| 1 |
ZHAO Yunliang, WANG Wei, ZHANG Yimin, et al. In-situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal[J]. Advanced Powder Technology, 2017, 28(3): 1103-1107.
|
| 2 |
LI Hongyi, FANG Haixing, WANG Kang, et al. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching[J]. Hydrometallurgy, 2015, 156: 124-135.
|
| 3 |
CAI Zhenlei, ZHANG Yimin. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology[J]. RSC Advances, 2017, 7(2): 36917-36922.
|
| 4 |
JI Yilong, SHEN Shaobo, LIU Jianhua, et al. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction[J]. Journal of Cleaner Production, 2017, 149: 1068-1078.
|
| 5 |
ZHANG Ying, ZHANG Ting’an, DREISINGER D, et al. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching[J]. Journal of Hazardous Materials, 2019, 369: 632-641.
|
| 6 |
PENG Hao, GUO Jing, ZHENG Xiaogang, et al. Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium[J]. Journal of Environmental Chemical Engineering, 2016, 256: 98-106.
|
| 7 |
LI Meng, ZHENG Shili, LIU Biao, et al. A clean and efficient method for recovery of vanadium from vanadium slag: nonsalt roasting and ammonium carbonate leaching processes[J]. Mineral Processing and Extractive Metallurgy Review, 2017, 38: 228-237.
|
| 8 |
LI Meng, LIU Biao, ZHENG Shili, et al. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching[J]. Journal of Cleaner Production, 2017, 149: 206-217.
|
| 9 |
ZHANG Xuefei, LIU Fengguo, XUE Xiangxin, et al. Effects of microwave and conventional blank roasting on oxidation behavior, microstructure and surface morphology of vanadium slag with high chromium content[J]. Journal of Alloys and Compounds, 2016, 686: 356-365.
|
| 10 |
张延安, 牟望重, 豆志河, 等. 转炉钒渣氧压酸浸过程V-Fe-H2O系的电位-pH图[J]. 中国有色金属学报, 2011, 21(11): 2936-2945.
|
|
ZHANG Yan’an, MOU Wangzhong, DOU Zhihe, et al. Potential-pH diagrams for V-Fe-H2O system during oxygen pressure acid leaching of vanadium-bearing converter slags[J]. Chinese Journal of Nonferrous Metals, 2011, 21(11): 2936-2945.
|
| 11 |
杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983: 523-674.
|
|
YANG Xianwan. Handbook of Thermodynamic Data in Aqueous Solutions at High Temperature[M]. Beijing: Metallurgical Industry Press, 1983: 523-674.
|
| 12 |
伊赫桑·巴伦. 纯物质热化学数据手册[M]. 程乃良, 牛四通, 徐桂英, 译. 北京: 科学出版社, 2003: 716-726.
|
|
BARIN I. Thermochemical data of pure substances[M]. CHENG Nailiang, NIU Sitong, XU Guiying, trans. Beijing: Science Press, 2003: 716-726.
|
| 13 |
CHEN B F, HUANG S, LIU B, et al. Thermodynamic analysis for separation of vanadium and chromium in V( )-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65. )-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65.
|
| 14 |
刘景文, 阳征斐, 周鹏, 等. V(Ⅴ)-Fe(Ⅲ)-S(Ⅵ)-H2O系热力学研究与钒铁分离方法理论[J]. 中国有色金属学报, 2020, 30(4): 912-919.
|
|
LIU Jingwen, YANG Zhengfei, ZHOU Peng, et al. V(Ⅴ)-Fe(Ⅲ)-S( )-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919. )-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919.
|
| 15 |
何伟. 黏土钒矿不磨不焙烧常压活化酸浸提钒的研究[D]. 昆明: 昆明理工大学, 2014.
|
|
HE Wei. Extraction of vanadium from clay vanadium ore by activated acid under normal pressure without grinding and roasting[D]. Kunming: Kunming University of Science and Technology, 2014.
|
 ), 叶国华1,2(
), 叶国华1,2( ), 陈子杨2, 谢禹1, 左琪2
), 陈子杨2, 谢禹1, 左琪2
 ), YE Guohua1,2(
), YE Guohua1,2( ), CHEN Ziyang2, XIE Yu1, ZUO Qi2
), CHEN Ziyang2, XIE Yu1, ZUO Qi2
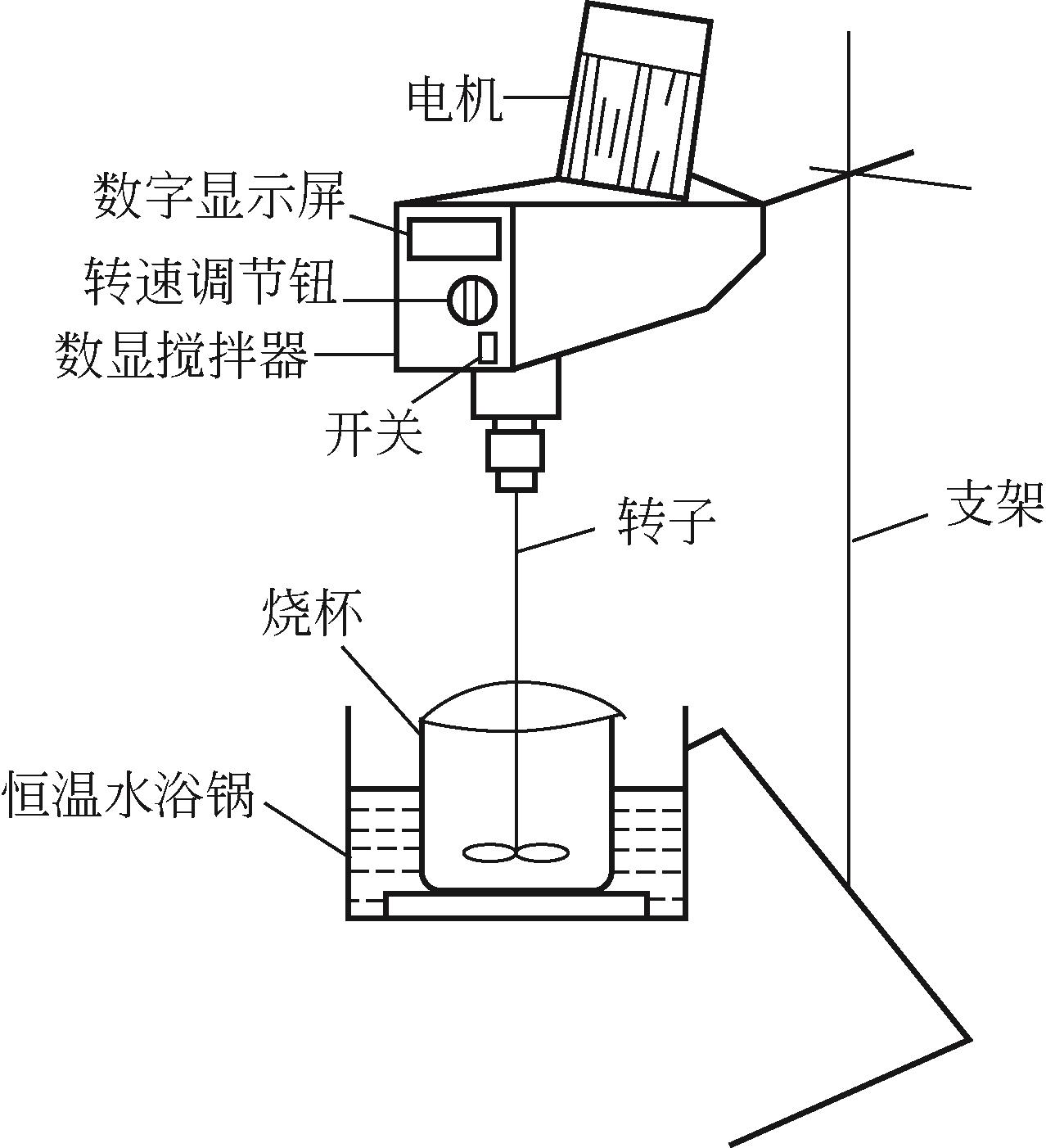
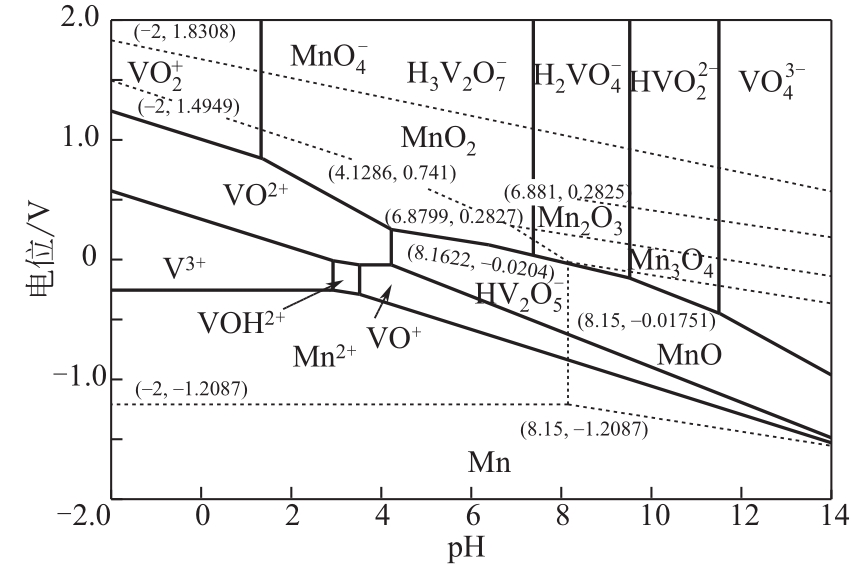

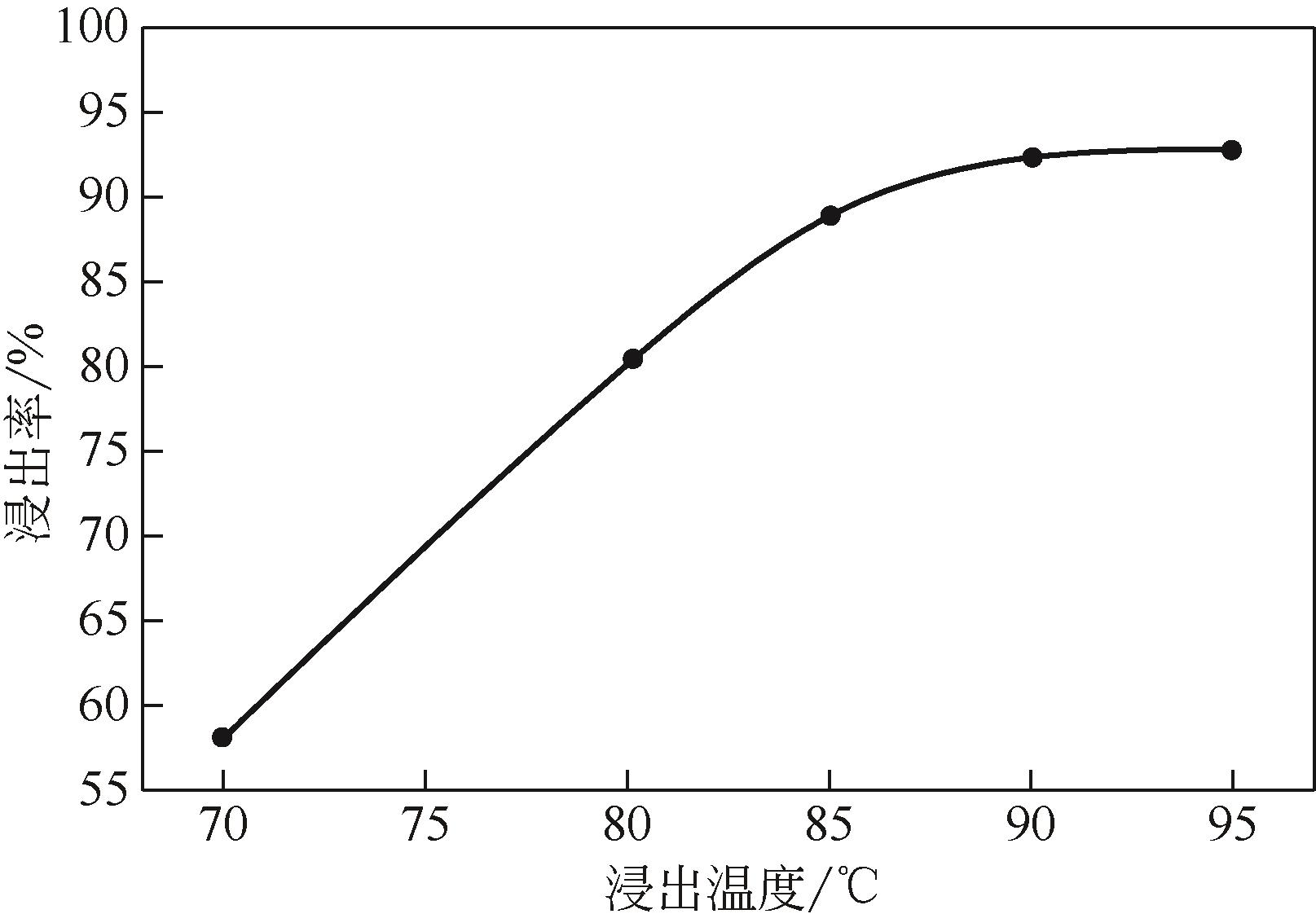
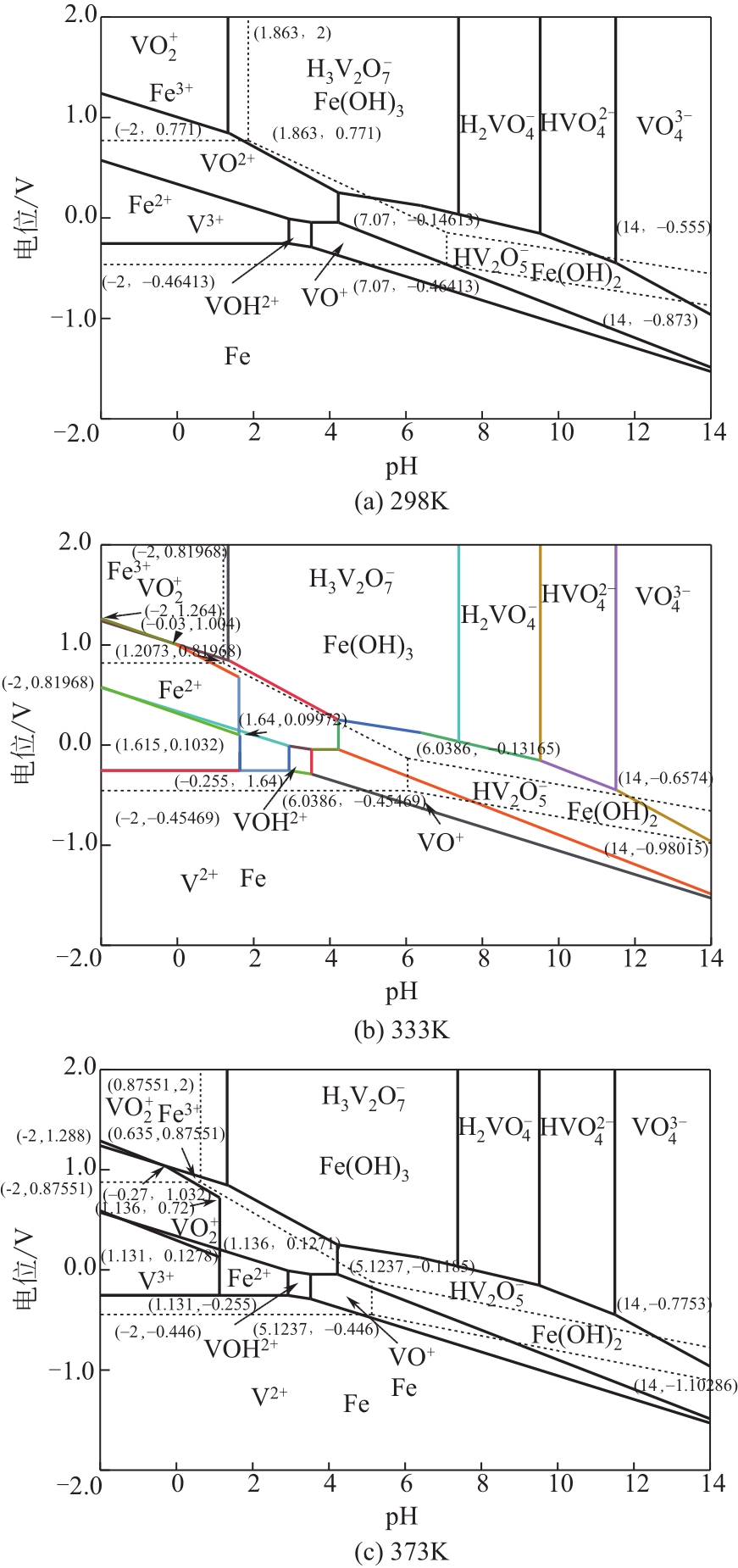
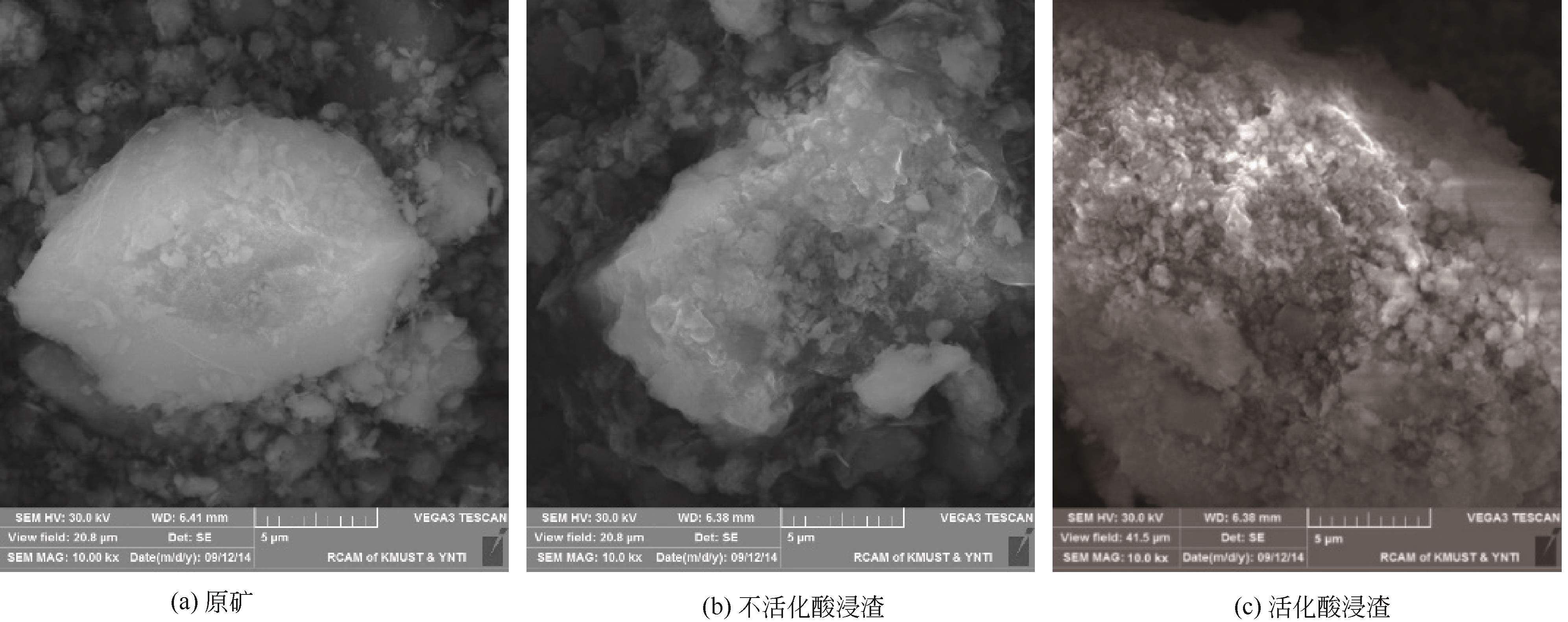

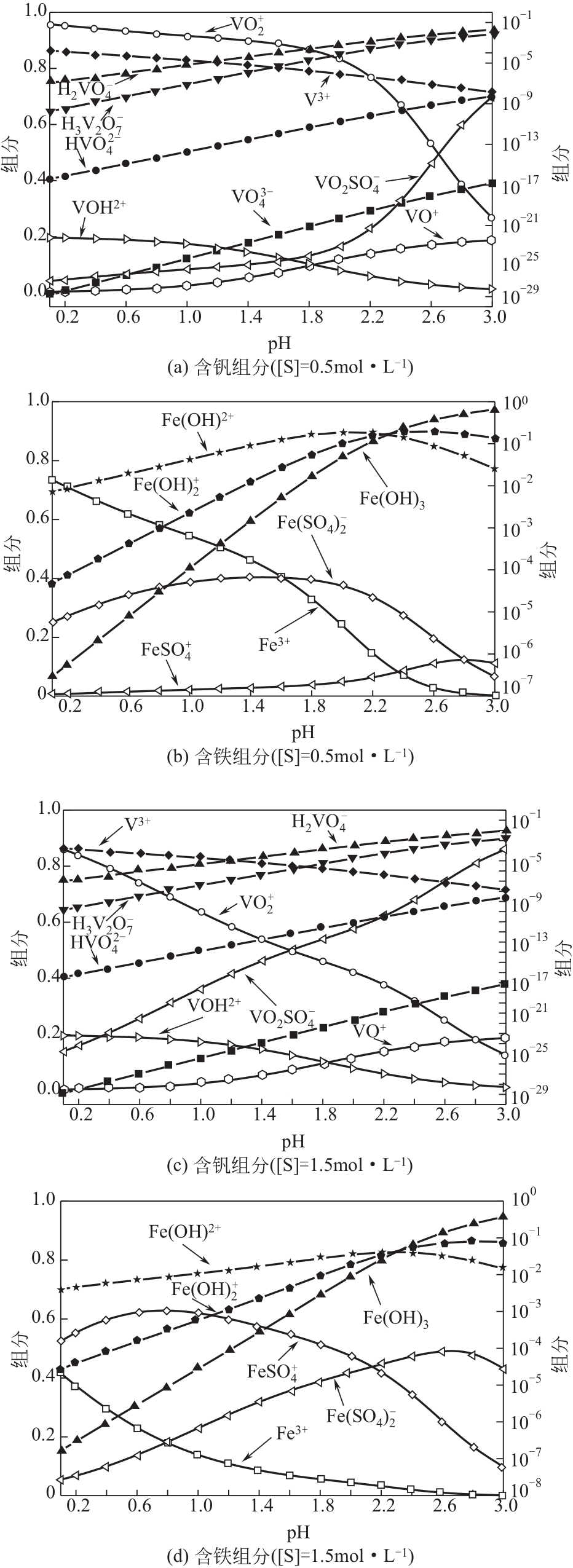
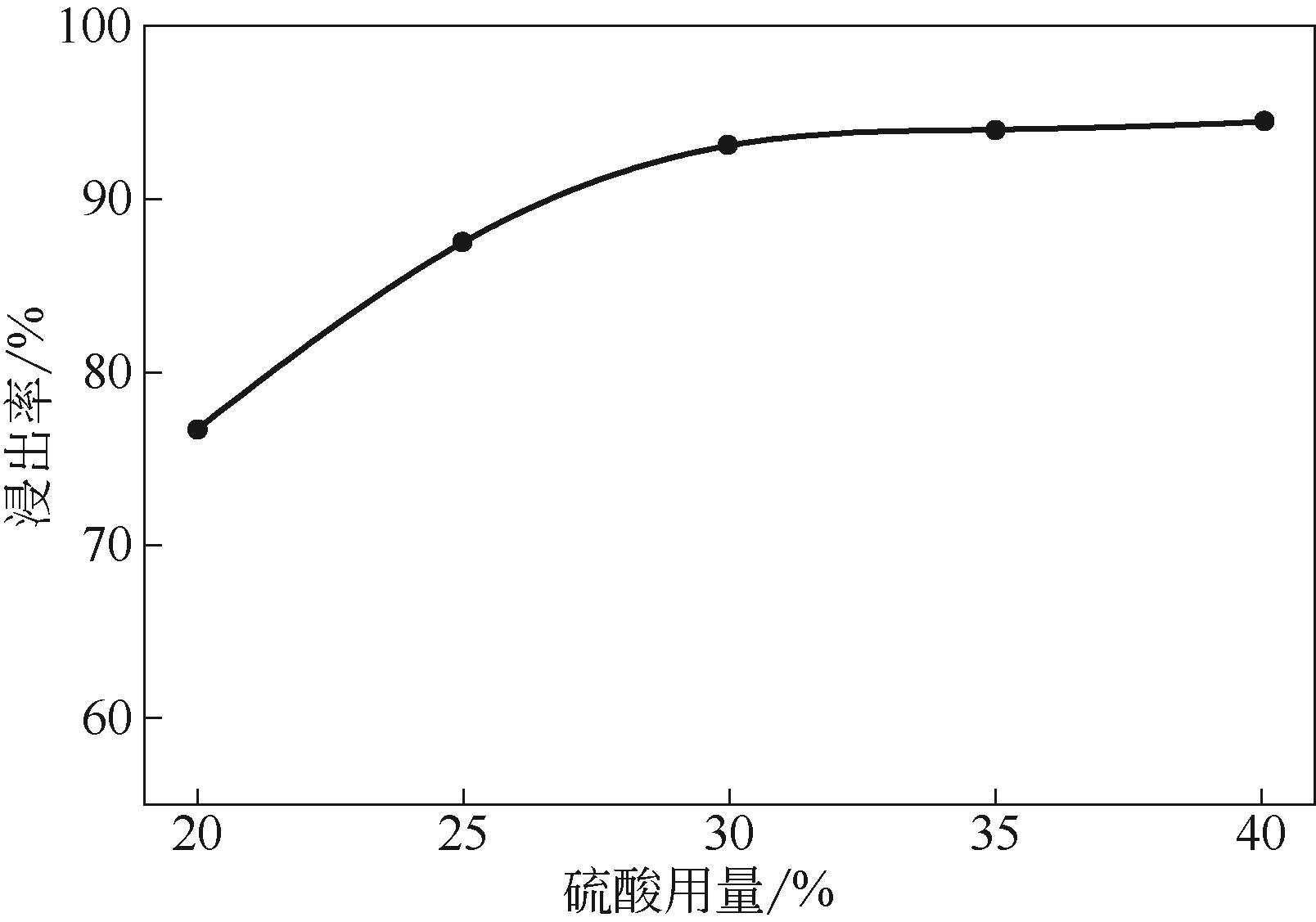
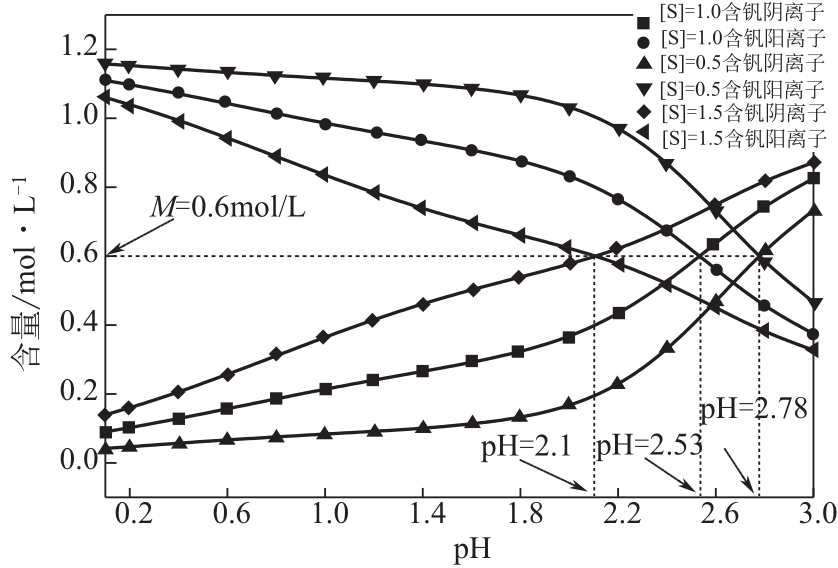
 )-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65.
)-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65. )-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919.
)-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919.