57.82 |
| ZrO280/CNTs | 57.31 | 59.23 | 77.45 |
| ZrO290/CNTs | 64.56 | 66.63 | 87.24 |
| ZrO2 | 74.15 | 74.03 | 100.00 |
| 催化剂样品 | ZrO2@MWCNTs(C)总用量/g | ||
|---|---|---|---|
| 保持催化剂 总量相同 | 保持氧化锆 用量相同 | 保持碳纳米管用量相同 | |
| MWCNTs(C) | 0.2000 | — | 0.0844 |
| ZrO220/CNTs | 0.2000 | 0.7289 | 0.1003 |
| ZrO240/CNTs | 0.2000 | 0.3061 | 0.1356 |
| ZrO260/CNTs | 0.2000 | 0.2000 | 0.2000 |
| ZrO280/CNTs | 0.2000 | 0.1493 | 0.3742 |
| ZrO290/CNTs | 0.2000 | 0.1325 | 0.6614 |
| ZrO2 | 0.2000 | 0.1154 | — |
表3 不同对照实验中复合催化剂ZrO2@MWCNTs(C)总用量
| 催化剂样品 | ZrO2@MWCNTs(C)总用量/g | ||
|---|---|---|---|
| 保持催化剂 总量相同 | 保持氧化锆 用量相同 | 保持碳纳米管用量相同 | |
| MWCNTs(C) | 0.2000 | — | 0.0844 |
| ZrO220/CNTs | 0.2000 | 0.7289 | 0.1003 |
| ZrO240/CNTs | 0.2000 | 0.3061 | 0.1356 |
| ZrO260/CNTs | 0.2000 | 0.2000 | 0.2000 |
| ZrO280/CNTs | 0.2000 | 0.1493 | 0.3742 |
| ZrO290/CNTs | 0.2000 | 0.1325 | 0.6614 |
| ZrO2 | 0.2000 | 0.1154 | — |
| 1 | JING Y X, GUO Y, XIA Q N, et al. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass[J]. Chem., 2019, 5(10): 2520-2546. |
| 2 | MIKA L T, CSÉFALVAY E, NÉMETH Á. Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability[J]. Chemical Reviews, 2018, 118(2): 505-613. |
| 3 | WEI Z J, LOU J T, LI Z B, et al. One-pot production of 2,5-dimethylfuran from fructose over Ru/C and a Lewis-Brønsted acid mixture in N,N-dimethylformamide[J]. Catalysis Science & Technology, 2016, 6(16): 6217-6225. |
| 4 | ZHANG S, YU Y F, SHENG K C, et al. Catalytic valorization of lignocellulosics: from bulk biofuels to value-added chemicals[J]. Biofuels, Bioproducts and Biorefining, 2021, 15(2): 592-608. |
| 5 | MARULLO S, RIZZO C, MELI A, et al. Ionic liquid binary mixtures, zeolites, and ultrasound irradiation: a combination to promote carbohydrate conversion into 5-hydroxymethylfurfural[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 5818-5826. |
| 6 | TEMPELMAN C, JACOBS U, HUT T, et al. Sn exchanged acidic ion exchange resin for the stable and continuous production of 5-HMF from glucose at low temperature[J]. Applied Catalysis A: General, 2019, 588: 117267. |
| 7 | ZHAO J, ZHOU C M, HE C, et al. Efficient dehydration of fructose to 5-hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts[J]. Catalysis Today, 2016, 264: 123-130. |
| 8 | KONWAR L J, MÄKI-ARVELA P, MIKKOLA J P. SO3H-containing functional carbon materials: synthesis, structure, and acid catalysis[J]. Chemical Reviews, 2019, 119(22): 11576-11630. |
| 9 | KOURIEH R, BENNICI S, MARZO M, et al. Investigation of the WO3/ZrO2 surface acidic properties for the aqueous hydrolysis of cellobiose[J]. Catalysis Communications, 2012, 19: 119-126. |
| 10 | ZHOU Y M, ZHANG L J, TAO S Y. Mesoporous ZrO2 nanopowder catalysts for the synthesis of 5-hydroxymethylfurfural[J]. ACS Applied Nano Materials, 2019, 2(8): 5125-5131. |
| 11 | BUINACHEV S, MASHKOVTSEV M A, ZHIRENKINA N, et al. A new approach for the synthesis of monodisperse zirconia powders with controlled particle size[J]. International Journal of Hydrogen Energy, 2021, 46(32): 16878-16887. |
| 12 | LIU C C, LEE S, SU D, et al. Controlling the particle size of ZrO2 nanoparticles in hydrothermally stable ZrO2/MWCNT composites[J]. Langmuir, 2012, 28(49): 17159-17167. |
| 13 | LI L, YAN B, LI H X, et al. Decreasing the acid value of pyrolysis oil via esterification using ZrO2/SBA-15 as a solid acid catalyst[J]. Renewable Energy, 2020, 146: 643-650. |
| 14 | LI X G, PANG C R, LI H, et al. Microwave energy inductive fluidized metal particles discharge behavior and its potential utilization in reaction intensification[J]. Chinese Journal of Chemical Engineering, 2021, 33: 256-267. |
| 15 | JIA X C, YU I K M, TSANG D C W, et al. Functionalized zeolite-solvent catalytic systems for microwave-assisted dehydration of fructose to 5-hydroxymethylfurfural[J]. Microporous and Mesoporous Materials, 2019, 284: 43-52. |
| 16 | CHHABRA T, BAHUGUNA A, DHANKHAR S S, et al. Sulfonated graphitic carbon nitride as a highly selective and efficient heterogeneous catalyst for the conversion of biomass-derived saccharides to 5-hydroxymethylfurfural in green solvents[J]. Green Chemistry, 2019, 21(21): 6012-6026. |
| 17 | WANG Q F, HAO J Q, ZHAO Z B. Microwave-assisted conversion of fructose to 5-hydroxymethylfurfural using sulfonated porous carbon derived from biomass[J]. Australian Journal of Chemistry, 2018, 71(1): 24. |
| 18 | QI X H, WATANABE M, AIDA T M, et al. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating[J]. Green Chemistry, 2008, 10(7): 799. |
| 19 | JI T, LI Z, LIU C, et al. Niobium-doped TiO2 solid acid catalysts: strengthened interfacial polarization, amplified microwave heating and enhanced energy efficiency of hydroxymethylfurfural production[J]. Applied Catalysis B: Environmental, 2019, 243: 741-749. |
| 20 | JI T, TU R, MU L W, et al. Enhancing energy efficiency in saccharide-HMF conversion with core/shell structured microwave responsive catalysts[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(5): 4352-4358. |
| 21 | LI H, ZHANG C Y, PANG C R, et al. The advances in the special microwave effects of the heterogeneous catalytic reactions[J]. Frontiers in Chemistry, 2020, 8: 355. |
| 22 | SONGO M, MOUTLOALI R, RAY S. Development of TiO2-carbon composite acid catalyst for dehydration of fructose to 5-hydroxymethylfurfural[J]. Catalysts, 2019, 9(2): 126. |
| 23 | LYU X Q, LI H, XIANG H Z, et al. Energy efficient production of 5-hydroxymethylfurfural (5-HMF) over surface functionalized carbon superstructures under microwave irradiation[J]. Chemical Engineering Journal, 2022, 428: 131143. |
| 24 | 吕孝琦, 李洪, 赵振宇, 等. 微波与碳基催化剂协同促进果糖制5-羟甲基糠醛[J]. 化工进展, 2022, 41(2): 637-647. |
| Xiaoqi LYU, LI Hong, ZHAO Zhenyu, et al. Microwave-assisted carbon-based catalysts for fructose dehydration to 5-hydroxymethylfurfural[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 637-647. | |
| 25 | WEN F S, ZHANG F, LIU Z Y. Investigation on microwave absorption properties for multiwalled carbon nanotubes/Fe/Co/Ni nanopowders as lightweight absorbers[J]. Journal of Physical Chemistry C, 2011, 115(29): 14025-14030. |
| 26 | AI Y J, LIU L, JING K, et al. Noncovalently functionalized carbon nanotubes immobilized Fe-Bi bimetallic oxides as a heterogeneous nanocatalyst for reduction of nitroaromatics[J]. Nano-Structures & Nano-Objects, 2017, 10: 116-124. |
| 27 | JI T, TU R, MU L W, et al. Structurally tuning microwave absorption of core/shell structured CNT/polyaniline catalysts for energy efficient saccharide-HMF conversion[J]. Applied Catalysis B: Environmental, 2018, 220: 581-588. |
| 28 | SHAN Y, GAO L. Synthesis and characterization of phase controllable ZrO2-carbon nanotube nanocomposites[J]. Nanotechnology, 2005, 16(6): 625-630. |
| 29 | LAU S K, DAG D, OZTURK S, et al. A comparison between the open-ended coaxial probe method and the parallel plate method for measuring the dielectric properties of low-moisture foods[J]. LWT, 2020, 130: 109719. |
| 30 | 赵振宇, 李洪, 李鑫钢, 等. 基于介电性质差异的微波诱导强化蒸馏分离[J]. 化工进展, 2020, 39(6): 2275-2283. |
| ZHAO Zhenyu, LI Hong, LI Xingang, et al. Microwave-induced enhancement of distillation separation based on dielectric properties difference[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2275-2283. | |
| 31 | MU S Y, LIU K, LI H, et al. Microwave-assisted synthesis of highly dispersed ZrO2 on CNTs as an efficient catalyst for producing 5-hydroxymethylfurfural (5-HMF)[J]. Fuel Processing Technology, 2022, 233: 107292. |
| 32 | ROMERO-SÁEZ M, DONGIL A B, BENITO N, et al. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: a comparison between two impregnation strategies[J]. Applied Catalysis B: Environmental, 2018, 237: 817-825. |
| 33 | QUAN Y H, ZHANG N, ZHANG Z L, et al. Enhanced performance of Ni catalysts supported on ZrO2 nanosheets for CO2 methanation: effects of support morphology and chelating ligands[J]. International Journal of Hydrogen Energy, 2021, 46(27): 14395-14406. |
| 34 | WU Y Q, WANG H J, WANG L Y, et al. Effect of iron on ZrO2-based catalysts for direct synthesis of isobutanol from syngas[J]. Fuel, 2021, 304: 121342. |
| 35 | ADDO NTIM S, MITRA S. Adsorption of arsenic on multiwall carbon nanotube-zirconia nanohybrid for potential drinking water purification[J]. Journal of Colloid and Interface Science, 2012, 375(1): 154-159. |
| 36 | GEORGIEVA I, DANCHOVA N, GUTZOV S, et al. DFT modeling, UV-vis and IR spectroscopic study of acetylacetone-modified zirconia sol-gel materials[J]. Journal of Molecular Modeling, 2012, 18(6): 2409-2422. |
| 37 | ANKU W W, OPPONG S O B, SHUKLA S K, et al. Palladium-doped-ZrO2-multiwalled carbon nanotubes nanocomposite: an advanced photocatalyst for water treatment[J]. Applied Physics A, 2016, 122(6): 1-8. |
| 38 | LI M F, ZHANG Q T, LUO B, et al. Lignin-based carbon solid acid catalyst prepared for selectively converting fructose to 5-hydroxymethylfurfural[J]. Industrial Crops and Products, 2020, 145: 111920. |
| 39 | WHITAKER M R, PARULKAR A, RANADIVE P, et al. Examining acid formation during the selective dehydration of fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide and water[J]. ChemSusChem, 2019, 12(10): 2211-2219. |
| 40 | QI X H, WATANABE M, AIDA T M, et al. Sulfated zirconia as a solid acid catalyst for the dehydration of fructose to 5-hydroxymethylfurfural[J]. Catalysis Communications, 2009, 10(13): 1771-1775. |
| 41 | WANG N N, YAO Y, LI W, et al. Catalytic dehydration of fructose to 5-hydroxymethylfurfural over a mesoscopically assembled sulfated zirconia nanoparticle catalyst in organic solvent[J]. RSC Advances, 2014, 4(100): 57164-57172. |
| 42 | LAM E, LUONG J H T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals[J]. ACS Catalysis, 2014, 4(10): 3393-3410. |
| 43 | ZHAO Z Y, SHEN X, LI H, et al. Frontispiz: watching microwave-induced microscopic hot spots via the thermosensitive fluorescence of europium/terbium mixed-metal organic complexes[J]. Angewandte Chemie, 2022, 134(6): e202280662. |
| 44 | ZHAO Z Y, LI H, ZHAO K, et al. Microwave-assisted synthesis of MOFs: rational design via numerical simulation[J]. Chemical Engineering Journal, 2022, 428: 131006. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||