化工进展 ›› 2022, Vol. 41 ›› Issue (11): 5887-5895.DOI: 10.16085/j.issn.1000-6613.2022-0037
氮掺杂碳限域的花状SnS催化CO2电还原制甲酸
黄鑫1( ), 刘成2, 唐如佳3, 韩欣欣3, 陈世霞1(
), 刘成2, 唐如佳3, 韩欣欣3, 陈世霞1( ), 王珺1
), 王珺1
- 1.南昌大学化学化工学院,江西 南昌 330031
2.南昌大学未来技术学院,江西 南昌 330031
3.南昌大学资源与环境学院,江西 南昌 330031
-
收稿日期:2022-01-06修回日期:2022-04-10出版日期:2022-11-25发布日期:2022-11-28 -
通讯作者:陈世霞 -
作者简介:黄鑫(1998—),女,硕士研究生,研究方向为电化学还原CO2。E-mail:1270583553@qq.com。 -
基金资助:国家自然科学基金(22008101);江西省自然科学基金(20212BAB213038);江西省研究生创新基金(YC2021-S045)
Nitrogen-doped carbon-confined flower-like SnS catalyst for electrochemical reduction of CO2 to HCOOH
HUANG Xin1( ), LIU Cheng2, TANG Rujia3, HAN Xinxin3, CHEN Shixia1(
), LIU Cheng2, TANG Rujia3, HAN Xinxin3, CHEN Shixia1( ), WANG Jun1
), WANG Jun1
- 1.School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, Jiangxi, China
2.School of Future Technology, Nanchang University, Nanchang 330031, Jiangxi, China
3.School of Resources & Environment, Nanchang University, Nanchang 330031, Jiangxi, China
-
Received:2022-01-06Revised:2022-04-10Online:2022-11-25Published:2022-11-28 -
Contact:CHEN Shixia
摘要:
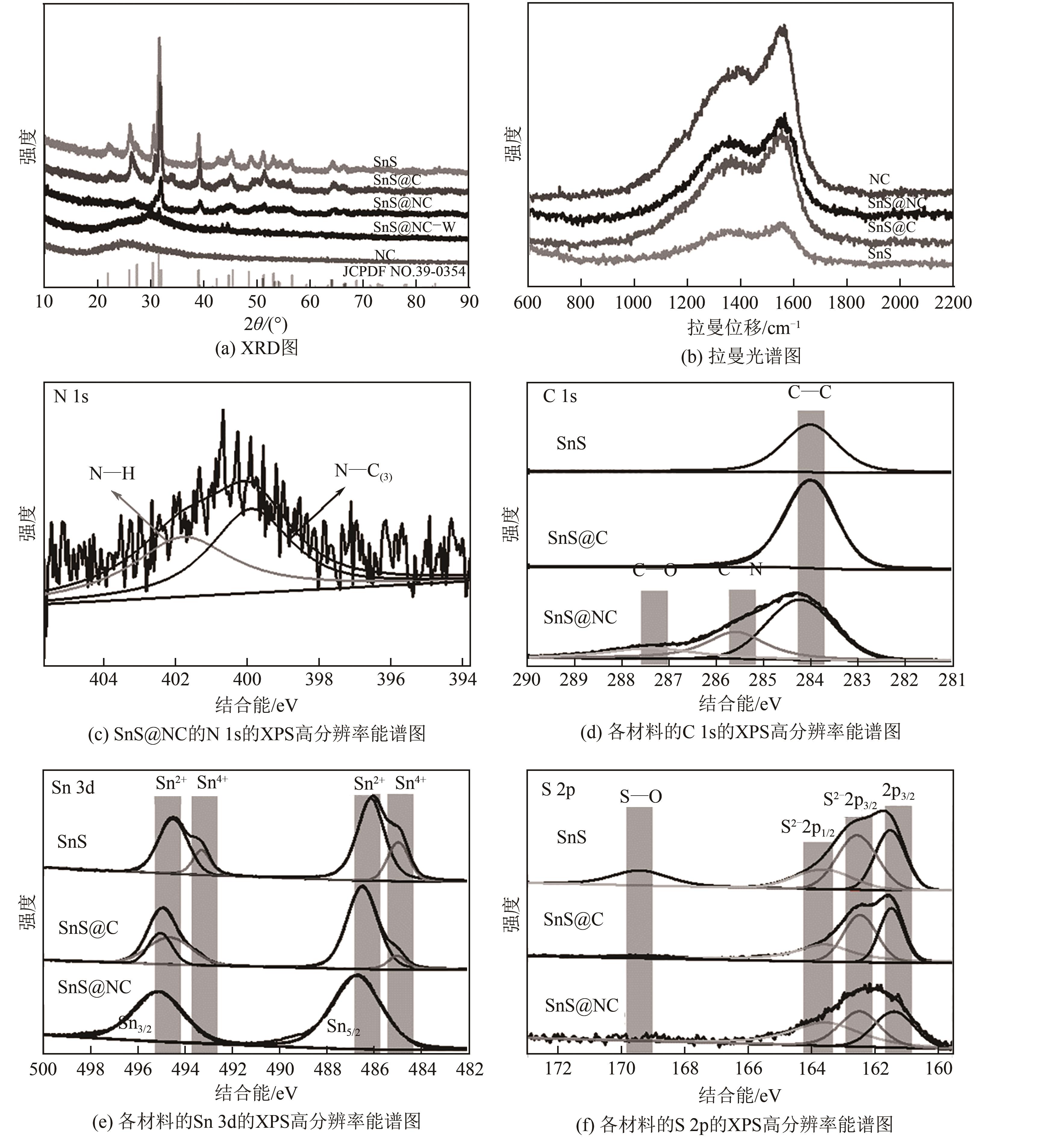
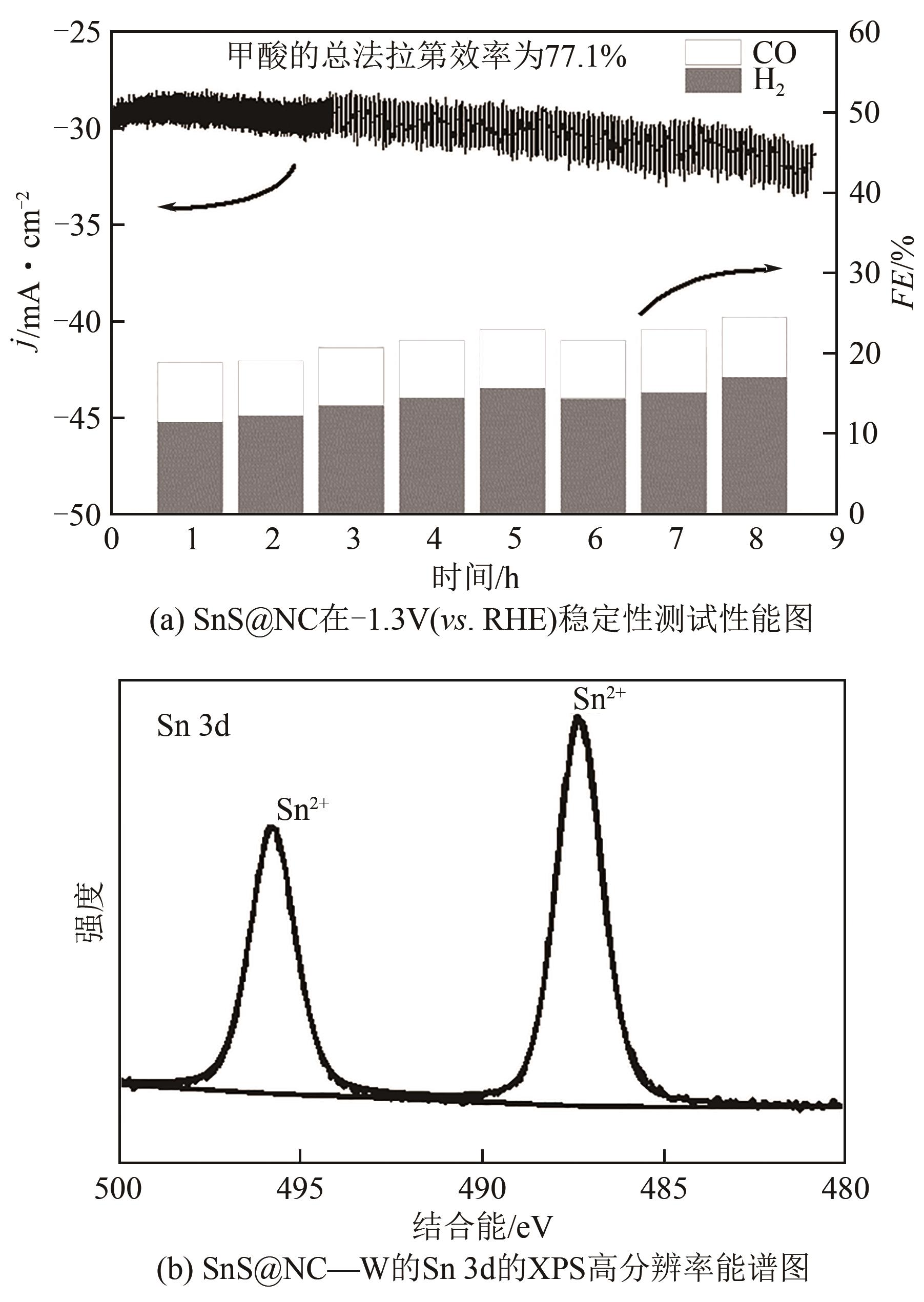
合理设计高效的电催化剂是二氧化碳电化学还原(CO2ER)为高附加值化学品和燃料的关键。本文利用水热-煅烧法制备了氮掺杂碳限域的花状SnS催化剂(SnS@NC)并研究了其电催化CO2的特性。基于超薄氮掺杂碳层的限域效应,SnS的层厚由原始的30nm缩减至20nm,电化学活性面积明显增强,同时氮掺杂碳层增强了对CO2的吸附和活化。SnS@NC催化CO2转化为甲酸的能力明显增强,在-1.3V(vs. RHE)的H型电解池中法拉第效率为81.2%,电流密度为29.5mA/cm2,本文为金属硫化物复合催化剂功能化提供了新策略。
中图分类号:
引用本文
黄鑫, 刘成, 唐如佳, 韩欣欣, 陈世霞, 王珺. 氮掺杂碳限域的花状SnS催化CO2电还原制甲酸[J]. 化工进展, 2022, 41(11): 5887-5895.
HUANG Xin, LIU Cheng, TANG Rujia, HAN Xinxin, CHEN Shixia, WANG Jun. Nitrogen-doped carbon-confined flower-like SnS catalyst for electrochemical reduction of CO2 to HCOOH[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5887-5895.
| 样品 | N/% | C/% | Sn/% | S/% |
|---|---|---|---|---|
| SnS | 2.09 | 64.22 | 18.11 | 15.15 |
| SnS@C | 2.29 | 82.40 | 7.23 | 8.08 |
| SnS@NC | 2.66 | 83.64 | 6.38 | 7.33 |
表1 XPS所测得的材料表层Sn、S、N、C各原子的占比
| 样品 | N/% | C/% | Sn/% | S/% |
|---|---|---|---|---|
| SnS | 2.09 | 64.22 | 18.11 | 15.15 |
| SnS@C | 2.29 | 82.40 | 7.23 | 8.08 |
| SnS@NC | 2.66 | 83.64 | 6.38 | 7.33 |
| 1 | KOTHANDARAMAN J, HELBEBRANT D J. Towards environmentally benign capture and conversion: heterogeneous metal catalyzed CO2 hydrogenation in CO2 capture solvents[J]. Green Chemistry, 2020, 22(3): 828-834. |
| 2 | BEHERA A, KAR A K, SRIVASTAVA R. Challenges and prospects in the selective photoreduction of CO2 to C1 and C2 products with nanostructured materials: a review[J]. Materials Horizons, 2022, 9: 607-639. |
| 3 | ZHANG W J, HU Y, MA L B, et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2018, 5(1): 1700275. |
| 4 | CHOI S Y, JEONG S K, KIM H J, et al. Electrochemical reduction of carbon dioxide to formate on tin-lead alloys[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1311-1318. |
| 5 | CHANG J F, FENG L G, LIU C P, et al. An effective Pd-Ni2P/C anode catalyst for direct formic acid fuel cells[J]. Angewandte Chemie, 2014, 126(1): 122-130. |
| 6 | ALVAREZ-GUERRA M, QUINTANILLA S, IRABIEN A. Conversion of carbon dioxide into formate using a continuous electrochemical reduction process in a lead cathode[J]. Chemical Engineering Journal, 2012, 207/208: 278-284. |
| 7 | ZHOU B W, KONG X H, VANKA S, et al. A GaN: Sn nanoarchitecture integrated on a silicon platform for converting CO2 to HCOOH by photoelectrocatalysis[J]. Energy & Environmental Science, 2019, 12(9): 2842-2848. |
| 8 | XING Y L, KONG X D, GUO X, et al. Bi@Sn core–shell structure with compressive strain boosts the electroreduction of CO2 into formic acid[J]. Advanced Science, 2020, 7(22): 1902989. |
| 9 | HUANG J J, CHEN S X, YANG F Q, et al. Nickel nanoparticles with narrow size distribution confined in nitrogen-doped carbon for efficient reduction of CO2 to CO[J]. Catalysis Letters, 2022, 152(2): 600-609. |
| 10 | BOK J, LEE S Y, LEE B H, et al. Designing atomically dispersed Au on tensile-strained Pd for efficient CO2 electroreduction to formate[J]. Journal of the American Chemical Society, 2021, 143(14): 5386-5395. |
| 11 | ZOUAOUI N, OSSONON B D, FAN M Y, et al. Electroreduction of CO2 to formate on amine modified Pb electrodes[J]. Journal of Materials Chemistry A, 2019, 7(18): 11272-11281. |
| 12 | PENG L W, WANG Y X, MASOOD I, et al. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction[J]. Applied Catalysis B: Environmental, 2020, 264: 118447. |
| 13 | ZHANG A, LIANG Y X, LI H P, et al. Harmonizing the electronic structures of the adsorbate and catalysts for efficient CO2 reduction[J]. Nano Letters, 2019, 19(9): 6547-6553. |
| 14 | LI J C, KUANG Y, MENG Y T, et al. Electroreduction of CO2 to formate on a copper-based electrocatalyst at high pressures with high energy conversion efficiency[J]. Journal of the American Chemical Society, 2020, 142: 7276-7282. |
| 15 | KIM M K, LEE H, WON J H, et al. Design of less than 1nm scale spaces on SnO2 nanoparticles for high-performance electrochemical CO2 reduction[J]. Advanced Functional Materials, 2022, 32: 2107349. |
| 16 | YANG Z J, YANG C Y, HAN J Y, et al. Boosting electrochemical CO2 reduction to formate using SnO2/graphene oxide with amide linkages[J]. Journal of Materials Chemistry A, 2021, 9: 19681-19686. |
| 17 | ZHANG B H, CHEN S, WULAN B, et al. Surface modification of SnO2 nanosheets via ultrathin N-doped carbon layers for improving CO2 electrocatalytic reduction[J]. Chemical Engineering Journal, 2021, 421: 130003. |
| 18 | WANG Q N, WU Y L, ZHU C Q, et al. Sn nanoparticles deposited onto a gas diffusion layer via impregnation-electroreduction for enhanced CO2 electroreduction to formate[J]. Electrochimica Acta, 2021, 369: 137662. |
| 19 | LI Q Q, ZHANG Y X, ZHANG X R, et al. Novel Bi, BiSn, Bi2Sn, Bi3Sn, and Bi4Sn catalysts for efficient electroreduction of CO2 to formic acid[J]. Industrial & Engineering Chemistry Research, 2019, 59(15): 6806-6814. |
| 20 | CHEN H L, CHEN J X, SI J C, et al. Ultrathin tin monosulfide nanosheets with the exposed (001) plane for efficient electrocatalytic conversion of CO2 into formate[J]. Chemical Science, 2020, 11(15): 3952-3958. |
| 21 | VANDAELE K, MOT B T, PUPO M, et al. Sn-based electrocatalyst stability: a crucial piece to the puzzle for the electrochemical CO2 reduction toward formic acid[J]. ACS Energy Letter, 2021, 6: 4317-4327. |
| 22 | MELCHIONNA M, FORNASIERO P, PRATO M, et al. Electrocatalytic CO2 reduction: role of the cross-talk at nano-carbon interfaces[J]. Energy & Environmental Science, 2021, 14(11): 5816-5833. |
| 23 | LI Z J, CAO A, ZHENG Q, et al. Elucidation of the synergistic effect of dopants and vacancies on promoted selectivity for CO2 electroreduction to formate[J]. Advanced Materials, 2020, 33(2): 2005113. |
| 24 | YE G Y, LIU S Q, HUANG K, et al. Domain-confined etching strategy to regulate defective sites in carbon for high-efficiency electrocatalytic oxygen reduction[J]. Advanced Functional Materials, 2022: 2111396. |
| 25 | LU Q, CHEN C, DI Q, et al. Dual role of pyridinic-N doping in carbon-coated Ni nanoparticles for highly efficient electrochemical CO2 reduction to CO over a wide potential range[J]. ACS Catalysis, 2022, 12(2): 1364-1374. |
| 26 | YU A, MA G M, ZHU L T, et al. Conversion of CO2 to defective porous carbons in one electro-redox cycle for boosting electrocatalytic H2O2 production[J]. Applied Catalysis B: Environmental, 2022, 307: 121161. |
| 27 | CHI SHAOYI, CHEN Q, ZHAO S S, et al. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide[J]. Journal of Materials Chemistry A, 2022, 10(9): 4653-4659. |
| 28 | LIU S, YANG H B, HUANG X, et al. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction[J]. Advanced Functional Materials, 2018, 28(21): 1800499. |
| 29 | SI Z, LYU Z Z, LU L H, et al. Nitrogen-doped graphene chainmail wrapped IrCo alloy particles on nitrogen-doped graphene nanosheet for highly active and stable full water splitting[J]. ChemCatChem, 2019, 11: 5457-6465. |
| 30 | CHEN J Y, WANG T T, WANG X Y, et al. Promoting electrochemical CO2 reduction via boosting activation of adsorbed intermediates on iron single-atom catalyst[J]. Advanced Functional Materials: 2022: 2110174. |
| 31 | CHEN J Y, LI Z G, WANG X Y, et al. Promoting CO2 electroreduction kinetics on atomically dispersed monovalent ZnI sites by rationally engineering proton-feeding centers[J]. Angewandte Chemie International Edition, 2022, 134 (7): e202111683. |
| 32 | ZHANG Y K, WANG X Y, ZHENG S X, et al. Hierarchical cross-linked carbon aerogels with transition metal-nitrogen sites for highly efficient industrial-level CO2 electroreduction[J]. Advanced Functional Materials, 2021, 31(45): 2104377. |
| 33 | WANG X Y, SANG X H, DONG C L, et al. Proton capture strategy for enhancing electrochemical CO2 reduction on atomically dispersed metal-nitrogen active sites[J]. Angewandte Chemie International Edition, 2021, 60(21): 11959-11965. |
| 34 | WANG X Y, FENG X H, LU W C, et al. A new strategy for accelerating dynamic proton transfer of electrochemical CO2 reduction at high current densities[J]. Advanced Functional Materials, 2021, 31(50): 2104243. |
| 35 | FAN Q K, ZHANG X, GE X H, et al. Manipulating Cu nanoparticle surface oxidation states tunes catalytic selectivity toward CH4 or C2+ products in CO2 electroreduction[J]. Advanced Energy Materials, 2021, 11(36): 2101424. |
| 36 | CHEN S X, LI Y W, BU Z G, et al. Boosting CO2-to-CO conversion on a robust single-atom copper decorated carbon catalyst by enhancing intermediate binding strength[J]. Journal of Materials Chemistry A, 2021, 9(3): 1705-1712. |
| 37 | HOU Y, LIANG Y L, SHI P C, et al. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity[J]. Applied Catalysis B: Environmental, 2020, 271: 118929. |
| 38 | CHEN Z P, ZHANG X X, JIAO M Y, et al. Engineering electronic structure of stannous sulfide by amino‐functionalized carbon: toward efficient electrocatalytic reduction of CO2 to formate[J]. Advanced Energy Materials, 2020, 10(8): 1903664. |
| 39 | VELASCO-VELEZ J J, JONES T, GAO D F, et al. The role of the copper oxidation state in the electrocatalytic reduction of CO2 into valuable hydrocarbons[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1485-1492. |
| 40 | LIU S B, TAO H B, ZENG L, et al. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates[J]. Journal of the American Chemical Society, 2017, 139: 2160-2163. |
| 41 | YANG F, WANG J, LIU L, et al. Synthesis of porous carbons with high N-content from shrimp shells for efficient CO2-capture and gas separation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15550-15559. |
| 42 | LI F W, CHEN L, XUE M Q, et al. Towards a better Sn: efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets[J]. Nano Energy, 2017, 31: 270-277. |
| 43 | RABIEE H, ZHANG X Q, GE L, et al. Tuning the product selectivity of the Cu hollow fiber gas diffusion electrode for efficient CO2 reduction to formate by controlled surface Sn electrodeposition[J]. ACS Applied Materials & Interfaces, 2020, 12(19): 21670-21681. |
| 44 | KUANG Z Y, ZHAO W N, PENG C, et al. Hierarchically porous SnO2 coupled organic carbon for CO2 electroreduction[J]. ChemSusChem, 2020, 13(22): 5896-5900. |
| 45 | GENG W H, CHEN W, LI G H, et al. Induced CO2 electroreduction to formic acid on metal-organic frameworks via node doping[J]. ChemSusChem, 2020, 13(16): 4035-4040. |
| 46 | YE K, CAO A, SHAO J Q, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity[J]. Science Bulletin, 2020, 65(19): 711-719. |
| 47 | LIUS Y, PAG F J, ZHANG Q Y, et al. Stable nanoporous Sn/SnO2 composites for efficient electroreduction of CO2 to formate over wide potential range[J]. Applied Materials Today, 2018, 13: 135-143. |
| 48 | DAIYAN R, LU X Y, NG Y H, et al. Surface engineered tin foil for electrocatalytic reduction of carbon dioxide to formate[J]. Catalysis Science &Technology, 2017, 7(12): 2542-2550. |
| 49 | ZHANG Y, ZHANG X L, BOND A M, et al. Identification of a new substrate effect that enhances the electrocatalytic activity of dendritic tin in CO2 reduction[J]. Physical Chemistry Chemical Physics, 2018, 20(8): 5936-5941. |
| 50 | KUMAR B, ATLAY, BRIAN J P, et al. Reduced SnO2 porous nanowires with a high density of grain boundaries as catalysts for efficient electrochemical CO2-into-HCOOH conversion[J]. Angewandte Chemie International Edition, 2017, 56(13): 3645-3649. |
| 51 | DONG W J, YOO C J, LEE J L. Monolithic nanoporous In-Sn aloy for electrochemical reduction of carbon doxide[J]. ACS Applied Materials & Interfaces, 2017, 9(50): 43575-43582. |
| 52 | HE J, YANG C, YANG X, et al. Hydrophobic electrocatalyst for the enhanced activity of oxygen reduction reaction through controllable liquid/gas/solid interface[J]. Applied Surface Science, 2020, 532: 147357. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [4] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [11] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||