化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5348-5359.DOI: 10.16085/j.issn.1000-6613.2020-2183
生物原油炼制: 副产物内循环及水热自催化
高传瑞1,2( ), 田纯焱1(
), 田纯焱1( ), 李志合2(
), 李志合2( ), 易维明2, 袁巧霞3, 付鹏1, 张玉春1, 李治宇1
), 易维明2, 袁巧霞3, 付鹏1, 张玉春1, 李治宇1
- 1.山东理工大学农业工程与食品科学学院,山东 淄博 255000
2.山东省清洁能源工程技术研究中心,山东 淄博 255000
3.华中农业大学工学院,湖北 武汉 430070
-
收稿日期:2020-11-02修回日期:2021-02-04出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:田纯焱,李志合 -
作者简介:高传瑞(1994—),男,硕士研究生,研究方向为生物质能源与材料。E-mail:842042822@qq.com 。 -
基金资助:国家自然科学基金(51706126);山东省自然科学基金(ZR2017BEE049);淄博市校城融合项目(2019ZBXC380);山东省高等学校青创科技支持计划(2019KJD013)
Biorefining of biocrude oil: recirculation of by-products and hydrothermal autocatalysis
GAO Chuanrui1,2( ), TIAN Chunyan1(
), TIAN Chunyan1( ), LI Zhihe2(
), LI Zhihe2( ), YI Weiming2, YUAN Qiaoxia3, FU Peng1, ZHANG Yuchun1, LI Zhiyu1
), YI Weiming2, YUAN Qiaoxia3, FU Peng1, ZHANG Yuchun1, LI Zhiyu1
- 1.School of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo 255000, Shandong, China
2.Shandong Research Center of Engineering & Technology for Clean Energy, Zibo 255000, Shandong, China
3.College of Engineering, Huazhong Agricultural University, Wuhan 430070, Hubei, China
-
Received:2020-11-02Revised:2021-02-04Online:2021-10-10Published:2021-10-25 -
Contact:TIAN Chunyan,LI Zhihe
摘要:
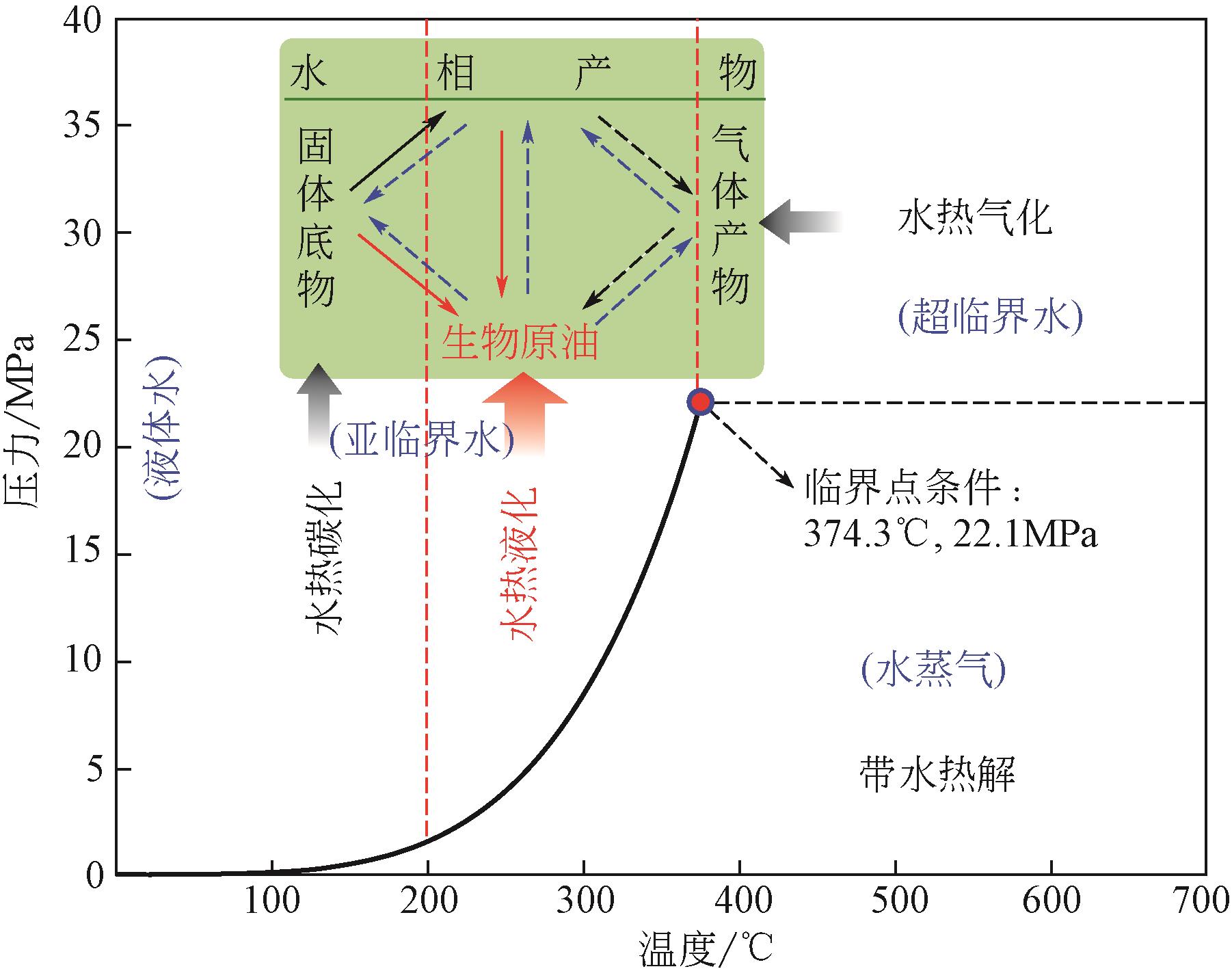
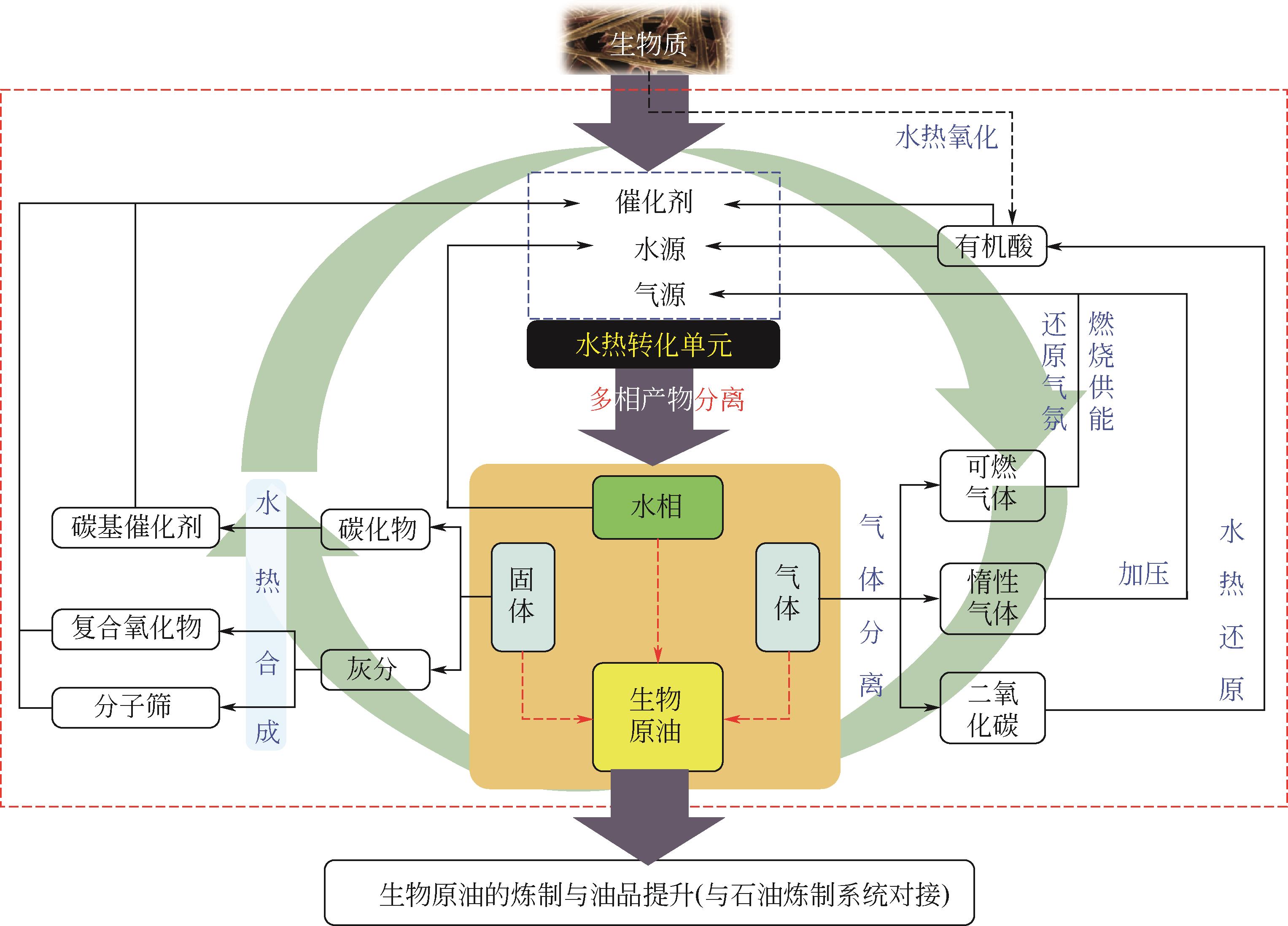
利用高温高压条件模拟石油生成的生物质水热液化技术可用于制备生物原油,以替代日益枯竭的石油资源,然而副产物处置问题制约了其可持续发展。解决该问题的方法首先是通过水热定向催化调控减少副产物,然后集成各种技术将副产物尽可能原位资源化。基于此并依据生物炼制的思想,本文对一种集成几种水热技术炼制生物原油的模式进行了讨论。依据生物质水热液化副产物的特性,通过对固体产物水热合成制备催化剂、水相产物回用产生有机酸、气体产物分离或彻底氧化后水热还原生产有机酸等,可实现副产物内循环并强化自催化生成生物原油。指出该模式符合绿色化工的理念,对于加快规模化生产可替代石油的生物原油、缓解能源危机具有重要的参考意义。
中图分类号:
引用本文
高传瑞, 田纯焱, 李志合, 易维明, 袁巧霞, 付鹏, 张玉春, 李治宇. 生物原油炼制: 副产物内循环及水热自催化[J]. 化工进展, 2021, 40(10): 5348-5359.
GAO Chuanrui, TIAN Chunyan, LI Zhihe, YI Weiming, YUAN Qiaoxia, FU Peng, ZHANG Yuchun, LI Zhiyu. Biorefining of biocrude oil: recirculation of by-products and hydrothermal autocatalysis[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5348-5359.
| 催化剂 | 原料 | 过程条件 | 产率提升 | 对生物原油催化效果 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | HHV | |||||
| 均相催化剂 | ||||||||||
| 氢氧化钾 | 玉米芯 | 340℃,60min | +49%TO | -22% | -9% | +67% | +33% | N/A | N/A | [ |
| 碳酸钾 | 木屑 | 280℃,15min | +136%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
| 碳酸钠 | 水葫芦 | -50%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ | |
| 微绿球藻 | 300℃,60min | -21%TO | +4% | -4% | -92% | +2% | +70% | -0.9% | [ | |
| 泡桐 | 300℃,10min | +18%HO | +5% | +2% | -13% | N/A | N/A | +7% | [ | |
| 螺旋藻 | 350℃,60min | -11%TO | +3% | +17% | -16% | -34% | +0.5% | -5% | [ | |
| +29%HO | -2% | +11% | +16% | -14% | -3% | +3% | [ | |||
| 甲酸 | 380℃,120min | +28%TO | -1% | -12% | +18% | -27% | N/A | -7% | [ | |
| 盐酸 | 纤维素 | 300℃,0min | +21%LO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
| 乙酸 | 小球藻蛋白 | 300℃,20min,8.5MPa | 降低LO | -7% | +81% | +3% | -10% | N/A | +18% | [ |
| 非均相催化剂 | ||||||||||
| 铁粉 | 泡桐 | 340℃,10min | +51%HO | +10% | +20% | -29% | N/A | N/A | +20% | [ |
| 硼酸钙石粉 | 山毛榉 | 300℃,0min | +89%HO | +4% | -4% | -6% | N/A | N/A | +3% | [ |
| 沸石 | +29%TO | -8% | -7% | +3% | +4% | -12% | -8% | [ | ||
| Pt/Al2O3 | -12%TO | +9% | +16% | -37% | -12% | N/A | +11% | [ | ||
| Ni/Al2O3 | -47%TO | +13% | +7% | -46% | -12% | N/A | +11% | |||
| Co/Mo/Al2O3 | -26%TO | +13% | +1% | -51% | +12% | N/A | +9% | |||
| CoMo/γ-Al2O3 | 微绿球藻 | 350℃,60min | +54%TO | +1% | +1% | -7% | +3% | -31% | +1% | [ |
| Ni/SiO2-Al2O3 | +44%TO | 0 | 0 | -6% | -11% | -100% | -1% | |||
| Pt/C | +34%TO | +1% | +6% | -8% | -3% | -14% | +3% | |||
| Ru/C | +43%TO | -4% | +1% | +2% | -17% | -63% | -3% | |||
| Pd/C | +63%TO | -3% | +6% | -2% | -7% | -38% | +0.3% | |||
| +4%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ | |||
| Pt/C | 280℃,30min | +6%HO | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Pd/Al2O3 | 小球藻 | +8%HO | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Ce/HZSM-5 | 300℃,20min | +47%LO | +15% | +52% | -10% | -97% | N/A | +40% | [ | |
| HZSM-5 | +3%LO | +4% | +25% | -2% | -38% | N/A | +15% | |||
| 螺旋藻 | 380℃,120min | +7%TO | -0.07% | -5% | -0.8% | +9% | N/A | -2% | [ | |
| Ni-Mo/Al2O3 | 微绿球藻 | 340℃,30min | +41%TO | +1% | +5% | -9% | -6% | N/A | +3% | [ |
| Ni-Cu/γ-Al2O3 | 纸铝塑 | 330℃,30min | +54%HO | +5% | -1% | -21% | N/A | N/A | +7% | [ |
| FeHZSM-5 | 玉米秸秆 | 340℃,30min | +25%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
表1 生物质水热催化制备生物原油的总结
| 催化剂 | 原料 | 过程条件 | 产率提升 | 对生物原油催化效果 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | HHV | |||||
| 均相催化剂 | ||||||||||
| 氢氧化钾 | 玉米芯 | 340℃,60min | +49%TO | -22% | -9% | +67% | +33% | N/A | N/A | [ |
| 碳酸钾 | 木屑 | 280℃,15min | +136%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
| 碳酸钠 | 水葫芦 | -50%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ | |
| 微绿球藻 | 300℃,60min | -21%TO | +4% | -4% | -92% | +2% | +70% | -0.9% | [ | |
| 泡桐 | 300℃,10min | +18%HO | +5% | +2% | -13% | N/A | N/A | +7% | [ | |
| 螺旋藻 | 350℃,60min | -11%TO | +3% | +17% | -16% | -34% | +0.5% | -5% | [ | |
| +29%HO | -2% | +11% | +16% | -14% | -3% | +3% | [ | |||
| 甲酸 | 380℃,120min | +28%TO | -1% | -12% | +18% | -27% | N/A | -7% | [ | |
| 盐酸 | 纤维素 | 300℃,0min | +21%LO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
| 乙酸 | 小球藻蛋白 | 300℃,20min,8.5MPa | 降低LO | -7% | +81% | +3% | -10% | N/A | +18% | [ |
| 非均相催化剂 | ||||||||||
| 铁粉 | 泡桐 | 340℃,10min | +51%HO | +10% | +20% | -29% | N/A | N/A | +20% | [ |
| 硼酸钙石粉 | 山毛榉 | 300℃,0min | +89%HO | +4% | -4% | -6% | N/A | N/A | +3% | [ |
| 沸石 | +29%TO | -8% | -7% | +3% | +4% | -12% | -8% | [ | ||
| Pt/Al2O3 | -12%TO | +9% | +16% | -37% | -12% | N/A | +11% | [ | ||
| Ni/Al2O3 | -47%TO | +13% | +7% | -46% | -12% | N/A | +11% | |||
| Co/Mo/Al2O3 | -26%TO | +13% | +1% | -51% | +12% | N/A | +9% | |||
| CoMo/γ-Al2O3 | 微绿球藻 | 350℃,60min | +54%TO | +1% | +1% | -7% | +3% | -31% | +1% | [ |
| Ni/SiO2-Al2O3 | +44%TO | 0 | 0 | -6% | -11% | -100% | -1% | |||
| Pt/C | +34%TO | +1% | +6% | -8% | -3% | -14% | +3% | |||
| Ru/C | +43%TO | -4% | +1% | +2% | -17% | -63% | -3% | |||
| Pd/C | +63%TO | -3% | +6% | -2% | -7% | -38% | +0.3% | |||
| +4%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ | |||
| Pt/C | 280℃,30min | +6%HO | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Pd/Al2O3 | 小球藻 | +8%HO | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Ce/HZSM-5 | 300℃,20min | +47%LO | +15% | +52% | -10% | -97% | N/A | +40% | [ | |
| HZSM-5 | +3%LO | +4% | +25% | -2% | -38% | N/A | +15% | |||
| 螺旋藻 | 380℃,120min | +7%TO | -0.07% | -5% | -0.8% | +9% | N/A | -2% | [ | |
| Ni-Mo/Al2O3 | 微绿球藻 | 340℃,30min | +41%TO | +1% | +5% | -9% | -6% | N/A | +3% | [ |
| Ni-Cu/γ-Al2O3 | 纸铝塑 | 330℃,30min | +54%HO | +5% | -1% | -21% | N/A | N/A | +7% | [ |
| FeHZSM-5 | 玉米秸秆 | 340℃,30min | +25%HO | N/A | N/A | N/A | N/A | N/A | N/A | [ |
| 原料 | 操作条件 | pH | TOC/mg·L-1 | TN/mg·L-1 | 参考文献 |
|---|---|---|---|---|---|
| 小球藻 | 260~300℃,30~90min | 7.77~8.29 | N/A | 11000~37000 | [ |
| 天然蓝藻 | 340℃,60min | N/A | 37.22 | 2215 | [ |
| 核桃壳 | 280℃,30min | N/A | 22900 | N/A | [ |
| 团集江蓠 | 350℃,15min | N/A | 2800 | 6400 | [ |
| 细江蓠 | 350℃,15min | N/A | 1750 | 2100 | [ |
| 微绿球藻 | 350℃,20min | 8.28 | 25.78 | 10.18 | [ |
| 等鞭金藻 | 350℃,20min | 6.8 | 17.24 | 8.08 | [ |
| 锯末 | 250℃,60min | 8.12 | N/A | N/A | [ |
表2 几种水热液化水相产物的特性
| 原料 | 操作条件 | pH | TOC/mg·L-1 | TN/mg·L-1 | 参考文献 |
|---|---|---|---|---|---|
| 小球藻 | 260~300℃,30~90min | 7.77~8.29 | N/A | 11000~37000 | [ |
| 天然蓝藻 | 340℃,60min | N/A | 37.22 | 2215 | [ |
| 核桃壳 | 280℃,30min | N/A | 22900 | N/A | [ |
| 团集江蓠 | 350℃,15min | N/A | 2800 | 6400 | [ |
| 细江蓠 | 350℃,15min | N/A | 1750 | 2100 | [ |
| 微绿球藻 | 350℃,20min | 8.28 | 25.78 | 10.18 | [ |
| 等鞭金藻 | 350℃,20min | 6.8 | 17.24 | 8.08 | [ |
| 锯末 | 250℃,60min | 8.12 | N/A | N/A | [ |
| 原料 | 操作条件 | 循环 次数 | 检测出的有机酸 | 回用效果 | 参考文献 |
|---|---|---|---|---|---|
| 白杨木 | 400℃,7.1~11.1h | 3 | N/A | 第1~3批次处理产油率上升,第4批次产率降低。水相中无机物和水溶性有机物随批次累积,灰分沉积率从6.2增长到12.6%。TOC含量从54.1g/L增加到136.2g/L | [ |
| 玉米秸秆 | 300℃,30min | 3 | 乙酸、丙酸 | 有机酸含量富集,促进酚酮类转化,生物原油产率提高了3.89%,固体产物中碳含量提高了0.8% | [ |
刚毛藻 江蓠 | 350℃,15min | 2 | N/A | 随着循环次数的增加,气体产物和液体产物的总量下降,固体沉积率下降,产油率分别增加了8.1%和8.89% | [ |
| 干酒糟 | 350℃,20min | 9 | 乙酸、乙酰丙酸、异丁酸、异戊酸 | 当循环第8次的时候,生物原油的产率从39.4%大幅增加到54.5%,同时循环后的水相体积更小,浓度更高。循环9次,生物原油的含氧量降低了52%。TOC和TN的浓度随循环次数逐渐增大 | [ |
| 海藻-锯末 | 250℃,60min | 1 | 乙酸、2-己酸 | 水相循环后,产油率由11.1%降到了9.2%,固体残渣生成增加,从31.3%~38.9%。通过回用能大幅度消减过程副产物 | [ |
| 大麦秸秆 | 300℃,15min | 3 | 乳酸,乙酸,2-羟基异丁酸, 2-丁烯二酸,2-羟基-3-甲基戊酸 | 循环3次后,产油率由34.9%增长到38.4%。循环前后生物原油的元素并无太大差异,热值有略微提升。水相有机物主要聚合为固体残渣导致循环后的固体残渣产率是未循环的两倍。固体残渣的碳含量随循环次数增加而增加,氧含量则相反,热值显著增加 | [ |
表3 水相产物回用对水热液化的影响
| 原料 | 操作条件 | 循环 次数 | 检测出的有机酸 | 回用效果 | 参考文献 |
|---|---|---|---|---|---|
| 白杨木 | 400℃,7.1~11.1h | 3 | N/A | 第1~3批次处理产油率上升,第4批次产率降低。水相中无机物和水溶性有机物随批次累积,灰分沉积率从6.2增长到12.6%。TOC含量从54.1g/L增加到136.2g/L | [ |
| 玉米秸秆 | 300℃,30min | 3 | 乙酸、丙酸 | 有机酸含量富集,促进酚酮类转化,生物原油产率提高了3.89%,固体产物中碳含量提高了0.8% | [ |
刚毛藻 江蓠 | 350℃,15min | 2 | N/A | 随着循环次数的增加,气体产物和液体产物的总量下降,固体沉积率下降,产油率分别增加了8.1%和8.89% | [ |
| 干酒糟 | 350℃,20min | 9 | 乙酸、乙酰丙酸、异丁酸、异戊酸 | 当循环第8次的时候,生物原油的产率从39.4%大幅增加到54.5%,同时循环后的水相体积更小,浓度更高。循环9次,生物原油的含氧量降低了52%。TOC和TN的浓度随循环次数逐渐增大 | [ |
| 海藻-锯末 | 250℃,60min | 1 | 乙酸、2-己酸 | 水相循环后,产油率由11.1%降到了9.2%,固体残渣生成增加,从31.3%~38.9%。通过回用能大幅度消减过程副产物 | [ |
| 大麦秸秆 | 300℃,15min | 3 | 乳酸,乙酸,2-羟基异丁酸, 2-丁烯二酸,2-羟基-3-甲基戊酸 | 循环3次后,产油率由34.9%增长到38.4%。循环前后生物原油的元素并无太大差异,热值有略微提升。水相有机物主要聚合为固体残渣导致循环后的固体残渣产率是未循环的两倍。固体残渣的碳含量随循环次数增加而增加,氧含量则相反,热值显著增加 | [ |
| 原料 | 操作条件 | 碳元素 /% | HHV /MJ·kg-1 | 灰分(质量分数) /% | 固体产物特性描述 | 参考文献 |
|---|---|---|---|---|---|---|
| 蓖麻渣 | 300℃,90min | 36.47 | N/A | N/A | 焦炭微观形貌为多孔的小尺寸颗粒,含有无定形炭和涡轮层炭,表层附着了残留矿物质。H/C和O/C比值降低,可作为优质的碳基材料 | [ |
| 死猪 | 400℃,120min | 17.67 | 21.3 | 0.02 | 猪肉灰分较少,因此固体产物几乎全部是焦炭,碳含量高达65.2%。热值为21.3MJ/kg,与某些煤热值相当。焦炭的比表面积与植物热解碳相当 | [ |
| 大麦秸秆 | 300℃,15min | 22.3 | 22.43 | N/A | 焦炭含碳率65.50%,具有较高的热值。粗糙多孔表面附着一层纤维素和半纤维素分子间脱水形成的微球,微球和多孔提升了比表面积增加了负载位点。水相循环增强脱水和缩合效应,使微球数量增多 | [ |
| 团集江蓠 | 350℃,15min | 14.3 | 13.1 | N/A | 大型藻类中的糖类含量较高,通常比微型藻类具有更高的焦炭生成量。由于灰分含量多导致了固体产物产率高 | [ |
| 滇池蓝藻 | 340℃,60min | N/A | N/A | 77.47 | 固体产物中含碳量低,主要成分是灰分,Al、Fe、Si、Ca是含量最多的元素。较多的灰分降低了固体产物的热值 | [ |
| 锯末-藻 | 250℃,60min | 16.6 | 16.2 | 7.67 | 使用藻类和锯末共液化,随着藻类的添加,固体残渣中C、H及热值均下降,N、O则相反 | [ |
| 小球藻 | 280℃,30min | N/A | N/A | N/A | 在催化剂作用下,原料中的P在Ca、Mg的作用下形成固体沉淀沉积在固体产物中促进了P的回收。部分碳和无机混合物沉积在催化剂表面和孔道内,使催化剂中毒 | [ |
表4 生物质水热液化固体产物特性
| 原料 | 操作条件 | 碳元素 /% | HHV /MJ·kg-1 | 灰分(质量分数) /% | 固体产物特性描述 | 参考文献 |
|---|---|---|---|---|---|---|
| 蓖麻渣 | 300℃,90min | 36.47 | N/A | N/A | 焦炭微观形貌为多孔的小尺寸颗粒,含有无定形炭和涡轮层炭,表层附着了残留矿物质。H/C和O/C比值降低,可作为优质的碳基材料 | [ |
| 死猪 | 400℃,120min | 17.67 | 21.3 | 0.02 | 猪肉灰分较少,因此固体产物几乎全部是焦炭,碳含量高达65.2%。热值为21.3MJ/kg,与某些煤热值相当。焦炭的比表面积与植物热解碳相当 | [ |
| 大麦秸秆 | 300℃,15min | 22.3 | 22.43 | N/A | 焦炭含碳率65.50%,具有较高的热值。粗糙多孔表面附着一层纤维素和半纤维素分子间脱水形成的微球,微球和多孔提升了比表面积增加了负载位点。水相循环增强脱水和缩合效应,使微球数量增多 | [ |
| 团集江蓠 | 350℃,15min | 14.3 | 13.1 | N/A | 大型藻类中的糖类含量较高,通常比微型藻类具有更高的焦炭生成量。由于灰分含量多导致了固体产物产率高 | [ |
| 滇池蓝藻 | 340℃,60min | N/A | N/A | 77.47 | 固体产物中含碳量低,主要成分是灰分,Al、Fe、Si、Ca是含量最多的元素。较多的灰分降低了固体产物的热值 | [ |
| 锯末-藻 | 250℃,60min | 16.6 | 16.2 | 7.67 | 使用藻类和锯末共液化,随着藻类的添加,固体残渣中C、H及热值均下降,N、O则相反 | [ |
| 小球藻 | 280℃,30min | N/A | N/A | N/A | 在催化剂作用下,原料中的P在Ca、Mg的作用下形成固体沉淀沉积在固体产物中促进了P的回收。部分碳和无机混合物沉积在催化剂表面和孔道内,使催化剂中毒 | [ |
| 原料 | 操作条件 | 气体产物体积分数①/% | 参考文献 | |||
|---|---|---|---|---|---|---|
| CO2 | CO | CH4 | H2 | |||
| 白杨木② | 400℃,60min | 60.8~63.6 | 2.7~3.3 | 4.2~4.6 | 28.8~32 | [ |
| 纤维素 | 380℃,30min,N2 | 5.32 | 11.32 | 0.37 | 15.43 | [ |
| 380℃,30min,H2 | 6.14 | 9.21 | 3.67 | 80.98 | ||
| 380℃,30min,CO | 12.63 | 66.94 | 1.74 | 18.69 | ||
| 甘蔗渣② | 345℃,30min | N/A | N/A | 5.46 | 2.31 | [ |
| 滇池蓝藻 | 340℃,60min,N2 | 81.43 | N/A | 0.7 | 0.05 | [ |
表5 几种生物质水热液化气体产物的成分
| 原料 | 操作条件 | 气体产物体积分数①/% | 参考文献 | |||
|---|---|---|---|---|---|---|
| CO2 | CO | CH4 | H2 | |||
| 白杨木② | 400℃,60min | 60.8~63.6 | 2.7~3.3 | 4.2~4.6 | 28.8~32 | [ |
| 纤维素 | 380℃,30min,N2 | 5.32 | 11.32 | 0.37 | 15.43 | [ |
| 380℃,30min,H2 | 6.14 | 9.21 | 3.67 | 80.98 | ||
| 380℃,30min,CO | 12.63 | 66.94 | 1.74 | 18.69 | ||
| 甘蔗渣② | 345℃,30min | N/A | N/A | 5.46 | 2.31 | [ |
| 滇池蓝藻 | 340℃,60min,N2 | 81.43 | N/A | 0.7 | 0.05 | [ |
| 1 | 何选明, 王春霞, 付鹏睿, 等. 水热技术在生物质转换中的研究进展[J]. 现代化工, 2014, 34(1): 26-29. |
| HE X M, WANG C X, FU P R, et al. Research development of hydrothermal technology for biomass transform utilization[J]. Modern Chemical Industry, 2014, 34(1): 26-29. | |
| 2 | 曲磊, 崔翔, 杨海平, 等. 微藻水热液化制取生物油的研究进展[J]. 化工进展, 2018, 37(8): 2962-2969. |
| QU Lei, CUI Xiang, YANG Haiping, et al. Review on the preparation of bio-oil by microalgae hydrothermal liquefaction[J]. Chemical Industry and Engineering Progress, 2018, 37(8): 2962-2969. | |
| 3 | KLEMMER M, MADSEN R B, HOULBERG K, et al. Effect of aqueous phase recycling in continuous hydrothermal liquefaction[J]. Industrial & Engineering Chemistry Research, 2016, 55: 12317-12325. |
| 4 | 黄照单. 预处理对低脂藻水热催化液化制备生物油的影响研究[D]. 上海: 华东师范大学, 2018. |
| HUANG Z D. The effect of pretreatment on catalytic hydrothermal liquefaction of low-lipid microalgae[D]. Shanghai: East China Normal University, 2018. | |
| 5 | PRADO J M, LACHOS-PEREZ D, FORSTER-CARNEIRO T, et al. Sub- and supercritical water hydrolysis of agricultural and food industry residues for the production of fermentable sugars: a review[J]. Food and Bioproducts Processing, 2016, 98: 95-123. |
| 6 | KHOO C G, LAM M K, MOHAMED A R, et al. Hydrochar production from high-ash low-lipid microalgal biomass via hydrothermal carbonization: effects of operational parameters and products characterization[J]. Environmental Research, 2020, 188: 109828. |
| 7 | VALDEZ P J, TOCCO V J, SAVAGE P E. A general kinetic model for the hydrothermal liquefaction of microalgae[J]. Bioresource Technology, 2014, 163: 123-127. |
| 8 | MAITY J P, BUNDSCHUH J, CHEN C Y, et al. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives—A mini review[J]. Energy, 2014, 78: 104-113. |
| 9 | 陈宇. 低脂微藻催化水热液化及过程原位分析的研究[D]. 北京: 清华大学, 2016. |
| CHEN Yu. Study on the catalytic hydrothermal liquefaction of low-lipid microalgae and process in situ analysis[D]. Beijing: Tsinghua University, 2016. | |
| 10 | 申瑞霞, 赵立欣, 冯晶, 等. 生物质水热液化产物特性与利用研究进展[J]. 农业工程学报, 2020, 36(2): 266-274. |
| SHEN Ruixia, ZHAO Lixin, FENG Jing, et al. Research progress on characteristics and utilization of products from hydrothermal liquefaction of biomass[J]. Transactions of the Chinese Society of Agricultural Engineering, 2020, 36(2): 266-274. | |
| 11 | YANG X, LYU H, CHEN K F, et al. Selective extraction of bio-oil from hydrothermal liquefaction of salix psammophila by organic solvents with different polarities through multistep extraction separation[J]. BioResources, 2014, 9(3): 5219-5233. |
| 12 | 韩鲁佳, 李彦霏, 刘贤, 等. 生物炭吸附水体中重金属机理与工艺研究进展[J]. 农业机械学报, 2017, 48(11): 1-11. |
| HAN Lujia, LI Yanfei, LIU Xian, et al. Review of biochar as adsorbent for aqueous heavy metal removal[J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(11): 1-11. | |
| 13 | BUNGAY H R. Biomass refining[J]. Science, 1982, 218(4573): 643-646. |
| 14 | ATALLA R H, BRADY J W, MATTHEWS J F, et al. Biomass recalcitrance-deconstructing the plant cell wall for bioenergy[M]// Persistent organic pollutants. Boston: Kluwer Academic Publishers, 2009: 7-9. |
| 15 | JIN F M, ENOMOTO H J. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: chemistry of acid/base-catalysed and oxidation reactions[J]. Energy & Environmental Science, 2011, 4(2): 382-397. |
| 16 | JIN F M. Hydrothermal reduction of carbon dioxide to low-carbon fuels[M]. Boca Raton: CRC Press, 2017: 223. |
| 17 | PETERSON A A, VOGEL F, LACHANCE R P, et al. Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies[J]. Energy & Environmental Science, 2008, 1: 32-65. |
| 18 | TITIRICI M M, WHITE R J, BUDARIN N B, et al. Sustainable carbon materials from hydrothermal processes[M]. New York: John Wiley & Sons, Ltd., 2013: 295-340. |
| 19 | 孙明荣, 刘晓欣, 谢文华, 等. 生物炼制经济的发展思路和展望[J]. 化工进展, 2017, 36(9): 3250-3256. |
| SUN Mingrong, LIU Xiaoxin, XIE Wenhua, et al. Present economical development and prospective of biorefineries[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3250-3256. | |
| 20 | 覃伟中, 朱兵, 李强, 等. 生物炼制的挑战与过程系统工程的机遇[J]. 化工进展, 2010, 29(5): 922-926. |
| QIN Weizhong, ZHU Bing, LI Qiang, et al. Challenges of biorefinery and opportunities for process systems engineering[J]. Chemical Industry and Engineering Progress, 2010, 29(5): 922-926. | |
| 21 | AKHTAR J, AMIN N A S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass[J]. Renewable and Sustainable Energy Reviews, 2011, 15(3): 1615-1624. |
| 22 | ZHOU Y, SCHIDEMAN L, YU G, et al. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling[J]. Energy & Environmental Science, 2013, 6(12): 3765-3779. |
| 23 | 唐受印, 戴友芝. 废水处理水热氧化技术[M]. 北京: 化学工业出版社, 2002: 1-3. |
| TANG Shouyin, DAI Youzhi. Hydrothermal oxidation technology for wastewater treatment [M]. Beijing: Chemical Industry Press, 2002: 1-3. | |
| 24 | ZHU Y H, ALBRECHT K O, ELLIOTT D C, et al. Development of hydrothermal liquefaction and upgrading technologies for lipid-extracted algae conversion to liquid fuels[J]. Algal Research, 2013, 2(4): 455-464. |
| 25 | 杨保祥, 何金勇, 张桂芳. 钒基材料制造[M]. 北京: 冶金工业出版社, 2014: 266. |
| YANG Baoxiang, HE Jinyong, ZHANG Guifang. Vanadium based material manufacture[M]. Beijing: Metallurgical Industry Press, 2014: 266. | |
| 26 | SHAKYA R, WHELEN J, ADHIKARI S, et al. Effect of temperature and Na2CO3 catalyst on hydrothermal liquefaction of algae[J]. Algal Research, 2015, 12: 80-90. |
| 27 | SINGH R, BALAGURUMURTHY B, PRAKASH A, et al. Catalytic hydrothermal liquefaction of water hyacinth[J]. Bioresource Technology, 2015, 178: 157-165. |
| 28 | LIU C Z, KONG L P, WANG Y Y, et al. Catalytic hydrothermal liquefaction of Spirulina to bio-oil in the presence of formic acid over palladium-based catalysts[J]. Algal Research, 2018, 33: 156-164. |
| 29 | JENA U, DAS K C, KASTNER J R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis[J]. Applied Energy, 2012, 98: 368-375. |
| 30 | YIN S D, TAN Z C. Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions[J]. Applied Energy, 2012, 92: 234-239. |
| 31 | ROSS A B, BILLER P, KUBACKI M L, et al. Hydrothermal processing of microalgae using alkali and organic acids[J]. Fuel, 2010, 89(9): 2234-2243. |
| 32 | YANG W C, LI X G, ZHANG D H, et al. Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids[J]. Energy Conversion and Management, 2017, 154: 336-343. |
| 33 | NITSOS C K, CHOLI-PAPADOPOULOU T, MATIS K A, et al. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(9): 4529-4544. |
| 34 | 吕春柳. 木薯秸秆两步水热自催化及强化预处理工艺研究[D]. 天津: 天津大学, 2018. |
| Chunliu LYU. Study on the two-stage hydrothermal auto-catalyic and strengthened pretreatment process of cassava straw[D]. Tianjin: Tianjin University, 2018. | |
| 35 | 闫莉. 基于玉米秸秆全组分利用的自催化水热预处理工艺[D]. 天津: 天津大学, 2014. |
| YAN Li. Auto-catalytic hydrothermal pretreatment process of corn stover based on full components use[D]. Tianjin: Tianjin University, 2014. | |
| 36 | DURAK H, GENEL S. Catalytic hydrothermal liquefaction of lactuca scariola with a heterogeneous catalyst: the investigation of temperature, reaction time and synergistic effect of catalysts[J]. Bioresource Technology, 2020, 309: 123375. |
| 37 | BILLER P, ROSS A B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content[J]. Bioresource Technology, 2011, 102(1): 215-225. |
| 38 | ZHENG M X, SCHIDEMAN L C, TOMMASO G, et al. Anaerobic digestion of wastewater generated from the hydrothermal liquefaction of Spirulina: Toxicity assessment and minimization[J]. Energy Conversion and Management, 2017, 141: 420-428. |
| 39 | KHAMPUANG K, BORERIBOON N, PRASASSARAKICH P. Alkali catalyzed liquefaction of corncob in supercritical ethanol-water[J]. Biomass and Bioenergy, 2015, 83: 460-466. |
| 40 | KARAGÖZ S, BHASKAR T, MUTO A, et al. Hydrothermal upgrading of biomass: effect of K2CO3 concentration and biomass/water ratio on products distribution[J]. Bioresource Technology, 2006, 97(1): 90-98. |
| 41 | SUN P Q, HENG M X, SUN S H, et al. Direct liquefaction of paulownia in hot compressed water: influence of catalysts[J]. Energy, 2010, 35(12): 5421-5429. |
| 42 | TEKIN K, KARAGÖZ S, BEKTAŞ S. Hydrothermal liquefaction of beech wood using a natural calcium borate mineral[J]. The Journal of Supercritical Fluids, 2012, 72: 134-139. |
| 43 | DUAN P G, SAVAGE P E. Hydrothermal liquefaction of a microalga with heterogeneous catalysts[J]. Industrial & Engineering Chemistry Research, 2011, 50(1): 52-61. |
| 44 | BILLER P, RILEY R, ROSS A B. Catalytic hydrothermal processing of microalgae: decomposition and upgrading of lipids[J]. Bioresource Technology, 2011, 102(7): 4841-4848. |
| 45 | YU G, ZHANG Y H, GUO B, et al. Nutrient flows and quality of bio-crude oil produced via catalytic hydrothermal liquefaction of low-lipid microalgae[J]. BioEnergy Research, 2014, 7(4): 1317-1328. |
| 46 | XU Y F, ZHENG X J, YU H Q, et al. Hydrothermal liquefaction of Chlorella pyrenoidosa for bio-oil production over Ce/HZSM-5[J]. Bioresource Technology, 2014, 156: 1-5. |
| 47 | LI H Y, HU J, ZHANG Z J, et al. Insight into the effect of hydrogenation on efficiency of hydrothermal liquefaction and physico-chemical properties of biocrude oil[J]. Bioresource Technology, 2014, 163: 143-151. |
| 48 | 朱怡彤. 纸铝塑复合材料在亚/超临界水中制油特性研究[D]. 西安: 西安理工大学, 2019. |
| ZHU Yitong. Liquefaction of paper-aluminum-plastic complex material to bio-oils in sub-/supercritical water[D]. Xi’an: Xi’an University of Technology, 2019. | |
| 49 | 张杨. 玉米秸秆催化液化制备生物油实验研究[D]. 沈阳: 沈阳航空航天大学, 2016. |
| ZHANG Yang. Hydrothermal catalytic liquefaction of corn stalk for preparation of bio-oil[D]. Shenyang: Shenyang Aerospace University, 2016. | |
| 50 | DUAN P G, SAVAGE P E. Upgrading of crude algal bio-oil in supercritical water[J]. Bioresource Technology, 2011, 102(2): 1899-1906. |
| 51 | YANG C, JIA L S, CHEN C P, et al. Bio-oil from hydro-liquefaction of Dunaliella Salina over Ni/REHY catalyst[J]. Bioresource Technology, 2011, 102(6): 4580-4584. |
| 52 | ZHANG J X, CHEN W T, ZHANG P, et al. Hydrothermal liquefaction of Chlorella pyrenoidosa in sub- and supercritical ethanol with heterogeneous catalysts[J]. Bioresource Technology, 2013, 133: 389-397. |
| 53 | RAMASWAMY S, HUANG H J, RAMARAO B V. Separation and purification technologies in biorefineries[M]. Chichester, UK:John Wiley & Sons, Ltd., 2013. |
| 54 | ANASTASAKIS K, BILLER P, MADSEN R, et al. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation[J]. Energies, 2018, 11(10): 2695. |
| 55 | ELLIOTT D C, SCHMIDT A J, HART T R, et al. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: grape pomace[J]. Biomass Conversion and Biorefinery, 2017, 7(4): 455-465. |
| 56 | USMAN M, CHEN H H, CHEN K F, et al. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: a review[J]. Green Chemistry, 2019, 21(7): 1553-1572. |
| 57 | GUO Y, YEH T, SONG W H, et al. A review of bio-oil production from hydrothermal liquefaction of algae[J]. Renewable and Sustainable Energy Reviews, 2015, 48: 776-790. |
| 58 | BILLER P, MADSEN R B, KLEMMER M, et al. Effect of hydrothermal liquefaction aqueous phase recycling on bio-crude yields and composition[J]. Bioresource Technology, 2016, 220: 190-199. |
| 59 | HAN Y, HOEKMAN S K, CUI Z, et al. Hydrothermal liquefaction of marine microalgae biomass using co-solvents[J]. Algal Research, 2019, 38: 101421. |
| 60 | DANDAMUDI K P R, MUPPANENI T, MARKOVSKI J S, et al. Hydrothermal liquefaction of green microalga Kirchneriella sp. under sub- and super-critical water conditions[J]. Biomass and Bioenergy, 2019, 120: 224-228. |
| 61 | PANISKO E, WIETSMA T, LEMMON T, et al. Characterization of the aqueous fractions from hydrotreatment and hydrothermal liquefaction of lignocellulosic feedstocks[J]. Biomass and Bioenergy, 2015, 74: 162-171. |
| 62 | GAI C, ZHANG Y H, CHEN W T, et al. Characterization of aqueous phase from the hydrothermal liquefaction of Chlorella pyrenoidosa[J]. Bioresource Technology, 2015, 184: 328-335. |
| 63 | TIAN C Y, LIU Z D, ZHANG Y H, et al. Hydrothermal liquefaction of harvested high-ash low-lipid algal biomass from Dianchi Lake: effects of operational parameters and relations of products[J]. Bioresource Technology, 2015, 184: 336-343. |
| 64 | DE CAPRARIIS B, DE FILIPPIS P, PETRULLO A, et al. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production[J]. Fuel, 2017, 208: 618-625. |
| 65 | PARSA M, JALILZADEH H, PAZOKI M, et al. Hydrothermal liquefaction of Gracilaria gracilis and Cladophora glomerata macro-algae for biocrude production[J]. Bioresource Technology, 2018, 250: 26-34. |
| 66 | HU Y L, FENG S H, BASSI A, et al. Improvement in bio-crude yield and quality through co-liquefaction of algal biomass and sawdust in ethanol-water mixed solvent and recycling of the aqueous by-product as a reaction medium[J]. Energy Conversion and Management, 2018, 171: 618-625. |
| 67 | PEDERSEN T H, GRIGORAS I F, HOFFMANN J, et al. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation[J]. Applied Energy, 2016, 162: 1034-1041. |
| 68 | 尹思媛, 田纯焱, 李艳美, 等. 水相循环对玉米秸秆水热液化成油特性影响的研究[J]. 燃料化学学报, 2020, 48(3): 275-285. |
| YIN Siyuan, TIAN Chunyan, LI Yanmei, et al. Effect of aqueous phase recirculation on characteristics of bio-crude oil formation during hydrothermal liquefaction of corn stalk[J]. Journal of Fuel Chemistry and Technology, 2020, 48(3): 275-285. | |
| 69 | 胡见波, 杜泽学, 闵恩泽. 生物质水热液化机理研究进展[J]. 石油炼制与化工, 2012, 43(4): 87-92. |
| HU Jianbo, DU Zexue, MIN Enze. Progress in research of reaction mechanism concerning hydrothermal liquefaction of biomass[J]. Petroleum Processing and Petrochemicals, 2012, 43(4): 87-92. | |
| 70 | DÃAZ-VáZQUEZ L M, ROJAS-PéREZ A, FUENTES-CARABALLO M, et al. Demineralization of sargassum spp. macroalgae biomass: selective hydrothermal liquefaction process for bio-oil production[J]. Frontiers in Energy Research, 2015, 3: 6. DOI:10.3389/fenrg.2015.00006. |
| 71 | ZHU Z, ROSENDAHL L, TOOR S S, et al. Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation[J]. Applied Energy, 2015, 137: 183-192. |
| 72 | 陈春红. 生物基水热炭材料的结构设计及其形成机理研究[D]. 杭州: 浙江大学, 2019. |
| CHEN Chunhong. Structural design of biomass-derived hydrothermal carbons and their formation mechanism investigations[D]. Hangzhou: Zhejiang University, 2019. | |
| 73 | 刘雨辰. 水热液化生物炭活化利用研究[D]. 上海: 复旦大学, 2014. |
| LIU Yuchen. Activating and utilization of hydrochar derived from hydrothermal liquefaction of biomass[D]. Shanghai: Fudan University, 2014. | |
| 74 | 张小娟, 胡建水, 智翠梅, 等. 超(亚)临界水处理白酒糟制备活性炭[J]. 化工时刊, 2019, 33(6): 1-6, 19. |
| ZHANG Xiaojuan, HU Jianshui, ZHI Cuimei, et al. Preparation of activated carbon from distiller's grains by sub-and supercritical water[J]. Chemical Industry Times, 2019, 33(6): 1-6, 19. | |
| 75 | KAUR R, GERA P, JHA M K, et al. Reaction parameters effect on hydrothermal liquefaction of castor (Ricinus Communis) residue for energy and valuable hydrocarbons recovery[J]. Renewable Energy, 2019, 141: 1026-1041. |
| 76 | ZHENG J L, ZHU M Q, WU H T. Alkaline hydrothermal liquefaction of swine carcasses to bio-oil[J]. Waste Management, 2015, 43: 230-238. |
| 77 | 徐文静. 多孔炭的制备、改性及其CO2吸附性能[D]. 大连: 大连理工大学, 2017. |
| XU Wenjing. The preparation and modification of porous carbon for CO2 capture[D]. Dalian: Dalian University of Technology, 2017. | |
| 78 | HAYASHI J, DE HORIKAWA T, TAKEDA I, et al. Preparing activated carbon from various nutshells by chemical activation with K2CO3[J]. Carbon, 2002, 40(13): 2381-2386. |
| 79 | LIMA I M, BOATENG A A, KLASSON K T. Physicochemical and adsorptive properties of fast-pyrolysis bio-chars and their steam activated counterparts[J]. Journal of Chemical Technology & Biotechnology, 2010, 85(11): 1515-1521. |
| 80 | LIU Z G, ZHANG F S. Removal of copper (Ⅱ) and phenol from aqueous solution using porous carbons derived from hydrothermal chars[J]. Desalination, 2011, 267(1): 101-106. |
| 81 | WANG L L, GUO Y P, ZOU B, et al. High surface area porous carbons prepared from hydrochars by phosphoric acid activation[J]. Bioresource Technology, 2011, 102(2): 1947-1950. |
| 82 | PETROVIĆ J T, STOJANOVIĆ M D, MILOJKOVIĆ J V, et al. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution[J]. Journal of Environmental Management, 2016, 182: 292-300. |
| 83 | 孙进, 陈启, 揭业斐. 水热催化液化制备生物油研究[J]. 中国石油和化工标准与质量, 2017, 37(18): 114-115. |
| SUN Jin, CHEN Qi, Yefei JIE. Study on hydrothermal catalytic liquefaction of bio-oil [J]. China Petroleum and Chemical Standard and Quality, 2017, 37(18): 114-115. | |
| 84 | LEUSBROCK I, METZ S J, REXWINKEL G, et al. The solubility of magnesium chloride and calcium chloride in near-critical and supercritical water[J]. The Journal of Supercritical Fluids, 2010, 53(1/2/3): 17-24. |
| 85 | 佟钰, 朱长军, 刘俊秀, 等. 低品位硅藻土的水热固化过程及其力学性能研究[J]. 硅酸盐通报, 2013, 32(3): 379-383. |
| TONG Yu, ZHU Changjun, LIU Junxiu, et al. Hydrothermal solidification of low-grade diatomite and its mechanical property[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(3): 379-383. | |
| 86 | 邓自祥, 杨建广, 李焌源, 等. 垂序商陆茎叶收获物“水热液化”脱除重金属及生物质转化[J]. 环境工程学报, 2014, 8(9): 3919-3926. |
| DENG Zixiang, YANG Jianguang, LI Junyuan, et al. Removal of heavy metals and upgrading crude bio-oil from Phytolacca Americana L. harvest using hydrothermal upgrading process[J]. Chinese Journal of Environmental Engineering, 2014, 8(9): 3919-3926. | |
| 87 | CHRISTENSEN P S, PENG G, VOGEL F, et al. Hydrothermal liquefaction of the microalgae phaeodactylum tricornutum: impact of reaction conditions on product and elemental distribution[J]. Energy & Fuels, 2014, 28(9): 5792-5803. |
| 88 | 王东. 高压反应釜水热液化制备生物油的实验研究[D]. 北京: 中国石油大学(北京), 2016. |
| WANG Dong. Experimental study on bio-oil production from hydrothermal liquefaction by the autoclave[D]. Beijing: China University of Petroleum (Beijing), 2016. | |
| 89 | JHA B, SINGH D N. A three step process for purification of fly ash zeolites by hydrothermal treatment[J]. Applied Clay Science, 2014, 90: 122-129. |
| 90 | LI G, WANG B D, SUN Q, et al. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method[J]. Microporous and Mesoporous Materials, 2017, 252: 105-115. |
| 91 | 张伟光, 张廷安, 冯伟, 等. 利用高铝粉煤灰种分法制备拟薄水铝石的基础研究[J]. 有色金属(冶炼部分), 2016(8): 22-26. |
| ZHANG W G, ZHANG T A, FENG W, et al. Basic research on preparation of pseudo-boehmite from high-alumina fly ash with seed precipitation method[J]. Nonferrous Metals (Extractive Metallurgy), 2016(8): 22-26. | |
| 92 | 段长平, 李微, 孙彤, 等. 沉淀法制备低维纳米AlO(OH)的工艺条件研究[J]. 材料导报, 2016, 30(10): 83-86, 112. |
| DUAN Changping, LI Wei, SUN Tong, et al. Study on the preparation technological condition of low-dimensional nanoscale AlO(OH) by precipitation method[J]. Materials Review, 2016, 30(10): 83-86, 112. | |
| 93 | ROBERTS G W, STURM B S M, HAMDEH U, et al. Promoting catalysis and high-value product streams by in situ hydroxyapatite crystallization during hydrothermal liquefaction of microalgae cultivated with reclaimed nutrients[J]. Green Chemistry, 2015, 17(4): 2560-2569. |
| 94 | XU D H, SAVAGE P E. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae[J]. Algal Research, 2014, 6: 1-7. |
| 95 | 伍超文, 吴诗勇, 彭文才, 等. 不同气氛下的纤维素水热液化过程[J]. 华东理工大学学报(自然科学版), 2011, 37(4): 430-434. |
| WU Chaowen, WU Shiyong, PENG Wencai, et al. Hydrothermal liquefaction of cellulose under different atmospheres[J]. Journal of East China University of Science and Technology (Natural Science Edition), 2011, 37(4): 430-434. | |
| 96 | 闫秋会, 孙冰洁, 张倩倩. 新型超临界水中煤气化制氢产物的CO2分离过程[J]. 化工进展, 2015, 34(1): 61-64, 107. |
| YAN Qiuhui, SUN Bingjie, ZHANG Qianqian. CO2 separation in hydrogen production by coal gasification in supercritical water[J]. Chemical Industry and Engineering Progress, 2015, 34(1): 61-64, 107. | |
| 97 | 张亚运. 木质纤维素热化学转化机理及裂解气体CO2和H2吸附分离的分子模拟研究[D]. 重庆: 重庆大学, 2017. |
| ZHANG Yayun. The mechanism of lignin cellulose thermal conversion and adsorption and separation of pyrolytic gases CO2 and H2 by molecular simulation[D]. Chongqing: Chongqing University, 2017. | |
| 98 | WANG Rui, HUANG Xinsong, LIU Tianfu, et al. Metal-organic frameworks for CO oxidation[J]. 高等学校化学学报, 2020, 41(10): 2174-2184. |
| WANG Rui, HUANG Xinsong, LIU Tianfu, et al. Metal-organic frameworks for CO oxidation[J]. Chemical Journal of Chinese Universities, 2020, 41(10): 2174-2184. | |
| 99 | GUO Y F, LIN J, SUN J, et al. Precursor effects on catalytic behaviors of copper-manganese-cerium ternary oxides pellets for low-temperature CO oxidation[J]. Catalysis Letters, 2020, 150(4): 979-991. |
| 100 | LI Z W, WANG H, ZHAO W W, et al. Enhanced catalytic activity of Au-CeO2/Al2O3 monolith for low-temperature CO oxidation[J]. Catalysis Communications, 2019, 129: 105729. |
| 101 | 王丽琼, 黄亮, 梁峰, 等. 单原子催化剂的制备、表征及催化性能[J]. 催化学报, 2017, 38(9): 1528-1539. |
| WANG Liqiong, HUANG Liang, LIANG Feng, et al. Preparation, characterization and catalytic performance of single-atom catalysts[J]. Chinese Journal of Catalysis, 2017, 38(9): 1528-1539. | |
| 102 | 吴文浩, 雷文, 王丽琼, 等. 单原子催化剂合成方法[J]. 化学进展, 2020, 32(1): 23-32. |
| WU Wenhao, LEI Wen, WANG Liqiong, et al. Preparation of single atom catalysts[J]. Progress in Chemistry, 2020, 32(1): 23-32. | |
| 103 | NJAGI E C, CHEN C H, GENUINO H, et al. Total oxidation of CO at ambient temperature using copper manganese oxide catalysts prepared by a redox method[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 103-110. |
| 104 | LV S, XIA G F, JIN C, et al. Low-temperature CO oxidation by Co3O4 nanocubes on the surface of Co(OH)2 nanosheets[J]. Catalysis Communications, 2016, 86: 100-103. |
| 105 | LIU J C, WANG Y G, LI J. Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria[J]. Journal of the American Chemical Society, 2017, 139(17): 6190-6199. |
| 106 | WANG C L, GU X K, YAN H, et al. Water-mediated mars-van krevelen mechanism for CO oxidation on ceria supported single-atom Pt1 catalyst[J]. ACS Catalysis, 2016, 7(1): 887-891. |
| 107 | ZHONG H, YAO H S, DUO J, et al. Pd/C-catalyzed reduction of NaHCO3 into CH3COOH with water as a hydrogen source[J]. Catalysis Today, 2016, 274: 28-34. |
| 108 | JIN F, ZENG X, LIU J, et al. Highly efficient and autocatalytic H2O dissociation for CO₂ reduction into formic acid with zinc[J]. Sci. Rep., 2014, 4: 4503. |
| 109 | 宋静文, 景镇子, 金放鸣. 金属锌水热法还原二氧化碳产乙酸的研究[J]. 科技创新与应用, 2017(6): 17-18. |
| SONG Jingwen, JING Zhenzi, JIN Fangming. Study on hydrothermal reduction of carbon dioxide to acetic acid by metal zinc [J]. Technology Innovation and Application, 2017(6): 17-18. | |
| 110 | 王伟. 植物性生物质废弃物水热转化制备生物油的基础研究[D]. 北京: 中国石油大学(北京), 2016. |
| WANG Wei. Basic research of bio-oil preparation from plant biomass waste of hydrothermal liquefaction[D]. Beijing: China University of Petroleum (Beijing), 2016. |
| [1] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [2] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [3] | 郑梦启, 王成业, 汪炎, 王伟, 袁守军, 胡真虎, 何春华, 王杰, 梅红. 菌藻共生技术在工业废水零排放中的应用与展望[J]. 化工进展, 2023, 42(8): 4424-4431. |
| [4] | 关红玲, 杨辉, 井红权, 刘玉琼, 谷守玉, 王好斌, 侯翠红. 木质素基控释材料及其在药物输送和肥料控释中的应用[J]. 化工进展, 2023, 42(7): 3695-3707. |
| [5] | 于丁一, 李圆圆, 王晨钰, 纪永升. pH响应性木质素水凝胶的制备及药物控释[J]. 化工进展, 2023, 42(6): 3138-3146. |
| [6] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [7] | 王雪, 徐期勇, 张超. 木质纤维素类生物质水热炭化机理及水热炭应用进展[J]. 化工进展, 2023, 42(5): 2536-2545. |
| [8] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| [9] | 杨自强, 李风海, 郭卫杰, 马名杰, 赵薇. 市政污泥热处理过程中磷迁移转化的研究进展[J]. 化工进展, 2023, 42(4): 2081-2090. |
| [10] | 万茂华, 张小红, 安兴业, 龙垠荧, 刘利琴, 管敏, 程正柏, 曹海兵, 刘洪斌. MXene在生物质基储能纳米材料领域中的应用研究进展[J]. 化工进展, 2023, 42(4): 1944-1960. |
| [11] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| [12] | 邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539. |
| [13] | 郑云武, 裴涛, 李冬华, 王继大, 李继容, 郑志锋. 金属氧化物活化P/HZSM-5催化生物质热解气重整制备富烃生物油[J]. 化工进展, 2023, 42(3): 1353-1364. |
| [14] | 宋叶, 陈玉卓, 宋云彩, 冯杰. 有机固废合成气原位净化催化剂设计及反应器分析[J]. 化工进展, 2023, 42(3): 1383-1396. |
| [15] | 杨程瑞雪, 黄琪媛, 冉建速, 崔耘通, 王健健. 磷酸修饰二氧化硅负载钯催化剂用于木质素衍生物高效水相低温加氢脱氧[J]. 化工进展, 2023, 42(10): 5179-5190. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||