化工进展 ›› 2022, Vol. 41 ›› Issue (6): 2981-2992.DOI: 10.16085/j.issn.1000-6613.2021-1551
羰基化合物直接还原胺化合成伯胺催化剂研究进展
吴静航1( ), 陈臣举1,2(
), 陈臣举1,2( ), 梁杰1,2(
), 梁杰1,2( ), 张春雷1,2(
), 张春雷1,2( )
)
- 1.上海师范大学化学与材料科学学院,上海 200234
2.上海绿色能源化工工程技术研究中心,上海 200234
-
收稿日期:2021-07-21修回日期:2021-11-19出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:陈臣举,梁杰,张春雷 -
作者简介:吴静航(1996—),男,硕士研究生,研究方向为工业催化。E-mail:1067280529@qq.com 。
Recent progress in the synthesis of primary amine via direct reductive amination of aldehydes and ketones
WU Jinghang1( ), CHEN Chenju1,2(
), CHEN Chenju1,2( ), LIANG Jie1,2(
), LIANG Jie1,2( ), ZHANG Chunlei1,2(
), ZHANG Chunlei1,2( )
)
- 1.College of Chemistry and Materials Science, Shanghai Normal University, Shanghai 200234, China
2.Shanghai Engineering Research Center of Green Energy Chemical Engineering, Shanghai 200234, China
-
Received:2021-07-21Revised:2021-11-19Online:2022-06-10Published:2022-06-21 -
Contact:CHEN Chenju,LIANG Jie,ZHANG Chunlei
摘要:
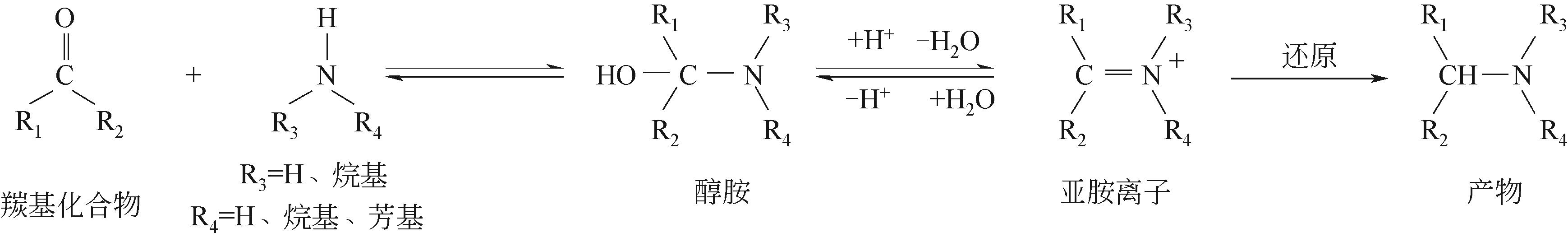
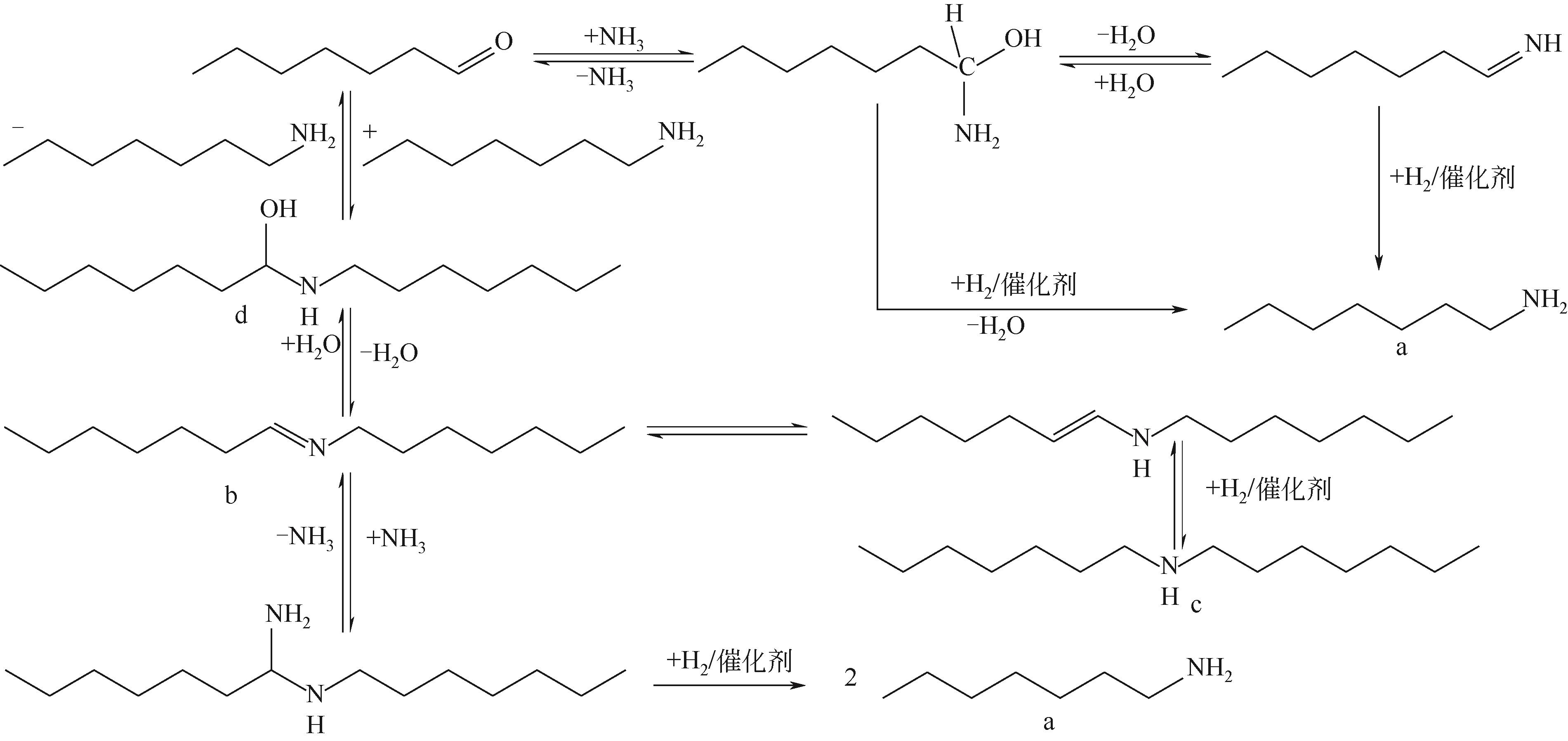
胺类化合物是一类重要的化工原料和中间体,在农药、医药、染料、高分子聚合物等领域有着广泛的应用。通过羰基化合物(醛或酮类)的还原胺化来制备胺类化合物是当前的研究热点。研究表明,贵金属基和非贵金属基的多相和均相催化剂均能够高效催化醛或酮类的还原胺化反应。本文对近年来羰基化合物直接还原胺化(或一锅法)合成伯胺的研究现状进行了综述,包括还原胺化反应、催化剂、反应条件、底物适用范围和催化作用机制等,其中重点阐述了直接还原胺化催化剂的研究进展。文章指出:通常多相催化剂具有活性高以及可重复使用等优点,而均相催化剂的优势在于催化效率高,伯胺选择性高;另一方面,以Pd、Rh、Ru等为代表的贵金属催化剂催化性能优异,但价格昂贵,因此可采用Co、Ni等性能同样优异但价格相对低廉的非贵金属催化剂以降低成本。文中提出,催化效率高、反应条件温和、普适性高的羰基化合物还原胺化催化剂应成为未来重点研究方向。
中图分类号:
引用本文
吴静航, 陈臣举, 梁杰, 张春雷. 羰基化合物直接还原胺化合成伯胺催化剂研究进展[J]. 化工进展, 2022, 41(6): 2981-2992.
WU Jinghang, CHEN Chenju, LIANG Jie, ZHANG Chunlei. Recent progress in the synthesis of primary amine via direct reductive amination of aldehydes and ketones[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2981-2992.
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| Rh/Al2O3[ | 80℃,氨水,2MPa H2,2h | 糠醛 | X糠醛=100%,S糠胺=91.5% |
| Rh/CeO2[ | 50℃,0.1MPa H2,14h | 苯甲醛 | X苯甲醛=100%,S苄胺=88% |
| Ru/γ-Al2O3[ | 80℃,0.4MPa NH3,3MPa H2,2h | 庚醛 | X庚醛=100%,S庚胺=94% |
| Ru/Nb2O5[ | 90℃,0.1MPa NH3,4MPa H2,4h | 糠醛 | X糠醛=100%,S糠胺=99% |
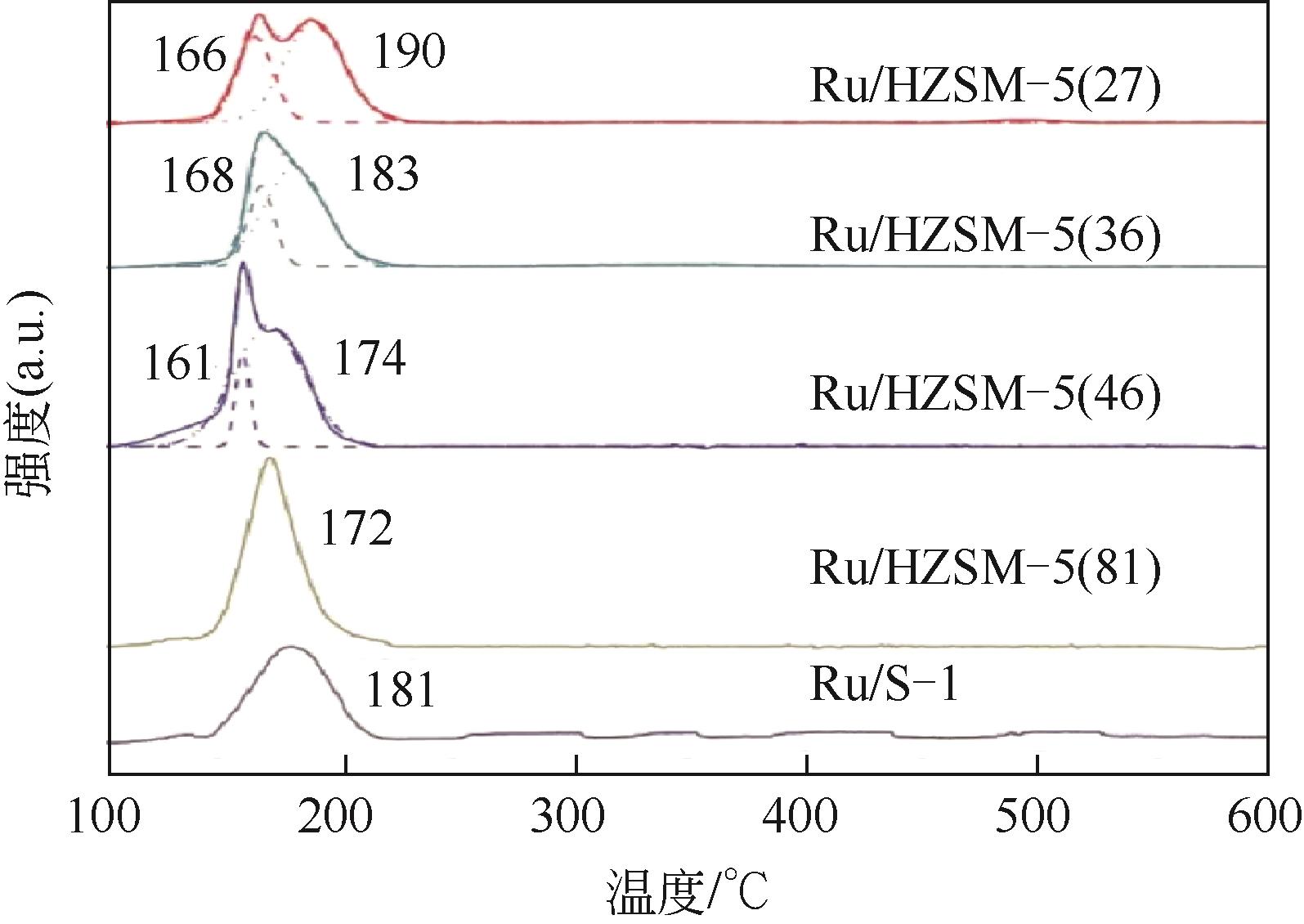
| Ru/HZSM-5[ | 80℃,NH3(7mol/L MeOH 溶液),3MPa H2,15min | 糠醛 | X糠醛=100%,S糠胺=76% |
| Ru/ZrO2[ | 85℃,氨水,2MPa H2,12h | 乙醇醛 | X乙醇醛=100%,S乙醇胺=93% |
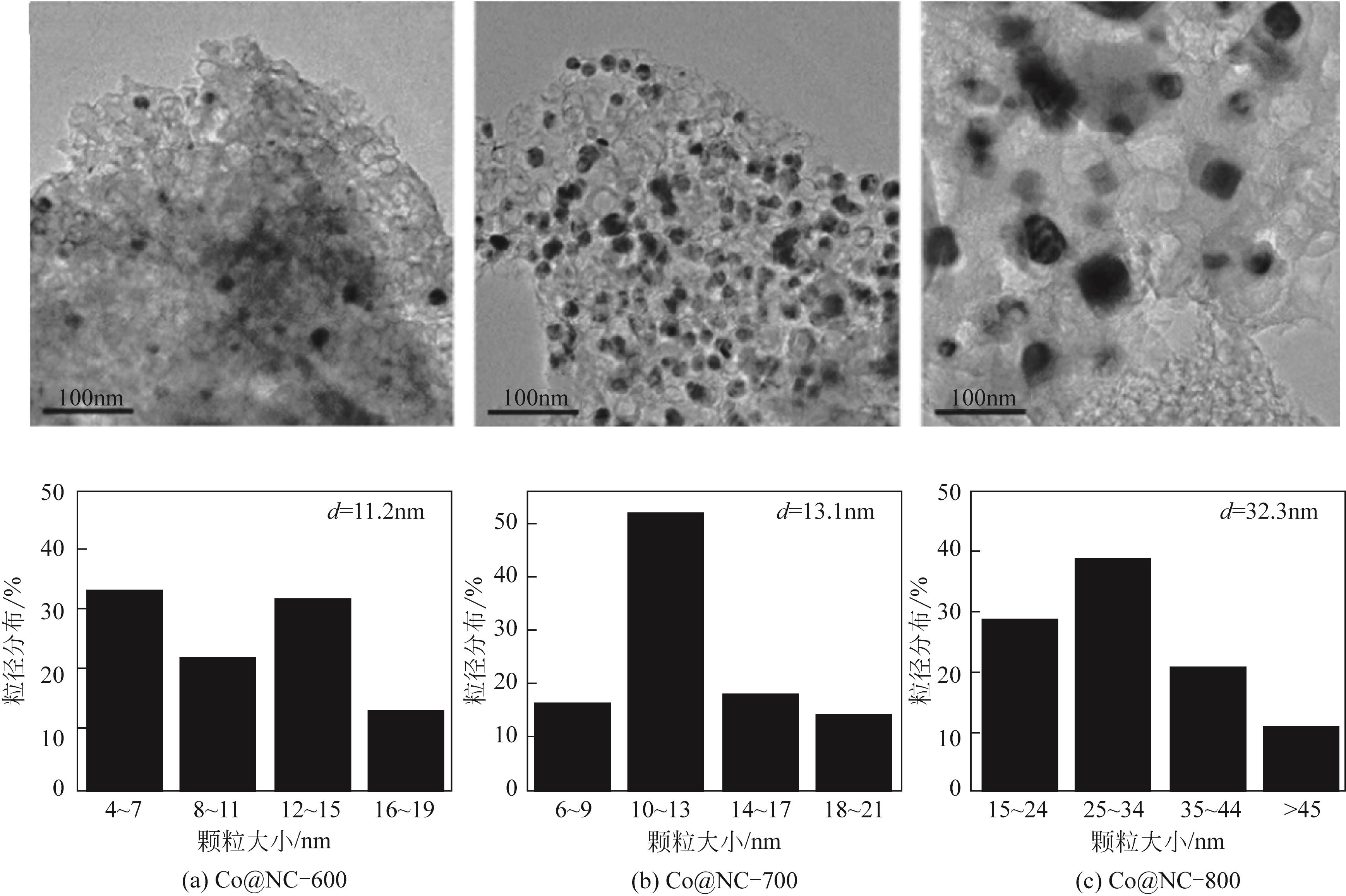
| Pt-MoO x /TiO2[ | 100℃,0.4MPa NH3,0.2MPa H2,20h | 2-金刚烷酮 | X2-金刚烷酮=100%,S2-金刚烷胺=93% |
| Pd/NPs[ | 室温,NH3,0.1MPa H2,3h | 苯甲醛 | X苯甲醛=100%,S苄胺=98% |
表1 负载型贵金属基催化剂催化还原胺化性能
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| Rh/Al2O3[ | 80℃,氨水,2MPa H2,2h | 糠醛 | X糠醛=100%,S糠胺=91.5% |
| Rh/CeO2[ | 50℃,0.1MPa H2,14h | 苯甲醛 | X苯甲醛=100%,S苄胺=88% |
| Ru/γ-Al2O3[ | 80℃,0.4MPa NH3,3MPa H2,2h | 庚醛 | X庚醛=100%,S庚胺=94% |
| Ru/Nb2O5[ | 90℃,0.1MPa NH3,4MPa H2,4h | 糠醛 | X糠醛=100%,S糠胺=99% |
| Ru/HZSM-5[ | 80℃,NH3(7mol/L MeOH 溶液),3MPa H2,15min | 糠醛 | X糠醛=100%,S糠胺=76% |
| Ru/ZrO2[ | 85℃,氨水,2MPa H2,12h | 乙醇醛 | X乙醇醛=100%,S乙醇胺=93% |
| Pt-MoO x /TiO2[ | 100℃,0.4MPa NH3,0.2MPa H2,20h | 2-金刚烷酮 | X2-金刚烷酮=100%,S2-金刚烷胺=93% |
| Pd/NPs[ | 室温,NH3,0.1MPa H2,3h | 苯甲醛 | X苯甲醛=100%,S苄胺=98% |
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| Co-DABCO-TPA@C-800[ | 120℃,0.5MPa NH3,4MPa H2,15h | 3,4-二甲氧基苯甲醛 | Y3,4-二甲氧基苄胺=88% |
| Co/GS@C[ | 120℃,0.5MPa NH3,4MPa H2,15h | 苯甲醛 | Y卞胺=88% |
| Co@NC-800[ | 130℃,氨水,1MPa H2,12h | 苯甲醛 | Y卞胺=96.9% |
| Co@NC[ | 110℃,氨水,2MPa H2,5h | 对甲氧基苯甲醛 | Y对甲氧基卞胺=94.5% |
| Ni/MC[ | 80℃,氨水,0.1MPa H2,2h | 苯甲醛 | Y苄胺=99.1% |
| Fe/(N)SiC[ | 140℃,氨水,6.5MPa H2,20h | 苯乙酮 | Y1-苯乙胺=99% |
| Ni/γ-Al2O3[ | 80℃,氨水,0.1MPa H2,20h | 苯甲醛 | Y苄胺=99% |
| Ni-TA@SiO2-800[ | 120℃,0.5MPa NH3,2MPa H2,15h | 邻溴苯甲醛 | Y邻溴卞胺=96% |
表2 负载型非贵金属基催化剂催化还原胺化反应性能
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| Co-DABCO-TPA@C-800[ | 120℃,0.5MPa NH3,4MPa H2,15h | 3,4-二甲氧基苯甲醛 | Y3,4-二甲氧基苄胺=88% |
| Co/GS@C[ | 120℃,0.5MPa NH3,4MPa H2,15h | 苯甲醛 | Y卞胺=88% |
| Co@NC-800[ | 130℃,氨水,1MPa H2,12h | 苯甲醛 | Y卞胺=96.9% |
| Co@NC[ | 110℃,氨水,2MPa H2,5h | 对甲氧基苯甲醛 | Y对甲氧基卞胺=94.5% |
| Ni/MC[ | 80℃,氨水,0.1MPa H2,2h | 苯甲醛 | Y苄胺=99.1% |
| Fe/(N)SiC[ | 140℃,氨水,6.5MPa H2,20h | 苯乙酮 | Y1-苯乙胺=99% |
| Ni/γ-Al2O3[ | 80℃,氨水,0.1MPa H2,20h | 苯甲醛 | Y苄胺=99% |
| Ni-TA@SiO2-800[ | 120℃,0.5MPa NH3,2MPa H2,15h | 邻溴苯甲醛 | Y邻溴卞胺=96% |
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| [Rh(COD)Cl]2/TPPTS[ | 135℃,氨水,6.5MPa H2,2h | 苯甲醛 | Y苄胺=86% |
| [Ru(CO)ClH(PPh3)3][ | 120℃,0.9MPa NH3,4MPa H2,16h | 苯乙酮 | Yα-苯乙胺=95% |
| [Ru(PPh3)3H(CO)Cl][ | 80℃,NH4I,3MPa H2,24h | 甲基酮环己酯 | Y(S)-(+)-1-环己基乙胺=78% |
| RuCl2(PPh3)3[ | 120℃,0.5~0.7MPa NH3,4MPa H2,24h | 苯甲醛 | Y卞胺=95% |
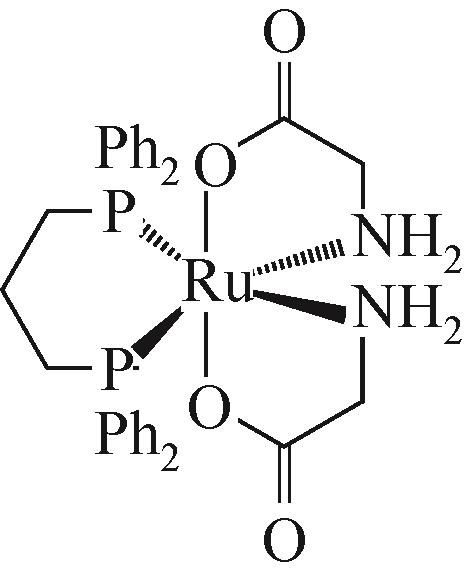
| Ru-1[ | 130℃,0.5MPa NH3,5MPa H2,12h | 苯甲醛 | Y苄胺=94% |
| Cp*Ir[ | 80℃,甲酸铵,4h | 苯乙酮 | Y1-苯乙胺=97% |
表3 不同均相贵金属基催化剂催化还原胺化反应性能
| 催化剂 | 反应条件 | 反应底物 | 反应结果 |
|---|---|---|---|
| [Rh(COD)Cl]2/TPPTS[ | 135℃,氨水,6.5MPa H2,2h | 苯甲醛 | Y苄胺=86% |
| [Ru(CO)ClH(PPh3)3][ | 120℃,0.9MPa NH3,4MPa H2,16h | 苯乙酮 | Yα-苯乙胺=95% |
| [Ru(PPh3)3H(CO)Cl][ | 80℃,NH4I,3MPa H2,24h | 甲基酮环己酯 | Y(S)-(+)-1-环己基乙胺=78% |
| RuCl2(PPh3)3[ | 120℃,0.5~0.7MPa NH3,4MPa H2,24h | 苯甲醛 | Y卞胺=95% |
| Ru-1[ | 130℃,0.5MPa NH3,5MPa H2,12h | 苯甲醛 | Y苄胺=94% |
| Cp*Ir[ | 80℃,甲酸铵,4h | 苯乙酮 | Y1-苯乙胺=97% |
| 1 | TALWAR D, SALGUERO N P, ROBERTSON C M, et al. Primary amines by transfer hydrogenative reductive amination of ketones by using cyclometalated IrⅢ catalysts[J]. Chemistry—A European Journal, 2014, 20(1): 245-252. |
| 2 | BÄHN S, IMM S, NEUBERT L, et al. The catalytic amination of alcohols[J]. ChemCatChem, 2011, 3(12): 1853-1864. |
| 3 | KOBAYASHI S, ISHITANI H. Catalytic enantioselective addition to imines[J]. Chemical Reviews, 1999, 99(5): 1069-1094. |
| 4 | MURUGESAN K, SENTHAMARAI T, CHANDRASHEKHAR V G, et al. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines[J]. Chemical Society Reviews, 2020, 49(17): 6273-6328. |
| 5 | 杨萍, 刘敏节, 张昊, 等. 硝基芳烃与醇还原胺化: 催化剂和催化机制[J]. 化学进展, 2020, 32(1): 72-83. |
| YANG Ping, LIU Minjie, ZHANG Hao, et al. Reductive amination of nitroarenes and alcohols: catalyst and catalytic mechanism[J]. Progress in Chemistry, 2020, 32(1): 72-83. | |
| 6 | GUNANATHAN C, MILSTEIN D. Selective synthesis of primary amines directly from alcohols and ammonia[J]. Angewandte Chemie International Edition, 2008, 47(45): 8661-8664. |
| 7 | 潘嘉晟, 王耀锋, 马爽爽, 等. 脂肪伯胺的合成及工业化研究进展[J]. 过程工程学报, 2021, 21(8): 905-917. |
| PAN Jiasheng, WANG Yaofeng, MA Shuangshuang, et al. Research progress on synthesis and industrialization of fatty primary amines[J]. The Chinese Journal of Process Engineering, 2021, 21(8): 905-917. | |
| 8 | YANG L C, WANG Y N, ZHANG Y, et al. Acid-assisted Ru-catalyzed enantioselective amination of 1,2-diols through borrowing hydrogen[J]. ACS Catalysis, 2017, 7(1): 93-97. |
| 9 | NOWROUZI N, JONAGHANI M Z. Highly selective mono-N-benzylation and amidation of amines with alcohols or carboxylic acids using the Ph2PCl/I2/imidazole reagent system[J]. Canadian Journal of Chemistry, 2012, 90(6): 498-509. |
| 10 | MARICHEV K O, TAKACS J M. Ruthenium-catalyzed amination of secondary alcohols using borrowing hydrogen methodology[J]. ACS Catalysis, 2016, 6(4): 2205-2210. |
| 11 | 滕燕燕. 纳米Pt催化剂上芳香硝基化合物选择加氢性能的研究[D]. 大连: 大连理工大学, 2019. |
| TENG Yanyan. Study on selective hydrogenation performance of aromatic nitro compounds over nano-Pt catalysts[D]. Dalian: Dalian University of Technology, 2019. | |
| 12 | SUN S T, DU M Y, ZHAO R X, et al. Zn(0)-catalysed mild and selective hydrogenation of nitroarenes[J]. Green Chemistry, 2020, 22(14): 4640-4644. |
| 13 | 曾云凤. 磷化镍催化腈类化合物选择性生成伯胺的研究[D]. 昆明: 云南师范大学, 2017. |
| ZENG Yunfeng. Selective hydrogenation of nitriles to primary amines catalyzed by Ni2P[D]. Kunming: Yunnan Normal University, 2017. | |
| 14 | SANAGAWA A, NAGASHIMA H. Hydrosilane reduction of nitriles to primary amines by cobalt-isocyanide catalysts[J]. Organic Letters, 2019, 21(1): 287-291. |
| 15 | 陈晓, 花文廷. 酰胺还原反应研究进展[J]. 化学通报, 2001, 64(12): 749-754. |
| CHEN Xiao, HUA Wenting. Recent developments on selective reduction of amides[J]. Chemistry Bulletin, 2001, 64(12): 749-754. | |
| 16 | 王建红, 李骞, 何丽华, 等. Gabriel合成胺反应的工艺改进[J]. 化学研究, 2010, 21(4): 48-51. |
| WANG Jianhong, LI Qian, HE Lihua, et al. Modification of process for synthesizing polyamine via Gabriel reaction[J]. Chemical Research, 2010, 21(4): 48-51. | |
| 17 | PAN X H, ZHAO D X, SHI Y, et al. Synthesis of S-3-hydroxyadamantylglycine ester by a Pd/C promoted mild leuckart-wallach reaction and an L-dibenzoyltartaric acid resolution[J]. Journal of Chemical Research, 2015, 39(2): 108-111. |
| 18 | KLINKENBERG J L, HARTWIG J F. Catalytic organometallic reactions of ammonia[J]. Angewandte Chemie International Edition, 2011, 50(1): 86-95. |
| 19 | LIN S K, MARCH J. March’s advanced organic chemistry: reactions, mechanisms, and structure, 5th edition[J]. Molecules, 2001, 6(12): 1064-1065. |
| 20 | 高爽. 过渡金属催化酮直接不对称还原胺化合成伯胺及其衍生物[D]. 哈尔滨:哈尔滨工业大学, 2019. |
| GAO Shuang. Transition metal-catalyzed direct asymmetric reductive amination of ketones for the synthesis of primary amines and their derivatives[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 21 | ABDEL-MAGID A F, CARSON K G, HARRIS B D, et al. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. studies on direct and indirect reductive amination procedures1[J]. The Journal of Organic Chemistry, 1996, 61(11): 3849-3862. |
| 22 | IRRGANG T, KEMPE R. Transition-metal-catalyzed reductive amination employing hydrogen[J]. Chemical Reviews, 2020, 120(17): 9583-9674. |
| 23 | WINANS C F. Hydrogenation of aldehydes in the presence of ammonia[J]. Journal of the American Chemical Society, 1939, 61(12): 3566-3567. |
| 24 | HASKELBERG L. Aminative reduction of ketones[J]. Journal of the American Chemical Society, 1948, 70(8): 2811-2812. |
| 25 | 王建强, 陈明明, 刘巍, 等. 固定床雷尼金属催化剂研究进展[J]. 化工进展, 2018, 37(11): 4280-4285. |
| WANG Jianqiang, CHEN Mingming, LIU Wei, et al. Advance in fixed-bed Raney metal catalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4280-4285. | |
| 26 | CHATTERJEE M, ISHIZAKA T, KAWANAMI H. Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: an environmentally friendly approach[J]. Green Chemistry, 2016, 18(2): 487-496. |
| 27 | 熊曼, 黄磊, 刘晓云, 等. 铑催化的还原胺化合成一级胺[J]. 南京工业大学学报(自然科学版), 2014, 36(4): 13-17, 44. |
| XIONG Man, HUANG Lei, LIU Xiaoyun, et al. Rhodium catalyzed reductive amination of aldehydes to primary amines with ammonium acetate[J]. Journal of Nanjing Tech University (Natural Science Edition), 2014, 36(4): 13-17, 44. | |
| 28 | DONG B, GUO X C, ZHANG B, et al. Heterogeneous Ru-based catalysts for one-pot synthesis of primary amines from aldehydes and ammonia[J]. Catalysts, 2015, 5(4): 2258-2270. |
| 29 | KOMANOYA T, KINEMURA T, KITA Y, et al. Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds[J]. Journal of the American Chemical Society, 2017, 139(33): 11493-11499. |
| 30 | GUO W J, TONG T, LIU X H, et al. Morphology-tuned activity of Ru/Nb2O5 catalysts for ketone reductive amination[J]. ChemCatChem, 2019, 11(16): 4130-4138. |
| 31 | DONG C L, WANG H T, DU H C, et al. Ru/HZSM-5 as an efficient and recyclable catalyst for reductive amination of furfural to furfurylamine[J]. Molecular Catalysis, 2020, 482: 110755. |
| 32 | LIANG G F, WANG A Q, LI L, et al. Production of primary amines by reductive amination of biomass-derived aldehydes/ketones[J]. Angewandte Chemie International Edition, 2017, 129(11): 3096-3100. |
| 33 | 黄龙俊, 鲍佳浩, 郭璐瑶, 等. Ru/ZrO2催化环己酮还原胺化制备环己胺工艺[J]. 化学反应工程与工艺, 2019, 35(3): 274-281. |
| HUANG Longjun, BAO Jiahao, GUO Luyao, et al. Study on Ru/ZrO2 catalyzed reductive amination of cyclohexanone to cyclohexylamine[J]. Chemical Reaction Engineering and Technology, 2019, 35(3): 274-281. | |
| 34 | NAKAMURA Y, KON K, TOUCHY A S, et al. Selective synthesis of primary amines by reductive amination of ketones with ammonia over supported Pt catalysts[J]. ChemCatChem, 2015, 7(6): 921-924. |
| 35 | JV X, SUN S T, ZHANG Q, et al. Efficient and mild reductive amination of carbonyl compounds catalyzed by dual-function palladium nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(3): 1618-1626. |
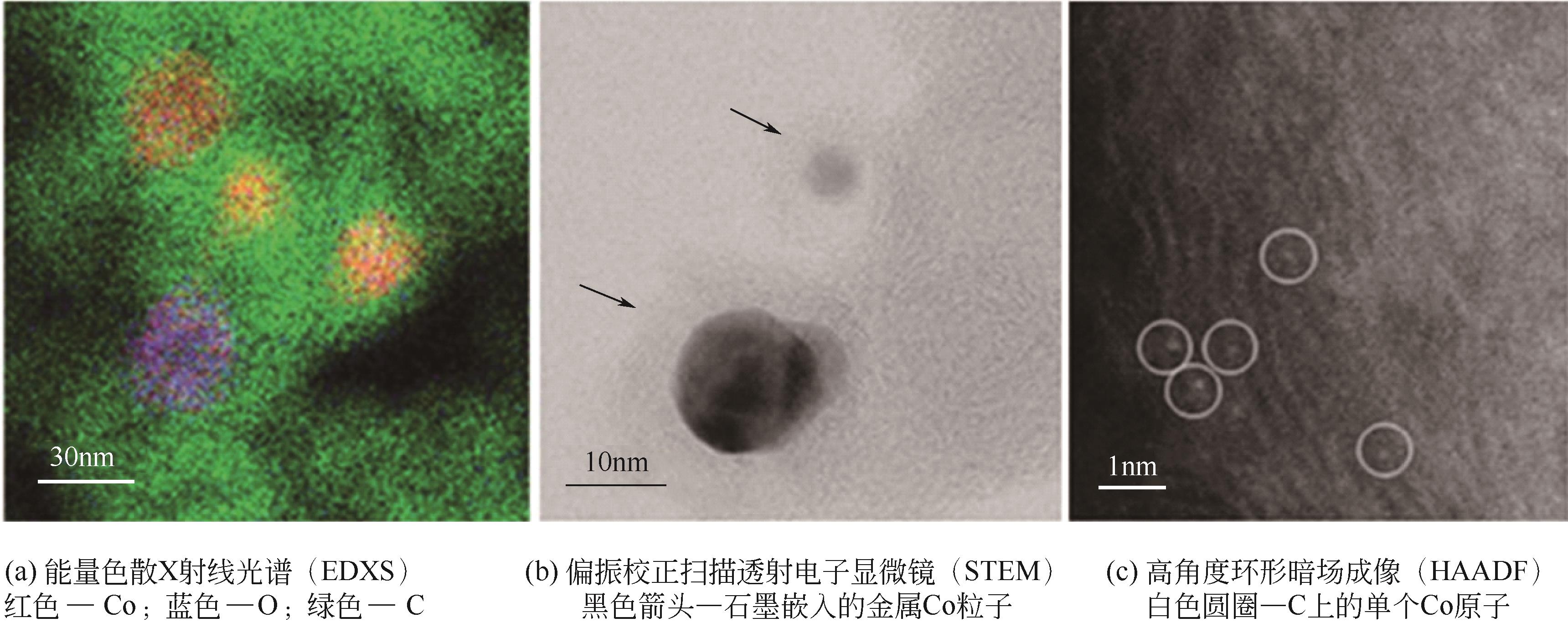
| 36 | JAGADEESH R V, MURUGESAN K, ALSHAMMARI A S, et al. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines[J]. Science, 2017, 358(6361): 326-332. |
| 37 | MURUGESAN K, CHANDRASHEKHAR V G, SENTHAMARAI T, et al. Reductive amination using cobalt-based nanoparticles for synthesis of amines[J]. Nature Protocols, 2020, 15(4): 1313-1337. |
| 38 | YUAN Z L, LIU B, ZHOU P, et al. Preparation of nitrogen-doped carbon supported cobalt catalysts and its application in the reductive amination[J]. Journal of Catalysis, 2019, 370: 347-356. |
| 39 | LIU J M, GUO W W, SUN H, et al. Reductive amination of carbonyl compounds with ammonia and hydrogenation of nitriles to primary amines with heterogeneous cobalt catalysts[J]. Chemical Research in Chinese Universities, 2019, 35(3): 457-462. |
| 40 | ZHANG Y M, YANG H M, CHI Q, et al. Nitrogen-doped carbon-supported nickel nanoparticles: a robust catalyst to bridge the hydrogenation of nitriles and the reductive amination of carbonyl compounds for the synthesis of primary amines[J]. ChemSusChem, 2019, 12(6): 1246-1255. |
| 41 | BÄUMLER C, BAUER C, KEMPE R. The synthesis of primary amines through reductive amination employing an iron catalyst[J]. ChemSusChem, 2020, 13(12): 3110-3114. |
| 42 | HAHN G, KUNNAS P, DE JONGE N, et al. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst[J]. Nature Catalysis, 2019, 2(1): 71-77. |
| 43 | MURUGESAN K, BELLER M, JAGADEESH R V. Reusable nickel nanoparticles-catalyzed reductive amination for selective synthesis of primary amines[J]. Angewandte Chemie International Edition, 2019, 58(15): 5064-5068. |
| 44 | 杜小宝. 芳香醛酮的绿色还原和三丙酮胺的还原胺化研究[D]. 天津: 天津大学, 2014. |
| DU Xiaobao. Studies on the green reduction of aromatic aldehydes and ketones and the reductive amination of triacetoneamine[D]. Tianjin: Tianjin University, 2014. | |
| 45 | GROSS T, SEAYAD A M, AHMAD M, et al. Synthesis of primary amines: first homogeneously catalyzed reductive amination with ammonia[J]. Organic Letters, 2002, 4(12): 2055-2058. |
| 46 | GALLARDO-DONAIRE J, ERNST M, TRAPP O, et al. Direct synthesis of primary amines via ruthenium-catalysed amination of ketones with ammonia and hydrogen[J]. Advanced Synthesis & Catalysis, 2016, 358(3): 358-363. |
| 47 | GHOSH T, ERNST M, HASHMI A S K, et al. Ruthenium catalyzed direct asymmetric reductive amination of simple aliphatic ketones using ammonium iodide and hydrogen[J]. European Journal of Organic Chemistry, 2020, 2020(30): 4796-4800. |
| 48 | SENTHAMARAI T, MURUGESAN K, SCHNEIDEWIND J, et al. Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines[J]. Nature Communications, 2018, 9: 4123. |
| 49 | PAN J S, ZHANG R, MA S S, et al. Easily synthesized Ru catalyst efficiently converts carbonyl compounds and ammonia into primary amines[J]. ChemistrySelect, 2020, 5(35): 10933-10938. |
| 50 | TANAKA K, MIKI T, MURATA K, et al. Reductive amination of ketonic compounds catalyzed by Cp*Ir(Ⅲ) complexes bearing a picolinamidato ligand[J]. The Journal of Organic Chemistry, 2019, 84(17): 10962-10977. |
| 51 | MURUGESAN K, WEI Z H, CHANDRASHEKHAR V G, et al. Homogeneous cobalt-catalyzed reductive amination for synthesis of functionalized primary amines[J]. Nature Communications, 2019, 10: 5443. |
| 52 | MURUGESAN K, WEI Z H, CHANDRASHEKHAR V G, et al. General and selective synthesis of primary amines using Ni-based homogeneous catalysts[J]. Chemical Science, 2020, 11(17): 4332-4339. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||