化工进展 ›› 2021, Vol. 40 ›› Issue (2): 990-997.DOI: 10.16085/j.issn.1000-6613.2020-0597
生物合成安丝菌素的研究进展
仲文雅( ), 俞汶佳, 谷艺明, 郭静, 樊博, 蔡志强(
), 俞汶佳, 谷艺明, 郭静, 樊博, 蔡志强( )
)
- 常州大学药学院,江苏 常州 213164
-
收稿日期:2020-04-15修回日期:2020-08-06出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:蔡志强 -
作者简介:仲文雅(1994—),女,硕士研究生,研究方向为生物制药与微生物发酵。E-mail:1959333715@qq.com 。 -
基金资助:国家自然科学基金(11275033);江苏省教育厅高校自然科学基金(19KJB180001)
Progress of ansamitocins by biosynthesis
Wenya ZHONG( ), Wenjia YU, Yiming GU, Jing GUO, Bo FAN, Zhiqiang CAI(
), Wenjia YU, Yiming GU, Jing GUO, Bo FAN, Zhiqiang CAI( )
)
- School of Pharmacy, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2020-04-15Revised:2020-08-06Online:2021-02-05Published:2021-02-09 -
Contact:Zhiqiang CAI
摘要:
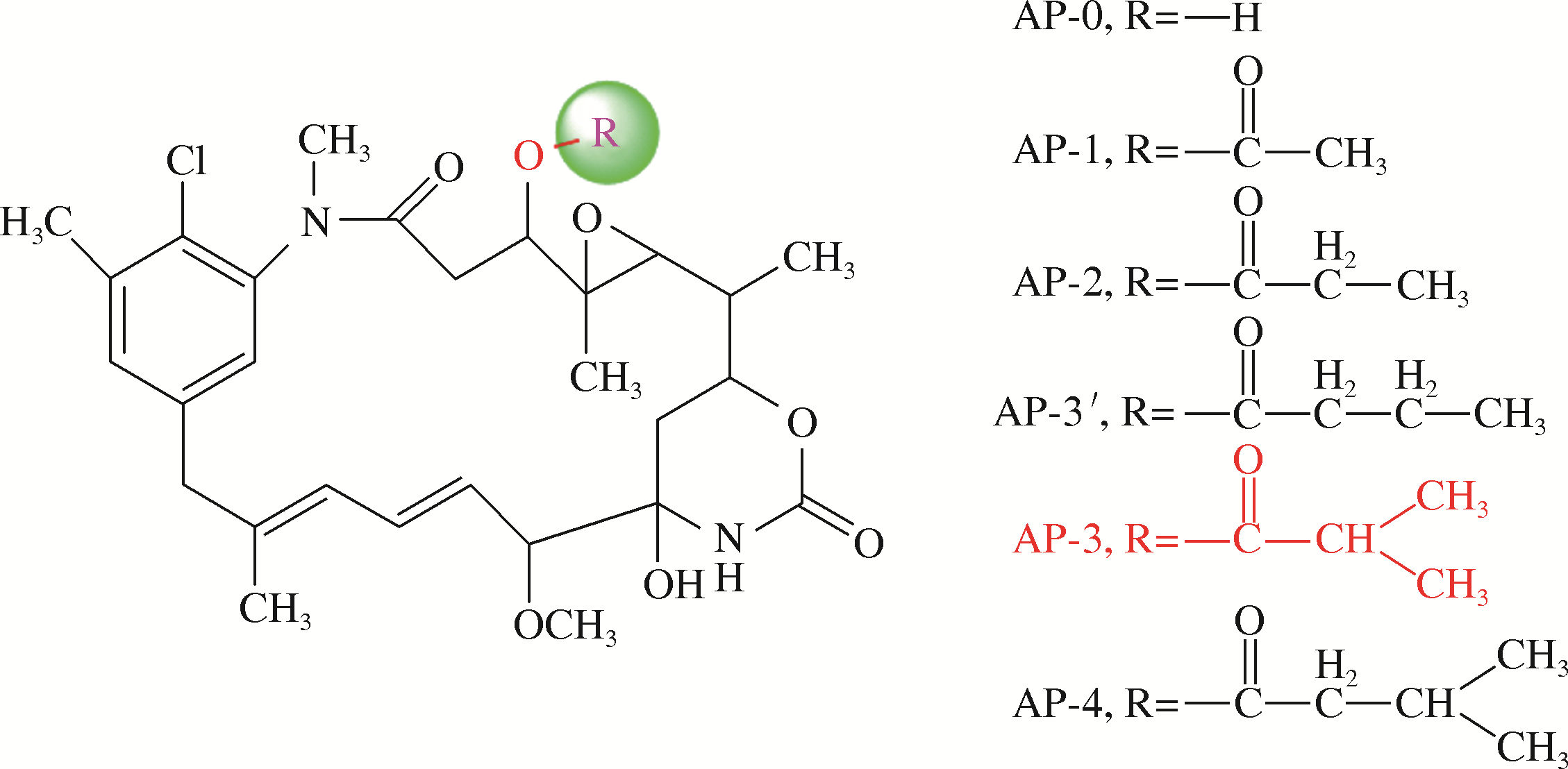
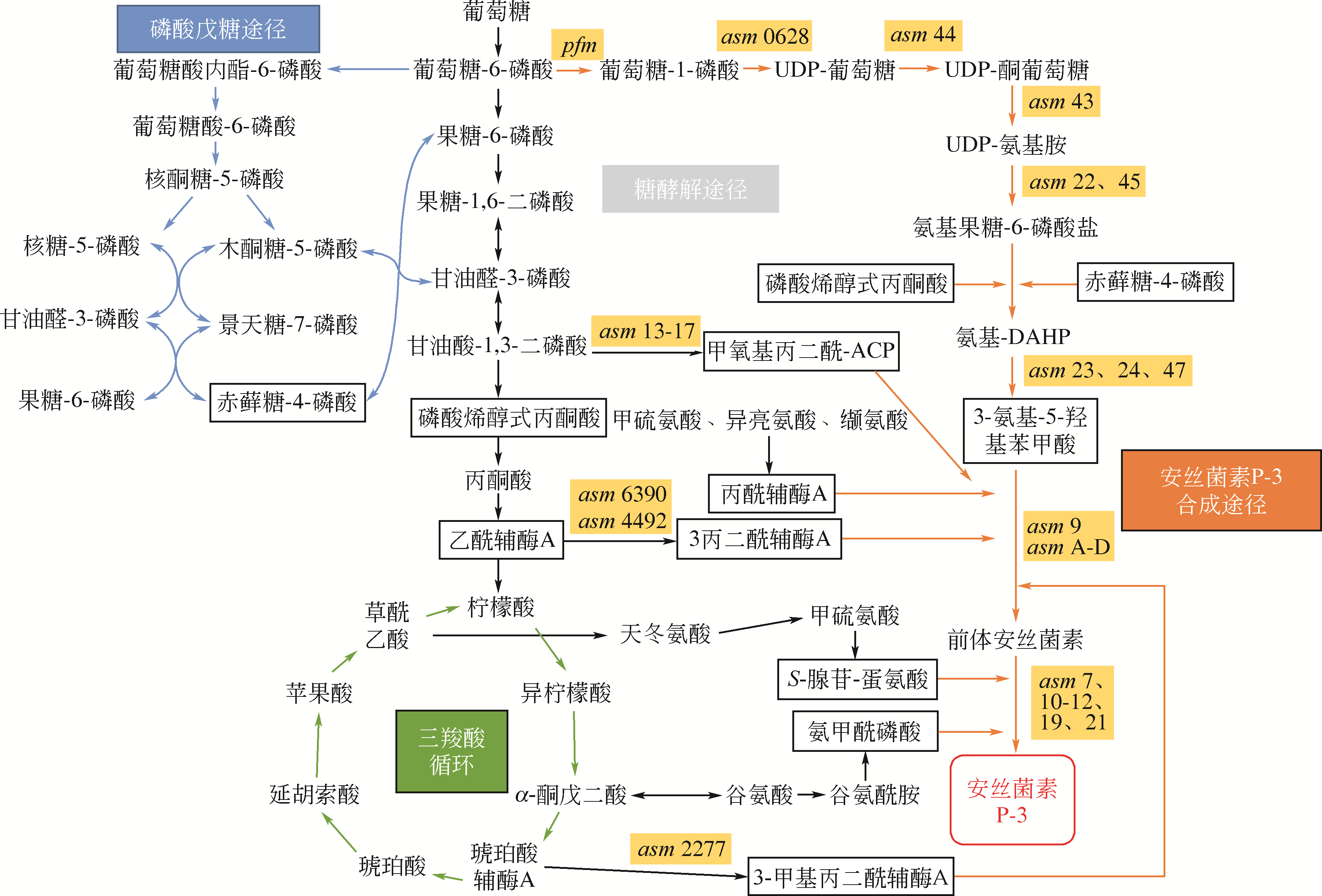
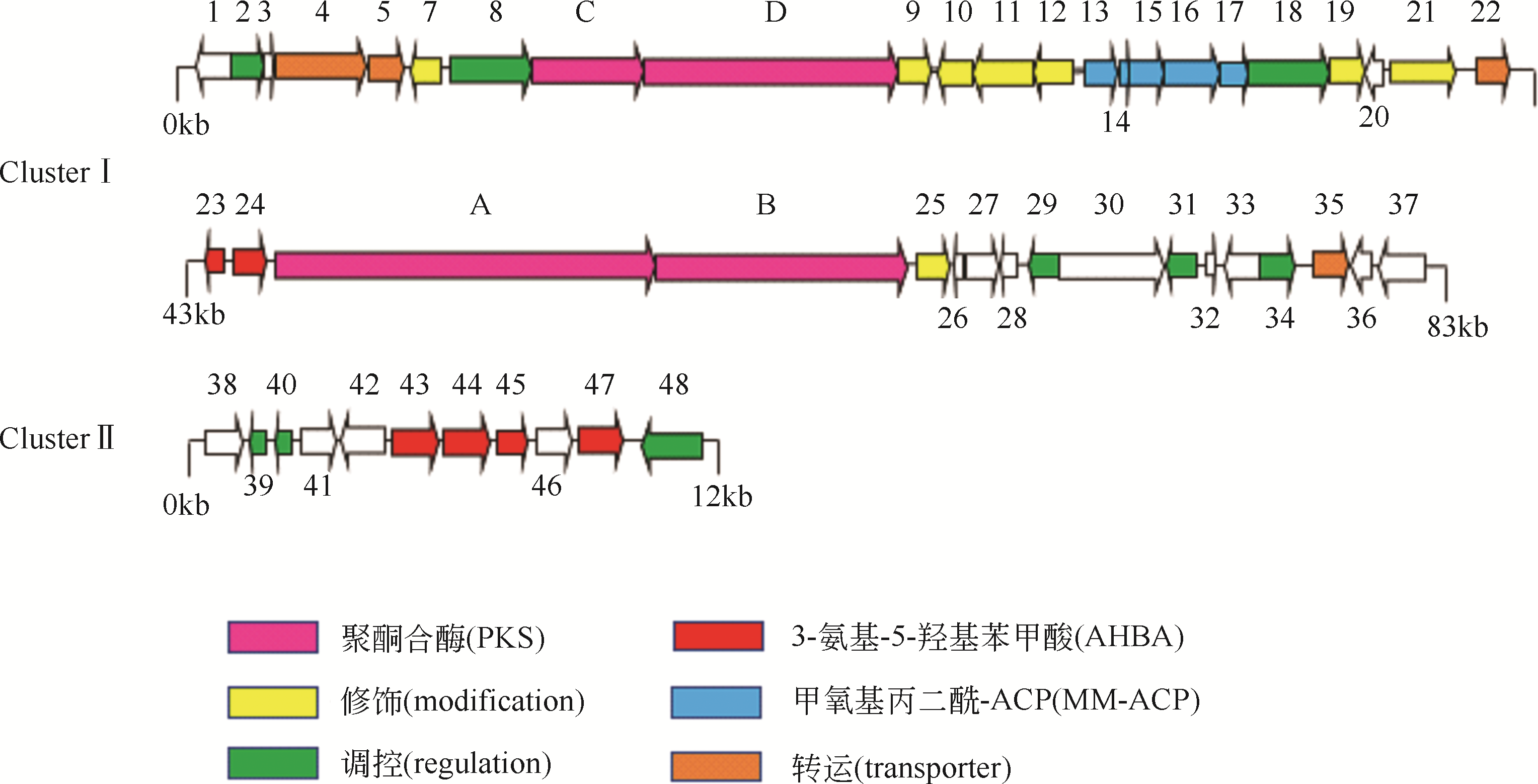
安丝菌素(Ansamitocin)是一种微生物来源的美登素类抗生素,具有独特的抗癌活性,其中AP-3在安丝菌素中所占比例最高,是发酵的目标产物,在临床上有靶向治疗乳腺癌的效果,因此具有重要的药用研究价值。但当前AP-3的微生物发酵产量不高,高昂的生产成本制约了其进一步的应用。本文在阐述安丝菌素生物合成及代谢调控机制的基础上,分析讨论了微生物发酵产安丝菌素过程中菌种选育、发酵复杂过程的控制与优化以及下游的分离纯化技术等研究进展。再对未来利用新型诱变技术、合成生物学技术与辅因子工程改造等方法选育高产安丝菌素的工程菌株,以及开发高效创新、低成本的分离纯化工艺进行了展望,以期显著地提高安丝菌素的发酵水平,为工业化安丝菌素的发展奠定坚实的基础。
中图分类号:
引用本文
仲文雅, 俞汶佳, 谷艺明, 郭静, 樊博, 蔡志强. 生物合成安丝菌素的研究进展[J]. 化工进展, 2021, 40(2): 990-997.
Wenya ZHONG, Wenjia YU, Yiming GU, Jing GUO, Bo FAN, Zhiqiang CAI. Progress of ansamitocins by biosynthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 990-997.
| 菌株 | 育种方法 | AP-3产量 | 参考文献 |
|---|---|---|---|
| A. pretiosum | 紫外诱变+化学诱变 | 150~370mg/L | [ |
| A. pretiosum | 基因工程 | 52mg/L,33mg/L | [ |
| A. pretiosumsp. auranticum strainSIPI-3-21 | 紫外诱变 | 367mg/L | [ |
| A. pretiosumsp. auranticum strainHF-064 | 常压常温等离子体诱变技术(ARTP)+化学诱变 | 287mg/L | [ |
| A. pretiosumsp. auranticum strainG3-368 | 化学诱变、原生质体制备与再生+基因组改组技术 | 132mg/L | [ |
| A. pretiosum sp. auranticum ATCC 31565 | 基因工程 | 70mg/L | [ |
| A. pretiosum | 基因工程 | 246mg/L | [ |
| Nocardiopsisansamitocini EGI80425 | 基因工程 | 12.6mg/L,7.4mg/L | [ |
| A. pretiosumATCC 31280 | 代谢工程 | 99mg/L | [ |
| A. pretiosumsp. auranticumstrainNXJ-22 | 代谢工程 | 141.8mg/L | [ |
表1 微生物发酵产AP-3菌株育种研究概况
| 菌株 | 育种方法 | AP-3产量 | 参考文献 |
|---|---|---|---|
| A. pretiosum | 紫外诱变+化学诱变 | 150~370mg/L | [ |
| A. pretiosum | 基因工程 | 52mg/L,33mg/L | [ |
| A. pretiosumsp. auranticum strainSIPI-3-21 | 紫外诱变 | 367mg/L | [ |
| A. pretiosumsp. auranticum strainHF-064 | 常压常温等离子体诱变技术(ARTP)+化学诱变 | 287mg/L | [ |
| A. pretiosumsp. auranticum strainG3-368 | 化学诱变、原生质体制备与再生+基因组改组技术 | 132mg/L | [ |
| A. pretiosum sp. auranticum ATCC 31565 | 基因工程 | 70mg/L | [ |
| A. pretiosum | 基因工程 | 246mg/L | [ |
| Nocardiopsisansamitocini EGI80425 | 基因工程 | 12.6mg/L,7.4mg/L | [ |
| A. pretiosumATCC 31280 | 代谢工程 | 99mg/L | [ |
| A. pretiosumsp. auranticumstrainNXJ-22 | 代谢工程 | 141.8mg/L | [ |
| 1 | HIGASHIDE E, ASAI M, OOTSU K, et al. Ansamitocin, a group of novel maytansinoid antibiotics withantitumour properties from Nocardia[J]. Nature, 1977, 270(5639): 721-722. |
| 2 | MA N, WEI L, FAN Y X, et al. Herterologoua expression characterization of soluble recombination 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase from Actinosynnema pretiosum ATCC 31565 through co-expression with chaperones in Escherichia coli[J]. Protein Expression and Purification, 2012, 82(2): 263-269. |
| 3 | AMIRI-KORDESTANI L, BLUMENTHAL G M, XU Q C, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer[J]. Clinical Cancer Research, 2014, 20(17): 4436-4441. |
| 4 | COLLIGNON P J, POWERS J H, CHILLER T M, et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: a criticalsteps for developing risk management strategies for the use of antimicrobials in food production animals[J]. Clinical Infectious Diseases, 2009, 49: 132-141. |
| 5 | 宁新娟. 安丝菌素生物合成瓶颈解析及产量提高[D]. 上海: 上海交通大学, 2017. |
| NING Xinjuan. Analysis of bottlenecks and production of ansinomycin biosynthesis[D]. Shanghai: Shanghai Jiao Tong University, 2017. | |
| 6 | MIYANAGA A. Structure and function of polyketide biosynthetic enzymes: various strategies for production of structurally diverse polyketides[J]. Bioscience, Biotechnology and Biochemistry, 2017, 81(12): 2227-2236. |
| 7 | YU T W, MÜLLER R, MULLER M, et al. Mutational analysis and reconstituted expression of the biosynthetic genes involved in the formation of 3-amino-5-hydroxybenzoic acid, the starter unit of rifamycin biosynthesis in Amycolatopsis mediterranei S699[J]. Journal of Biotechnology and Chemistry, 2001, 276:12546-12555. |
| 8 | MOSS S J, BAI L Q, TOELZER S, et al. Identification of asm19 as an acyltransferase attaching the biologically essential ester side chain of ansamitocins using N-desmethyl-4,5-desepoxymaytansinol, not maytansinol, as its substrate[J]. Journal of the American Chemical Society, 2002, 124(23): 6544-6545. |
| 9 | SPITELLER P, BAI L Q, SHANG G D, et al. The post-polyketide synthase modification steps in the biosynthesis of the antitumor agent ansamitocin by Actinosynnema pretiosum[J]. Journal of the American Chemical Society, 2003, 125(47): 14236-14237. |
| 10 | YU T W, BAI L Q, CLADE D, et al. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum[J]. Proceedings of the National Academy of Sciences, 2002, 99(12):7968-7973. |
| 11 | KAKAHAMA K, IZAWA M, ASAI M, et al. Microbiol conversion of ansamitocin[J]. Journal of Antibiot, 1981, 34(12):1581-1586. |
| 12 | ASAI M, HATANO K, HIGASHIDE E. Method for producing antibiotic C-15003 by culturing nocardia:US4162940[P]. 1979-01-30. |
| 13 | CHUNG J, BYNG G S, SNOHOMISH W A. Mutant Actinosynnema pretiosum strain with increased maytansinoid production: US7192750[P]. 2003-01-15. |
| 14 | NG D, CHIN H K, WONG V V T. Constitutive over expression of asm2 and asm39 increases AP-3 production in the actinomycete Actinosynnema pretiosum[J]. Journal of Industrial Microbiology and Biotechnology, 2009, 36(11): 1345-1351. |
| 15 | FAN Y X, ZHAO M J, WEI L J, et al. Enhancement of UDPG synthetic pathway improves ansamitocin production in Actinosynnem pretiosum[J]. Applied Microbiology and Biotechnology, 2016, 100(6): 2651-2662. |
| 16 | FAN Y X, HU F X, WEI L J, et al. Effects of modulation of pentose-phosphate pathway on biosynthesis of ansamitocins in Actinosynnema pretiosum[J]. Journal of Biotechnology, 2016, 230: 3-10. |
| 17 | DU Z Q, ZHANG Y, QIAN Z G, et al. Combination of traditional mutation and metabolic engineering to enhance ansamitocin P-3 production in Actinosynnema pretiosum[J]. Biotechnology and Bioengineering, 2017, 114(12): 2794-2806. |
| 18 | DU Z Q, ZHONG J J. Rational approach to improve ansamitocin P-3 production by integrating pathway engineering and substrate feeding in Actinosynnema pretiosum[J]. Biotechnology and Bioengineering, 2018, 115(10): 2456-2466. |
| 19 | 荣艳, 郭静, 苏春, 等. 安丝菌素P-3生产菌株Actinosynnema pretiosum ssp. auranticum的诱变育种及其发酵培养基优化[J]. 化工进展, 2018, 37(10): 295-301. |
| RONG Yan, GUO Jing, SU Chun, et al. Mutation breeding and optimization of fermentation medium for ansamitocinP-3 production from Actinosynnema pretiosum ssp. auranticum[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3988-3994. | |
| 20 | 伊丹, 王岩, 陈少欣. 安丝菌素P-3 的发酵工艺[J]. 中国医药工业杂志, 2012, 43(4): 260-262. |
| YI Dan, WANG Yan, CHEN Shaoxin. Fermentation process of ansinomycin P-3[J]. China Journal of Pharmaceutical Industry, 2012, 43(4): 260-262. | |
| 21 | 张金龙, 李芳. 高产安丝菌素的珍贵束丝放线菌菌种选育[J]. 发酵科技通讯, 2015, 44(4): 43-47. |
| ZHANG Jinlong, LI Fang. Breeding of enhanced ansamitocin producing strains of Actinosynnema pretiosum[J]. Bulletin of Fermentation Science and Technology, 2015, 44(4): 43-47. | |
| 22 | 梁美贤. 安丝菌素AP-3高产菌株选育及发酵条件优化[D].福州: 福建师范大学, 2016. |
| LIANG Meixian. Breeding and optimization of fermentation conditions for anmycin AP-3 high yield strain[D]. Fuzhou: Fujian Normal University, 2016. | |
| 23 | ZHAO M, FAN Y, WEI L, et al. Effects of the methylmalonyl-CoA metabolic pathway on ansamitocin production in Actinosynnema pretiosum[J]. Applied Biochemistry and Biotechnology, 2017, 181(3): 1167-1178. |
| 24 | NING X, WANG X, WU Y, et al. Identification and engineering of post-PKS modification bottlenecks for ansamitocin P-3 titer improvement in Actinosynnema pretiosum subsp. pretiosum ATCC 31280[J]. Biotechnology Journal, 2017, 12(11): 41-48. |
| 25 | 成筱钰, 王欣然, 康前进, 等. 安丝菌素产生菌拟诺卡氏菌EGI80425接合转移体系的建立与应用[J]. 基因组学与应用生物学, 2020(3): 1163-1171. |
| CHENG Xiaoyu, WANG Xinran, KANG Qianjin, et al. Establishment and application of an anomycin-producing strain Nocardiopsis ansamitocini EGI80425 junction transfer system[J]. Genomics and Applied Biology, 2020(3): 1163-1171. | |
| 26 | 于丽洁, 白林泉, 宁新娟, 等. 增强聚酮合酶基因转录水平的高产安丝菌素菌株及其制备方法: CN107881139[P]. 2018-04-06. |
| YU Lijie, BAI Linquan, NING Xinjuan, et al. High-yielding anmycin strain that enhances the transcription level of polyketide synthase gene and its preparation method: CN107881139[P]. 2018-04-06. | |
| 27 | 于丽洁, 白林泉, 宁新娟, 等. 增强转录水平的高产安丝菌素菌株及其制备方法: CN107881137[P]. 2018-04-06. |
| YU Lijie, BAI Linquan, NING Xinjuan, et al. Highly-producing anmycin strains with enhanced transcription levels and preparation methods: CN107881137[P]. 2018-04-06. | |
| 28 | BANDI S, KIM Y, CHANG Y K, et al. Construction of asm2 deletion mutant of Actinosynnema pretiosum and medium optimization for ansamitocin P-3 production using statistical approach[J]. Journal of Microbiology and Biotechnology, 2006, 16(9):1338-1346. |
| 29 | GAO Y, FAN Y, NAMBOU K, et al. Enhancement of ansamitocin P-3 production in Actinosynnema pretiosum by a synergistic effect of glycerol and glucose[J]. Journal of Industrial Microbiology and Biotechnology, 2014, 41(1):143-152. |
| 30 | FAN Y X, GAO Y, ZHOU J, et al. Process optimization with alternative carbon sources and modulation of secondary metabolism for enhanced ansamitocin P-3 production in Actinosynnema pretiosum[J]. Journal of Biotechnology, 2014, 192:1-10. |
| 31 | HATANO K, HIGASHIDE E, AKIYAMA S I, et al. Selective accumulation of ansamitocins P-2, P-3 and P-4 and biosynthetic origins of their acyl moieties[J]. Agricultural and Biological Chemistry, 1984, 48(7):1721-1729. |
| 32 | LIN J X, BAI L Q, DENGZ X, et al. Enhanced production of ansamitocin P-3 by addition of isobutanol in fermentation of Actinosynnema pretiosum[J]. Bioresource Technology, 2010, 102(2):1863-1868. |
| 33 | LIN J X, ZHONG J J. Proteomic studies on anti-tumor agent ansamitocin P-3 producer Actinosynnema pretiosumin response to ammonium and isobutanol[J]. Bioprocess and Biosystems Engineering, 2017, 40(7):1133-1139. |
| 34 | 李婷兰, 韦柳静, 范宇翔, 等. 奇迹束丝放线菌合成安丝菌素过程中支链氨基酸的添加效果分析[J]. 华东理工大学学报(自然科学版),2013, 39(6): 675-680. |
| LI Tinglan, WEI Liujing, FAN Yuxiang, et al. Analysis of the effect of adding branched chain amino acids in the process of synthesis of anomycin by miracle beam Actinomyces[J]. Journal of East China University of Science and Technology (Natural Science Edition), 2013, 39(6): 675-680. | |
| 35 | JIA Y L, ZHONG J J. Enhanced production of ansamitocin P-3 by addition of Mg2+ in fermentation of Actinosynnema pretiosum[J]. Bioresource Technology, 2011, 102(21): 10147-10150. |
| 36 | 南艳妮. 安丝菌素固体发酵及安丝菌素P-0制备[D]. 北京: 北京协和医学院, 2015. |
| Yanni NAN. Ansicin solid fermentation and preparation of ansinomycin P-0[D]. Beijing: Peking Union Medical College, 2015. | |
| 37 | 周少敏, 郭静, 王怡, 等. 珍贵橙色束丝放线菌固体发酵合成安丝菌素P-3的工艺探索[J]. 化工进展, 2018, 37(12): 4837-4844. |
| ZHOU Shaomin, GUO Jing, WANG Yi, et al. Optimal process of solidstate fermentation for ansamitocin P-3 production by Actinosynnema pretiosum[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4837-4844. | |
| 38 | 林锦霞. 橙色珍贵束丝放线菌发酵生产抗癌药物安丝菌素P-3的研究[D]. 上海: 华东理工大学, 2011. |
| LIN Jinxia. Study on the production of anticancer drug ansinomycin P-3 by the fermentation of orange precious actinomycetes[D]. Shanghai: East China University of Science and Technology, 2011. | |
| 39 | 朱晓媛.微生物发酵法制备安丝菌素P-3的研究[D]. 长沙: 中南林业科技大学, 2014. |
| ZHU Xiaoyuan. Study on preparation of ansinomycin P-3 by microbial fermentation[D]. Changsha: Central South University of Forestry and Technology, 2014. | |
| 40 | 沈晓放, 伊丹, 陈少欣. 珍贵橙色束丝放线菌生产安丝菌素P-3的发酵工艺[J]. 中国医药工业杂志, 2017, 48(10): 1449-1453. |
| SHEN Xiaofang, YI Dan, CHEN Shaoxin. Fermentation process of Ansamitocin P-3 by Actinosynnema Pretiosum[J]. China Journal of Pharmaceutical Industry, 2017, 48 (10): 1449-1453. | |
| 41 | 朱晓媛, 黎继烈, 韦晓菊, 等. 基于遗传算法的安丝菌素P-3分批发酵动力学研究[J]. 中国酿造, 2013, 32(12): 101-104. |
| ZHU Xiaoyuan, LI Jilie, WEI Xiaoju, et al. Kinetics of batch fermentation of aspirin P-3 based on genetic algorithm[J]. China Brewing, 2013, 32 (12): 101-104. | |
| 42 | LI Jian, SUN Renliang, NING Xinjuan, et al. Genome-Scale metabolic model of Actinosynnema pretiosum ATCC 31280 and its application for Ansamitocin P-3 production improvement[J]. Genes, 2018, 9(7):364-370. |
| 43 | 王凯, 李婷, 师瑞芳, 等. 链霉菌生物活性物质分离纯化技术研究进展[J]. 食品工业科技, 2015(14): 373-378. |
| WANG Kai, LI Ting, SHI Ruifang, et al. Research progress on separation and purification technology of bioactive substances from Streptomyces[J]. Science and Technology of Food Industry, 2015(14): 373-378. | |
| 44 | 杨金亮, 姚于勤, 陈俐娟, 等. 从珍贵橙色束丝放线菌发酵液中分离纯化安丝菌素的方法: CN101928291[P]. 2010-12-29. |
| YANG Jinliang, YAO Yuqin, CHEN Lijuan, et al. Separation and purification of anthracycline from the fermentation broth of Actinosynnema Pretiosum: CN101928291[P]. 2010-12-29. | |
| 45 | 毛思雨, 陈宏, 陈丽, 等. 放线菌FIM06-0063产生的安莎类化合物的分离、纯化及其结构鉴定[J]. 中国抗生素杂志, 2013, 38(1): 36-40. |
| MAO Siyu, CHEN Hong, CHEN Li, et al. Isolation, purification, and structure identification of ansamycin compounds from Actinomycetes FIM06-0063[J]. Chinese Journal of Antibiotics, 2013, 38(1): 36-40. | |
| 46 | 吴兴可, 祁荃, 卞婷婷, 等. 珍贵橙色束丝放线菌发酵产安丝菌素P-3的分离纯化工艺优化[J]. 化工进展, 2020, 39(3): 1122-1128. |
| WU Xingke, QI Quan, BIAN Tingting, et al. Optimization of separation and purification process of ansamitocin P-3 from fermentation broth of Actinosynnema pretiosum ssp. auranticum[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1122-1128. |
| [1] | 刘宇龙, 姚俊虎, 舒闯闯, 佘跃惠. 磁性Fe3O4纳米颗粒的生物合成及其在提高采收率中的应用[J]. 化工进展, 2023, 42(5): 2464-2474. |
| [2] | 司银芳, 胡语婕, 张凡, 董浩, 佘跃惠. 生物合成氧化锌纳米颗粒材料及其抗菌应用[J]. 化工进展, 2023, 42(4): 2013-2023. |
| [3] | 冯阳阳, 赵众从, 杨文博, 胡琳琪, 张文达, 佘跃惠. 微生物合成金属纳米颗粒及在稠油催化降黏中的应用研究进展[J]. 化工进展, 2021, 40(4): 2215-2226. |
| [4] | 吴兴可,祁荃,卞婷婷,刘苏煜,谷艺明,郭静,蔡志强. 珍贵橙色束丝放线菌发酵产安丝菌素P-3的分离纯化工艺优化[J]. 化工进展, 2020, 39(3): 1122-1128. |
| [5] | 程荡, 陈芬儿. 连续流微反应技术在药物合成中的应用研究进展[J]. 化工进展, 2019, 38(01): 556-575. |
| [6] | 程申, 张颂红, 贠军贤. α-酮异己酸的生物合成研究进展[J]. 化工进展, 2018, 37(12): 4821-4829. |
| [7] | 富敏霞, 祝铃钰, 贠军贤. 乳酸脱氢酶的分离纯化及其催化合成苯乳酸的研究进展[J]. 化工进展, 2018, 37(12): 4814-4820. |
| [8] | 于新磊, 毛雨丰, 张晓霞, 陆凌雪, 王智文, 陈涛. 生物法生产3-羟基丙酸研究进展[J]. 化工进展, 2018, 37(11): 4427-4436. |
| [9] | 王晓静, 魏琦峰, 任秀莲. 乙醇酸和聚乙醇酸的制备与分离研究进展[J]. 化工进展, 2018, 37(09): 3577-3584. |
| [10] | 张翔, 张彦昊, 刘孝永, 辛雪, 王易芬, 陈蕾蕾, 周庆新, 赵双枝. 产壳聚糖酶菌株ncps116发酵条件优化及其酶学性质[J]. 化工进展, 2018, 37(06): 2354-2363. |
| [11] | 倪正, 关今韬, 沈绍传, 贠军贤. 苯乳酸的微生物合成及分离研究进展[J]. 化工进展, 2016, 35(11): 3627-3633. |
| [12] | 张晓华, 姜岩, 岳希权, 张贤明. 生物表面活性剂驱油研究进展[J]. 化工进展, 2016, 35(07): 2033-2040. |
| [13] | 许旭旭1,毛 政2,吴 斌1,孙洪林1,何冰芳1. 高速逆流色谱法分离纯化内吗啡肽-1 [J]. 化工进展, 2011, 30(9): 2064-. |
| [14] | 黄锦标1,尚龙安2. 聚羟基烷酸酯的生物合成研究进展 [J]. 化工进展, 2011, 30(9): 2041-. |
| [15] | 李金娟,赵 林,谭 欣,黄 宇,柳听义. 利用淀粉酸化废水驯化活性污泥合成聚-?-羟基脂肪酸酯及其表征 [J]. 化工进展, 2011, 30(7): 1618-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||