化工进展 ›› 2023, Vol. 42 ›› Issue (5): 2464-2474.DOI: 10.16085/j.issn.1000-6613.2022-1246
磁性Fe3O4纳米颗粒的生物合成及其在提高采收率中的应用
- 长江大学石油工程学院,湖北 武汉 430100
-
收稿日期:2022-07-04修回日期:2022-08-23出版日期:2023-05-10发布日期:2023-06-02 -
通讯作者:佘跃惠 -
作者简介:刘宇龙(1995—),男,博士研究生,研究方向为生物纳米解堵增注和提高采收率。E-mail:yulong13220@163.com。 -
基金资助:国家自然科学基金(51634008);国家重大科技专项(2017ZX05009-004)
Biosynthesis and EOR application of magnetic Fe3O4 NPs
LIU Yulong( ), YAO Junhu, SHU Chuangchuang, SHE Yuehui(
), YAO Junhu, SHU Chuangchuang, SHE Yuehui( )
)
- College of Petroleum Engineering, Yangtze University, Wuhan 430100, Hubei, China
-
Received:2022-07-04Revised:2022-08-23Online:2023-05-10Published:2023-06-02 -
Contact:SHE Yuehui
摘要:
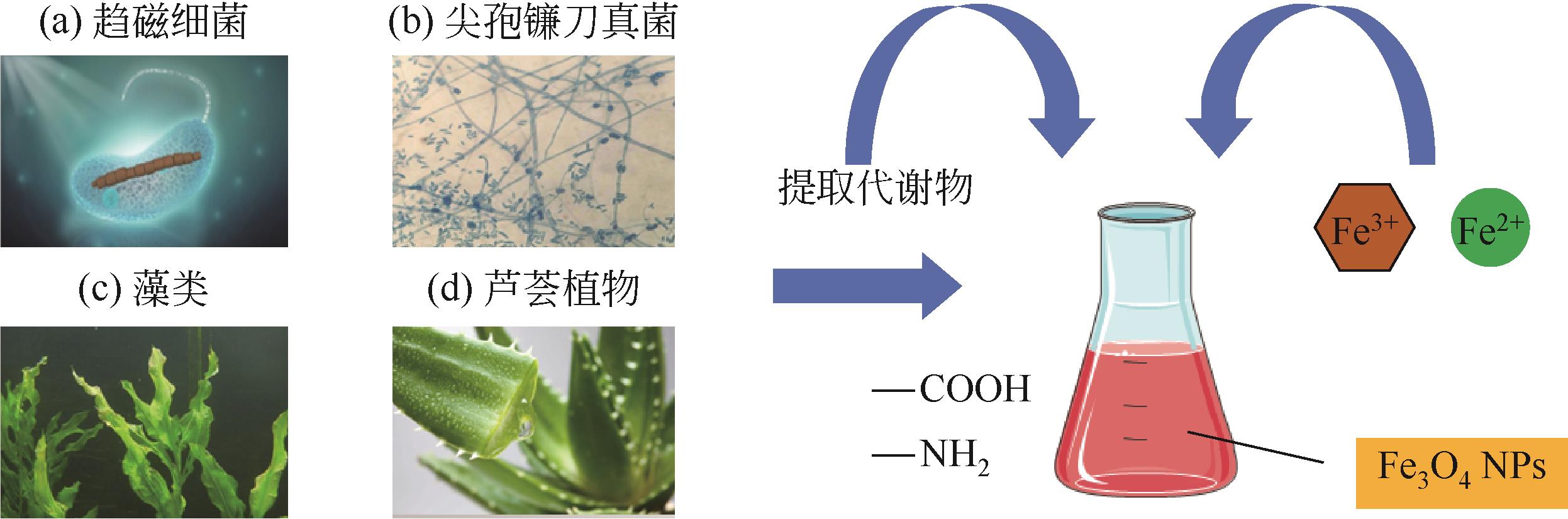
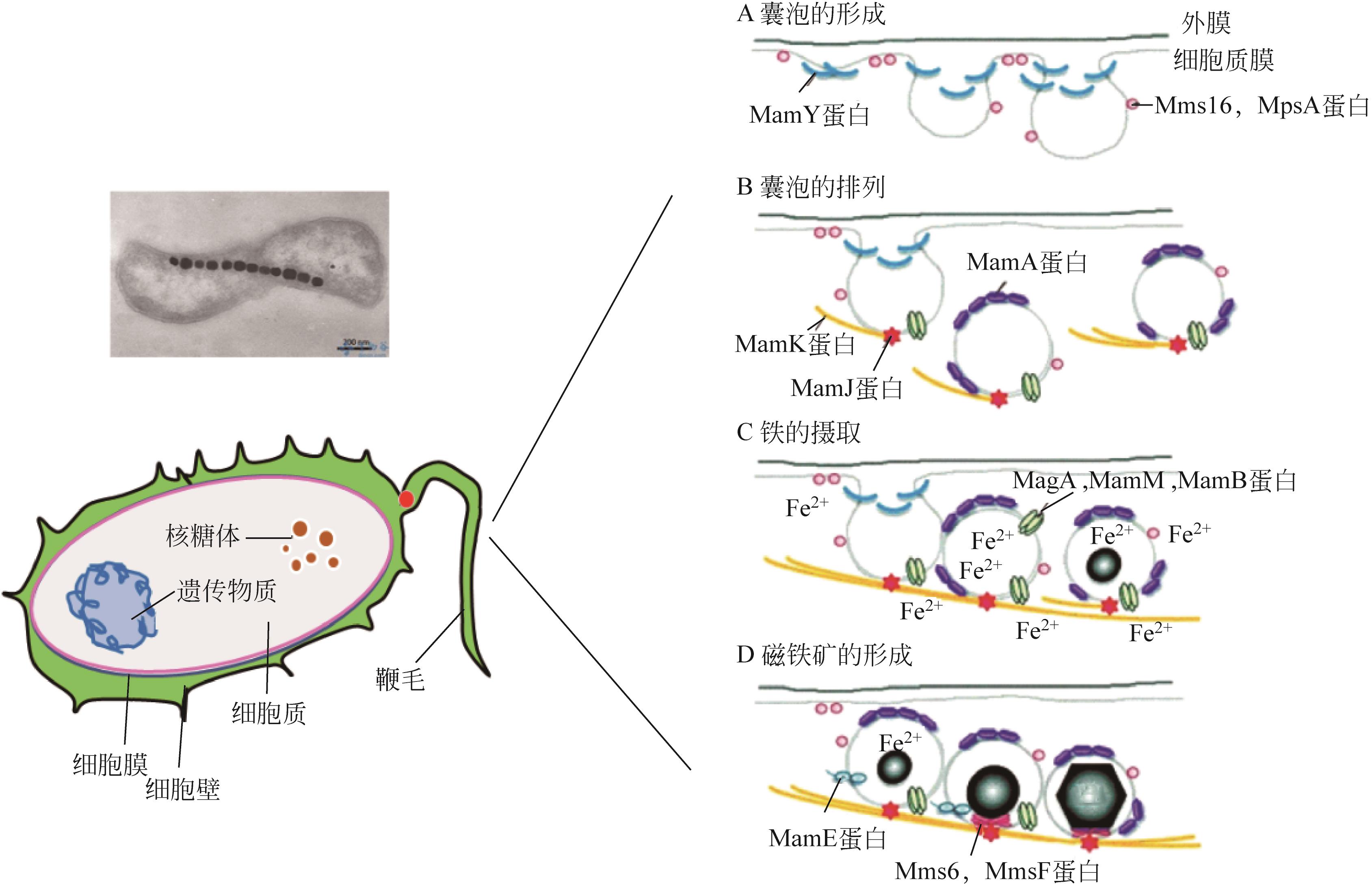
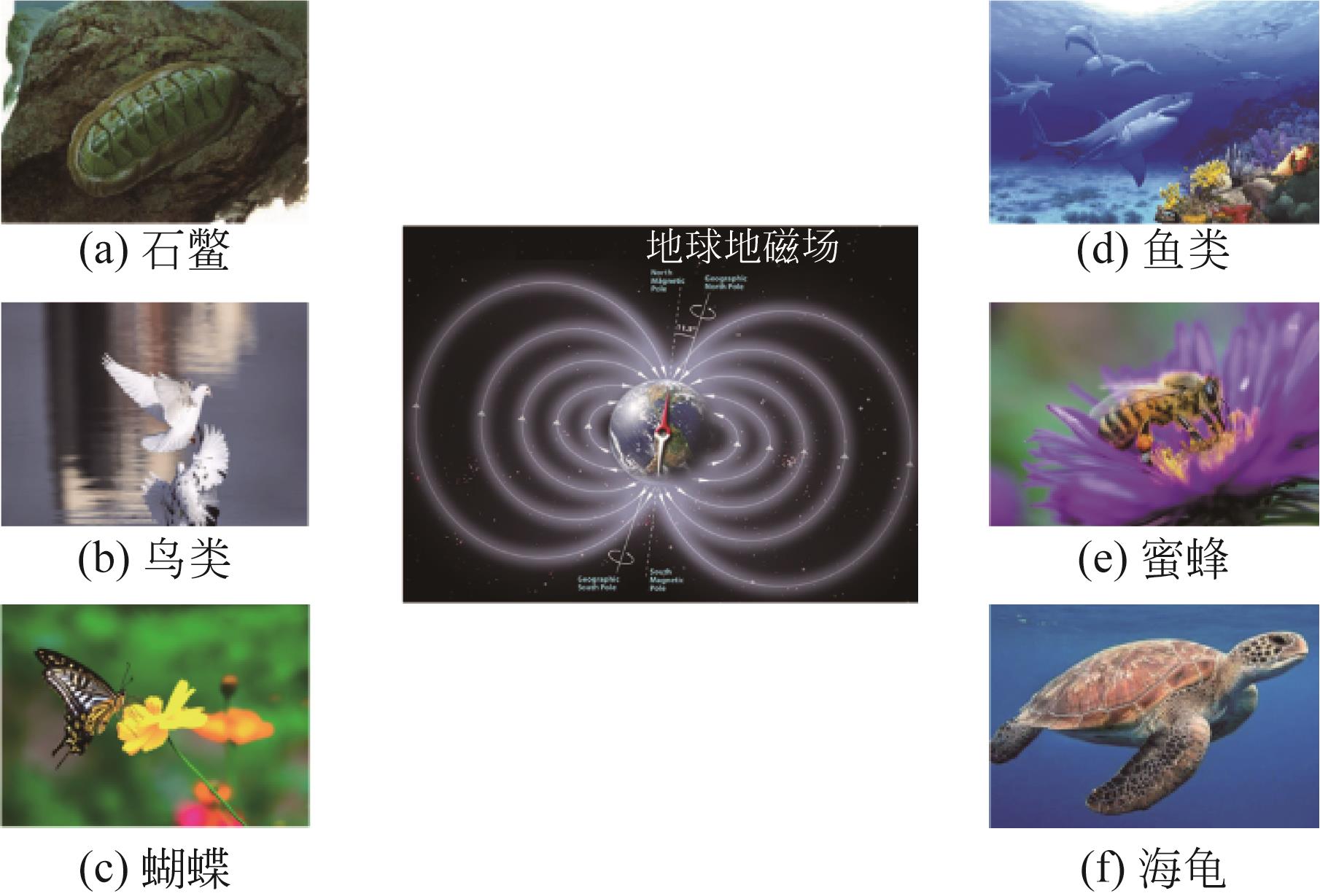
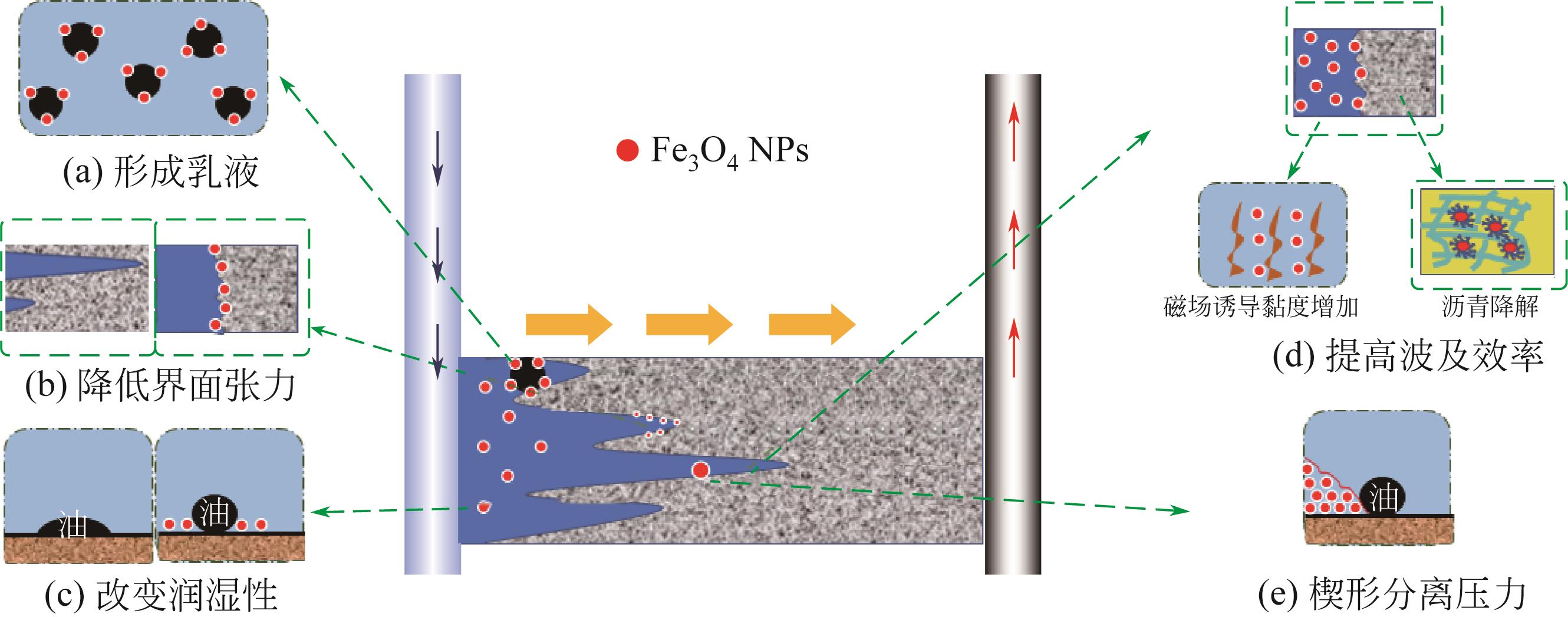
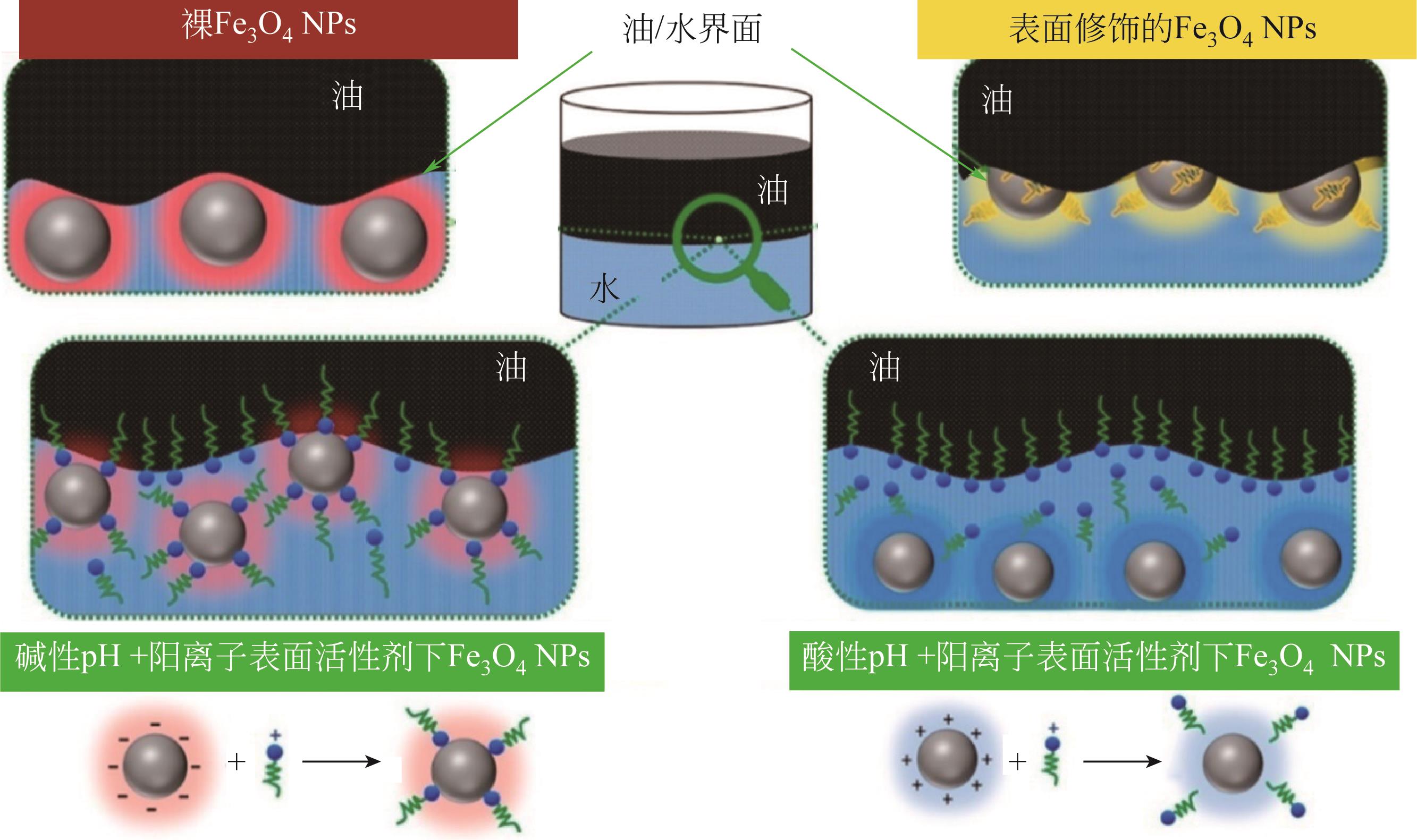
纳米Fe3O4具有独特的性质,如超顺磁性、尺寸小和低毒性,且易在外磁场下分离,近几年来在石油开发领域受到广泛的关注。本文综述了磁性Fe3O4纳米颗粒(NPs)的生物合成及其在提高采收率(EOR)的应用,首先介绍了微生物、植物提取液和动物合成磁铁矿:微生物的合成主要包括两个过程,一是对金属离子的吸附,二是还原矿化作用;植物提取液的合成依赖水溶性的一些代谢物,如多酚、生物碱和柠檬酸等的作用;动物体中也发现了磁铁矿,可以感应地磁场从而判断方向。然后阐述了Fe3O4 NPs的EOR机理和应用:Fe3O4 NPs可以将油滴包裹形成稳定的乳液,降低界面张力,改变润湿性,通过增加驱替流体的黏度和降低重油的黏度来提高波及效率以及楔形结构产生分离压力。在此作用下,总结了磁性Fe3O4在EOR方面的应用。
中图分类号:
引用本文
刘宇龙, 姚俊虎, 舒闯闯, 佘跃惠. 磁性Fe3O4纳米颗粒的生物合成及其在提高采收率中的应用[J]. 化工进展, 2023, 42(5): 2464-2474.
LIU Yulong, YAO Junhu, SHU Chuangchuang, SHE Yuehui. Biosynthesis and EOR application of magnetic Fe3O4 NPs[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2464-2474.
| 细菌 | 铁源 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|
| 放线杆菌 | K3Fe(CN)6/K4Fe(CN)6 | 50~150 | 均匀的立方颗粒 | [ |
| 硫还原地杆菌 | FeCl3 | 10.6~15 | 球形 | [ |
| 硝酸盐厌氧铁氧化菌BoFeN1 | FeCl2 | 55±15 | 球状 | [ |
| 铁还原菌:Thermoanaerobacter ethanolicus(TOR-39)和Shewanella loiha(PV-4) | FeCl3 | 35 | 八面体均匀晶体 | [ |
| 枯草芽孢杆菌 | Fe2O3 | 60~80 | 立方尖晶石,球形 | [ |
| 趋磁细菌:Magnetospirillum magneticum菌株AMB-1 | FeCl3和FeCl2 | 30~40 | 形态、尺寸均匀 | [ |
表1 细菌合成Fe3O4 NPs [13]
| 细菌 | 铁源 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|
| 放线杆菌 | K3Fe(CN)6/K4Fe(CN)6 | 50~150 | 均匀的立方颗粒 | [ |
| 硫还原地杆菌 | FeCl3 | 10.6~15 | 球形 | [ |
| 硝酸盐厌氧铁氧化菌BoFeN1 | FeCl2 | 55±15 | 球状 | [ |
| 铁还原菌:Thermoanaerobacter ethanolicus(TOR-39)和Shewanella loiha(PV-4) | FeCl3 | 35 | 八面体均匀晶体 | [ |
| 枯草芽孢杆菌 | Fe2O3 | 60~80 | 立方尖晶石,球形 | [ |
| 趋磁细菌:Magnetospirillum magneticum菌株AMB-1 | FeCl3和FeCl2 | 30~40 | 形态、尺寸均匀 | [ |
| 分类 | 微生物 | 铁离子 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|---|
| 真菌 | 黄萎病菌 | FeCl3 | 10~40 | 立方形 | [ |
| 黑曲霉真菌 | FeCl3 | 8 | 球形 | [ | |
| 尖孢镰刀菌 | FeCl3 | 26.78 | 形状不规则 | [ | |
| 藻类 | 褐藻水:Padina pavonica和Sargassum acinarium | FeCl3 | 10~19.5,21.6~27.4 | — | [ |
| 褐藻:尾藻水 | FeCl3 | 18±4 | 立方体 | [ | |
| 海藻Kappaphycus alvarezii | FeCl2·4H2O和FeCl3·6H2O | 14.7 | 球形 | [ | |
| 微藻(螺旋藻属) | FeCl2·4H2O和FeCl3·6H2O | 45 | 球形 | [ |
表2 真菌和藻类合成Fe3O4 NPs [13]
| 分类 | 微生物 | 铁离子 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|---|
| 真菌 | 黄萎病菌 | FeCl3 | 10~40 | 立方形 | [ |
| 黑曲霉真菌 | FeCl3 | 8 | 球形 | [ | |
| 尖孢镰刀菌 | FeCl3 | 26.78 | 形状不规则 | [ | |
| 藻类 | 褐藻水:Padina pavonica和Sargassum acinarium | FeCl3 | 10~19.5,21.6~27.4 | — | [ |
| 褐藻:尾藻水 | FeCl3 | 18±4 | 立方体 | [ | |
| 海藻Kappaphycus alvarezii | FeCl2·4H2O和FeCl3·6H2O | 14.7 | 球形 | [ | |
| 微藻(螺旋藻属) | FeCl2·4H2O和FeCl3·6H2O | 45 | 球形 | [ |
| 植物 | 部位 | 铁源 | 粒径/nm | 参考文献 |
|---|---|---|---|---|
大麦 酸模 | 叶子 | FeCl3·6H2O | 30,10~40 | [ |
| 安第斯黑莓 | 叶子 | FeSO4·7H2O | 54.5±24.6 | [ |
| 西番莲 | 果实 | FeCl3·6H2O | 22.3±3 | [ |
| 苜蓿 | — | FeNH4(SO4)2·12H2O | <5 | [ |
| 车前草皮 | 叶子 | FeCl3·6H2O | 50 | [ |
| 芦荟植物提取液 | 叶子 | Fe(C5H8O2)3 | 6~35 | [ |
| 西瓜 | 果皮 | FeCl3·6H2O | <20 | [ |
| 桉树 | 叶子 | FeCl3·6H2O | 80 | [ |
| 柑橘 | 果皮 | FeCl3,FeCl2·4H2O | 50~200 | [ |
| 大豆 | 豆芽 | Fe(NH4)2(SO4)2·6H2O,FeCl3·6H2O | 8 | [ |
表3 植物提取液合成Fe3O4 NPs[5]
| 植物 | 部位 | 铁源 | 粒径/nm | 参考文献 |
|---|---|---|---|---|
大麦 酸模 | 叶子 | FeCl3·6H2O | 30,10~40 | [ |
| 安第斯黑莓 | 叶子 | FeSO4·7H2O | 54.5±24.6 | [ |
| 西番莲 | 果实 | FeCl3·6H2O | 22.3±3 | [ |
| 苜蓿 | — | FeNH4(SO4)2·12H2O | <5 | [ |
| 车前草皮 | 叶子 | FeCl3·6H2O | 50 | [ |
| 芦荟植物提取液 | 叶子 | Fe(C5H8O2)3 | 6~35 | [ |
| 西瓜 | 果皮 | FeCl3·6H2O | <20 | [ |
| 桉树 | 叶子 | FeCl3·6H2O | 80 | [ |
| 柑橘 | 果皮 | FeCl3,FeCl2·4H2O | 50~200 | [ |
| 大豆 | 豆芽 | Fe(NH4)2(SO4)2·6H2O,FeCl3·6H2O | 8 | [ |
| 类型 | 尺寸 /nm | 浓度 | 驱替介质 | EOR机制 | RF/% | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
黏度 /mPa·s | IFT /mN·m-1 | 接触角 | ||||||
| Fe3O4@十二烷基三甲基溴化铵(CTAB) | — | — | 碳酸钙颗粒 | — | 30→1 | 90°→<30° | 35 | [ |
| Fe3O4@SiO2@黄原胶 | — | 1500mg/L | 碳酸岩 | 0.89→1.93 | 28.3→8.6 | 134°→34° | — | [ |
| Fe3O4@柠檬酸盐聚合物 | 47 | 400mg/L | 砂岩 | 0.99→1.08 | 11.23→7.92 | 160°→114° | 26 | [ |
| Fe3O4@柠檬酸 | 20~26 | 0.8%~2%(质量分数) | 微模型 | — | 37.47→16.71 | 106°→41° | 22 | [ |
| Fe3O4@C | 60 | 100mg/L | 砂岩 | — | 24.3→1×10-4 | 54°→10° | 30 | [ |
| Fe3O4@SiO2 | 30 | 0.05%(质量分数) | 碳酸岩砂 | 1.09→1.19 | 39→17.5 | 137°→40° | 24 | [ |
| Fe3O4@壳聚糖 | — | 0.03%(质量分数) | 填砂管 | 264→161 | 22.49→14.47 | 145°→90° | 10.8 | [ |
| Fe3O4 | <80 | 0.8%(质量分数) | 砂岩 | — | — | 50.44°→29.7° | 15.38 | [ |
| Fe3O4@SiO2 | 25~30 | 0.1%(质量分数) | 玻璃微模型 | 1.09→1.19 | 25→21 | 120°→106° | 13.2 | [ |
| Pd功能化磁铁矿 | — | — | — | 催化降黏 | 90 | [ | ||
表4 磁性Fe3O4 NPs的EOR总结[44]
| 类型 | 尺寸 /nm | 浓度 | 驱替介质 | EOR机制 | RF/% | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
黏度 /mPa·s | IFT /mN·m-1 | 接触角 | ||||||
| Fe3O4@十二烷基三甲基溴化铵(CTAB) | — | — | 碳酸钙颗粒 | — | 30→1 | 90°→<30° | 35 | [ |
| Fe3O4@SiO2@黄原胶 | — | 1500mg/L | 碳酸岩 | 0.89→1.93 | 28.3→8.6 | 134°→34° | — | [ |
| Fe3O4@柠檬酸盐聚合物 | 47 | 400mg/L | 砂岩 | 0.99→1.08 | 11.23→7.92 | 160°→114° | 26 | [ |
| Fe3O4@柠檬酸 | 20~26 | 0.8%~2%(质量分数) | 微模型 | — | 37.47→16.71 | 106°→41° | 22 | [ |
| Fe3O4@C | 60 | 100mg/L | 砂岩 | — | 24.3→1×10-4 | 54°→10° | 30 | [ |
| Fe3O4@SiO2 | 30 | 0.05%(质量分数) | 碳酸岩砂 | 1.09→1.19 | 39→17.5 | 137°→40° | 24 | [ |
| Fe3O4@壳聚糖 | — | 0.03%(质量分数) | 填砂管 | 264→161 | 22.49→14.47 | 145°→90° | 10.8 | [ |
| Fe3O4 | <80 | 0.8%(质量分数) | 砂岩 | — | — | 50.44°→29.7° | 15.38 | [ |
| Fe3O4@SiO2 | 25~30 | 0.1%(质量分数) | 玻璃微模型 | 1.09→1.19 | 25→21 | 120°→106° | 13.2 | [ |
| Pd功能化磁铁矿 | — | — | — | 催化降黏 | 90 | [ | ||
| 1 | 李杨. 铁强化厌氧水解酸化微生物种间氢传递及其调控[D]. 大连: 大连理工大学, 2017. |
| LI Yang. Interspecific hydrogen transfer and its regulation in Fe-enhanced anaerobic hydrolysis and acidification microorganisms[D]. Dalian : Dalian University of Technology, 2017. | |
| 2 | 秦曦. 微生物电催化耦合磁铁矿负载型生物炭强化污泥厌氧消化机理研究[D]. 上海: 华东师范大学, 2022. |
| QIN Xi. Study on mechanism of microbial electrocatalysis coupled with magnetite supported biochar to enhance anaerobic digestion of sludge[D]. Shanghai: East China Normal University, 2022. | |
| 3 | 冯阳阳, 赵众从, 杨文博, 等. 微生物合成金属纳米颗粒及在稠油催化降黏中的应用研究进展[J]. 化工进展, 2021, 40(4): 2215-2226. |
| FENG Yangyang, ZHAO Zhongcong, YANG Wenbo, et al. Microbial natural synthetic metal nanoparticles and the application in heavy oil catalytic viscosity reduction[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2215-2226. | |
| 4 | 翟旭东, 徐政. 利用生物技术制备纳米材料的研究新进展[J]. 中国粉体技术, 2010, 16(2): 76-82. |
| ZHAI Xudong, XU Zheng. New development of nano-sized materials synthesis by bio-technology[J]. China Powder Science and Technology, 2010, 16(2): 76-82. | |
| 5 | 刘清, 邓真宁, 滑熠龙, 等. 纳米铁的绿色合成及其在环境中的应用研究进展[J]. 化工进展, 2020, 39(5): 1950-1963. |
| LIU Qing, DENG Zhenning, HUA Yilong, et al. Green synthesis of Fe nanoparticles and their environmental applications[J]. Chemical Industry and Engineering Progress,2020,39(5): 1950-1963. | |
| 6 | ZHONG Xun, CHEN Jiating, AN Ran, et al. A state-of-the-art review of nanoparticle applications with a focus on heavy oil viscosity reduction[J]. Journal of Molecular Liquids, 2021, 344: 117845. |
| 7 | 刘清, 张美, 韩科昌, 等. 生物法合成纳米铁的研究进展[J]. 环境工程, 2015, 33(3): 163-167. |
| LIU Qing, ZHANG Mei, HAN Kechang, et al. Research progress on biosynthesis of iron nanoparticles[J]. Environmental Engineering, 2015, 33(3): 163-167. | |
| 8 | MAROUZI Somayeh, SABOURI Zahra, DARROUDI Majid. Greener synthesis and medical applications of metal oxide nanoparticles[J]. Ceramics International, 2021, 47(14): 19632-19650. |
| 9 | ABHILASH, REVATI K, PANDEY B D. Microbial synthesis of iron-based nanomaterials—A review[J]. Bulletin of Materials Science, 2011, 34(2): 191-198. |
| 10 | ARAKAKI Atsushi, SHIMIZU Katsuhiko, Mayumi ODA, et al. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: organic molecular control of self-organization of hybrids[J]. Organic & Biomolecular Chemistry, 2015, 13(4): 974-989. |
| 11 | BAZYLINSKI D A, FRANKEL R B, KONHAUSER K O. Modes of biomineralization of magnetite by microbes[J]. Geomicrobiology Journal, 2007, 24(6): 465-475. |
| 12 | BHARDE Atul, WANI Aijaz, SHOUCHE Yogesh, et al. Bacterial aerobic synthesis of nanocrystalline magnetite[J]. Journal of the American Chemical Society, 2005, 127(26): 9326-9327. |
| 13 | SAIF S, ADIL S F, CHAUDHRY A, et al. Microbial synthesis of magnetic nanomaterials[M]. Agri-Waste and Microbes for Production of Sustainable Nanomaterials. Amsterdam: Elsevier, 2022: 323-356. |
| 14 | BYRNE J M, MUHAMADALI H, COKER V S, et al. Scale-up of the production of highly reactive biogenic magnetite nanoparticles using Geobacter sulfurreducens [J]. Journal of the Royal Society Interface, 2015, 12(107): 20150240. |
| 15 | MIOT Jennyfer, LI Jinhua, BENZERARA Karim, et al. Formation of single domain magnetite by green rust oxidation promoted by microbial anaerobic nitrate-dependent iron oxidation[J]. Geochimica et Cosmochimica Acta, 2014, 139: 327-343. |
| 16 | ROH Y, LAUF R J, MCMILLAN A D, et al. Microbial synthesis and the characterization of metal-substituted magnetites[J]. Solid State Communications, 2001, 118(10): 529-534. |
| 17 | SUNDARAM P A, AUGUSTINE R, KANNAN M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil[J]. Biotechnology and Bioprocess Engineering, 2012, 17(4): 835-840. |
| 18 | PROZOROV T, MALLAPRAGADA S K, NARASIMHAN B, et al. Protein-mediated synthesis of uniform superparamagnetic magnetite nanocrystals[J]. Advanced Functional Materials, 2007, 17(6): 951-957. |
| 19 | BHARDE Atul, RAUTARAY Debabrata, BANSAL Vipul, et al. Extracellular biosynthesis of magnetite using fungi[J]. Small, 2006, 2(1): 135-141. |
| 20 | ABDEEN Mai, SABRY Soraya, GHOZLAN Hanan, et al. Microbial-physical synthesis of Fe and Fe3O4 magnetic nanoparticles using Aspergillus niger YESM1 and supercritical condition of ethanol[J]. Journal of Nanomaterials, 2016, 2016: 1-7. |
| 21 | BALAKRISHNAN G S, RAJENDRAN K, KALIRAJAN J. Microbial synthesis of magnetite nanoparticles for arsenic removal[J]. Journal of Applied Biology & Biotechnology, 2020, 8(3): 7-5. |
| 22 | EL-KASSAS H Y, ALY-ELDEEN M A, GHARIB S M. Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: characterization and application for lead bioremediation[J]. Acta Oceanologica Sinica, 2016, 35(8): 89-98. |
| 23 | MAHDAVI M, NAMVAR F, AHMAD M B, et al. Green biosynthesis and characterization of magnetic iron oxide (Fe₃O₄) nanoparticles using seaweed (Sargassum muticum) aqueous extract[J]. Molecules, 2013, 18(5): 5954-5964. |
| 24 | YEW Y P, SHAMELI K, MIYAKE M, et al. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (kappaphycus alvarezii) extract[J]. Nanoscale Research Letters, 2016, 11(1): 276. |
| 25 | HAWEZY H J S, SDIQ K H, QADR V A, et al. Biosynthesis of magnetite-nanoparticles using microalgae (Spirulina sp. and Spirogyra sp.) [J]. Indian Journal of Public Health Research & Development, 2020, 11(7): 1023-1027. |
| 26 | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| SUN Wentao, LI Chun. Design and construction of microbial cell factory for biosynthesis of plant natural products[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1202-1214. | |
| 27 | MAKAROV V V, MAKAROVA S S, LOVE A J, et al. Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants[J]. Langmuir, 2014, 30(20): 5982-5988. |
| 28 | KUMAR Brajesh, SMITA Kumari, CUMBAL Luis, et al. Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf[J]. Materials Chemistry and Physics, 2016, 179: 310-315. |
| 29 | KUMAR Brajesh, SMITA Kumari, CUMBAL Luis, et al. Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication[J]. Journal of Saudi Chemical Society, 2014, 18(4): 364-369. |
| 30 | HERRERA-BECERRA R, ZORRILLA C, ASCENCIO J A. Production of iron oxide nanoparticles by a biosynthesis method: An environmentally friendly route[J]. The Journal of Physical Chemistry C, 2007, 111(44): 16147-16153. |
| 31 | VENKATESWARLU S, RAO Y S, BALAJI T, et al. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract[J]. Materials Letters, 2013, 100: 241-244. |
| 32 | PHUMYING Santi, LABUAYAI Sarawuth, THOMAS Chunpen, et al. Aloe vera plant-extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe3O4) nanoparticles[J]. Applied Physics A, 2013, 111(4): 1187-1193. |
| 33 | PRASAD C, GANGADHARA S, VENKATESWARLU P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity[J]. Applied Nanoscience, 2016, 6(6): 797-802. |
| 34 | CAO Dan, JIN Xiaoying, GAN Li, et al. Removal of phosphate using iron oxide nanoparticles synthesized by eucalyptus leaf extract in the presence of CTAB surfactant[J]. Chemosphere, 2016, 159: 23-31. |
| 35 | 柳检. 典型富集植物对铅的吸收和耐受机制研究[D]. 北京: 中国地质科学院, 2019. |
| LIU Jian. Mechanism of lead uptake and tolerance in typical accumulator plants[D]. Beijing: Chinese Academy of Geological Sciences, 2019. | |
| 36 | EHRAMPOUSH M H, MIRIA M, SALMANI M H, et al. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract[J]. Journal of Environmental Health Science and Engineering, 2015, 13(1): 84. |
| 37 | CAI Yan, SHEN Yuhua, XIE Anjian, et al. Green synthesis of soya bean sprouts-mediated superparamagnetic Fe3O4 nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 2010, 322(19): 2938-2943. |
| 38 | 钱霞. 蜜蜂中磁铁矿纳米粒子的合成[J]. 原子与分子物理学报, 2021, 38(5): 77-81. |
| QIAN Xia. Synthesis of nano-magnetite particles in the honeybees[J]. Journal of Atomic and Molecular Physics, 2021, 38(5): 77-81. | |
| 39 | HSU C Y, KO F Y, LI C W, et al. Magnetoreception system in honeybees (Apis mellifera) [J]. PlosOne, 2007, 2(4): e395. |
| 40 | 尧德中,熊茜桃. 生物合成磁铁矿及其应用[J]. 大自然探索, 1995(2): 63-66. |
| YAO Dezhong, XIONG Qiantao. Biogenic magnetite and its application[J]. Exploration of Nature, 1995(2): 63-66. | |
| 41 | 刘小峰, 史远. 鸟类磁感受的生物物理机制研究进展[J]. 生物物理学报, 2009, 25(4): 247-254. |
| LIU Xiaofeng, SHI Yuan. Progress in the research on the biophysical mechanism underlying avian magnetoreception[J]. Acta Biophysica Sinica, 2009, 25(4): 247-254. | |
| 42 | ZHOU Kaibo, ZHOU Xiang, LIU Jie, et al. Application of magnetic nanoparticles in petroleum industry: a review[J]. Journal of Petroleum Science and Engineering, 2020, 188: 106943. |
| 43 | PEREIRA M L, MAIA K C B, SILVA W C, et al. Fe3O4 nanoparticles as surfactant carriers for enhanced oil recovery and scale prevention[J]. ACS Applied Nano Materials, 2020, 3(6): 5762-5772. |
| 44 | YAKASAI F, JAAFAR M Z, BANDYOPADHYAY S, et al. Application of iron oxide nanoparticles in oil recovery—A critical review of the properties, formulation, recent advances and prospects[J]. Journal of Petroleum Science and Engineering, 2022, 208: 109438. |
| 45 | SUN Xiaofei, ZHANG Yanyu, CHEN Guangpeng, et al. Application of nanoparticles in enhanced oil recovery: a critical review of recent progress[J]. Energies, 2017, 10(3): 345. |
| 46 | BINKS B P, YIN D. Pickering emulsions stabilized by hydrophilic nanoparticles: in situ surface modification by oil[J]. Soft Matter, 2016, 12(32): 6858-6867. |
| 47 | KAZEMZADEH Y, DEHDARI B, ETEMADAN Z, et al. Experimental investigation into Fe3O4/SiO2 nanoparticle performance and comparison with other nanofluids in enhanced oil recovery[J]. Petroleum Science, 2019, 16(3): 578-590. |
| 48 | DIVANDARI H, HEMMATI-SARAPARDEH A, SCHAFFIE M, et al. Integrating functionalized magnetite nanoparticles with low salinity water and surfactant solution: Interfacial tension study[J]. Fuel, 2020, 281: 118641. |
| 49 | KONDIPARTY Kirti, NIKOLOV Alex, WU Stanley, et al. Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure: Statics analysis and experiments[J]. Langmuir, 2011, 27(7): 3324-3335. |
| 50 | AMROUCHE F, GOMARI S R, ISLAM M, et al. A novel hybrid technique to enhance oil production from oil-wet carbonate reservoirs by combining a magnetic field with alumina and iron oxide nanoparticles[J]. Journal of Cleaner Production, 2021, 281: 124891. |
| 51 | ZHANG H, RAMAKRISHNAN T S, NIKOLOV A, et al. Enhanced oil displacement by nanofluid's structural disjoining pressure in model fractured porous media[J]. Journal of Colloid and Interface Science, 2018, 511: 48-56. |
| 52 | ESMAEILNEZHAD E, CHOI H J, SCHAFFIE M, et al. Characteristics and applications of magnetized water as a green technology[J]. Journal of Cleaner Production, 2017, 161: 908-921. |
| 53 | ADIL M, LEE K, MOHD Z H, et al. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR)[J]. PLoS One, 2018, 13(2): e0193518. |
| 54 | ESMAEILNEZHAD E, VAN I E, CHON O H, et al. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field[J]. Journal of Industrial and Engineering Chemistry, 2018, 58: 319-327. |
| 55 | KOTHARI Nikita, RAINA Bhavna, CHANDAK Krishna, et al. Application of ferrofluid for enhanced surfactant flooding in EOR[C]//SPE EUROPEC/EAGE Annual Conference and Exhibition. OnePetro, 2010. |
| 56 | OMAJALI J B, HART A, WALKER M, et al. In-situ catalytic upgrading of heavy oil using dispersed bionanoparticles supported on gram-positive and gram-negative bacteria[J]. Applied Catalysis B: Environmental, 2017, 203: 807-819. |
| 57 | 杨方源, 赵尉伶, 孙英, 等. 表面活性剂修饰磁性纳米颗粒的除砷效果研究[J]. 新疆农业大学学报, 2021, 44(3): 188-195. |
| YANG Fangyuan, ZHAO Yuling, SUN Ying, et al. Study on the arsenic removal effect of surfactant-modified magnetic nanoparticles[J]. Journal of Xinjiang Agricultural University, 2021, 44(3): 188-195. | |
| 58 | ALI J, MANSHAD A K, IMANI I, et al. Greenly synthesized Magnetite@SiO2@Xanthan nanocomposites and its application in enhanced oil recovery: IFT reduction and wettability alteration[J]. Arabian Journal for Science and Engineering, 2020, 45(9): 7751-7761. |
| 59 | IZADI N, KOOCHI M M, AMROLLAHI A, et al. Investigation of functionalized polyelectrolyte polymer-coated Fe3O4 nanoparticles stabilized in high salinity brine at high temperatures as an EOR agent[J]. Journal of Petroleum Science and Engineering, 2019, 178: 1079-1091. |
| 60 | DIVANDARI Hassan, Abdolhossein HEMMATI-SARAPARDEH, SCHAFFIE Mahin, et al. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: experimental study and mechanistic understanding[J]. Journal of Petroleum Science and Engineering, 2019, 174: 425-436. |
| 61 | BETANCUR S, CARRASCO-MARÍN F, PÉREZ-CADENAS A F, et al. Effect of magnetic iron core–carbon shell nanoparticles in chemical enhanced oil recovery for ultralow interfacial tension region[J]. Energy & Fuels, 2019, 33(5): 4158-4168. |
| 62 | KAZEMZADEH Yousef, SHARIFI Mohammad, RIAZI Masoud, et al. Potential effects of metal oxide/SiO2 nanocomposites in EOR processes at different pressures[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 559: 372-384. |
| 63 | REZVANI Hosein, RIAZI Masoud, TABAEI Morteza, et al. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 544: 15-27. |
| [1] | 司银芳, 胡语婕, 张凡, 董浩, 佘跃惠. 生物合成氧化锌纳米颗粒材料及其抗菌应用[J]. 化工进展, 2023, 42(4): 2013-2023. |
| [2] | 冯阳阳, 赵众从, 杨文博, 胡琳琪, 张文达, 佘跃惠. 微生物合成金属纳米颗粒及在稠油催化降黏中的应用研究进展[J]. 化工进展, 2021, 40(4): 2215-2226. |
| [3] | 于洋, 刘琦, 彭勃, 吕静. 微生物降解稠油中沥青质的研究进展[J]. 化工进展, 2021, 40(3): 1574-1585. |
| [4] | 仲文雅, 俞汶佳, 谷艺明, 郭静, 樊博, 蔡志强. 生物合成安丝菌素的研究进展[J]. 化工进展, 2021, 40(2): 990-997. |
| [5] | 王晨伊,刘琦,彭勃,吕静. Surfactin生物表面活性剂及其驱油研究进展[J]. 化工进展, 2019, 38(9): 4012-4019. |
| [6] | 李原,狄勤丰,华帅,张景楠,叶峰,王文昌. 纳米流体对储层润湿性反转提高石油采收率研究进展[J]. 化工进展, 2019, 38(08): 3612-3620. |
| [7] | 程申, 张颂红, 贠军贤. α-酮异己酸的生物合成研究进展[J]. 化工进展, 2018, 37(12): 4821-4829. |
| [8] | 于新磊, 毛雨丰, 张晓霞, 陆凌雪, 王智文, 陈涛. 生物法生产3-羟基丙酸研究进展[J]. 化工进展, 2018, 37(11): 4427-4436. |
| [9] | 杨兆中, 朱静怡, 李小刚, 费阳, 徐彬予. 纳米颗粒稳定泡沫在油气开采中的研究进展[J]. 化工进展, 2017, 36(05): 1675-1681. |
| [10] | 倪正, 关今韬, 沈绍传, 贠军贤. 苯乳酸的微生物合成及分离研究进展[J]. 化工进展, 2016, 35(11): 3627-3633. |
| [11] | 李 森,王海峰. 电化学法处理冷却循环水技术的应用[J]. 化工进展, 2013, 32(10): 2514-2517. |
| [12] | 沈秋莹1,罗利军1,王 娟2,刘拥军2,潘学军1,蒋峰芝2. 仿生吸附剂去除水中环境内分泌干扰物的研究进展[J]. 化工进展, 2012, 31(02 ): 428-434. |
| [13] | 黄锦标1,尚龙安2. 聚羟基烷酸酯的生物合成研究进展 [J]. 化工进展, 2011, 30(9): 2041-. |
| [14] | 李金娟,赵 林,谭 欣,黄 宇,柳听义. 利用淀粉酸化废水驯化活性污泥合成聚-?-羟基脂肪酸酯及其表征 [J]. 化工进展, 2011, 30(7): 1618-. |
| [15] | 井淑波,朱万春,管景奇,王国甲. 环境友好氧化剂催化氧化合成己二酸研究进展 [J]. 化工进展, 2009, 28(1): 62-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||