化工进展 ›› 2022, Vol. 41 ›› Issue (6): 2993-3001.DOI: 10.16085/j.issn.1000-6613.2021-1467
富氮类共价有机骨架材料COF-MC催化Knoevenagel缩合反应的应用
吕杰琼( ), 谢晖, 高永平, 连丽丽, 王希越, 张浩, 高文秀(
), 谢晖, 高永平, 连丽丽, 王希越, 张浩, 高文秀( ), 娄大伟(
), 娄大伟( )
)
- 吉林化工学院化学与制药工程学院,吉林 吉林 132022
-
收稿日期:2021-07-12修回日期:2021-10-06出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:高文秀,娄大伟 -
作者简介:吕杰琼(1997—),女,硕士研究生,研究方向为催化化学。E-mail:lvjieqiong0518@163.com 。 -
基金资助:吉林省科技发展计划(20190303116SF);吉林省产业技术研究与开发项目(2020C028-1);吉林省教育厅科研规划项目(JJKH20200240KJ)
Application of nitrogen-rich covalent organic framework material COF-MC catalyzing Knoevenagel condensation reaction
LYU Jieqiong( ), XIE Hui, GAO Yongping, LIAN Lili, WANG Xiyue, ZHANG Hao, GAO Wenxiu(
), XIE Hui, GAO Yongping, LIAN Lili, WANG Xiyue, ZHANG Hao, GAO Wenxiu( ), LOU Dawei(
), LOU Dawei( )
)
- School of Chemical and Pharmaceutical Engineering, Jilin Institute of Chemical Technology, Jilin 132022, Jilin, China
-
Received:2021-07-12Revised:2021-10-06Online:2022-06-10Published:2022-06-21 -
Contact:GAO Wenxiu,LOU Dawei
摘要:
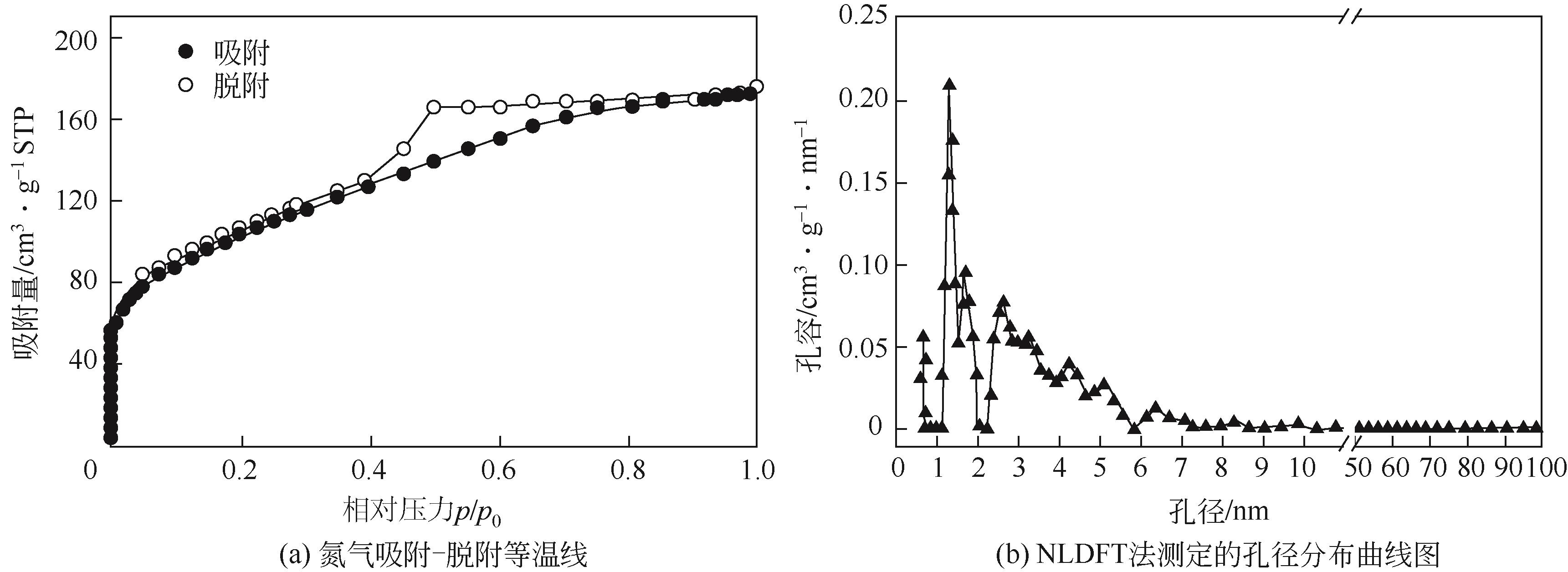
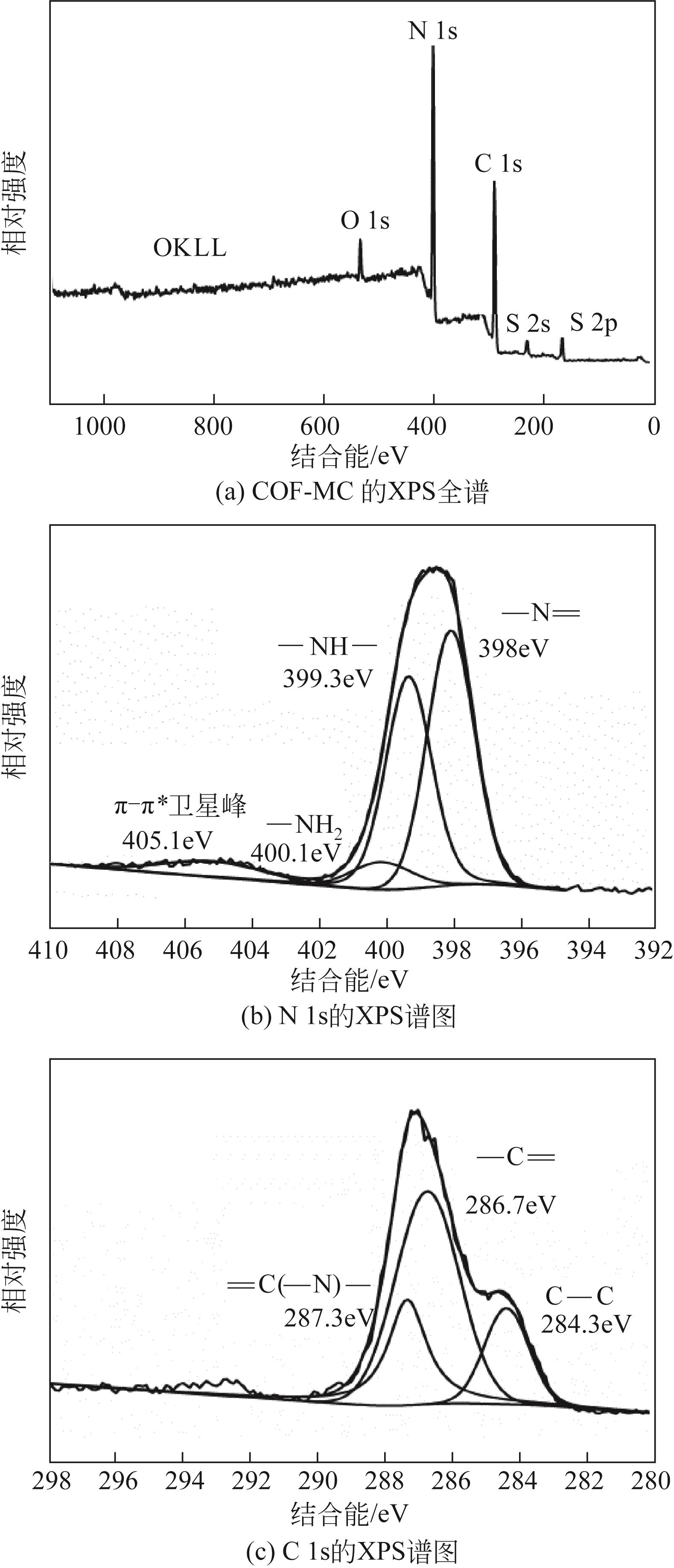
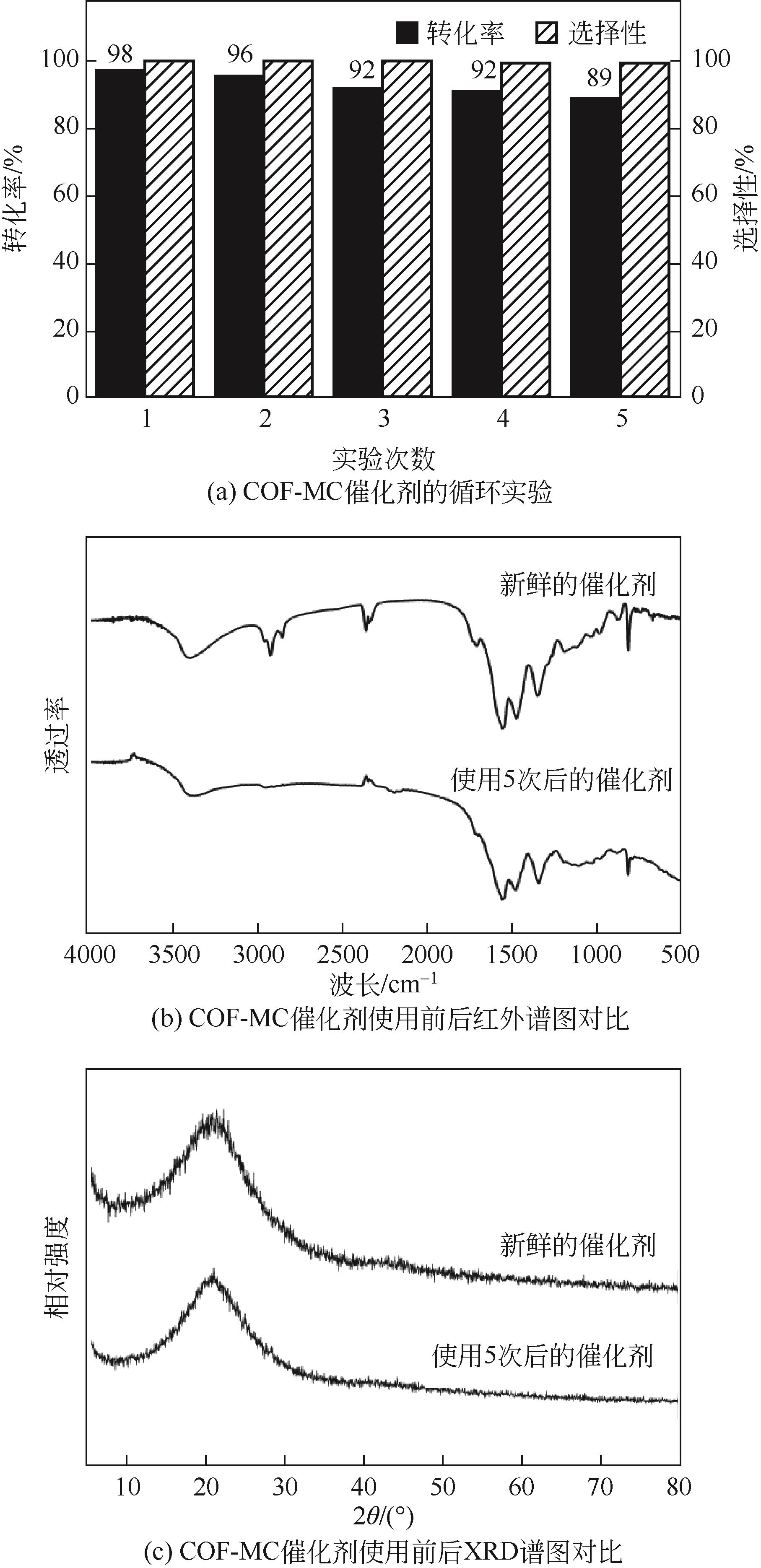
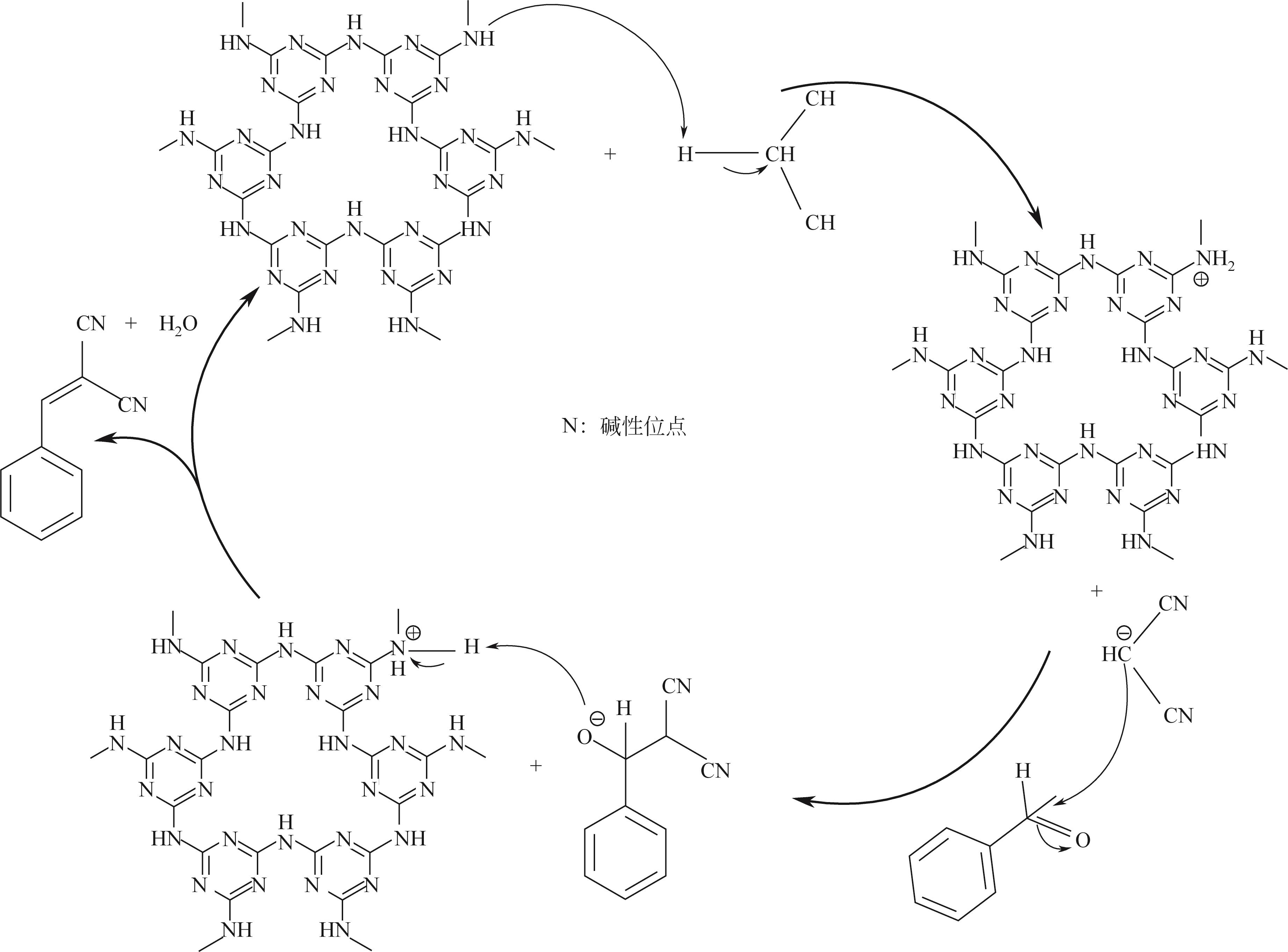
以三聚氰胺和三聚氯氰为前体,采用溶剂热法制备富氮类共价有机骨架材料 COF-MC[含氮量为55%(质量分数)]。利用傅里叶变换红外光谱(FTIR)、X射线衍射(XRD)、N2吸附-脱附、扫描电子显微镜(SEM)、热重(TGA)和X射线光电子能谱(XPS)测试手段对COF-MC进行表征。通过对COF-MC在苯甲醛和丙二腈Knoevenagel缩合反应中催化性能的评价,考察反应条件与催化性能的关系,并对 Knoevenagel缩合反应的碱性催化机理进行了初步的探讨。实验结果表明,COF-MC作为催化剂在氮气环境中,无需借助其他溶剂,80℃回流搅拌2h后,苯甲醛转化率为98%,苄烯丙二腈选择性在99.9%以上。反应后的催化剂可以通过简单的热过滤分离,重复使用4次后苯甲醛转化率仍可达89%,且催化剂中无金属离子的参与,避免了金属对环境的污染。
中图分类号:
引用本文
吕杰琼, 谢晖, 高永平, 连丽丽, 王希越, 张浩, 高文秀, 娄大伟. 富氮类共价有机骨架材料COF-MC催化Knoevenagel缩合反应的应用[J]. 化工进展, 2022, 41(6): 2993-3001.
LYU Jieqiong, XIE Hui, GAO Yongping, LIAN Lili, WANG Xiyue, ZHANG Hao, GAO Wenxiu, LOU Dawei. Application of nitrogen-rich covalent organic framework material COF-MC catalyzing Knoevenagel condensation reaction[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2993-3001.
| 元素质量分数/% | 不同种类氮质量分数/% | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | S | N | —N | —NH— | —NH2 | π-π* 卫星峰 | |
| 35.96 | 3.71 | 5.10 | 55.23 | 48.23 | 39.24 | 6.72 | 5.81 | |
表1 COF-MC中元素及不同种类氮质量分数
| 元素质量分数/% | 不同种类氮质量分数/% | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | S | N | —N | —NH— | —NH2 | π-π* 卫星峰 | |
| 35.96 | 3.71 | 5.10 | 55.23 | 48.23 | 39.24 | 6.72 | 5.81 | |
| 序号 | 催化剂 | n(苯甲醛)∶n(丙二腈) | 转化率 /% | 选择性 /% | TOF×10-3 /mol·g-1·h-1 |
|---|---|---|---|---|---|
| 1 | 无 | 1∶1 | 7 | >99.9 | |
| 2 | COF-MC | 1∶1 | 92 | >99.9 | 92 |
| 3 | COF-MC | 1∶1.5 | 100 | >99.9 | 100 |
表2 催化剂及底物比对Knoevenagel缩合反应的影响
| 序号 | 催化剂 | n(苯甲醛)∶n(丙二腈) | 转化率 /% | 选择性 /% | TOF×10-3 /mol·g-1·h-1 |
|---|---|---|---|---|---|
| 1 | 无 | 1∶1 | 7 | >99.9 | |
| 2 | COF-MC | 1∶1 | 92 | >99.9 | 92 |
| 3 | COF-MC | 1∶1.5 | 100 | >99.9 | 100 |
| 序号 | 催化剂用量/mg | 转化率/% | 选择性/% | TOF |
|---|---|---|---|---|
| 1 | 50 | 100 | >99.9 | 100 |
| 2 | 40 | 100 | >99.9 | 125 |
| 3 | 30 | 99 | >99.9 | 163 |
| 4 | 20 | 98 | >99.9 | 242 |
| 5 | 10 | 80 | >99.9 | 402 |
表3 催化剂用量对Knoevenagel 缩合反应的影响
| 序号 | 催化剂用量/mg | 转化率/% | 选择性/% | TOF |
|---|---|---|---|---|
| 1 | 50 | 100 | >99.9 | 100 |
| 2 | 40 | 100 | >99.9 | 125 |
| 3 | 30 | 99 | >99.9 | 163 |
| 4 | 20 | 98 | >99.9 | 242 |
| 5 | 10 | 80 | >99.9 | 402 |
| 序号 | 温度/℃ | 时间/h | 转化率/% | 选择性/% | TOF×10-3/mol·g-1·h-1 |
|---|---|---|---|---|---|
| 1 | 60 | 2 | 79 | >99.9 | 196 |
| 2 | 70 | 2 | 95 | >99.9 | 238 |
| 3 | 80 | 2 | 98 | >99.9 | 244 |
| 4 | 90 | 2 | 99 | >99.9 | 248 |
| 5 | 80 | 0.5 | 61 | >99.9 | 610 |
| 6 | 80 | 1 | 75 | >99.9 | 375 |
| 7 | 80 | 1.5 | 94 | >99.9 | 313 |
| 8 | 80 | 2.5 | 98 | >99.9 | 196 |
表4 反应温度及时间对苯甲醛与丙二腈Knoevenagel缩合反应的影响
| 序号 | 温度/℃ | 时间/h | 转化率/% | 选择性/% | TOF×10-3/mol·g-1·h-1 |
|---|---|---|---|---|---|
| 1 | 60 | 2 | 79 | >99.9 | 196 |
| 2 | 70 | 2 | 95 | >99.9 | 238 |
| 3 | 80 | 2 | 98 | >99.9 | 244 |
| 4 | 90 | 2 | 99 | >99.9 | 248 |
| 5 | 80 | 0.5 | 61 | >99.9 | 610 |
| 6 | 80 | 1 | 75 | >99.9 | 375 |
| 7 | 80 | 1.5 | 94 | >99.9 | 313 |
| 8 | 80 | 2.5 | 98 | >99.9 | 196 |
| 序号 | 催化剂 | 溶剂 | 温度/℃ | 时间/h | 产率/% | TOF×10-3/mol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | COF-MC | 无 | 80 | 2 | 98 | 244 | 本文 |
| 2 | COF-HNU14 | 无 | 25 | 5 | 99 | 50 | [ |
| 3 | A2B2-Pro-COF | 甲苯/H2O | 60 | 3 | 81 | 27 | [ |
| 4 | COF-366-R | 甲苯 | 60 | 6 | 81 | 25 | [ |
| 5 | GO/COF | 无 | 室温 | 1/6 | 98 | 392 | [ |
| 6 | Co-MOF/COF | 无 | 25 | 1/6 | 93 | 372 | [ |
| 7 | Zn-MOF/COF | 无 | 25 | 1/6 | 99 | 396 | [ |
| 8 | NC-700 | H2O/乙醇 | 40 | 1 | > 99 | 50 | [ |
| 9 | 壳聚糖 | 乙醇 | 40 | 6 | > 99 | 7 | [ |
| 10 | N-GO-1.00 | CH3CN | 40 | 4 | 96.5 | 24 | [ |
| 11 | Cyt@SBA-15 | 乙醇 | 室温 | 1 | 99 | 99 | [ |
| 12 | UiO-66-NH-RNH2 | 甲苯 | 室温 | 2 | 97 | 46 | [ |
表5 与已报道的苯甲醛和丙二腈 Knoevenagel 缩合反应催化剂比较
| 序号 | 催化剂 | 溶剂 | 温度/℃ | 时间/h | 产率/% | TOF×10-3/mol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | COF-MC | 无 | 80 | 2 | 98 | 244 | 本文 |
| 2 | COF-HNU14 | 无 | 25 | 5 | 99 | 50 | [ |
| 3 | A2B2-Pro-COF | 甲苯/H2O | 60 | 3 | 81 | 27 | [ |
| 4 | COF-366-R | 甲苯 | 60 | 6 | 81 | 25 | [ |
| 5 | GO/COF | 无 | 室温 | 1/6 | 98 | 392 | [ |
| 6 | Co-MOF/COF | 无 | 25 | 1/6 | 93 | 372 | [ |
| 7 | Zn-MOF/COF | 无 | 25 | 1/6 | 99 | 396 | [ |
| 8 | NC-700 | H2O/乙醇 | 40 | 1 | > 99 | 50 | [ |
| 9 | 壳聚糖 | 乙醇 | 40 | 6 | > 99 | 7 | [ |
| 10 | N-GO-1.00 | CH3CN | 40 | 4 | 96.5 | 24 | [ |
| 11 | Cyt@SBA-15 | 乙醇 | 室温 | 1 | 99 | 99 | [ |
| 12 | UiO-66-NH-RNH2 | 甲苯 | 室温 | 2 | 97 | 46 | [ |
| 1 | APPATURI J N, PULINGAM T, RAJABATHAR J R, et al. Acid-base bifunctional SBA-15 as an active and selective catalyst for synthesis of ethyl α-cyanocinnamate via Knoevenagel condensation[J]. Microporous and Mesoporous Materials, 2021, 320: 111091. |
| 2 | PATEL D, VITHALANI R, MODI C K. Highly efficient FeNP-embedded hybrid bifunctional reduced graphene oxide for Knoevenagel condensation with active methylene compounds[J]. New Journal of Chemistry, 2020, 44(7): 2868-2881. |
| 3 | 陈琳, 谢蓉蓉, 关丽, 等. 苯并-α-吡喃酮类生物活性物质的合成[J]. 化工进展, 2014, 33(8): 2160-2164. |
| CHEN Lin, XIE Rongrong, GUAN Li, et al. Synthesis of some biologically active coumarins[J]. Chemical Industry and Engineering Progress, 2014, 33(8): 2160-2164. | |
| 4 | YANG Y, WANG D, JIANG P F, et al. Structure-induced Lewis-base Ga4B2O9 and its superior performance in Knoevenagel condensation reaction[J]. Molecular Catalysis, 2020, 490: 110914. |
| 5 | RUBAN S M, SATHISH C I, RAMADASS K, et al. Ordered mesoporous carbon nitrides with tuneable nitrogen contents and basicity for Knoevenagel condensation[J]. ChemCatChem, 2021, 13(1): 468-474. |
| 6 | 高朋召, 吴迪, 郑航博, 等. 有机胺改性对ZIF-8催化Knoevenagel缩合反应活性的影响[J]. 湖南大学学报(自然科学版), 2020, 47(8): 124-132. |
| GAO Pengzhao, WU Di, ZHENG Hangbo, et al. Effect of amine modification on catalytic activity of ZIF-8 in Knoevenagel condensation reaction[J]. Journal of Hunan University (Natural Sciences), 2020, 47(8): 124-132. | |
| 7 | 颜世强, 郭伟, 王文笙, 等. 锌-脯氨酸复合物催化的水相Knoevenagel缩合[J]. 有机化学, 2019, 39(5): 1469-1474. |
| YAN Shiqiang, GUO Wei, WANG Wensheng, et al. Zinc-proline complex catalyzed Knoevenagel condensation in water[J]. Chinese Journal of Organic Chemistry, 2019, 39(5): 1469-1474. | |
| 8 | ŞEN B, AKDERE E H, ŞAVK A, et al. A novel thiocarbamide functionalized graphene oxide supported bimetallic monodisperse Rh-Pt nanoparticles (RhPt/TC@GO NPs) for Knoevenagel condensation of aryl aldehydes together with malononitrile[J]. Applied Catalysis B: Environmental, 2018, 225: 148-153. |
| 9 | JOHARIAN M, MORSALI A, AZHDARI TEHRANI A, et al. Water-stable fluorinated metal–organic frameworks (F-MOFs) with hydrophobic properties as efficient and highly active heterogeneous catalysts in aqueous solution[J]. Green Chemistry, 2018, 20(23): 5336-5345. |
| 10 | BAHUGUNA A, KUMAR A, CHHABRA T, et al. Potassium-functionalized graphitic carbon nitride supported on reduced graphene oxide as a sustainable catalyst for Knoevenagel condensation[J]. ACS Applied Nano Materials, 2018, 1(12): 6711-6723. |
| 11 | 李航, 付海, 班大明, 等. 蒙脱土负载KF催化Knoevenagel缩合反应[J]. 福建师范大学学报(自然科学版), 2019, 35(4): 37-43. |
| LI Hang, FU Hai, BAN Daming, et al. Montmorillonite loading KF catalyzed Knoevenagel condensation reaction[J]. Journal of Fujian Normal University (Natural Science Edition), 2019, 35(4): 37-43. | |
| 12 | 李琳琳, 龚维, 付海, 等. 介孔分子筛KF-SBA-15的制备及其催化Knoevenagel反应[J]. 化学工业与工程, 2020, 37(3): 17-22. |
| LI Linlin, GONG Wei, FU Hai,et al. Preparation of KF-SBA-15 and its catalytic Knoevenagel reaction[J]. Chemical Industry and Engineering, 2020, 37(3): 17-22. | |
| 13 | TANGALE N P, SONAR S K, NIPHADKAR P S, et al. Hierarchical K/LTL zeolites: synthesis by alkali treatment, characterization and catalytic performance in Knoevenagel condensation reaction[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 128-136. |
| 14 | COTE A P, BENIN A I, OCKWIG N W, et al. Porous, crystalline, covalent organic frameworks[J]. Science, 2005, 310(5751): 1166-1170. |
| 15 | 刘祎, 汪明旺, 吕宏凌, 等. 共价有机骨架聚合物功能膜制备方法的研究进展[J]. 化工进展, 2021, 40(8): 4360-4370. |
| LIU Yi, WANG Mingwang, Hongling LYU, et al. Research progress in the preparation method of covalent organic framework polymers (COFs) functional membranes[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4360-4370. | |
| 16 | 张安睿, 艾玥洁. 共价有机框架(COFs)材料的结构控制及其在环境化学中的应用[J]. 化学进展, 2020, 32(10): 1564-1581. |
| ZHANG Anrui, AI Yuejie. Structure control of covalent organic frameworks(COFs) and their applications in environmental chemistry[J]. Progress in Chemistry, 2020, 32(10): 1564-1581. | |
| 17 | WU X C, WANG B W, YANG Z Q, et al. Novel imine-linked covalent organic frameworks: preparation, characterization and application[J]. Journal of Materials Chemistry A, 2019, 7(10): 5650-5655. |
| 18 | MONEHZADEH F, RAFIEE Z. Application of GO/COF as a novel, efficient and recoverable catalyst in the Knoevenagel reaction[J]. Applied Organometallic Chemistry, 2020, 34(6): e5631. |
| 19 | APPATURI J N, RATTI R, PHOON B L, et al. A review of the recent progress on heterogeneous catalysts for Knoevenagel condensation[J]. Dalton Transactions, 2021, 50(13): 4445-4469. |
| 20 | QI S C, WU J K, LU J, et al. Underlying mechanism of CO2 adsorption onto conjugated azacyclo-copolymers: N-doped adsorbents capture CO2 chiefly through acid–base interaction?[J]. Journal of Materials Chemistry A, 2019, 7(30): 17842-17853. |
| 21 | 焦莉, 徐金妹, 张秋亚, 等. 氨基修饰片状氮化碳的制备及光催化性能[J]. 化工进展, 2020, 39(5): 1866-1874. |
| JIAO Li, XU Jinmei, ZHANG Qiuya, et al. Preparation and photocatalytic activity of amino-modified sheet-like carbon nitride[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1866-1874. | |
| 22 | WANG Z Z, LYU P, HU Y, et al. Thermal degradation study of intumescent flame retardants by TG and FTIR: melamine phosphate and its mixture with pentaerythritol[J]. Journal of Analytical and Applied Pyrolysis, 2009, 86(1): 207-214. |
| 23 | HU X W, LONG Y, FAN M Y, et al. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis[J]. Applied Catalysis B: Environmental, 2019, 244: 25-35. |
| 24 | BHUNIA M K, MELISSEN S, PARIDA M R, et al. Dendritic tip-on polytriazine-based carbon nitride photocatalyst with high hydrogen evolution activity[J]. Chemistry of Materials, 2015, 27(24): 8237-8247. |
| 25 | WANG H F, WANG C Y, YANG Y F, et al. H3PW12O40 /mpg-C3N4 as an efficient and reusable bifunctional catalyst in one-pot oxidation–Knoevenagel condensation tandem reaction[J]. Catalysis Science & Technology, 2017, 7(2): 405-417. |
| 26 | LIU H H, CHEN D L, WANG Z Q, et al. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance[J]. Applied Catalysis B: Environmental, 2017, 203: 300-313. |
| 27 | HUA S X, QU D, AN L, et al. Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route[J]. Applied Catalysis B: Environmental, 2019, 240: 253-261. |
| 28 | CHAUDHARY M, SINGH L, REKHA P, et al. Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine[J]. Chemical Engineering Journal, 2019, 378: 122236. |
| 29 | ZHAO Y L, ZHAO Y, QIU J K, et al. Facile grafting of imidazolium salt in covalent organic frameworks with enhanced catalytic activity for CO2 fixation and the Knoevenagel reaction[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(50): 18413-18419. |
| 30 | HAO W J, CHEN D, LI Y S, et al. Facile synthesis of porphyrin based covalent organic frameworks via an A2B2 monomer for highly efficient heterogeneous catalysis[J]. Chemistry of Materials, 2019, 31(19): 8100-8105. |
| 31 | HU J Y, ZANCA F, MCMANUS G J, et al. Catalyst-enabled in situ linkage reduction in imine covalent organic frameworks[J]. ACS Applied Materials & Interfaces, 2021, 13(18): 21740-21747. |
| 32 | RAHMATI E, RAFIEE Z. Synthesis of Co-MOF/COF nanocomposite: application as a powerful and recoverable catalyst in the Knoevenagel reaction[J]. Journal of Porous Materials, 2021, 28(1): 19-27. |
| 33 | RAFIEE Z. Fabrication of efficient Zn-MOF/COF catalyst for the Knoevenagel condensation reaction[J]. Journal of the Iranian Chemical Society, 2021, 18(10): 2657-2664. |
| 34 | SAKTHIVEL B, DHAKSHINAMOORTHY A. Chitosan as a reusable solid base catalyst for Knoevenagel condensation reaction[J]. Journal of Colloid and Interface Science, 2017, 485: 75-80. |
| 35 | XUE B, ZHU J G, LIU N, et al. Facile functionalization of graphene oxide with ethylenediamine as a solid base catalyst for Knoevenagel condensation reaction[J]. Catalysis Communications, 2015, 64: 105-109. |
| 36 | RAJABI F, FAYYAZ F, LUQUE R. Cytosine-functionalized SBA-15 mesoporous nanomaterials: synthesis, characterization and catalytic applications[J]. Microporous and Mesoporous Materials, 2017, 253: 64-70. |
| 37 | LUAN Y, QI Y, GAO H Y, et al. A general post-synthetic modification approach of amino-tagged metal–organic frameworks to access efficient catalysts for the Knoevenagel condensation reaction[J]. Journal of Materials Chemistry A, 2015, 3(33): 17320-17331. |
| 38 | ZHANG L N, WANG H, SHEN W Z, et al. Controlled synthesis of graphitic carbon nitride and its catalytic properties in Knoevenagel condensations[J]. Journal of Catalysis, 2016, 344: 293-302. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||