化工进展 ›› 2022, Vol. 41 ›› Issue (10): 5541-5548.DOI: 10.16085/j.issn.1000-6613.2021-2475
一株产低温脂肪酶酵母菌的鉴定及酶学性质
- 1.南阳师范学院生命科学与农业工程学院,河南 南阳 473061
2.河南天冠企业集团有限公司车用生物燃料技术国家重点实验室,河南 南阳 473000
-
收稿日期:2021-12-03修回日期:2022-02-12出版日期:2022-10-20发布日期:2022-10-21 -
通讯作者:惠丰立 -
作者简介:史程风(1996—),女,硕士研究生,研究方向为生物质能源。E-mail:2712835812@qq.com。 -
基金资助:车用生物燃料技术国家重点实验室开放课题(2013021)
Identification of a cold-adapted lipase-producing yeast and its enzyme characterization
SHI Chengfeng1( ), JIA Ranran1, YAN Zhenli2, HUI Fengli1(
), JIA Ranran1, YAN Zhenli2, HUI Fengli1( )
)
- 1.College of Life Science and Agricultural Engineering, Nanyang Normal University, Nanyang 473061, Henan, China
2.State Key Laboratory of Vehicle Biofuel Technology, Henan Tianguan Group Company, Limited, Nanyang 473000, Henan, China
-
Received:2021-12-03Revised:2022-02-12Online:2022-10-20Published:2022-10-21 -
Contact:HUI Fengli
摘要:
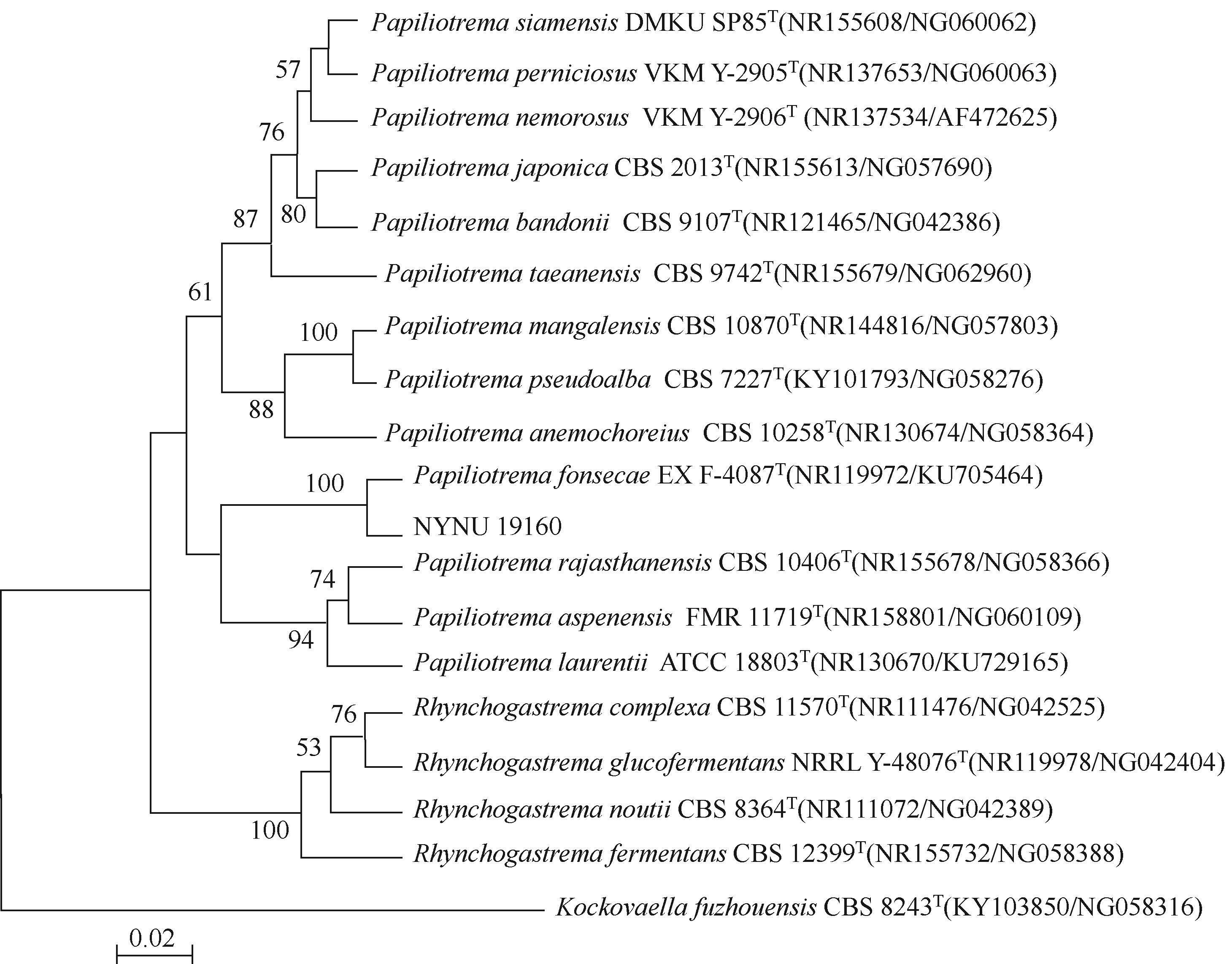
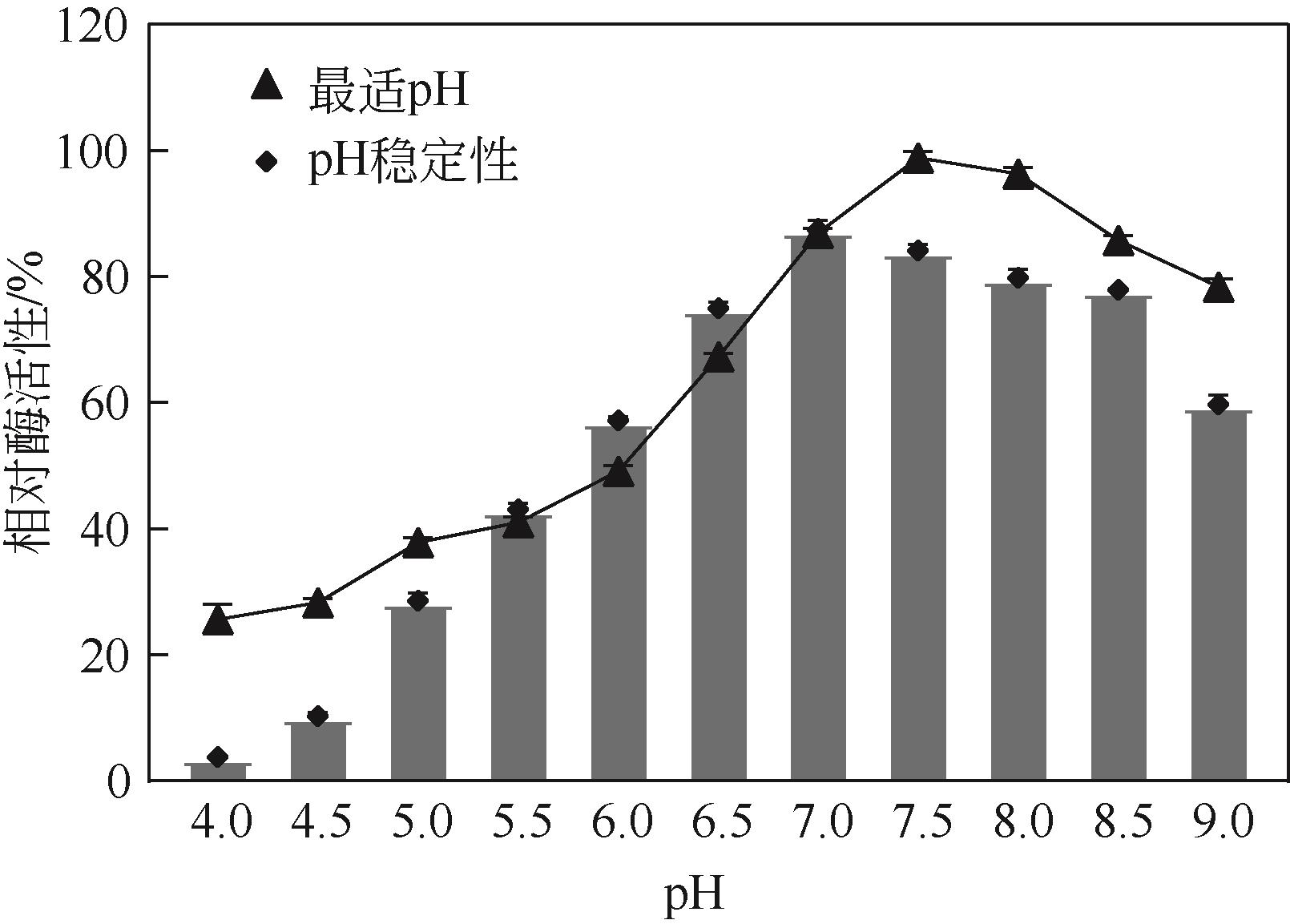
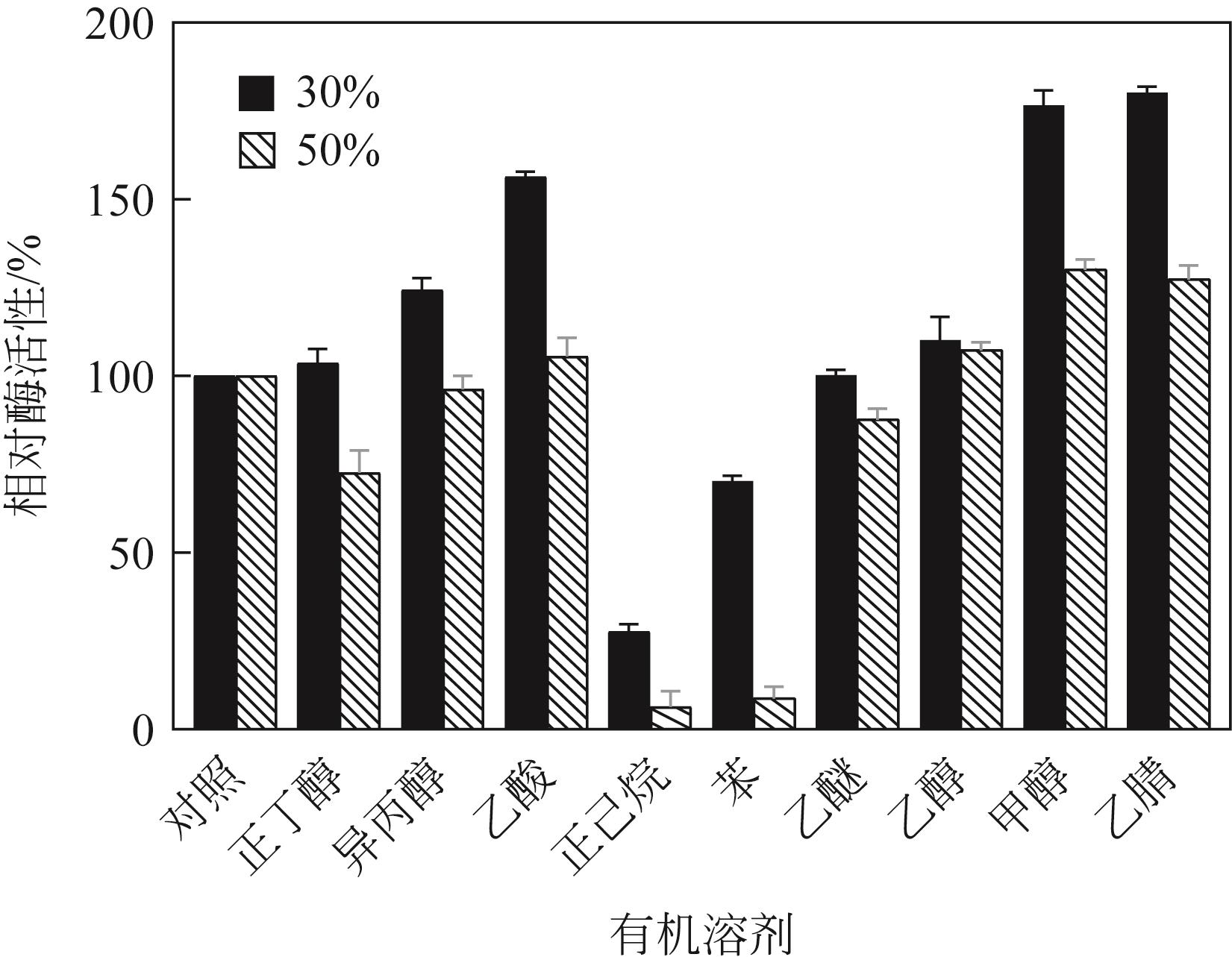
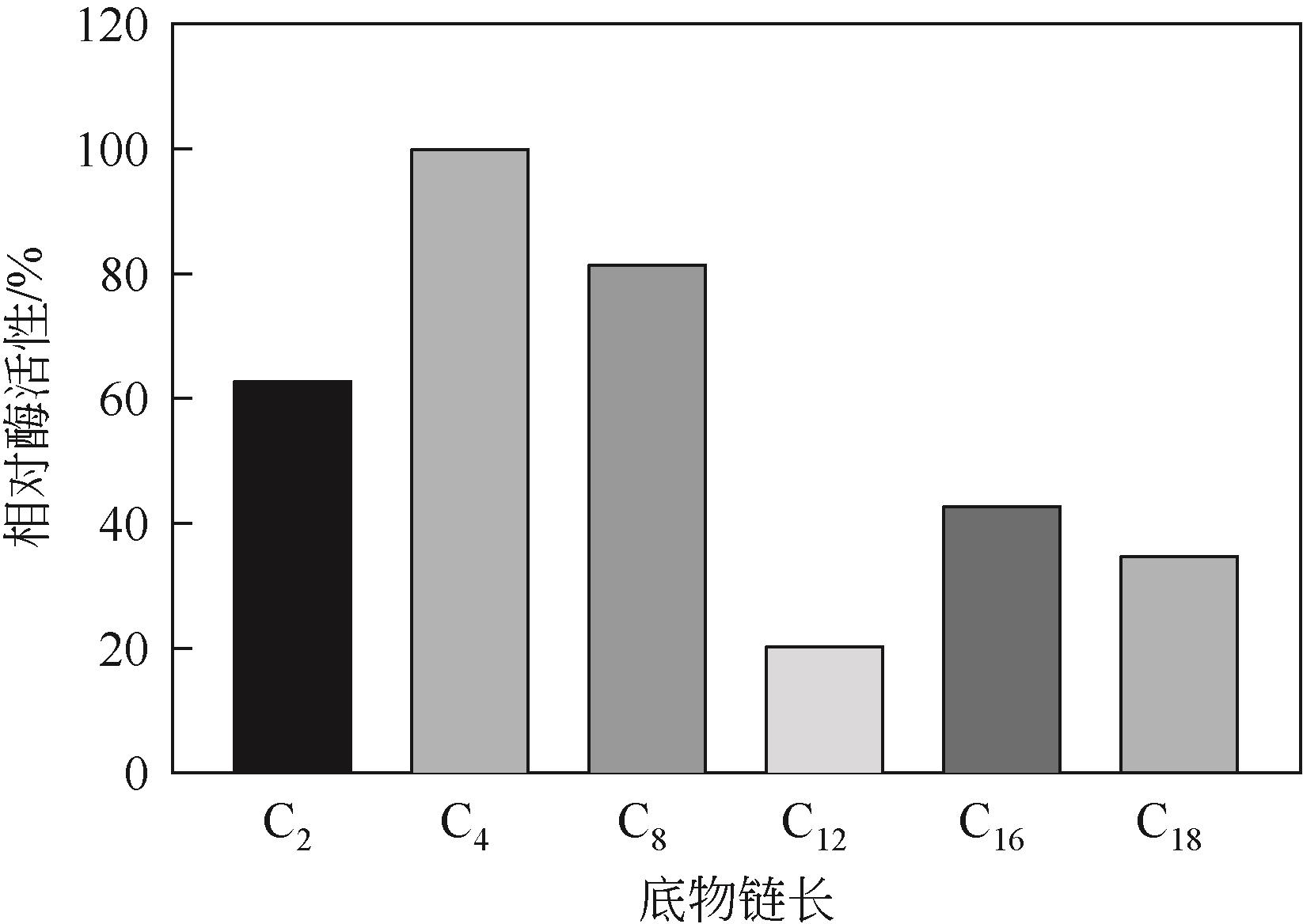
低温脂肪酶已成为工业低温工艺良好候选物,在生物质能源、食品、皮制品、废水处理等领域发挥重要作用。本文从实验室保藏的55株产低温脂肪酶酵母菌株中筛选出一株高产菌株NYNU 19160,通过形态学、生理生化以及ITS和26S rDNA序列分析,将该菌株鉴定为Papiliotrema fonsecae。经过硫酸铵分级沉淀、透析、浓缩将该脂肪酶纯化后,对酶学性质进行了研究。结果表明,该脂肪酶最适反应温度为20℃,最适作用pH为7.5,属于低温碱性脂肪酶;Cu2+显著促进该酶的水解活性,而Li+表现为显著抑制作用;有机溶剂乙腈、甲醇、乙酸对酶活性有较强的促进作用,而苯和正己烷则抑制了该酶的活性;该酶对对硝基苯酚丁酸脂(pNPC4)底物表现出较强特异性。
中图分类号:
引用本文
史程风, 贾冉冉, 阎振丽, 惠丰立. 一株产低温脂肪酶酵母菌的鉴定及酶学性质[J]. 化工进展, 2022, 41(10): 5541-5548.
SHI Chengfeng, JIA Ranran, YAN Zhenli, HUI Fengli. Identification of a cold-adapted lipase-producing yeast and its enzyme characterization[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5541-5548.
| 菌株编号 | 初筛酶活性 | 复筛酶活性/U·mL-1 | 菌株编号 | 初筛酶活性 | 复筛酶活性/U·mL-1 |
|---|---|---|---|---|---|
| NYNU 18124 | ++++ | 17.72±4.95 | NYNU 18335 | +++ | 9.13±3.78 |
| NYNU 19166 | ++++ | 19.51±3.08 | NYNU 18316 | +++ | 9.05±4.17 |
| NYNU 18314 | +++ | 12.88±1.75 | NYNU 18457 | ++ | 8.88±3.04 |
| NYNU 18320 | ++ | 9.26±3.82 | NYNU 1833 | ++ | 8.72±5.88 |
| NYNU 1812141 | +++ | 20.43±2.32 | NYNU 18315 | ++ | 8.55±1.55 |
| NYNU 19160 | ++++ | 34.47±3.03 | NYNU 18455 | ++ | 7.88±2.98 |
表1 不同的菌株脂肪酶活性
| 菌株编号 | 初筛酶活性 | 复筛酶活性/U·mL-1 | 菌株编号 | 初筛酶活性 | 复筛酶活性/U·mL-1 |
|---|---|---|---|---|---|
| NYNU 18124 | ++++ | 17.72±4.95 | NYNU 18335 | +++ | 9.13±3.78 |
| NYNU 19166 | ++++ | 19.51±3.08 | NYNU 18316 | +++ | 9.05±4.17 |
| NYNU 18314 | +++ | 12.88±1.75 | NYNU 18457 | ++ | 8.88±3.04 |
| NYNU 18320 | ++ | 9.26±3.82 | NYNU 1833 | ++ | 8.72±5.88 |
| NYNU 1812141 | +++ | 20.43±2.32 | NYNU 18315 | ++ | 8.55±1.55 |
| NYNU 19160 | ++++ | 34.47±3.03 | NYNU 18455 | ++ | 7.88±2.98 |
| 糖发酵 | 结果 | 氮源同化 | 结果 | 碳源同化 | 结果 | 生长测试 | 结果 |
|---|---|---|---|---|---|---|---|
| D-葡萄糖 | - | 乙胺 | + | D-葡萄糖 | + | 维生素基础培养基 | + |
| D-半乳糖 | - | 尸胺 | - | D-半乳糖 | + | 生长在4℃ | + |
| L-山梨糖 | - | 肌酐 | - | L-山梨糖 | - | 生长在10℃ | + |
| 麦芽糖 | - | 硝酸盐 | + | 蔗糖 | + | 生长在15℃ | + |
| α-D-吡喃葡糖苷 | - | D-色氨酸 | + | 木糖醇 | + | 生长在20℃ | + |
| 蔗糖 | - | 亚硝酸盐 | + | D-麦芽糖 | + | 生长在25℃ | + |
| D-海藻糖 | - | L-赖氨酸 | - | 5-酮-D-葡糖糖盐 | + | 生长在30℃ | + |
| 蜜二糖 | - | 氨基葡萄糖 | + | 甲醇 | - | 生长在35℃ | + |
| 乳糖 | - | 咪唑 | w | D,L-乳酸 | - | 生长在40℃ | - |
| 纤维二塘 | - | 肌酸 | + | 水杨苷 | + | 生长在45℃ | - |
| 松三糖 | - | 核糖醇 | d | 0.01%放线菌酮 | w | ||
| D-木糖 | - | L-阿拉伯糖醇 | + | 0.1%放线菌酮 | - | ||
| D-绵子糖 | - | 柠檬酸 | d | 1%乙酸 | - | ||
| 菊粉 | - | 乙醇 | d | ||||
| 可溶性淀粉 | - | D-阿拉伯糖 | w |
表2 菌株NYNU 19160的生理生化特征
| 糖发酵 | 结果 | 氮源同化 | 结果 | 碳源同化 | 结果 | 生长测试 | 结果 |
|---|---|---|---|---|---|---|---|
| D-葡萄糖 | - | 乙胺 | + | D-葡萄糖 | + | 维生素基础培养基 | + |
| D-半乳糖 | - | 尸胺 | - | D-半乳糖 | + | 生长在4℃ | + |
| L-山梨糖 | - | 肌酐 | - | L-山梨糖 | - | 生长在10℃ | + |
| 麦芽糖 | - | 硝酸盐 | + | 蔗糖 | + | 生长在15℃ | + |
| α-D-吡喃葡糖苷 | - | D-色氨酸 | + | 木糖醇 | + | 生长在20℃ | + |
| 蔗糖 | - | 亚硝酸盐 | + | D-麦芽糖 | + | 生长在25℃ | + |
| D-海藻糖 | - | L-赖氨酸 | - | 5-酮-D-葡糖糖盐 | + | 生长在30℃ | + |
| 蜜二糖 | - | 氨基葡萄糖 | + | 甲醇 | - | 生长在35℃ | + |
| 乳糖 | - | 咪唑 | w | D,L-乳酸 | - | 生长在40℃ | - |
| 纤维二塘 | - | 肌酸 | + | 水杨苷 | + | 生长在45℃ | - |
| 松三糖 | - | 核糖醇 | d | 0.01%放线菌酮 | w | ||
| D-木糖 | - | L-阿拉伯糖醇 | + | 0.1%放线菌酮 | - | ||
| D-绵子糖 | - | 柠檬酸 | d | 1%乙酸 | - | ||
| 菊粉 | - | 乙醇 | d | ||||
| 可溶性淀粉 | - | D-阿拉伯糖 | w |
| 样品 | 总蛋白质质量 /μg | 总酶活性 /U | 回收率 /% | 比活性 /U·mg-1 | 纯化 倍数 |
|---|---|---|---|---|---|
| 粗酶液 | 65691.7 | 3034.3 | 100 | 46.2 | 1.0 |
| 一级沉淀上清液 | 54470.7 | 2881.5 | 94.9 | 52.9 | 1.1 |
| 浓缩后 | 10387 | 1017.2 | 33.5 | 97.9 | 2.1 |
表3 菌株NYNU 19160低温脂肪酶纯化结果
| 样品 | 总蛋白质质量 /μg | 总酶活性 /U | 回收率 /% | 比活性 /U·mg-1 | 纯化 倍数 |
|---|---|---|---|---|---|
| 粗酶液 | 65691.7 | 3034.3 | 100 | 46.2 | 1.0 |
| 一级沉淀上清液 | 54470.7 | 2881.5 | 94.9 | 52.9 | 1.1 |
| 浓缩后 | 10387 | 1017.2 | 33.5 | 97.9 | 2.1 |
| 1 | DAVIS B G, BOYER V. Biocatalysis and enzymes in organic synthesis[J]. Natural Product Reports, 2001, 18(6): 618-640. |
| 2 | JOSEPH B, RAMTEKE P W, THOMAS G. Cold active microbial lipases: some hot issues and recent developments[J]. Biotechnology Advances, 2008, 26(5): 457-470. |
| 3 | 陆小波, 魏云林. 低温酶及其冷适应性机制研究进展[J]. 生物技术通报, 2006, 58(4): 51-53, 58. |
| LU Xiaobo, WEI Yunlin. Progress in the study of cold-adapted enzyme and their cold-adaptation mechanisms[J]. Biotechnology Bulletin, 2006, 58(4): 51-53, 58. | |
| 4 | RASHID N, SHIMADA Y, EZAKI S, et al. Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A[J]. Applied and Environmental Microbiology, 2001, 67(9): 4064-4069. |
| 5 | 达文燕, 杨霞霞, 张爱梅. 低温酶及产低温脂肪酶微生物研究概述[J]. 甘肃农业, 2013(13): 14-16. |
| Wenyan DA, YANG Xiaxia, ZHANG Aimei. A review of cryogenic enzymes and microorganisms producing cryogenic lipase[J]. Gansu Agriculture, 2013 (13): 14-16. | |
| 6 | 张学林, 唐湘华, 李俊俊, 等. 低温脂肪酶酶促酯交换制备生物柴油[J]. 中国油脂, 2013, 38(2): 66-68. |
| ZHANG Xuelin, TANG Xianghua, LI Junjun, et al. Preparation of biodiesel by enzymatic transesterification with low temperature lipase[J]. China Oils and Fats, 2013, 38(2): 66-68. | |
| 7 | 薛永强, 张辉华, 王达, 等. 短链脂肪酸对肠道健康的调控机制及在动物生产中的应用[J]. 饲料工业, 2020, 41(19): 18-22. |
| XUE Yongqiang, ZHANG Huihua, WANG Da, et al. Regulation mechanism of short-chain fatty acids on intestinal health and their application in animal production[J]. Feed Industry, 2020, 41(19): 18-22. | |
| 8 | 蔡懿鑫, 蒋慧姣, 孔祥峰, 等. 短链脂肪酸的生理功能及其在仔猪生产中的应用[J]. 中国畜牧杂志, 2019, 55(11): 30-34. |
| CAI Yixin, JIANG Huijiao, KONG Xiangfeng, et al. Physiological function of short-chain fatty acids and their application in piglet production[J]. Chinese Journal of Animal Science, 2019, 55(11): 30-34. | |
| 9 | ARAVINDAN R, ANBUMATHI P, VIRUTHAGIRI T. Lipase applications in food industry[J]. Indian Journal of Biotechnology, 2007, 6(2): 141-158. |
| 10 | 陈贵元, 魏云林. 低温脂肪酶的研究现状与应用前景[J]. 生物技术通报, 2006 (2): 29-32. |
| CHEN Guiyuan, WEI Yunlin. The research status and application prospect of cdd-adapted lipase[J]. Biotechnology Bulletin, 2006(2): 29-32. | |
| 11 | HASSAN S W M, LATIF H H ABD EL, ALI S M. Production of cold-active lipase by free and immobilized marine Bacillus cereus HSS: application in wastewater treatment[J]. Frontiers in Microbiology, 2018, 9: 2377. |
| 12 | BOETIUS A, ANESIO A M, DEMING J W, et al. Microbial ecology of the cryosphere: sea ice and glacial habitats[J]. Nature Reviews Microbiology, 2015, 13(11): 677-690. |
| 13 | 唐兵, 唐晓峰, 彭珍荣. 嗜冷菌研究进展[J]. 微生物学杂志, 2002, 22(1): 51-53. |
| TANG Bing, TANG Xiaofeng, PENG Zhenrong. Andvance in psychrophiles study[J]. Journal of Microbiology, 2002, 22(1): 51-53. | |
| 14 | 谢玉婷, 查代明, 石红璆, 等. Burkholderia sp. JXJ-16低温耐有机溶剂脂肪酶产酶条件优化及粗酶酶学性质[J]. 食品工业科技, 2017, 38(4): 207-213. |
| XIE Yuting, Daiming CHA, SHI Hongqiu, et al. Optimization of enzyme production conditions and enzymatic properties of cold-adapted and organic solvent-tolerant lipase from Burkholderia sp. JXJ-16[J]. Science and Technology of Food Industry, 2017, 38(4): 207-213. | |
| 15 | 王春雨, 迟乃玉, 张庆芳, 等. 低温脂肪酶的分离纯化及酶学性质[J].食品与生物技术学报, 2013, 32(8): 809-813. |
| WANG Chunyu, CHI Naiyu, ZHANG Qingfang, et al. Purification and enzymatic properties of cryogenic lipase[J]. Journal of Food Science and Biotechnology, 2013, 32(8): 809-813. | |
| 16 | PARK S Y, KIM J Y, BAE J H, et al. Optimization of culture conditions for production of a novel cold-active lipase from Pichia lynferdii NRRL Y-7723[J]. Agricultural and Food Chemistry, 2013, 61(4): 882-886. |
| 17 | ABHAS K, MAHARANA, SHIV M. A cold and organic solvent tolerant lipase produced by antarctic strain Rhodotorula sp. Y-23[J]. Journal of Basic Microbiology, 2018, 58(4): 331-342. |
| 18 | 王培培, 刘晓宁, 林国秀, 等. 低温脂肪酶产生菌的筛选、产酶发酵及粗酶性质研究[J]. 工业微生物, 2011, 41(6): 78-84. |
| WANG Peipei, LIU Xiaoning, LIN Guoxiu, et al. Screening, fermentation and crude enzyme properties of low temperature lipase producing bacteria[J]. Industrial Microbiology, 2011, 41(6): 78-84. | |
| 19 | 陈贵元, 季秀玲, 林连兵, 等. 低温脂肪酶产生菌筛选与鉴定、产酶条件及酶学性质研究[J]. 云南大学学报(自然科学版), 2010, 32(1): 108-113. |
| CHEN Guiyuan, JI Xiuling, LIN Lianbing, et al. Screening and identification of low temperature lipase-producing bacteria, enzyme-producing conditions and enzymatic properties[J]. Journal of Yunnan University, 2010, 32(1): 108-113. | |
| 20 | 王兴吉, 贾仁洁, 王克芬, 等. 低温脂肪酶菌株的诱变筛选及酶学特性研究[J]. 安徽农业科学, 2019, 47(1): 1-3, 9. |
| WANG Xingji, JIA Renjie, WANG Kefen, et al. Mutagenesis screening of low temperature lipase strain and its enzymatic characteristics[J]. Anhui Agricultural Sciences, 2019, 47(1): 1-3, 9. | |
| 21 | TANAKA D, YONEDA S, YAMASHIRO Y, et al. Characterization of a new cold-adapted lipase from Pseudomonas sp. TK-3[J]. Applied Biochemistry and Biotechnology, 2012, 168(2): 327-338. |
| 22 | 刘元利, 陈吉祥, 李彦林, 等. 一株产低温脂肪酶沙雷氏菌的鉴定、基因表达及酶学性质[J]. 中国食品学报, 2018, 18(6): 121-129. |
| LIU Yuanli, CHEN Jixiang, LI Yanlin, et al. Identification, gene expression and enzyme characterization of Serratia sp. producing cold adapted lipase[J]. Journal of Chinese Institute of Food Science and Technology, 2018, 18(6): 121-129. | |
| 23 | 萧能, 余瑞元, 袁明秀, 等. 生物化学实验原理和方法[M]. 北京: 北京大学出版社, 2005. |
| XIAO neng, YU ruiyuan, YUAN mingxiu, et al. Principles and methods of biochemical experiments[M]. Beijing: Peking University Press, 2005. | |
| 24 | MARGESIN R, FELLER G, HÄMMERLE M, et al. A colorimetric method for the determination of lipase activity in soil[J]. Biotechnology Letters, 2002, 24(1): 27-33. |
| 25 | KURTZMAN C P, FELL J W, BOEKHOUT T, et al. Methods for isolation, phenotypic characterization and maintenance of yeasts[M]// The Yeasts. Amsterdam: Elsevier, 2011: 87-110. |
| 26 | FONSECA Á, BOEKHOUT T, FELL J W. Cryptococcus Vuillemin (1901)[M]. 5th ed. Amsterdam: Elsevier, 2011: 1659-1737. |
| 27 | THOMPSON J D, GIBSON T J, PLEWNIAK F, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools nucleic[J]. Nucleic Acids Research, 1997, 25(24): 4876-4882. |
| 28 | 李司宇, 刘雪, 王文婧, 等. 微生物进化树构建方法[J]. 现代农业科技, 2019(19): 249-250, 253. |
| LI Siyu, LIU Xue, WANG Wenjing, et al. Construction of microbial evolutionary trees[J]. Modern Agricultural Science and Technology, 2019(19): 249-250, 253. | |
| 29 | SAITOU N, NEI M. The neighbor-joining method: a new method for reconstructing phylogenetic trees[J]. Molecular Biology and Evolution, 1987, 4(4): 406-425. |
| [1] | 孙鲁芹, 卢会霞, 王建友. 电渗析/超滤内在耦合过程分离蛋清中溶菌酶[J]. 化工进展, 2023, 42(5): 2262-2271. |
| [2] | 王哲, 余颖, 石永杰, 杨顺, 陈久洲, 崔希利, 邢华斌. 尿素包合法分离费托轻质油中的正构烃[J]. 化工进展, 2023, 42(2): 677-683. |
| [3] | 毛停停, 李双福, 黄李茗铭, 周川玲, 韩凯. 面向水处理与有机溶剂回收的太阳能界面蒸发系统与材料[J]. 化工进展, 2023, 42(1): 178-193. |
| [4] | 齐亚兵, 贾宏磊. 熔融结晶技术分离纯化有机化合物的研究进展[J]. 化工进展, 2023, 42(1): 373-385. |
| [5] | 黄晔, 言行, 吴巧薇, 柴小涛, 潘恭赢, 张金峰, 李相前. 环孢菌素A柱层析硅胶再生工艺[J]. 化工进展, 2022, 41(S1): 461-468. |
| [6] | 马镓莉, 卢会霞, 苗晓雪. 基于膜技术分离纯化乳清蛋白的研究进展[J]. 化工进展, 2022, 41(6): 2826-2838. |
| [7] | 孔庆强, 黄显虹, 王振兵, 郭晓倩, 谢莉婧, 苏方远, 孙国华, 陈成猛. 超级电容器用活性炭国产化关键化学与化工问题[J]. 化工进展, 2021, 40(9): 5088-5096. |
| [8] | 张春伟, 张学军, 赵阳. 应用于空分纯化系统的相变储热器建模及分析[J]. 化工进展, 2021, 40(6): 3099-3106. |
| [9] | 李阳, 朱晨辉, 范代娣. 重组胶原蛋白的绿色生物制造及其应用[J]. 化工进展, 2021, 40(3): 1262-1275. |
| [10] | 仲文雅, 俞汶佳, 谷艺明, 郭静, 樊博, 蔡志强. 生物合成安丝菌素的研究进展[J]. 化工进展, 2021, 40(2): 990-997. |
| [11] | 陈亮, 田飞豹, 辛秀兰, 兰蓉, 于然, 吴志明, 梁浩. 核黄素超声结晶工艺优化[J]. 化工进展, 2021, 40(2): 1018-1024. |
| [12] | 邹俊康, 鲍宗必, 杨启炜, 张治国, 任其龙, 杨亦文. 甘油磷酰胆碱的分析、制备及纯化研究进展[J]. 化工进展, 2021, 40(11): 6295-6304. |
| [13] | 梁高峰, 陈学青, 王德武, 张少峰, 刘燕, 张继军. 淘洗六水氯化铝过程中晶体沉降速度与纯化规律分析[J]. 化工进展, 2021, 40(1): 386-393. |
| [14] | 赵鑫, 熊健力, 任叶琳, 杨家鑫, 李伟, 韩雪容. 细菌纤维素合成与鉴定研究综述[J]. 化工进展, 2020, 39(S2): 262-268. |
| [15] | 饶轶晟, 杨晓健, 张红, 胡国涛. 湿法磷酸净化脱砷的研究现状[J]. 化工进展, 2020, 39(S1): 219-224. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||