化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5491-5498.DOI: 10.16085/j.issn.1000-6613.2020-2225
NO2硝化正己烷及其理论计算
李星彦1( ), 王墨1, 戴璇1, 彭新华1, 唐双凌2(
), 王墨1, 戴璇1, 彭新华1, 唐双凌2( )
)
- 1.南京理工大学化学与化工学院,江苏 南京 210094
2.南京理工大学环境与生物工程学院,江苏 南京 210094
-
收稿日期:2020-11-06修回日期:2021-03-16出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:唐双凌 -
作者简介:李星彦(1996—),男,硕士研究生,研究方向为有机化学。E-mail:15061130209@163.com 。 -
基金资助:国家乏燃料后处理科研专项
Nitration of n-hexane with NO2 and theoretical calculation
LI Xingyan1( ), WANG Mo1, DAI Xuan1, PENG Xinhua1, TANG Shuangling2(
), WANG Mo1, DAI Xuan1, PENG Xinhua1, TANG Shuangling2( )
)
- 1.School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
2.School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
-
Received:2020-11-06Revised:2021-03-16Online:2021-10-10Published:2021-10-25 -
Contact:TANG Shuangling
摘要:
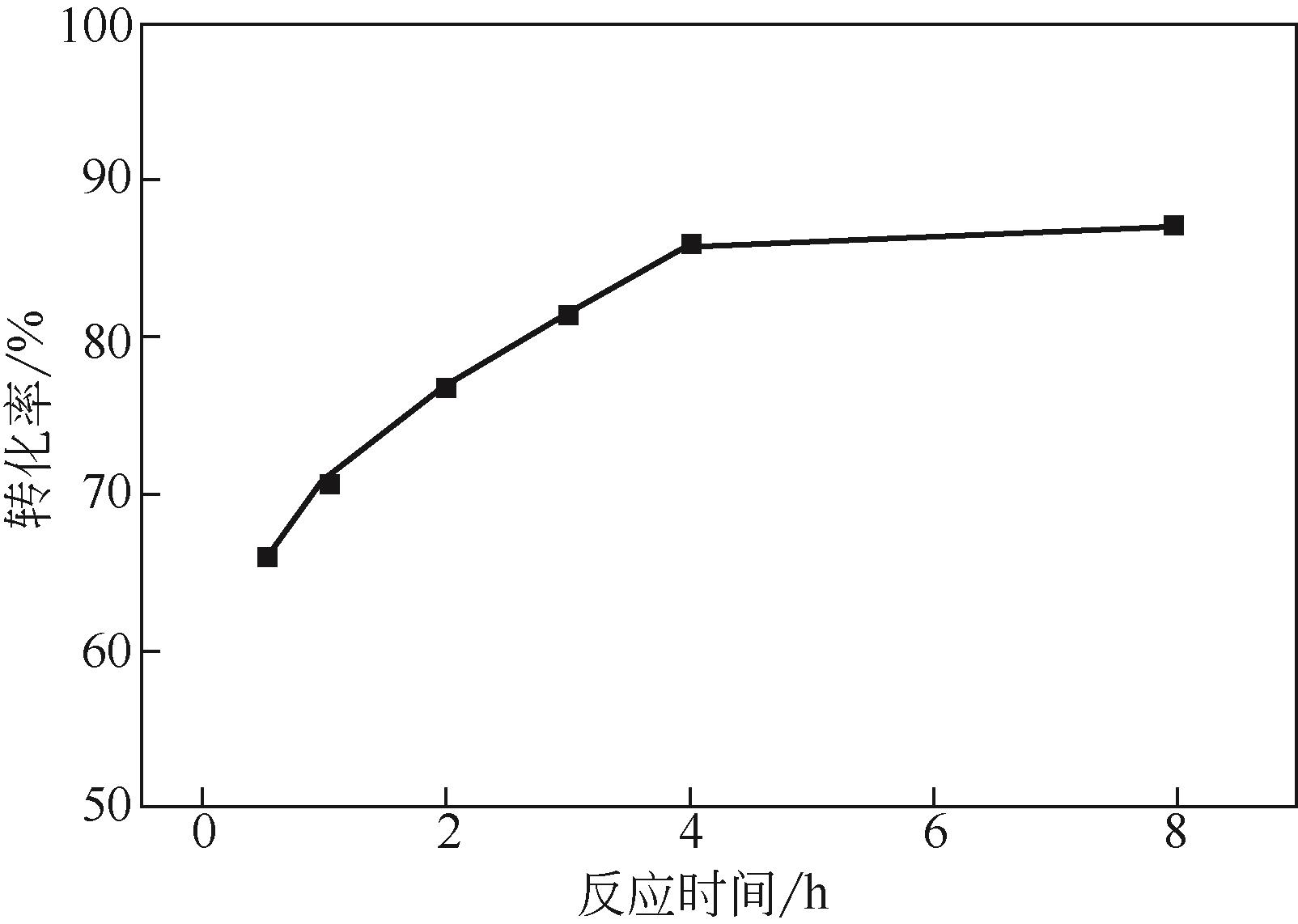
对NO2硝化正己烷的反应进行了研究,分别考察了反应温度、摩尔比和反应时间的影响。结果表明:在反应温度为120℃、正己烷与NO2摩尔比为1∶2、反应时间为4h的反应条件下,正己烷转化率可达85.9%。通过密度泛函理论(DFT)研究了NO2硝化正己烷的反应机理,在B3LYP/6-311++G(3df,2pd)//B3LYP/6-31G*计算水平下精确计算了三个可能反应途径的活化能(Ea)。计算结果表明:该反应决速步骤为NO2中O原子进攻正己烷中的H原子,其中2-硝基己烷和3-硝基己烷为主要产物,且计算结果与实验结果一致。分子几何结构、原子电荷和IR振动频率的数据表明C—H键的断裂和N—H键的形成是一个协同过程,参与硝化反应的原子C(5)、H(7)、O(22)、O(23)和N(21)的分子几何参数及其原子电荷有明显的变化。
中图分类号:
引用本文
李星彦, 王墨, 戴璇, 彭新华, 唐双凌. NO2硝化正己烷及其理论计算[J]. 化工进展, 2021, 40(10): 5491-5498.
LI Xingyan, WANG Mo, DAI Xuan, PENG Xinhua, TANG Shuangling. Nitration of n-hexane with NO2 and theoretical calculation[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5491-5498.
| 序号 | 温度 /℃ | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 100 | 29.6 | 1.2 | 58.0 | 40.9 |
| 2 | 110 | 58.9 | 1.4 | 57.6 | 41.0 |
| 3 | 120 | 64.3 | 2.3 | 56.5 | 41.2 |
| 4 | 130 | 64.8 | 1.9 | 57.2 | 40.9 |
| 5 | 140 | 65.3 | 2.1 | 56.5 | 41.4 |
表1 不同反应温度对正己烷硝化反应①的影响
| 序号 | 温度 /℃ | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 100 | 29.6 | 1.2 | 58.0 | 40.9 |
| 2 | 110 | 58.9 | 1.4 | 57.6 | 41.0 |
| 3 | 120 | 64.3 | 2.3 | 56.5 | 41.2 |
| 4 | 130 | 64.8 | 1.9 | 57.2 | 40.9 |
| 5 | 140 | 65.3 | 2.1 | 56.5 | 41.4 |
| 序号 | 正己烷∶NO2 (摩尔比) | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 1∶0.5 | 32.0 | 1.3 | 55.9 | 42.9 |
| 2 | 1∶1 | 64.3 | 2.3 | 56.5 | 41.2 |
| 3 | 1∶1.5 | 73.0 | 2.7 | 57.0 | 41.4 |
| 4 | 1∶2 | 85.9 | 2.0 | 54.3 | 43.7 |
| 5 | 1∶2.5 | 86.1 | 1.8 | 52.2 | 46.0 |
表2 不同摩尔比对正己烷硝化反应①的影响
| 序号 | 正己烷∶NO2 (摩尔比) | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 1∶0.5 | 32.0 | 1.3 | 55.9 | 42.9 |
| 2 | 1∶1 | 64.3 | 2.3 | 56.5 | 41.2 |
| 3 | 1∶1.5 | 73.0 | 2.7 | 57.0 | 41.4 |
| 4 | 1∶2 | 85.9 | 2.0 | 54.3 | 43.7 |
| 5 | 1∶2.5 | 86.1 | 1.8 | 52.2 | 46.0 |
| 进攻位置 | 总能量/kJ·mol-1 | ||
|---|---|---|---|
| 途径(1) | 途径(2) | 途径(3) | |
| 1-C | |||
| ER | -1160803.565 | -1160803.155 | -1160803.708 |
| ETS | -1160694.314 | -1160701.600 | -1160538.883 |
| Ea | 109.252 | 101.556 | 264.825 |
| 2-C | |||
| ER | -1160803.595 | -1160803.618 | -1160799.290 |
| ETS | -1160710.242 | -1160715.722 | -1160524.374 |
| Ea | 93.354 | 87.896 | 274.916 |
| 3-C | |||
| ER | -1160803.507 | -1160802.418 | -1160803.395 |
| ETS | -1160708.007 | -1160713.692 | -1160532.478 |
| Ea | 95.499 | 88.727 | 270.917 |
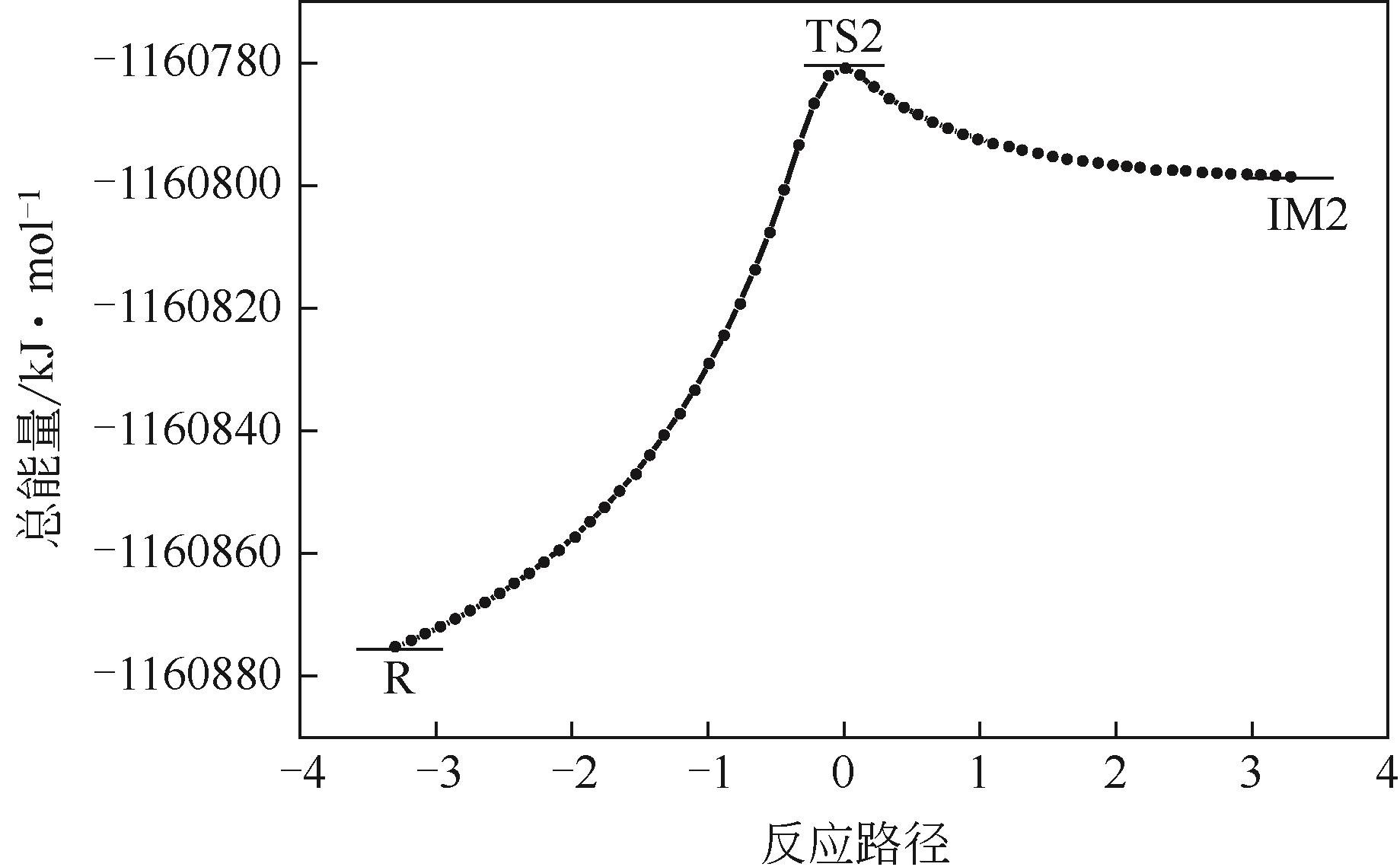
表3 B3LYP/6-311++G(3df,2pd)// B3LYP/6-31G*方法计算各可能反应路径中NO2硝化正己烷各反应物、过渡态能量(E)和活化能(Ea)
| 进攻位置 | 总能量/kJ·mol-1 | ||
|---|---|---|---|
| 途径(1) | 途径(2) | 途径(3) | |
| 1-C | |||
| ER | -1160803.565 | -1160803.155 | -1160803.708 |
| ETS | -1160694.314 | -1160701.600 | -1160538.883 |
| Ea | 109.252 | 101.556 | 264.825 |
| 2-C | |||
| ER | -1160803.595 | -1160803.618 | -1160799.290 |
| ETS | -1160710.242 | -1160715.722 | -1160524.374 |
| Ea | 93.354 | 87.896 | 274.916 |
| 3-C | |||
| ER | -1160803.507 | -1160802.418 | -1160803.395 |
| ETS | -1160708.007 | -1160713.692 | -1160532.478 |
| Ea | 95.499 | 88.727 | 270.917 |
| 几何参数 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|
键长/? C(1)—C(5) C(5)—C(8) C(8)—C(11) C(5)—H(6) C(5)—H(7) H(7)—O(22) O(22)—N(21) N(21)—O(23) | 1.531 1.532 1.537 1.097 1.089(0.3525) 1.991(0.0050) 1.205 1.204 | 1.508 1.511 1.536 1.095 1.412(0.1889) 1.190(0.0185) 1.319 1.205 | 1.496 1.499 1.538 1.091 1.988(0.0728) 1.009(0.1121) 1.372 1.197 |
键角/(°) C(1)—C(5)—C(8) C(5)—C(8)—C(11) C(8)—C(11)—C(14) C(11)—C(14)—C(17) C(1)—C(5)—H(7) C(8)—C(5)—H(7) | 115.4 113.0 113.3 113.7 109.0 107.8 | 118.3 114.6 113.2 113.0 104.1 103.2 | 121.6 114.2 113.3 113.1 98.3 100.5 |
二面角/(°) C(5)—N(21)—O(22)—O(23) H(7)—N(21)—O(22)—O(23) | -1.0 -7.7 | -0.9 -1.2 | -0.3 -0.9 |
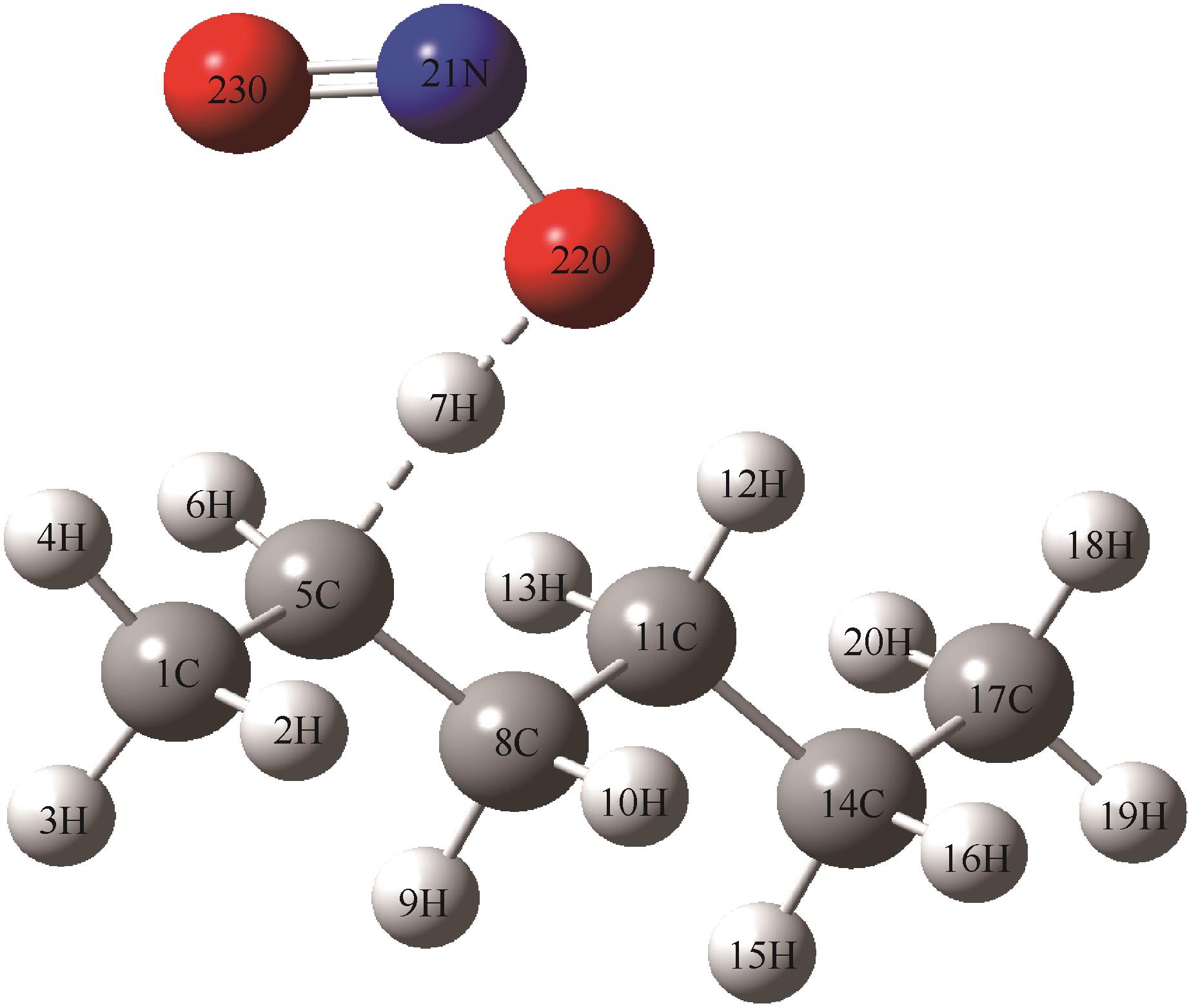
表4 NO2中O原子进攻正己烷2-位H反应的部分分子几何参数(键长、键角和二面角)的变化
| 几何参数 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|
键长/? C(1)—C(5) C(5)—C(8) C(8)—C(11) C(5)—H(6) C(5)—H(7) H(7)—O(22) O(22)—N(21) N(21)—O(23) | 1.531 1.532 1.537 1.097 1.089(0.3525) 1.991(0.0050) 1.205 1.204 | 1.508 1.511 1.536 1.095 1.412(0.1889) 1.190(0.0185) 1.319 1.205 | 1.496 1.499 1.538 1.091 1.988(0.0728) 1.009(0.1121) 1.372 1.197 |
键角/(°) C(1)—C(5)—C(8) C(5)—C(8)—C(11) C(8)—C(11)—C(14) C(11)—C(14)—C(17) C(1)—C(5)—H(7) C(8)—C(5)—H(7) | 115.4 113.0 113.3 113.7 109.0 107.8 | 118.3 114.6 113.2 113.0 104.1 103.2 | 121.6 114.2 113.3 113.1 98.3 100.5 |
二面角/(°) C(5)—N(21)—O(22)—O(23) H(7)—N(21)—O(22)—O(23) | -1.0 -7.7 | -0.9 -1.2 | -0.3 -0.9 |
| 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) | 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|---|---|---|---|
| C(1) | -0.4413 | -0.4516 | -0.4750 | H(13) | 0.1241 | 0.1320 | 0.1320 |
| H(2) | 0.1398 | 0.1604 | 0.1569 | C(14) | -0.2464 | -0.2500 | -0.2497 |
| H(3) | 0.1405 | 0.1624 | 0.1612 | H(15) | 0.1289 | 0.1307 | 0.1311 |
| H(4) | 0.1414 | 0.1758 | 0.1728 | H(16) | 0.1299 | 0.1347 | 0.1343 |
| C(5) | -0.2490 | -0.2535 | -0.1665 | C(17) | -0.4413 | -0.4430 | -0.4431 |
| H(6) | 0.1291 | 0.1722 | 0.1512 | H(18) | 0.1410 | 0.1468 | 0.1468 |
| H(7) | 0.1230 | 0.3422 | 0.4022 | H(19) | 0.1405 | 0.1445 | 0.1442 |
| C(8) | -0.2436 | -0.2546 | -0.2697 | H(20) | 0.1401 | 0.1429 | 0.1424 |
| H(9) | 0.1250 | 0.1509 | 0.1475 | N(21) | 0.4838 | 0.3091 | 0.2935 |
| H(10) | 0.1261 | 0.1484 | 0.1447 | O(22) | -0.2365 | -0.3971 | -0.4660 |
| C(11) | -0.2494 | -0.2572 | -0.2590 | O(23) | -0.2340 | -0.2966 | -0.2765 |
| H(12) | 0.1282 | 0.1508 | 0.1446 |
表5 NO2中O原子进攻正己烷2-位H原子反应中原子自然电荷的变化 (e)
| 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) | 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|---|---|---|---|
| C(1) | -0.4413 | -0.4516 | -0.4750 | H(13) | 0.1241 | 0.1320 | 0.1320 |
| H(2) | 0.1398 | 0.1604 | 0.1569 | C(14) | -0.2464 | -0.2500 | -0.2497 |
| H(3) | 0.1405 | 0.1624 | 0.1612 | H(15) | 0.1289 | 0.1307 | 0.1311 |
| H(4) | 0.1414 | 0.1758 | 0.1728 | H(16) | 0.1299 | 0.1347 | 0.1343 |
| C(5) | -0.2490 | -0.2535 | -0.1665 | C(17) | -0.4413 | -0.4430 | -0.4431 |
| H(6) | 0.1291 | 0.1722 | 0.1512 | H(18) | 0.1410 | 0.1468 | 0.1468 |
| H(7) | 0.1230 | 0.3422 | 0.4022 | H(19) | 0.1405 | 0.1445 | 0.1442 |
| C(8) | -0.2436 | -0.2546 | -0.2697 | H(20) | 0.1401 | 0.1429 | 0.1424 |
| H(9) | 0.1250 | 0.1509 | 0.1475 | N(21) | 0.4838 | 0.3091 | 0.2935 |
| H(10) | 0.1261 | 0.1484 | 0.1447 | O(22) | -0.2365 | -0.3971 | -0.4660 |
| C(11) | -0.2494 | -0.2572 | -0.2590 | O(23) | -0.2340 | -0.2966 | -0.2765 |
| H(12) | 0.1282 | 0.1508 | 0.1446 |
| 频率 | 反应物 | 过渡态 | 中间体 | 频率 | 反应物 | 过渡态 | 中间体 |
|---|---|---|---|---|---|---|---|
| υ1 | 5(0) | -1417(355) | 16(0) | υ33 | 1290(0) | 1209(18) | 1233(8) |
| υ2 | 14(0) | 31(0) | 34(1) | υ34 | 1297(1) | 1231(16) | 1270(1) |
| υ3 | 19(0) | 48(0) | 40(0) | υ35 | 1306(0) | 1272(3) | 1295(0) |
| υ4 | 49(0) | 57(0) | 71(0) | υ36 | 1345(0) | 1294(1) | 1303(1) |
| υ5 | 52(0) | 93(0) | 79(0) | υ37 | 1355(5) | 1299(1) | 1345(15) |
| υ6 | 71(0) | 117(1) | 93(0) | υ38 | 1368(0) | 1341(3) | 1358(3) |
| υ7 | 86(1) | 130(0) | 125(1) | υ39 | 1384(3) | 1358(1) | 1374(1) |
| υ8 | 98(0) | 139(0) | 126(0) | υ40 | 1385(0) | 1369(1) | 1380(8) |
| υ9 | 124(0) | 166(0) | 148(1) | υ41 | 1452(1) | 1374(10) | 1386(3) |
| υ10 | 148(0) | 208(0) | 166(2) | υ42 | 1454(0) | 1385(2) | 1433(1) |
| υ11 | 232(0) | 239(0) | 208(4) | υ43 | 1460(0) | 1433(0) | 1450(5) |
| υ12 | 243(0) | 250(0) | 245(0) | υ44 | 1468(9) | 1450(7) | 1454(10) |
| υ13 | 286(0) | 301(0) | 295(0) | υ45 | 1468(1) | 1457(0) | 1457(0) |
| υ14 | 352(0) | 362(0) | 354(0) | υ46 | 1469(3) | 1459(5) | 1467(0) |
| υ15 | 448(0) | 438(0) | 449(0) | υ47 | 1477(0) | 1467(1) | 1469(6) |
| υ16 | 710(4) | 475(1) | 565(24) | υ48 | 1482(3) | 1469(6) | 1480(3) |
| υ17 | 721(5) | 706(6) | 651(14) | υ49 | 1654(296) | 1480(3) | 1634(177) |
| υ18 | 723(1) | 729(1) | 710(2) | υ50 | 2890(1) | 1571(270) | 2814(28) |
| υ19 | 783(1) | 770(0) | 760(2) | υ51 | 2893(6) | 2844(16) | 2856(30) |
| υ20 | 861(3) | 813(11) | 829(70) | υ52 | 2903(0) | 2890(17) | 2899(1) |
| υ21 | 879(0) | 866(0) | 864(2) | υ53 | 2907(94) | 2901(3) | 2902(4) |
| υ22 | 880(0) | 881(4) | 879(4) | υ54 | 2911(19) | 2907(32) | 2910(61) |
| υ23 | 979(0) | 887(1) | 889(1) | υ55 | 2919(72) | 2921(13) | 2922(34) |
| υ24 | 981(1) | 981(12) | 913(270) | υ56 | 2919(13) | 2923(48) | 2926(3) |
| υ25 | 1017(0) | 989(2) | 965(1) | υ57 | 2920(2) | 2928(3) | 2945(14) |
| υ26 | 1027(0) | 1025(13) | 986(0) | υ58 | 2935(1) | 2953(35) | 2949(43) |
| υ27 | 1042(4) | 1036(16) | 1026(1) | υ59 | 2949(90) | 2963(12) | 2985(56) |
| υ28 | 1122(0) | 1061(83) | 1036(4) | υ60 | 2981(15) | 2984(28) | 2990(41) |
| υ29 | 1173(0) | 1078(3) | 1084(0) | υ61 | 2982(126) | 2985(50) | 2992(7) |
| υ30 | 1217(0) | 1120(19) | 1095(2) | υ62 | 2985(46) | 2990(41) | 3016(44) |
| υ31 | 1235(5) | 1140(10) | 1135(1) | υ63 | 2986(42) | 3012(19) | 3024(381) |
| υ32 | 1272(0) | 1165(29) | 1199(0) |
表6 NO2中O原子进攻正己烷2-位H原子反应过程中振动频率的变化 (cm-1)
| 频率 | 反应物 | 过渡态 | 中间体 | 频率 | 反应物 | 过渡态 | 中间体 |
|---|---|---|---|---|---|---|---|
| υ1 | 5(0) | -1417(355) | 16(0) | υ33 | 1290(0) | 1209(18) | 1233(8) |
| υ2 | 14(0) | 31(0) | 34(1) | υ34 | 1297(1) | 1231(16) | 1270(1) |
| υ3 | 19(0) | 48(0) | 40(0) | υ35 | 1306(0) | 1272(3) | 1295(0) |
| υ4 | 49(0) | 57(0) | 71(0) | υ36 | 1345(0) | 1294(1) | 1303(1) |
| υ5 | 52(0) | 93(0) | 79(0) | υ37 | 1355(5) | 1299(1) | 1345(15) |
| υ6 | 71(0) | 117(1) | 93(0) | υ38 | 1368(0) | 1341(3) | 1358(3) |
| υ7 | 86(1) | 130(0) | 125(1) | υ39 | 1384(3) | 1358(1) | 1374(1) |
| υ8 | 98(0) | 139(0) | 126(0) | υ40 | 1385(0) | 1369(1) | 1380(8) |
| υ9 | 124(0) | 166(0) | 148(1) | υ41 | 1452(1) | 1374(10) | 1386(3) |
| υ10 | 148(0) | 208(0) | 166(2) | υ42 | 1454(0) | 1385(2) | 1433(1) |
| υ11 | 232(0) | 239(0) | 208(4) | υ43 | 1460(0) | 1433(0) | 1450(5) |
| υ12 | 243(0) | 250(0) | 245(0) | υ44 | 1468(9) | 1450(7) | 1454(10) |
| υ13 | 286(0) | 301(0) | 295(0) | υ45 | 1468(1) | 1457(0) | 1457(0) |
| υ14 | 352(0) | 362(0) | 354(0) | υ46 | 1469(3) | 1459(5) | 1467(0) |
| υ15 | 448(0) | 438(0) | 449(0) | υ47 | 1477(0) | 1467(1) | 1469(6) |
| υ16 | 710(4) | 475(1) | 565(24) | υ48 | 1482(3) | 1469(6) | 1480(3) |
| υ17 | 721(5) | 706(6) | 651(14) | υ49 | 1654(296) | 1480(3) | 1634(177) |
| υ18 | 723(1) | 729(1) | 710(2) | υ50 | 2890(1) | 1571(270) | 2814(28) |
| υ19 | 783(1) | 770(0) | 760(2) | υ51 | 2893(6) | 2844(16) | 2856(30) |
| υ20 | 861(3) | 813(11) | 829(70) | υ52 | 2903(0) | 2890(17) | 2899(1) |
| υ21 | 879(0) | 866(0) | 864(2) | υ53 | 2907(94) | 2901(3) | 2902(4) |
| υ22 | 880(0) | 881(4) | 879(4) | υ54 | 2911(19) | 2907(32) | 2910(61) |
| υ23 | 979(0) | 887(1) | 889(1) | υ55 | 2919(72) | 2921(13) | 2922(34) |
| υ24 | 981(1) | 981(12) | 913(270) | υ56 | 2919(13) | 2923(48) | 2926(3) |
| υ25 | 1017(0) | 989(2) | 965(1) | υ57 | 2920(2) | 2928(3) | 2945(14) |
| υ26 | 1027(0) | 1025(13) | 986(0) | υ58 | 2935(1) | 2953(35) | 2949(43) |
| υ27 | 1042(4) | 1036(16) | 1026(1) | υ59 | 2949(90) | 2963(12) | 2985(56) |
| υ28 | 1122(0) | 1061(83) | 1036(4) | υ60 | 2981(15) | 2984(28) | 2990(41) |
| υ29 | 1173(0) | 1078(3) | 1084(0) | υ61 | 2982(126) | 2985(50) | 2992(7) |
| υ30 | 1217(0) | 1120(19) | 1095(2) | υ62 | 2985(46) | 2990(41) | 3016(44) |
| υ31 | 1235(5) | 1140(10) | 1135(1) | υ63 | 2986(42) | 3012(19) | 3024(381) |
| υ32 | 1272(0) | 1165(29) | 1199(0) |
| 1 | SIMPSON M F, LAW J D. Nuclear fuel reprocessing[M]//Nuclear Energy. New York: Springer, 2018: 187-204. |
| 2 | 姜圣阶, 任凤仪, 等. 核燃料后处理工学[M]. 北京: 原子能出版社, 1995. |
| JIANG Shengjie, REN Fengyi, et al. Nuclear fuel reprocessing engineering[M]. Beijing: Atomic Energy Press, 1995. | |
| 3 | COLVEN T J, NICHOLS G M, SIDDALL T H. TNX evaporator incident January 12, 1953. interim technical report[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 4 | DURANT W S. Red oil explosion at the Savannah river plant[R]. DPMS-83-142, Savannah River Laboratory: 1984. |
| 5 | SEGE G. Overconcentration in initial operation of uranium evaporator, 321 Building[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 6 | DAVIS W J, BALDWIN W H, MESERVEY A B. Chemistry of the intercycle evaporator incident of November 20, 1959[R]. Office of Scientific and Technical Information (OSTI), 1960. |
| 7 | ANDREEV G, ANTIPIN Y, BENNETT B, et al. The radiological accident in the reprocessing plant at Tomsk[R]. International Atomic Energy Agency, Austria: 1998. |
| 8 | MINCHER B J, MODOLO G, MEZYK S P. Review article: the effects of radiation chemistry on solvent extraction: 1. Conditions in acidic solution and a review of TBP radiolysis[J]. Solvent Extraction and Ion Exchange, 2009, 27(1): 1-25. |
| 9 | 许明霞. 乏燃料后处理厂“红油”爆炸安全分析[J]. 核安全, 2011(1): 22-27. |
| XU Mingxia. Safety analysis on explosion of “red oils” in spent fuel reprocessing plants[J]. Nuclear safety, 2011(1): 22-27. | |
| 10 | KUMAR S, KUMAR R, KOGANTI S B. Studies on reaction runaways for Urex/Purex solvent-nitric acid and red-oil synthesis[C]//Oak Ridge National Laboratory,International Solvent Extraction Conference2008. 9ISEC-2008. Tucson, USA. 2008. |
| 11 | KUMAR S, SINHA P K, KAMACHI MUDALI U, et al. Thermal decomposition of red-oil/nitric acid mixtures in adiabatic conditions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2011, 289(2): 545-549. |
| 12 | SMITHA V S, SURIANARAYANAN M, SESHADRI H, et al. Thermal behavior pattern of tributyl phosphate under adiabatic conditions[J]. Journal of Thermal Analysis and Calorimetry, 2013, 111(1): 849-856. |
| 13 | CHANDRAN K, SAHOO T K, MURALIDARAN P, et al. Calorimetric studies on the thermal decomposition of tri n-butyl phosphate-nitric acid systems[J]. Journal of Thermal Analysis and Calorimetry, 2012, 110(2): 879-890. |
| 14 | TALLENT O K, MAILEN J C, DODSON K E. Purex diluent chemical degradation[J]. Nuclear Technology, 1985, 71(2): 417-425. |
| 15 | ISHIHARA T, OHWADA K. Chemical degradation of kerosene diluent with nitric acid[J]. Journal of Nuclear Science and Technology, 2008, 3(1): 20-26. |
| 16 | OHWADA K. On the identification of hydroxamic acids formed by nitric acid degradation of kerosene and i-dodecane[J]. Journal of Nuclear Science and Technology, 1968, 5(4): 163-167. |
| 17 | 周春俐, 王墨, 唐洪彬, 等. 正己烷与正十二烷在硝酸体系中的化学行为[J]. 核化学与放射化学, 2021, 43(2): 122-128. |
| ZHOU Chunli, WANG Mo, TANG Hongbin, et al. Chemical behavior of n-hexane and n-dodecane in nitric acid system[J]. Journal of Nuclear and Radiochemistry, 2021, 43(2): 122-128. | |
| 18 | WAGNER R M. Investigation of explosive characteristics of purex solvent decomposition products (Red oil)[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 19 | WILBOURN R G. Safety aspects of solvent nitration in HTGR fuel reprocessing[R]. Office of Scientific and Technical Information (OSTI), 1977. |
| 20 | HOU Y, BAREFIELD E K, TEDDER D W, et al. Thermal decomposition of nitrated tributyl phosphate[J]. Nuclear Technology, 1996, 113(3): 304-315. |
| 21 | SAKAGUCHI S, NISHIWAKI Y, KITAMURA T, et al. Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalimide[J]. Angewandte Chemie International Edition, 2001, 40(1): 222-224. |
| 22 | NISHIWAKI Y, SAKAGUCHI S, ISHII Y. An efficient nitration of light alkanes and the alkyl side-chain of aromatic compounds with nitrogen dioxide and nitric acid catalyzed by N-hydroxyphthalimide[J]. The Journal of Organic Chemistry, 2002, 67(16): 5663-5668. |
| 23 | SUZUKI H, MURASHIMA T, KOZAI I, et al. Ozone-mediated nitration of alkylbenzenes and related compounds with nitrogen dioxide[J]. Journal of the Chemical Society, Perkin Transactions 1, 1993(14): 1591. |
| 24 | PENG X, SUZUKI H, LU C. Zeolite-assisted nitration of neat toluene and chlorobenzene with a nitrogen dioxide/molecular oxygen system. Remarkable enhancement of para-selectivity[J]. Tetrahedron Letters, 2001, 42(26): 4357-4359. |
| 25 | DONG J, JIN B, SUN P. Palladium-catalyzed direct ortho-nitration of azoarenes using NO2 as nitro source[J]. Organic Letters, 2014, 16(17): 4540-4542. |
| 26 | 许晓娟, 肖鹤鸣, 贡雪东, 等. NO2气相硝化金刚烷的计算研究[J]. 化学学报, 2006, 64(4): 306-312. |
| XU Xiaojuan, XIAO Heming, GONG Xuedong, et al. Computational studies on the nitration of adamantane with NO2[J]. Acta Chimica Sinica, 2006, 64(4): 306-312. |
| [1] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [2] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [3] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [4] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [5] | 吴毅恒, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 镓改性HZSM-5催化低碳烷烃芳构化研究进展[J]. 化工进展, 2023, 42(10): 5162-5178. |
| [6] | 王庆宏, 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂. 天然矿物催化氧化水中难降解有机污染物研究进展[J]. 化工进展, 2023, 42(1): 417-434. |
| [7] | 潘杰, 王明新, 高生旺, 夏训峰, 韩雪. 氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑[J]. 化工进展, 2022, 41(8): 4204-4212. 唑[J]. 化工进展, 2022, 41(8): 4204-4212. |
| [8] | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| [9] | 王龙, 刘永峰, 毕贵军, 宋金瓯. 基于量子化学计算柴油在CO2/O2氛围下的燃烧特性[J]. 化工进展, 2022, 41(6): 2948-2958. |
| [10] | 王恩华, 靳丽丽, 高善彬, 迟克彬, 段爱军. 正构烷烃临氢异构化催化剂研究进展[J]. 化工进展, 2022, 41(6): 2967-2980. |
| [11] | 孙德贇, 胡艳宏, 刘鹏, 唐茂, 胡泽, 柳召刚, 吴锦绣. 不同铈盐体系(硝酸盐、硫酸盐、氯化盐)中CTAB与Ce3+的相互作用机理[J]. 化工进展, 2022, 41(6): 3212-3220. |
| [12] | 韩京京, 谭涓, 刘靖, 刘宇. 小晶粒ZSM-22的可控合成及其催化长链正构生物烷烃制航空煤油性能[J]. 化工进展, 2022, 41(4): 1916-1924. |
| [13] | 甄建政, 聂士松, 潘世元, 吕维扬, 姚玉元. 多维度碳基负载金属催化剂活化PMS降解水中污染物的研究进展[J]. 化工进展, 2022, 41(4): 1858-1872. |
| [14] | 熊哲, 邓伟, 刘佳, 汪雪棚, 徐俊, 江龙, 苏胜, 汪一, 胡松, 向军. 生物油非催化热转化过程中受热结焦特性研究进展[J]. 化工进展, 2022, 41(4): 1802-1813. |
| [15] | 徐铭骏, 郭兆春, 李立, 朱紫琦, 张倩, 洪俊明. 纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料[J]. 化工进展, 2022, 41(2): 1043-1053. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||