化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5747-5771.DOI: 10.16085/j.issn.1000-6613.2020-2041
吸附法净化室内甲醛研究进展
- 南京邮电大学材料科学与工程学院,江苏 南京 210023
-
收稿日期:2020-10-10修回日期:2020-11-27出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:王琼 -
作者简介:肖康(1985—),男,高级实验师,研究方向为室内甲醛净化、环境催化。E-mail:iamkxiao@njupt.edu.cn 。 -
基金资助:南京邮电大学校级科研基金(NY 219057);南京邮电大学实验室工作研究课题(2020XSG11)
Progress in research on adsorption for abatement of indoor formaldehyde
- School of Materials Science & Engineering, Nanjing University of Posts and Telecommunications, Nanjing 210023, Jiangsu, China
-
Received:2020-10-10Revised:2020-11-27Online:2021-10-10Published:2021-10-25 -
Contact:WANG Qiong
摘要:
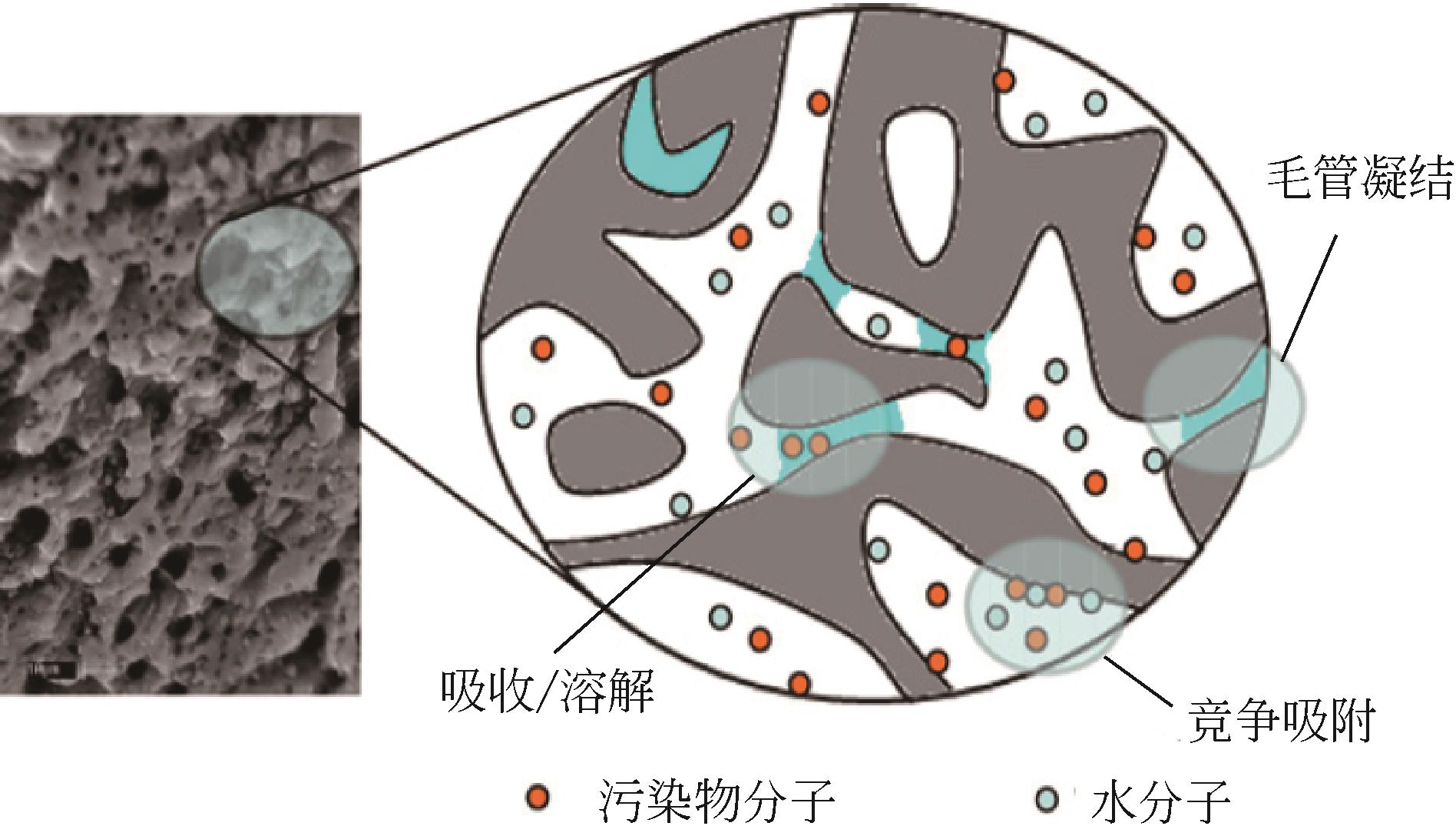
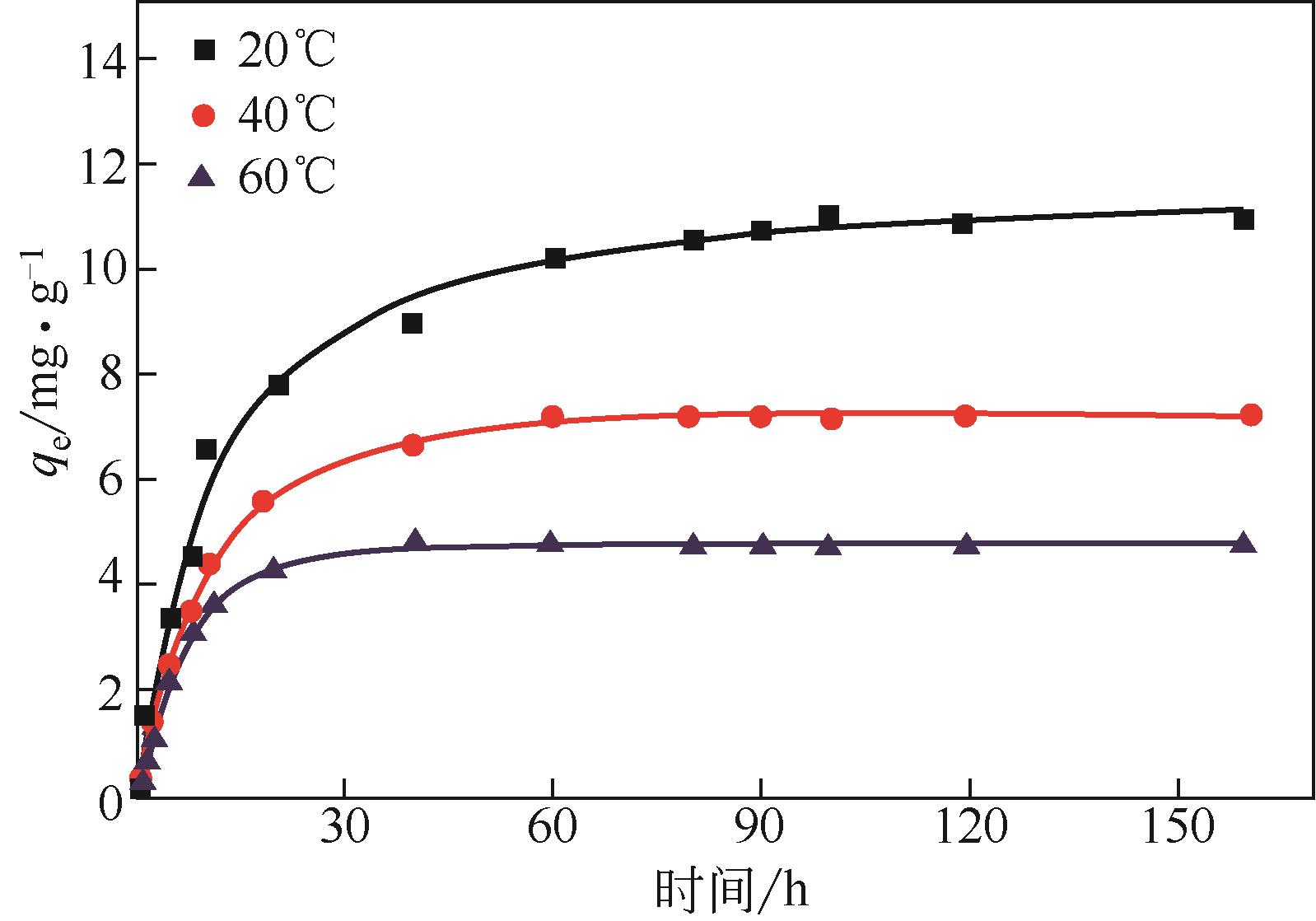
吸附法技术成熟、操作简单、现有系统完善,且一次性固定投资小,被广泛应用于室内气相污染物的净化处理。本文简单综述了吸附法在净化室内甲醛方面的研究,从原生碳吸附剂、改性碳吸附剂、无机非碳基吸附剂和有机非碳基吸附剂几个方面介绍了不同类型吸附剂的研究现状,对比了吸附性能,简要介绍了甲醛与吸附剂表面的作用机理,并详细总结了影响吸附效果的主要因素。指出整体上改性碳吸附剂性能最优,其他类型吸附剂之间差异不大,并与商品吸附剂没有明显差别;吸附剂结构和表面物化性质是影响吸附效果的首要因素,其织构特征、表面酸碱性、表面含氧/含杂原子官能团以及表面第二相等均对吸附效果产生重要影响。评述了吸附条件如甲醛浓度、吸附温度和湿度、吸附剂粒径等同样影响最终吸附效果。
中图分类号:
引用本文
肖康, 王琼. 吸附法净化室内甲醛研究进展[J]. 化工进展, 2021, 40(10): 5747-5771.
XIAO Kang, WANG Qiong. Progress in research on adsorption for abatement of indoor formaldehyde[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5747-5771.
| 原生碳吸附剂 | 织构特征 | 条件① | 效率①② | 关键性质 | 文献 |
|---|---|---|---|---|---|
| 活性炭AC | SBET 942m2/g;Vmicro 0.358cm3/g;Vmeso 0.101cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa(等价于0.012~2400mg/m3) | 亨利常数:23%(质量分数)/hPa;吸附量2mg/g(12mg/m3) | — | [ |
| 糠炭 | SBET 120.3~466.9m2/g;VT 0.11~0.35cm3/g;Vmicro 0.02~0.12cm3/g;灰分质量分数 39.8%~58.8% | 静态:1g,C0=1.2mg/m3,5L,22~24℃ | 12min内净化完全 | 自含的碱性K、Ca无机化合物 | [ |
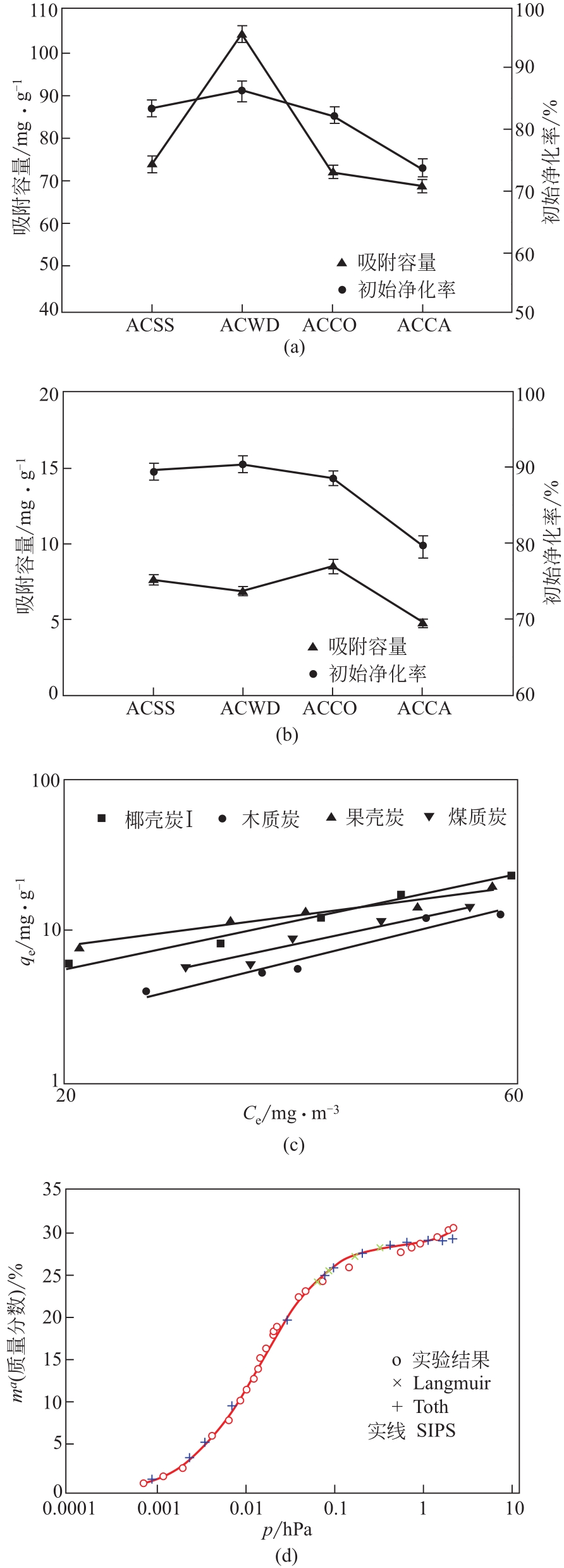
污泥活性炭 ACSS | SBET 509.88m2/g;Smicro 353.83m2/g;VT 0.301cm3/g;Vmicro 0.161cm3/g;D 4.85nm | 动态:0.4g,Cin=498mg/m3或0.4mg/m3,100mL/min,30℃ | 74.27mg/g(498mg/m3);7.62mg/g(0.41mg/m3) | 高SBET,高微孔比例,表面亲水官能团 | [ |
| 聚丙烯腈碳纤维PAN-ACNFs | SBET 375~268m2/g;Smicro 241.9~427.5m2/g③;N质量分数18%~22% | 动态:0.05g,Cin=13.2mg/m3,100mL/min | tbr=10.5h,1.5mg/g(干燥环境) | 高N含量 | [ |
| 介孔炭CMK-3 | SBET 1178m2/g;Smicro 142m2/g;VT 1.28cm3/g;Vmicro 0.05cm3/g;D 3.8nm | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>50% | 高SBET | [ |
纳米网络 结构炭 | SBET(1059±19)m2/g;Smicro(773±42)m2/g;Smeso(287±35)m2/g;VT(0.9±0.02)cm3/g | 静态:C0=(4.5±0.5)mg/m3, 室温 | 120.3mg/g(18h内) | 高SBET,相互交联的微孔-介孔-大孔结构 | [ |
表1 室内甲醛净化用原生碳吸附剂示例
| 原生碳吸附剂 | 织构特征 | 条件① | 效率①② | 关键性质 | 文献 |
|---|---|---|---|---|---|
| 活性炭AC | SBET 942m2/g;Vmicro 0.358cm3/g;Vmeso 0.101cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa(等价于0.012~2400mg/m3) | 亨利常数:23%(质量分数)/hPa;吸附量2mg/g(12mg/m3) | — | [ |
| 糠炭 | SBET 120.3~466.9m2/g;VT 0.11~0.35cm3/g;Vmicro 0.02~0.12cm3/g;灰分质量分数 39.8%~58.8% | 静态:1g,C0=1.2mg/m3,5L,22~24℃ | 12min内净化完全 | 自含的碱性K、Ca无机化合物 | [ |
污泥活性炭 ACSS | SBET 509.88m2/g;Smicro 353.83m2/g;VT 0.301cm3/g;Vmicro 0.161cm3/g;D 4.85nm | 动态:0.4g,Cin=498mg/m3或0.4mg/m3,100mL/min,30℃ | 74.27mg/g(498mg/m3);7.62mg/g(0.41mg/m3) | 高SBET,高微孔比例,表面亲水官能团 | [ |
| 聚丙烯腈碳纤维PAN-ACNFs | SBET 375~268m2/g;Smicro 241.9~427.5m2/g③;N质量分数18%~22% | 动态:0.05g,Cin=13.2mg/m3,100mL/min | tbr=10.5h,1.5mg/g(干燥环境) | 高N含量 | [ |
| 介孔炭CMK-3 | SBET 1178m2/g;Smicro 142m2/g;VT 1.28cm3/g;Vmicro 0.05cm3/g;D 3.8nm | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>50% | 高SBET | [ |
纳米网络 结构炭 | SBET(1059±19)m2/g;Smicro(773±42)m2/g;Smeso(287±35)m2/g;VT(0.9±0.02)cm3/g | 静态:C0=(4.5±0.5)mg/m3, 室温 | 120.3mg/g(18h内) | 高SBET,相互交联的微孔-介孔-大孔结构 | [ |
| 改性碳吸附剂 | 改性剂 | 改善/提升的性质 | 条件① | 效率 | 文献 |
|---|---|---|---|---|---|
| 介孔炭CMK-3-H2SO4 | H2SO4 | 表面含O官能团有所增加 | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>50% | [ |
| 介孔炭CMK-3-NH3 | NH3 | 表面含N官能团增加 | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>70% | [ |
| 改性碳织物 | 双氰胺/青霉素G/硫脲/尿素 | 微孔增加,表面含N/S官能团增加 | 动态:0.130g,Cin=1.32~1.54mg/m3,25℃ | tbr=109min,最优1.56mg/g(双氰胺改性) | [ |
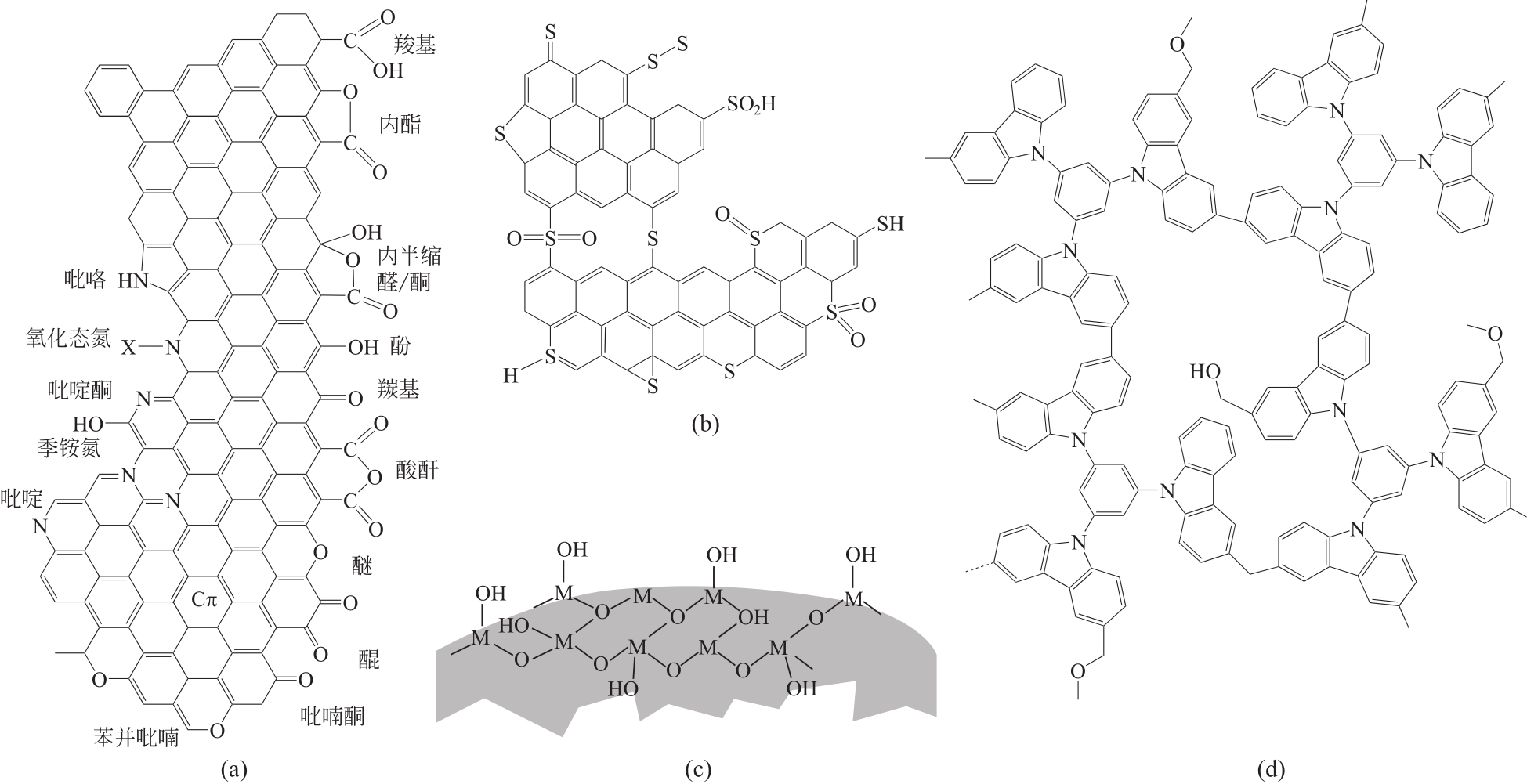
| 木质炭BAX | 三聚氰胺(BAX-M) 硫脲(BAX-T) | 增加表面含N/S官能团 | 动态:1.3cm3(0.3g),粒径1~2mm颗粒,Cin 约1.2mg/m3,400mL/min,25℃ | BAX-M:0.66mg/g(干燥气),0.57mg/g(潮湿气); BAX-T:0.40mg/g(干燥气),0.34mg/g(潮湿气) | [ |
| 竹炭 | Ag纳米颗粒(AC-Ag) Cu纳米颗粒(AC-Cu) | 表面纳米颗粒[约4%(质量分数)] | 静态:0.2g,25℃,10mL | AC-Ag:0.425mg/g;AC-Cu:0.341mg/g;高于未改性AC 0.264mg/g | [ |
Ag改性粒状活性炭 Ag-GAC | Ag纳米颗粒 | 表面Ag纳米颗 粒(1‰±0.03‰,质量分数) | 动态:1cm直径,4cm高的装填量,Cin=(10.8±1.2)mg/m3,停留时间(tr)0.25s或0.5s,室温 | tr=0.25s:tbr=630min,2.16mg HCHO去除量; tr=0.5s:tbr=1200min,3.94mg HCHO去除量; (原GAC去除量仅1.63mg) | [ |
| Ag改性活性炭Ag-AC | Ag纳米颗粒 | 表面Ag纳米颗粒(平均粒径5.44nm) | 动态:1cm直径、3cm高的装填量,多个评价条件,Cin 120~1200mg/m3,350~500mL/min,室温 | Ag-AC甲醛净化量是原AC的3.36倍 | [ |
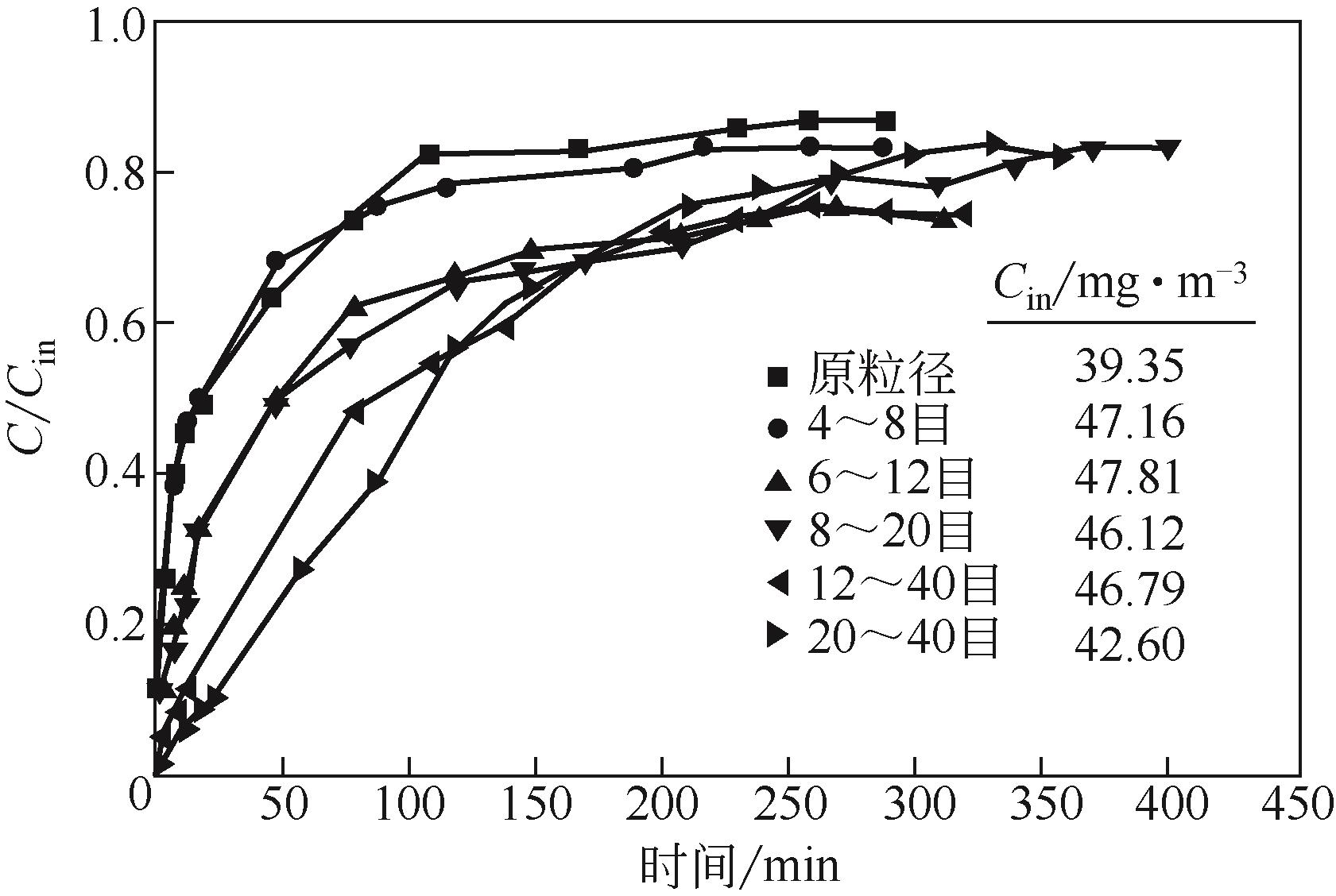
| 改性活性炭 | Na2CO3,NaHSO3 | 表面HSO3? | 静态:30g或40g吸附剂,C0=0.2~0.3mg/m3,12.66m3,环境条件 | 4h内甲醛清除率96%,优于未改性活性炭,且不会二次释放 | [ |
| KMnO4改性活性炭 | KMnO4 | C | 静态:0.5g吸附剂,5μL甲醛溶液,C0约8.25mg/m3,环境条件 | 4h内甲醛浓度降至2.854mg/m3,好于未改性的7.258mg/m3 | [ |
| KMnO4改性球形中孔炭 | KMnO4 | C | 动态:1g吸附剂 | 穿透吸附量(Cout/Cin=5%)30.55mg/g,饱和吸附量(Cout/Cin=95%)66.04mg/g,分别是改性前的5.2倍和3.4倍 | [ |
| KMnO4改性活性炭 | KMnO4 | 表面MnOx | 静态:液相吸附,1g吸附剂,50mL 600mg/L的甲醛溶液,25℃,24h | 5.51mg/g(0.079mol/LKMnO4溶液,650℃热处理),是未改性活性炭的3.3倍 | [ |
| MnOx/GAC | KMnO4 | 表面MnOx | 静态:0.5g吸附剂,C0约50mg/m3,0.5625m3,25℃ | 23.76mg/g(0.98% MnOx,380℃热处理),是未改性活性炭的2倍 | [ |
表2 室内甲醛净化用改性碳吸附剂吸附剂示例
| 改性碳吸附剂 | 改性剂 | 改善/提升的性质 | 条件① | 效率 | 文献 |
|---|---|---|---|---|---|
| 介孔炭CMK-3-H2SO4 | H2SO4 | 表面含O官能团有所增加 | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>50% | [ |
| 介孔炭CMK-3-NH3 | NH3 | 表面含N官能团增加 | 静态:0.07g,C0=1.2mg/m3,10L,30℃ | 80min净化率>70% | [ |
| 改性碳织物 | 双氰胺/青霉素G/硫脲/尿素 | 微孔增加,表面含N/S官能团增加 | 动态:0.130g,Cin=1.32~1.54mg/m3,25℃ | tbr=109min,最优1.56mg/g(双氰胺改性) | [ |
| 木质炭BAX | 三聚氰胺(BAX-M) 硫脲(BAX-T) | 增加表面含N/S官能团 | 动态:1.3cm3(0.3g),粒径1~2mm颗粒,Cin 约1.2mg/m3,400mL/min,25℃ | BAX-M:0.66mg/g(干燥气),0.57mg/g(潮湿气); BAX-T:0.40mg/g(干燥气),0.34mg/g(潮湿气) | [ |
| 竹炭 | Ag纳米颗粒(AC-Ag) Cu纳米颗粒(AC-Cu) | 表面纳米颗粒[约4%(质量分数)] | 静态:0.2g,25℃,10mL | AC-Ag:0.425mg/g;AC-Cu:0.341mg/g;高于未改性AC 0.264mg/g | [ |
Ag改性粒状活性炭 Ag-GAC | Ag纳米颗粒 | 表面Ag纳米颗 粒(1‰±0.03‰,质量分数) | 动态:1cm直径,4cm高的装填量,Cin=(10.8±1.2)mg/m3,停留时间(tr)0.25s或0.5s,室温 | tr=0.25s:tbr=630min,2.16mg HCHO去除量; tr=0.5s:tbr=1200min,3.94mg HCHO去除量; (原GAC去除量仅1.63mg) | [ |
| Ag改性活性炭Ag-AC | Ag纳米颗粒 | 表面Ag纳米颗粒(平均粒径5.44nm) | 动态:1cm直径、3cm高的装填量,多个评价条件,Cin 120~1200mg/m3,350~500mL/min,室温 | Ag-AC甲醛净化量是原AC的3.36倍 | [ |
| 改性活性炭 | Na2CO3,NaHSO3 | 表面HSO3? | 静态:30g或40g吸附剂,C0=0.2~0.3mg/m3,12.66m3,环境条件 | 4h内甲醛清除率96%,优于未改性活性炭,且不会二次释放 | [ |
| KMnO4改性活性炭 | KMnO4 | C | 静态:0.5g吸附剂,5μL甲醛溶液,C0约8.25mg/m3,环境条件 | 4h内甲醛浓度降至2.854mg/m3,好于未改性的7.258mg/m3 | [ |
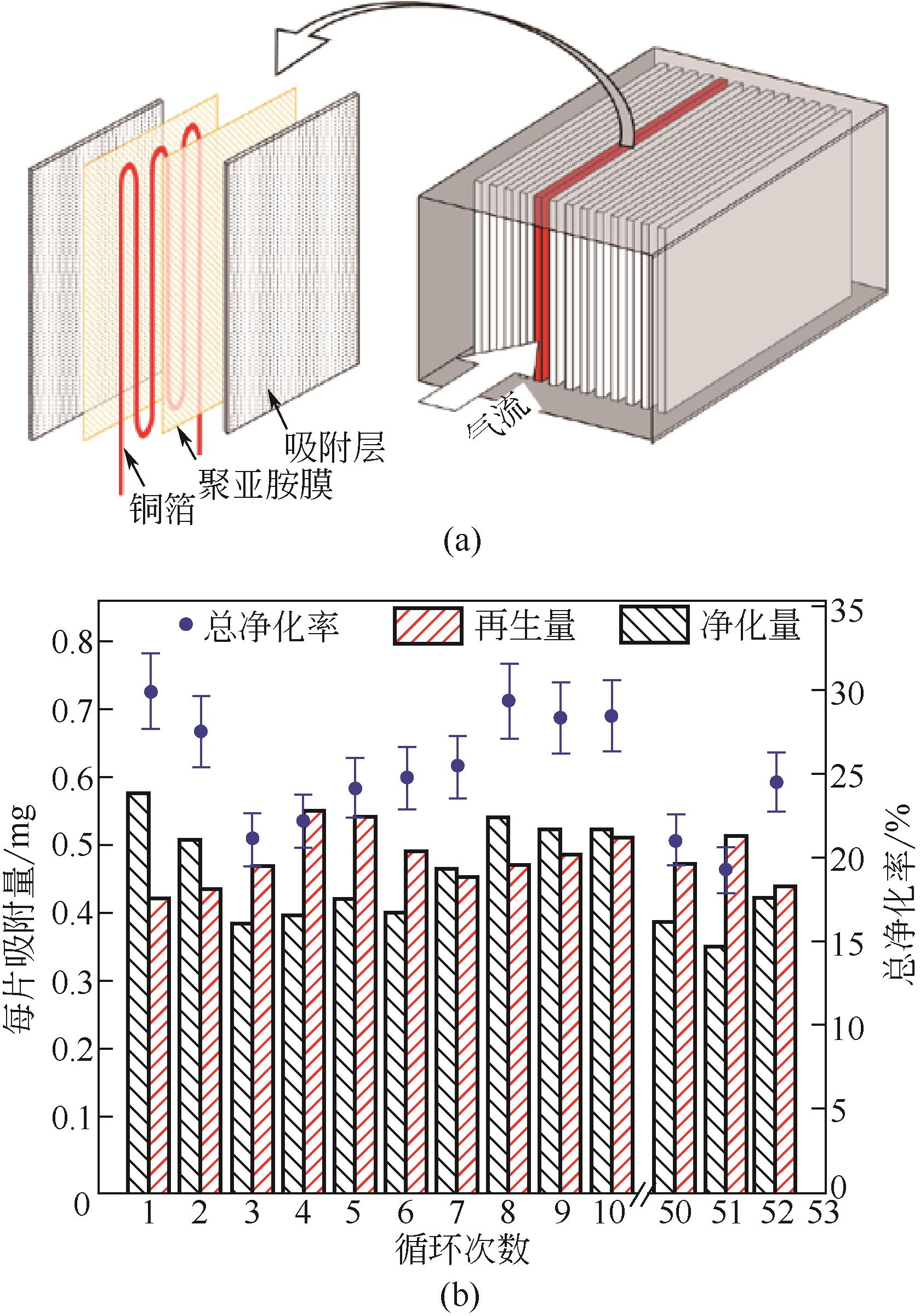
| KMnO4改性球形中孔炭 | KMnO4 | C | 动态:1g吸附剂 | 穿透吸附量(Cout/Cin=5%)30.55mg/g,饱和吸附量(Cout/Cin=95%)66.04mg/g,分别是改性前的5.2倍和3.4倍 | [ |
| KMnO4改性活性炭 | KMnO4 | 表面MnOx | 静态:液相吸附,1g吸附剂,50mL 600mg/L的甲醛溶液,25℃,24h | 5.51mg/g(0.079mol/LKMnO4溶液,650℃热处理),是未改性活性炭的3.3倍 | [ |
| MnOx/GAC | KMnO4 | 表面MnOx | 静态:0.5g吸附剂,C0约50mg/m3,0.5625m3,25℃ | 23.76mg/g(0.98% MnOx,380℃热处理),是未改性活性炭的2倍 | [ |
| 非碳基吸附剂 | 织构特征 | 条件① | 效率①② | 关键性质 | 文献 |
|---|---|---|---|---|---|
| 复合氧化物MMOs(ZrO2/SiO2,TiO2/SiO2) | SBET 4~1115m2/g;VT 0.01~0.64cm3/g;D 2.3~3.1nm | 动态:0.3g,Cin 约204mg/m3,40mL/min,25℃ | 30~87mg/g | 表面羟基,表面酸性 | [ |
| 阳离子型八面沸石分子筛(NaY,KY,NaX,CuX) | SBET 653~749m2/g;Vmicro 0.304~0.348cm3/g;Daperture 0.74nm | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:(235~1720)%/hPa;12mg/m3时吸附量24~120mg/g | 平衡阳离子 | [ |
| Linda A分子筛 (3A) | SBET 497m2/g;Vmicro 0.23cm3/g;Daperture 0.3nm | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:183%/hPa;12mg/m3时吸附量7m2/g | — | [ |
| 介孔氧化硅SBA-15 | SBET 595m2/g;Vmicro 0.085cm3/g;Vmeso 0.422cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:34%/hPa;12mg/m3时吸附量3.3m2/g | — | [ |
| 有机金属框架MOF Ga-MIL-53 | SBET 560m2/g;Vmicro 0.470cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:1.6%/hPa;12mg/m3时吸附量0.2m2/g | — | [ |
| 氨基化介孔氧化硅SiO2-NH2 | SBET 8~237m2/g;Smeso 6~198m2/g;Smicro 0~109m2/g;VT 0.038~0.859cm3/g;Vmeso 0.038~0.859cm3/g;Vmicro 0~0.089cm3/g;D 6~19nm | 静态:5%~37 %(质量分数)甲醛溶液挥发产生HCHO气体 | 488~1208m2/g (AEAP修饰最佳) | 表面氨基,高SBET,大孔径 | [ |
| 多孔氧化铝Al2O3 | SBET 264~347m2/g;VT 0.25~0.86cm3/g;D 3.44~9.73nm | 动态:0.1g,Cin=1200mg/m3,50mL/min,室温 | 9.85~20.50m2/g | 高SBET和孔容 | [ |
| SiO2空心微米管 | SBET 896m2/g;VT 0.69cm3/g; D 3.1nm | 静态:0.1g,C0 约360mg/m3,室温 | 20.65m2/g(P0.3-50) | TEPA引入的表面N/氨基 | [ |
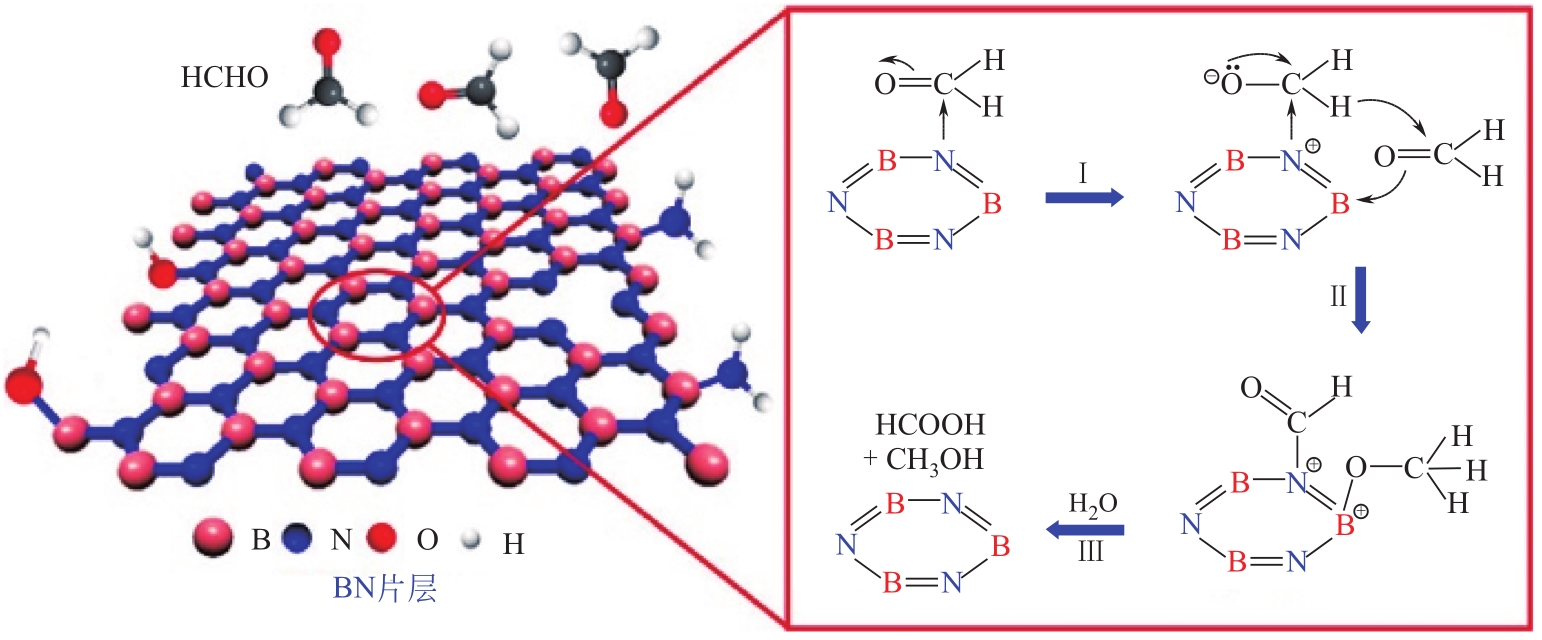
| 海绵状石墨烯型氮化硼p-BN | SBET 627m2/g;VT 0.42cm3/g; D 2.7nm | 静态:C0=24mg/m3,25℃ | 19m2/g | 高SBET,表面羟基和氨基,表面Lewis酸碱对(催化甲醛歧化) | [ |
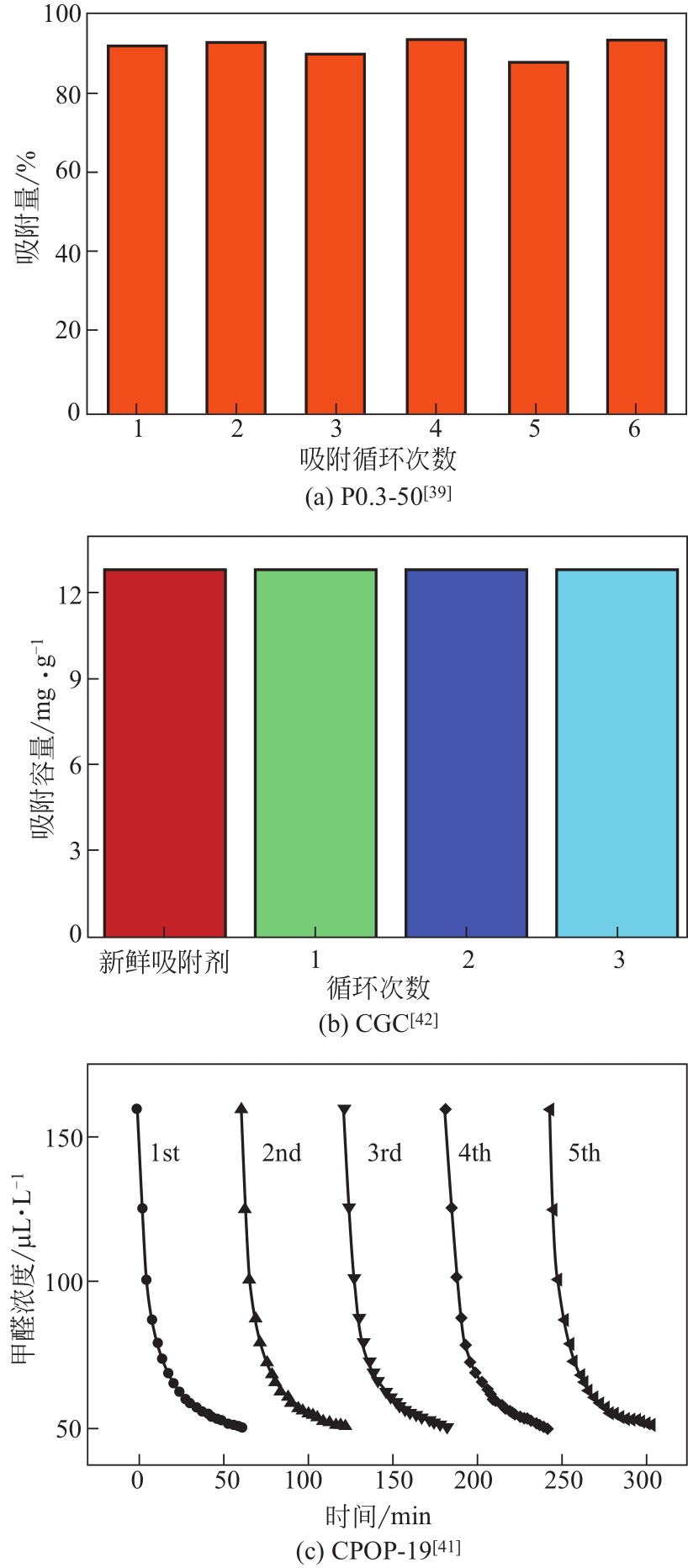
| 咔唑基多孔有机聚合物CPOPs | SBET 1130m2/g;Smicro 490m2/g;VT 0.80cm3/g;D 0.59nm | 静态平衡吸附:25℃,常压 | 7.8~11.2m2/g (CPOP-19最好,至少可循环使用5次) | 高SBET,高N含量(可能) | [ |
| β-环糊精修饰的壳聚糖CGC | SBET 10.8m2/g;VT 0.02cm3/g; D 7.81nm | 动态:0.5g,Cin=46.1mg/m3, 28mL/min,20℃ | 15.5m2/g | 表面氨基和羟基 | [ |
表3 室内甲醛净化用非碳基吸附剂示例
| 非碳基吸附剂 | 织构特征 | 条件① | 效率①② | 关键性质 | 文献 |
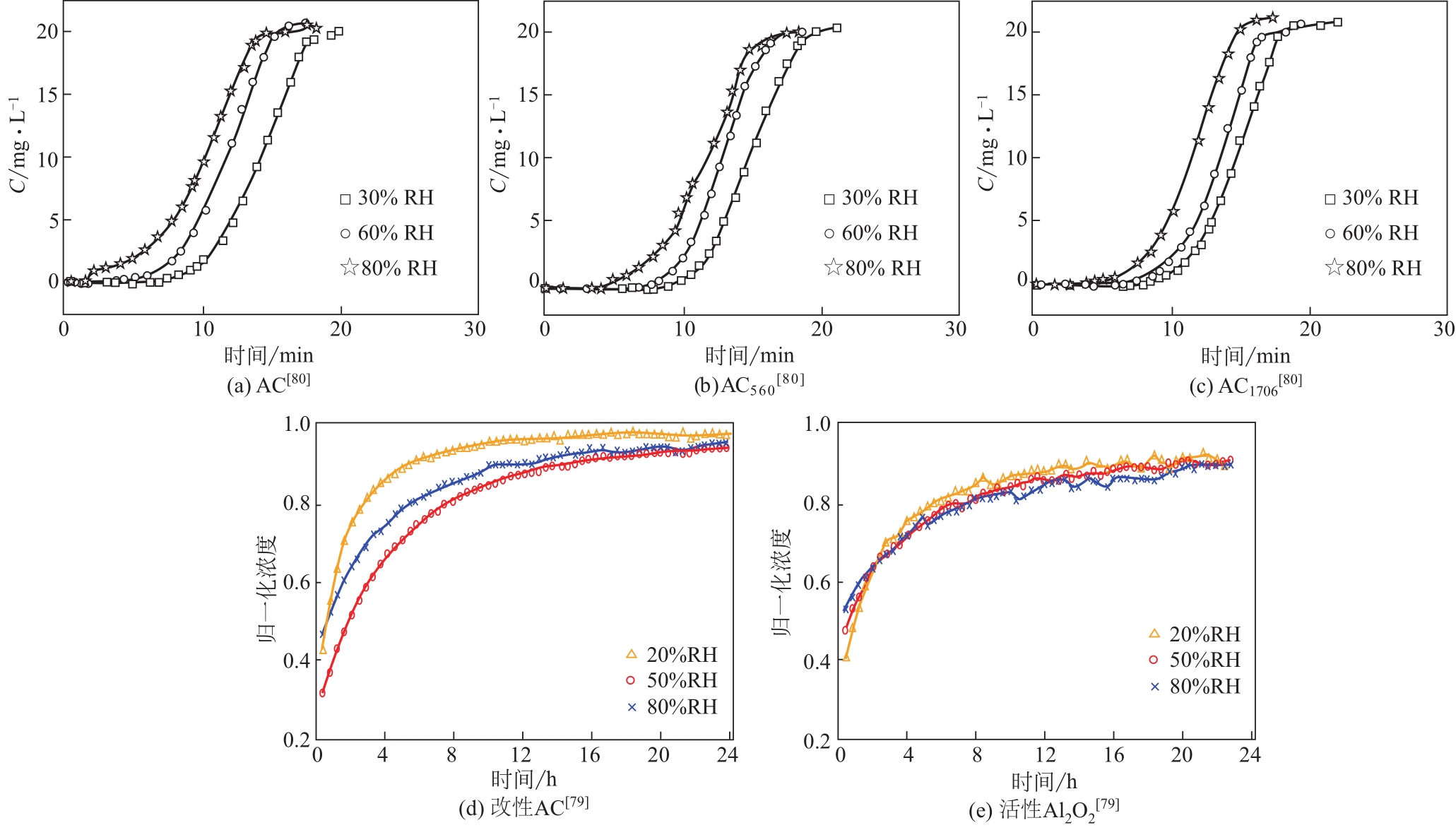
|---|---|---|---|---|---|
| 复合氧化物MMOs(ZrO2/SiO2,TiO2/SiO2) | SBET 4~1115m2/g;VT 0.01~0.64cm3/g;D 2.3~3.1nm | 动态:0.3g,Cin 约204mg/m3,40mL/min,25℃ | 30~87mg/g | 表面羟基,表面酸性 | [ |
| 阳离子型八面沸石分子筛(NaY,KY,NaX,CuX) | SBET 653~749m2/g;Vmicro 0.304~0.348cm3/g;Daperture 0.74nm | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:(235~1720)%/hPa;12mg/m3时吸附量24~120mg/g | 平衡阳离子 | [ |
| Linda A分子筛 (3A) | SBET 497m2/g;Vmicro 0.23cm3/g;Daperture 0.3nm | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:183%/hPa;12mg/m3时吸附量7m2/g | — | [ |
| 介孔氧化硅SBA-15 | SBET 595m2/g;Vmicro 0.085cm3/g;Vmeso 0.422cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:34%/hPa;12mg/m3时吸附量3.3m2/g | — | [ |
| 有机金属框架MOF Ga-MIL-53 | SBET 560m2/g;Vmicro 0.470cm3/g | TGA上的平衡吸脱附:25℃,10?5~2hPa (0.012~2400mg/m3) | 亨利常数:1.6%/hPa;12mg/m3时吸附量0.2m2/g | — | [ |
| 氨基化介孔氧化硅SiO2-NH2 | SBET 8~237m2/g;Smeso 6~198m2/g;Smicro 0~109m2/g;VT 0.038~0.859cm3/g;Vmeso 0.038~0.859cm3/g;Vmicro 0~0.089cm3/g;D 6~19nm | 静态:5%~37 %(质量分数)甲醛溶液挥发产生HCHO气体 | 488~1208m2/g (AEAP修饰最佳) | 表面氨基,高SBET,大孔径 | [ |
| 多孔氧化铝Al2O3 | SBET 264~347m2/g;VT 0.25~0.86cm3/g;D 3.44~9.73nm | 动态:0.1g,Cin=1200mg/m3,50mL/min,室温 | 9.85~20.50m2/g | 高SBET和孔容 | [ |
| SiO2空心微米管 | SBET 896m2/g;VT 0.69cm3/g; D 3.1nm | 静态:0.1g,C0 约360mg/m3,室温 | 20.65m2/g(P0.3-50) | TEPA引入的表面N/氨基 | [ |
| 海绵状石墨烯型氮化硼p-BN | SBET 627m2/g;VT 0.42cm3/g; D 2.7nm | 静态:C0=24mg/m3,25℃ | 19m2/g | 高SBET,表面羟基和氨基,表面Lewis酸碱对(催化甲醛歧化) | [ |
| 咔唑基多孔有机聚合物CPOPs | SBET 1130m2/g;Smicro 490m2/g;VT 0.80cm3/g;D 0.59nm | 静态平衡吸附:25℃,常压 | 7.8~11.2m2/g (CPOP-19最好,至少可循环使用5次) | 高SBET,高N含量(可能) | [ |
| β-环糊精修饰的壳聚糖CGC | SBET 10.8m2/g;VT 0.02cm3/g; D 7.81nm | 动态:0.5g,Cin=46.1mg/m3, 28mL/min,20℃ | 15.5m2/g | 表面氨基和羟基 | [ |
| 类型 | 吸附剂 | 甲醛浓度/×10-6 | 甲醛分压/Pa | 吸附量/mg·g?1 | 分配系数/mg·g?1?Pa?1 | 文献 |
|---|---|---|---|---|---|---|
| 商品活性炭 | 椰壳炭Ⅰ-20~40目 | 31.38 | 3.14 | 11.68 | 3.72 | [ |
| 椰壳炭Ⅰ-30~60目 | 33.78 | 3.38 | 11.53 | 3.41 | ||
| 果壳炭-20~40目 | 28.72 | 2.87 | 9.76 | 3.40 | ||
| 果壳炭-30~60目 | 34.28 | 3.43 | 9.63 | 2.81 | ||
| 煤质炭-20~40目 | 33.30 | 3.33 | 8.41 | 2.53 | ||
| 煤质炭-30~60目 | 34.94 | 3.49 | 8.08 | 2.32 | ||
| 木质炭-20~40目 | 30.12 | 3.01 | 7.00 | 2.33 | ||
| 木质炭-30~60目 | 33.47 | 3.35 | 6.98 | 2.08 | ||
| 椰壳炭Ⅱ-原粒径 | 32.99 | 3.30 | 5.01 | 1.52 | ||
| 椰壳炭Ⅱ-4~8目 | 39.30 | 3.93 | 5.78 | 1.47 | ||
| 椰壳炭Ⅱ-6~12目 | 39.84 | 3.98 | 9.03 | 2.27 | ||
| 椰壳炭Ⅱ-8~20目 | 38.43 | 3.84 | 9.45 | 2.46 | ||
| 椰壳炭Ⅱ-12~40目 | 38.99 | 3.90 | 11.08 | 2.84 | ||
| 椰壳炭Ⅱ-20~40目 | 35.50 | 3.55 | 10.87 | 3.06 | ||
| 商品活性炭 | AC-KHL | 3.75 | 0.38 | 6.60 | 17.37 | [ |
| AC-YP50 | 3.75 | 0.38 | 56.10 | 147.63 | ||
| 商品椰壳炭 | ACCO | 415 | 41.50 | 约71.00 | 1.71 | [ |
| 0.34 | 0.034 | 约8.50 | 250.00 | |||
| 商品木质炭 | ACWD | 415 | 41.50 | 约105.00 | 2.53 | [ |
| 0.34 | 0.034 | 约7.00 | 205.88 | |||
| 商品煤质炭 | ACCA | 415 | 41.50 | 约69.00 | 1.66 | [ |
| 0.34 | 0.034 | 约5.00 | 147.06 | |||
| 商品活性炭 | AC | — | 200.00 | 20.00 | 0.10 | [ |
| 含硫聚合物基炭 | PSC | 1.22 | 0.12 | 0.31 | 2.58 | [ |
| 商品椰壳炭 | S208 | 0.94 | 0.094 | 0.31 | 3.30 | [ |
| 商品木质炭 | BAX1500 | 1.18 | 0.12 | 0.25 | 2.08 | [ |
| 市售果壳炭 | AC | 41.67 | 4.17 | 12.56 | 3.01 | [ |
| 人造丝基活性碳纤维 | ACF0 | 80 | 8.00 | 237.77 | 29.72 | [ |
| H-723-0.5h | 80 | 8.00 | 763.51 | 95.44 | ||
| H-823-0.5h | 80 | 8.00 | 629.93 | 78.74 | ||
| H-1123-0.5h | 80 | 8.00 | 600.89 | 75.11 | ||
| H-1223-0.5h | 80 | 8.00 | 875.56 | 109.45 | ||
| PAN基活性碳纤维 | FE100 | 20 | 2.00 | 14.34 | 7.17 | [ |
| FE200 | 20 | 2.00 | 8.22 | 4.11 | ||
| FE300 | 20 | 2.00 | 3.81 | 1.91 | ||
| FE400 | 20 | 2.00 | 3.27 | 1.64 | ||
| 沥青基活性碳纤维 | OG5A | 20 | 2.00 | 0.60 | 0.30 | [ |
| OG7A | 20 | 2.00 | 0.54 | 0.27 | ||
| OG15A | 20 | 2.00 | 0.30 | 0.15 | ||
| 人造丝基活性碳纤维 | KF1500 | 20 | 2.00 | 1.05 | 0.53 | [ |
| 污泥活性炭 | ACSS | 415 | 41.50 | 74.27 | 1.79 | [ |
| 0.34 | 0.034 | 7.62 | 224.12 | |||
| 骨焦 | BC | 20 | 2.00 | 154.43 | 77.22 | [ |
| 50 | 5.00 | 212.31 | 42.46 | |||
| 100 | 10.00 | 251.42 | 25.14 | |||
| 200 | 20.00 | 275.78 | 13.79 | |||
| 纳米多孔炭 | NNSCs | 3.75 | 0.38 | 120.30 | 316.58 | [ |
| 改性木质炭 | BAX-M | 0.94 | 0.094 | 0.40 | 4.26 | [ |
| BAX-T | 0.94 | 0.094 | 0.66 | 7.02 | ||
| 改性果壳炭 | 0.49%MnOx/AC | 41.67 | 4.17 | 16.06 | 3.85 | [ |
| 0.98%MnOx/AC | 41.67 | 4.17 | 23.76 (25℃) | 5.70 | ||
| 41.67 | 4.17 | 24.01 (20℃) | 5.76 | |||
| 41.67 | 4.17 | 25.33 (15℃) | 6.07 | |||
| 41.67 | 4.17 | 28.65 (10℃) | 6.87 | |||
| 4.59%MnOx/AC | 41.67 | 4.17 | 21.41 | 5.13 | ||
| 10.28%MnOx/AC | 41.67 | 4.17 | 14.35 | 3.44 | ||
| 改性碳织物 | CC | 1.25 | 0.13 | 0.56 | 4.31 | [ |
| CC-HT | 1.12 | 0.11 | 0.53 | 4.82 | ||
| CC-U | 1.25 | 0.13 | 0.73 | 5.62 | ||
| CC-T | 1.28 | 0.13 | 0.68 | 5.23 | ||
| CC-P | 1.10 | 0.11 | 0.80 | 7.27 | ||
| CC-D | 1.15 | 0.12 | 1.56 | 13.00 | ||
| 改性竹炭 | AC-Ag | 2.4 | 0.24 | 0.083 | 0.35 | [ |
| 3.5 | 0.35 | 0.17 | 0.49 | |||
| 5.0 | 0.50 | 0.26 | 0.52 | |||
| 6.0 | 0.60 | 0.34 | 0.57 | |||
| 8.0 | 0.80 | 0.43 | 0.54 | |||
| 11.9 | 1.19 | 0.51 | 0.43 | |||
| AC-Cu | 2.6 | 0.26 | 0.084 | 0.32 | ||
| 4.2 | 0.42 | 0.17 | 0.40 | |||
| 5.6 | 0.56 | 0.25 | 0.45 | |||
| 7.4 | 0.74 | 0.34 | 0.46 | |||
| 8.0 | 0.80 | 0.34 | 0.43 | |||
| 12.9 | 1.29 | 0.51 | 0.40 | |||
| AC | 3.0 | 0.30 | 0.086 | 0.29 | ||
| 5.0 | 0.50 | 0.17 | 0.34 | |||
| 7.4 | 0.74 | 0.26 | 0.35 | |||
| 8.0 | 0.80 | 0.26 | 0.33 | |||
| 12.9 | 1.29 | 0.43 | 0.33 | |||
| 17.9 | 1.79 | 0.51 | 0.28 | |||
| 改性骨焦 | 改性BC | 20 | 2.00 | 164.12 | 82.06 | [ |
| 50 | 5.00 | 225.55 | 45.11 | |||
| 100 | 10.00 | 266.67 | 26.67 | |||
| 200 | 20.00 | 291.91 | 14.60 | |||
| 多孔氧化铝 | Al2O3-blank | 1000 | 100.00 | 12.91 | 0.13 | [ |
| Al2O3-P123 | 1000 | 100.00 | 16.71 | 0.17 | ||
| Al2O3-SDS | 1000 | 100.00 | 11.21 | 0.11 | ||
| Al2O3-CTAB | 1000 | 100.00 | 8.03 | 0.08 | ||
| 复合氧化物 | SiO2 | 170 | 17.00 | 24.00 | 1.41 | [ |
| Ti/Si (15) | 170 | 17.00 | 36.00 | 2.12 | ||
| Ti/Si (25) | 170 | 17.00 | 51.00 | 3.00 | ||
| Ti/Si (50) | 170 | 17.00 | 45.00 | 2.65 | ||
| Ti/Si (75) | 170 | 17.00 | 30.00 | 1.76 | ||
| Zr/Si (15) | 170 | 17.00 | 63.00 | 3.71 | ||
| Zr/Si (25) | 170 | 17.00 | 87.00 | 5.12 | ||
| Zr/Si (50) | 170 | 17.00 | 81.00 | 4.76 | ||
| Zr/Si (75) | 170 | 17.00 | 33.00 | 1.94 | ||
| 改性SiO2中空微米管 | P0.3-50 | 300 | 30.00 | 20.65 | 0.69 | [ |
| 介孔氧化硅 | SBA-15 | — | 200.00 | 3.30 | 0.02 | [ |
| 分子筛 | NaY (市售) | — | 200.00 | 327.80 | 1.64 | [ |
| KY | — | 200.00 | 236.60 | 1.18 | ||
| NaX (市售) | — | 200.00 | 303.20 | 1.52 | ||
| CuX | — | 200.00 | 283.10 | 1.42 | ||
| 3A | — | 200.00 | 161.40 | 0.81 | ||
| MOFs | MIL-101(Cr) | 170 | 17.00 | 99.00 | 5.82 | [ |
| Ga MIL-53 | — | 200.00 | 0.20 | 0.00 | [ | |
| 有机吸附剂 | CGC | 38.4 | 3.84 | 15.50 | 4.04 | [ |
| 多孔有机聚合物 | CPOP-16 | 50 | 5.00 | 7.80 | 1.56 | [ |
| CPOP-17 | 50 | 5.00 | 8.10 | 1.62 | ||
| CPOP-18 | 50 | 5.00 | 9.30 | 1.86 | ||
| CPOP-19 | 50 | 5.00 | 11.20 | 2.24 | ||
| 海绵状石墨烯型氮化硼 | p-BN | 20 | 2.00 | 19.00 | 9.50 | [ |
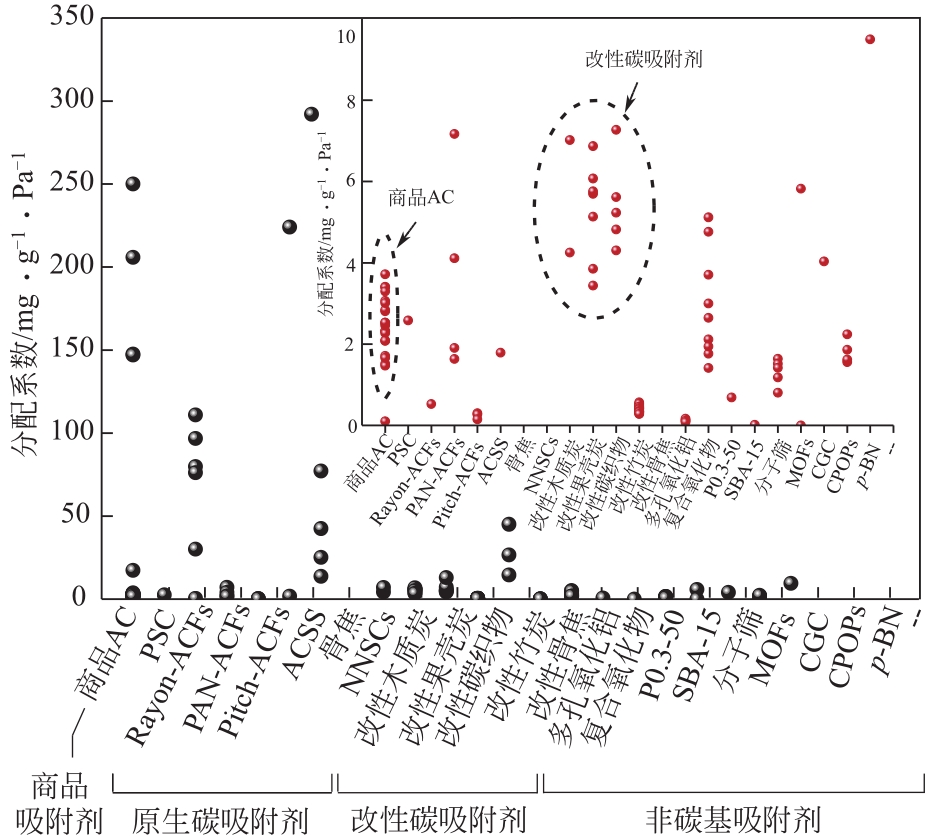
表4 不同吸附剂的甲醛吸附量和分配系数
| 类型 | 吸附剂 | 甲醛浓度/×10-6 | 甲醛分压/Pa | 吸附量/mg·g?1 | 分配系数/mg·g?1?Pa?1 | 文献 |
|---|---|---|---|---|---|---|
| 商品活性炭 | 椰壳炭Ⅰ-20~40目 | 31.38 | 3.14 | 11.68 | 3.72 | [ |
| 椰壳炭Ⅰ-30~60目 | 33.78 | 3.38 | 11.53 | 3.41 | ||
| 果壳炭-20~40目 | 28.72 | 2.87 | 9.76 | 3.40 | ||
| 果壳炭-30~60目 | 34.28 | 3.43 | 9.63 | 2.81 | ||
| 煤质炭-20~40目 | 33.30 | 3.33 | 8.41 | 2.53 | ||
| 煤质炭-30~60目 | 34.94 | 3.49 | 8.08 | 2.32 | ||
| 木质炭-20~40目 | 30.12 | 3.01 | 7.00 | 2.33 | ||
| 木质炭-30~60目 | 33.47 | 3.35 | 6.98 | 2.08 | ||
| 椰壳炭Ⅱ-原粒径 | 32.99 | 3.30 | 5.01 | 1.52 | ||
| 椰壳炭Ⅱ-4~8目 | 39.30 | 3.93 | 5.78 | 1.47 | ||
| 椰壳炭Ⅱ-6~12目 | 39.84 | 3.98 | 9.03 | 2.27 | ||
| 椰壳炭Ⅱ-8~20目 | 38.43 | 3.84 | 9.45 | 2.46 | ||
| 椰壳炭Ⅱ-12~40目 | 38.99 | 3.90 | 11.08 | 2.84 | ||
| 椰壳炭Ⅱ-20~40目 | 35.50 | 3.55 | 10.87 | 3.06 | ||
| 商品活性炭 | AC-KHL | 3.75 | 0.38 | 6.60 | 17.37 | [ |
| AC-YP50 | 3.75 | 0.38 | 56.10 | 147.63 | ||
| 商品椰壳炭 | ACCO | 415 | 41.50 | 约71.00 | 1.71 | [ |
| 0.34 | 0.034 | 约8.50 | 250.00 | |||
| 商品木质炭 | ACWD | 415 | 41.50 | 约105.00 | 2.53 | [ |
| 0.34 | 0.034 | 约7.00 | 205.88 | |||
| 商品煤质炭 | ACCA | 415 | 41.50 | 约69.00 | 1.66 | [ |
| 0.34 | 0.034 | 约5.00 | 147.06 | |||
| 商品活性炭 | AC | — | 200.00 | 20.00 | 0.10 | [ |
| 含硫聚合物基炭 | PSC | 1.22 | 0.12 | 0.31 | 2.58 | [ |
| 商品椰壳炭 | S208 | 0.94 | 0.094 | 0.31 | 3.30 | [ |
| 商品木质炭 | BAX1500 | 1.18 | 0.12 | 0.25 | 2.08 | [ |
| 市售果壳炭 | AC | 41.67 | 4.17 | 12.56 | 3.01 | [ |
| 人造丝基活性碳纤维 | ACF0 | 80 | 8.00 | 237.77 | 29.72 | [ |
| H-723-0.5h | 80 | 8.00 | 763.51 | 95.44 | ||
| H-823-0.5h | 80 | 8.00 | 629.93 | 78.74 | ||
| H-1123-0.5h | 80 | 8.00 | 600.89 | 75.11 | ||
| H-1223-0.5h | 80 | 8.00 | 875.56 | 109.45 | ||
| PAN基活性碳纤维 | FE100 | 20 | 2.00 | 14.34 | 7.17 | [ |
| FE200 | 20 | 2.00 | 8.22 | 4.11 | ||
| FE300 | 20 | 2.00 | 3.81 | 1.91 | ||
| FE400 | 20 | 2.00 | 3.27 | 1.64 | ||
| 沥青基活性碳纤维 | OG5A | 20 | 2.00 | 0.60 | 0.30 | [ |
| OG7A | 20 | 2.00 | 0.54 | 0.27 | ||
| OG15A | 20 | 2.00 | 0.30 | 0.15 | ||
| 人造丝基活性碳纤维 | KF1500 | 20 | 2.00 | 1.05 | 0.53 | [ |
| 污泥活性炭 | ACSS | 415 | 41.50 | 74.27 | 1.79 | [ |
| 0.34 | 0.034 | 7.62 | 224.12 | |||
| 骨焦 | BC | 20 | 2.00 | 154.43 | 77.22 | [ |
| 50 | 5.00 | 212.31 | 42.46 | |||
| 100 | 10.00 | 251.42 | 25.14 | |||
| 200 | 20.00 | 275.78 | 13.79 | |||
| 纳米多孔炭 | NNSCs | 3.75 | 0.38 | 120.30 | 316.58 | [ |
| 改性木质炭 | BAX-M | 0.94 | 0.094 | 0.40 | 4.26 | [ |
| BAX-T | 0.94 | 0.094 | 0.66 | 7.02 | ||
| 改性果壳炭 | 0.49%MnOx/AC | 41.67 | 4.17 | 16.06 | 3.85 | [ |
| 0.98%MnOx/AC | 41.67 | 4.17 | 23.76 (25℃) | 5.70 | ||
| 41.67 | 4.17 | 24.01 (20℃) | 5.76 | |||
| 41.67 | 4.17 | 25.33 (15℃) | 6.07 | |||
| 41.67 | 4.17 | 28.65 (10℃) | 6.87 | |||
| 4.59%MnOx/AC | 41.67 | 4.17 | 21.41 | 5.13 | ||
| 10.28%MnOx/AC | 41.67 | 4.17 | 14.35 | 3.44 | ||
| 改性碳织物 | CC | 1.25 | 0.13 | 0.56 | 4.31 | [ |
| CC-HT | 1.12 | 0.11 | 0.53 | 4.82 | ||
| CC-U | 1.25 | 0.13 | 0.73 | 5.62 | ||
| CC-T | 1.28 | 0.13 | 0.68 | 5.23 | ||
| CC-P | 1.10 | 0.11 | 0.80 | 7.27 | ||
| CC-D | 1.15 | 0.12 | 1.56 | 13.00 | ||
| 改性竹炭 | AC-Ag | 2.4 | 0.24 | 0.083 | 0.35 | [ |
| 3.5 | 0.35 | 0.17 | 0.49 | |||
| 5.0 | 0.50 | 0.26 | 0.52 | |||
| 6.0 | 0.60 | 0.34 | 0.57 | |||
| 8.0 | 0.80 | 0.43 | 0.54 | |||
| 11.9 | 1.19 | 0.51 | 0.43 | |||
| AC-Cu | 2.6 | 0.26 | 0.084 | 0.32 | ||
| 4.2 | 0.42 | 0.17 | 0.40 | |||
| 5.6 | 0.56 | 0.25 | 0.45 | |||
| 7.4 | 0.74 | 0.34 | 0.46 | |||
| 8.0 | 0.80 | 0.34 | 0.43 | |||
| 12.9 | 1.29 | 0.51 | 0.40 | |||
| AC | 3.0 | 0.30 | 0.086 | 0.29 | ||
| 5.0 | 0.50 | 0.17 | 0.34 | |||
| 7.4 | 0.74 | 0.26 | 0.35 | |||
| 8.0 | 0.80 | 0.26 | 0.33 | |||
| 12.9 | 1.29 | 0.43 | 0.33 | |||
| 17.9 | 1.79 | 0.51 | 0.28 | |||
| 改性骨焦 | 改性BC | 20 | 2.00 | 164.12 | 82.06 | [ |
| 50 | 5.00 | 225.55 | 45.11 | |||
| 100 | 10.00 | 266.67 | 26.67 | |||
| 200 | 20.00 | 291.91 | 14.60 | |||
| 多孔氧化铝 | Al2O3-blank | 1000 | 100.00 | 12.91 | 0.13 | [ |
| Al2O3-P123 | 1000 | 100.00 | 16.71 | 0.17 | ||
| Al2O3-SDS | 1000 | 100.00 | 11.21 | 0.11 | ||
| Al2O3-CTAB | 1000 | 100.00 | 8.03 | 0.08 | ||
| 复合氧化物 | SiO2 | 170 | 17.00 | 24.00 | 1.41 | [ |
| Ti/Si (15) | 170 | 17.00 | 36.00 | 2.12 | ||
| Ti/Si (25) | 170 | 17.00 | 51.00 | 3.00 | ||
| Ti/Si (50) | 170 | 17.00 | 45.00 | 2.65 | ||
| Ti/Si (75) | 170 | 17.00 | 30.00 | 1.76 | ||
| Zr/Si (15) | 170 | 17.00 | 63.00 | 3.71 | ||
| Zr/Si (25) | 170 | 17.00 | 87.00 | 5.12 | ||
| Zr/Si (50) | 170 | 17.00 | 81.00 | 4.76 | ||
| Zr/Si (75) | 170 | 17.00 | 33.00 | 1.94 | ||
| 改性SiO2中空微米管 | P0.3-50 | 300 | 30.00 | 20.65 | 0.69 | [ |
| 介孔氧化硅 | SBA-15 | — | 200.00 | 3.30 | 0.02 | [ |
| 分子筛 | NaY (市售) | — | 200.00 | 327.80 | 1.64 | [ |
| KY | — | 200.00 | 236.60 | 1.18 | ||
| NaX (市售) | — | 200.00 | 303.20 | 1.52 | ||
| CuX | — | 200.00 | 283.10 | 1.42 | ||
| 3A | — | 200.00 | 161.40 | 0.81 | ||
| MOFs | MIL-101(Cr) | 170 | 17.00 | 99.00 | 5.82 | [ |
| Ga MIL-53 | — | 200.00 | 0.20 | 0.00 | [ | |
| 有机吸附剂 | CGC | 38.4 | 3.84 | 15.50 | 4.04 | [ |
| 多孔有机聚合物 | CPOP-16 | 50 | 5.00 | 7.80 | 1.56 | [ |
| CPOP-17 | 50 | 5.00 | 8.10 | 1.62 | ||
| CPOP-18 | 50 | 5.00 | 9.30 | 1.86 | ||
| CPOP-19 | 50 | 5.00 | 11.20 | 2.24 | ||
| 海绵状石墨烯型氮化硼 | p-BN | 20 | 2.00 | 19.00 | 9.50 | [ |
| 分子筛 | 甲醛吸附量②/mg·g?1 | 对应液态甲醛体积③/cm3·g?1 | 对应多聚甲醛体积④/cm3·g?1 | 分子筛比体积/cm3·g?1 | 分子筛孔容/cm3·g?1 |
|---|---|---|---|---|---|
| NaY | 327.8 | 0.402 | 0.230 | 0.341 | 0.325 |
| KY | 236.6 | 0.290 | 0.167 | 0.324 | 0.304 |
| NaX | 303.2 | 0.372 | 0.213 | 0.335 | 0.348 |
| CuX | 283.1 | 0.347 | 0.199 | 0.379 | 0.317 |
| 3A | 161.4 | 0.198 | 0.114 | 0.185 | 0.230 |
表5 分子筛甲醛吸附量、对应液态甲醛和多聚甲醛体积①
| 分子筛 | 甲醛吸附量②/mg·g?1 | 对应液态甲醛体积③/cm3·g?1 | 对应多聚甲醛体积④/cm3·g?1 | 分子筛比体积/cm3·g?1 | 分子筛孔容/cm3·g?1 |
|---|---|---|---|---|---|
| NaY | 327.8 | 0.402 | 0.230 | 0.341 | 0.325 |
| KY | 236.6 | 0.290 | 0.167 | 0.324 | 0.304 |
| NaX | 303.2 | 0.372 | 0.213 | 0.335 | 0.348 |
| CuX | 283.1 | 0.347 | 0.199 | 0.379 | 0.317 |
| 3A | 161.4 | 0.198 | 0.114 | 0.185 | 0.230 |
| 样品 | SBET/m2·g?1 | Smicro/m2·g?1 | SBET?Smicro/m2·g?1 | 甲醛蒸气吸附量③/mg·g?1 | 水蒸气吸附量③/mg·g?1 |
|---|---|---|---|---|---|
| ACF0 | 1267 | 1057 | 210 | 237.77 | 156.63 |
| H-723-0.5h② | 1714 | 1000 | 714 | 763.51 | 364.17 |
| H-823-0.5h② | 1434 | 1017 | 417 | 629.93 | 316.03 |
| H-1123-0.5h② | 1346 | 850 | 496 | 600.89 | 291.41 |
| H-1223-0.5h② | 2121 | 1304 | 817 | 875.56 | 367.36 |
表6 不同人造丝基ACNFs织构特征及对应甲醛和水蒸气吸附量①
| 样品 | SBET/m2·g?1 | Smicro/m2·g?1 | SBET?Smicro/m2·g?1 | 甲醛蒸气吸附量③/mg·g?1 | 水蒸气吸附量③/mg·g?1 |
|---|---|---|---|---|---|
| ACF0 | 1267 | 1057 | 210 | 237.77 | 156.63 |
| H-723-0.5h② | 1714 | 1000 | 714 | 763.51 | 364.17 |
| H-823-0.5h② | 1434 | 1017 | 417 | 629.93 | 316.03 |
| H-1123-0.5h② | 1346 | 850 | 496 | 600.89 | 291.41 |
| H-1223-0.5h② | 2121 | 1304 | 817 | 875.56 | 367.36 |
| 1 | JONES A P. Indoor air quality and health[J]. Atmospheric Environment, 1999, 33(28): 4535-4564. |
| 2 | 刘建龙, 谭超毅, 张国强. 长沙市居民在不同室内环境中停留时间的调查研究[J]. 制冷空调与电力机械, 2008, 29(6): 32-35. |
| LIU Jianlong, TAN Chaoyi, ZHANG Guoqiang. Study on stopover time of resident in different indoor environment in Changsha City[J]. Refrigeration Air Conditioning & Electric Power Machinery, 2008, 29(6): 32-35. | |
| 3 | KOSTIAINEN R. Volatile organic-compounds in the indoor air of normal and sick houses[J]. Atmospheric Environment, 1995, 29(6): 693-702. |
| 4 | 王立鑫, 赵彬, 刘聪, 等. 中国室内SVOC污染问题评述[J]. 科学通报, 2010, 55(11): 967-977. |
| WANG Lixin, ZHAO Bin, LIU Chong, et al. Indoor SVOC pollution in China:a review[J]. Chinese Science Bulletin, 2010, 55(11): 967-977. | |
| 5 | WESCHLER C J. Changes in indoor pollutants since the 1950s[J]. Atmospheric Environment, 2009, 43(1): 153-169. |
| 6 | GHAFFARIANHOSEINI A, ALWAER H, OMRANY H, et al. Sick building syndrome: are we doing enough?[J]. Architectural Science Review, 2018, 61(3): 99-121. |
| 7 | REDLICH C A, SPARER J, CULLEN M R. Sick-building syndrome[J]. The Lancet, 1997, 349(9057): 1013-1016. |
| 8 | 张淑娟, 黄耀棠. 利用植物净化室内甲醛污染的研究进展[J]. 生态环境学报, 2010, 19(12): 3006-3013. |
| ZHANG Shujuan, HUANG Yaotang. Research progress on the elimination of indoor formaldehyde pollution by plants[J]. Ecology and Environmental Sciences, 2010, 19(12): 3006-3013. | |
| 9 | FAROOQUI M Y H. Formaldehyde[J]. Journal of Applied Toxicology, 1983, 3(5): 264-265. |
| 10 | UNEP-PUBLICATIONS. Formaldehyde. SIDS Assessment Report 2002[R]. [2018-10-15]. http://www.inchem.org/documents/sids/sids/FORMALDEHYDE.pdf. |
| 11 | SALTHAMMER T, MENTESE S, MARUTZKY R. Formaldehyde in the indoor environment[J]. Chemical Reviews, 2010, 110(4): 2536-2572. |
| 12 | WHO. Formaldehyde. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 88, 2006[R]. [2018-10-15]. http://www.inchem.org/documents/iarc/vol88/volume.pdf. |
| 13 | 郝丽娟. 自如深陷“甲醛门”[J]. 质量与认证, 2018(12): 38-40. |
| HAO Lijuan. Ziroom company deeply surrounded by the “formaldehyde room event”[J]. China Quality Certification. 2018(12): 38-40. | |
| 14 | 于潇, 崔晓丽. 长租公寓甲醛超标, 租户权益谁来维护[J]. 方圆, 2018(17): 62-65. |
| YU Xiao, CUI Xiaoli. Formaldehyde out of limits severely in long-term rental apartment, who will pay the rights and interests for tenements?[J]. Fangyuan Magazine, 2018(17): 62-65. | |
| 15 | YANG C, MIAO G, PI Y, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. |
| 16 | ZHANG X Y, GAO B, CREAMER A E, et al. Adsorption of VOCs onto engineered carbon materials: a review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. |
| 17 | NA C J, YOO M J, TSANG D C W, et al. High-performance materials for effective sorptive removal of formaldehyde in air[J]. Journal of Hazardous Materials, 2019, 366: 452-465. |
| 18 | SURESH S, BANDOSZ T J. Removal of formaldehyde on carbon-based materials: a review of the recent approaches and findings[J]. Carbon, 2018, 137: 207-221. |
| 19 | PEI J, ZHANG J S. Critical review of catalytic oxidization and chemisorption methods for indoor formaldehyde removal[J]. HVAC&R Research, 2011, 17(4): 476-503. |
| 20 | BELLAT J P, BEZVERKHYY I, WEBER G, et al. Capture of formaldehyde by adsorption on nanoporous materials[J]. Journal of Hazardous Materials, 2015, 300: 711-717. |
| 21 | KUMAGAI S, SASAKI K, SHIMIZU Y, et al. Formaldehyde and acetaldehyde adsorption properties of heat-treated rice husks[J]. Separation and Purification Technology, 2008, 61(3): 398-403. |
| 22 | WEN Q B, LI C T, CAI Z H, et al. Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde[J]. Bioresour Technol., 2011, 102(2): 942-947. |
| 23 | LEE K J, SHIRATORI N, LEE G H, et al. Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent[J]. Carbon, 2010, 48(15): 4248-4255. |
| 24 | AN H B, YU M J, KIM J M, et al. Indoor formaldehyde removal over CMK-3[J]. Nanoscale Res. Lett., 2012, 7: 7. |
| 25 | ZHANG W, CHEN L, XU L, et al. Advanced nanonetwork-structured carbon materials for high-performance formaldehyde capture[J]. Journal of Colloid and Interface Science, 2019, 537: 562-568. |
| 26 | DE FALCO G, BARCZAK M, MONTAGNARO F, et al. A new generation of surface active carbon textiles as reactive adsorbents of indoor formaldehyde[J]. ACS Appl. Mater. Interfaces, 2018, 10(9): 8066-8076. |
| 27 | DE FALCO G, LI W, CIMINO S, et al. Role of sulfur and nitrogen surface groups in adsorption of formaldehyde on nanoporous carbons[J]. Carbon, 2018, 138: 283-291. |
| 28 | RENGGA W D P, SUDIBANDRIYO M, NASIKIN M. Adsorption of low-concentration formaldehyde from air by silver and copper nano-particles attached on bamboo-based activated carbon[J]. International Journal of Chemical Engineering and Applications, 2013, 4(5): 332-336. |
| 29 | SHIN S, SONG J. Modeling and simulations of the removal of formaldehyde using silver nano-particles attached to granular activated carbon[J]. Journal of Hazardous Materials, 2011, 194: 385-392. |
| 30 | RENGGA W D P, CHAFIDZ A, SUDIBANDRIYO M, et al. Silver nano-particles deposited on bamboo-based activated carbon for removal of formaldehyde[J]. Journal of Environmental Chemical Engineering, 2017, 5(2): 1657-1665. |
| 31 | 董春欣, 孙胜龙, 杨秋昕, 等. 改性活性炭吸附室内甲醛气体的应用研究[J]. 吉林化工学院学报, 2011, 28(3): 28-31. |
| DONG Chunxin, SUN Shenglong, YANG Qiuxin, et al. Research on application of modified activated carbon in adsorbing indoor formaldehyde[J]. Journal of Jilin Institute of Chemical Technology, 2011, 28(3): 28-31. | |
| 32 | 何萌, 袁琳, 曲可琪, 等. 高锰酸钾改性活性炭对甲醛吸附性能的研究[J]. 广州化工, 2017, 45(14): 93-95. |
| HE Meng, YUAN Lin, QU Keqi, et al. Study on formaldehyde adsorption performance of potassium permanganate modified activated carbon[J]. Guangzhou Chemical Industry, 2017, 45(14): 93-95. | |
| 33 | 蒋昕楠, 孔振凯, 王际童, 等. 高锰酸钾改性球形中孔炭的甲醛吸附性能[J]. 环境工程学报, 2018, 12(6): 1676-1682. |
| JIANG Xinnan, KONG Zhenkai, WANG Jitong, et al. Formaldehyde adsorption performance of KMnO4 modified mesoporous carbon spheres[J]. Chinese Journal of Environmental Engineering, 2018, 12(6): 1676-1682. | |
| 34 | 姜良艳, 周仕学, 王文超, 等. 活性炭负载锰氧化物用于吸附甲醛[J]. 环境科学学报, 2008, 28(2): 337-341. |
| JIANG Liangyan, ZHOU Shixue, WANG Wenchao, et al. Adsorption of formaldehyde by activated carbon loaded with manganese oxides[J]. Acta Scientiae Circumstantiae, 2008, 28(2): 337-341. | |
| 35 | 周昕彦, 张芃, 蒋文, 等. 锰氧化物改性活性炭去除空气中甲醛[J]. 环境工程学报, 2015, 9(12): 5965-5972. |
| ZHOU Xinyan, ZHANG Peng, JIANG Wen, et al. MnOx/GAC for removal of formaldehyde in air[J]. Chinese Journal of Environmental Engineering, 2015, 9(12): 5965-5972. | |
| 36 | KRISHNAMURTHY A, THAKKAR H, ROWNAGHI A A, et al. Adsorptive removal of formaldehyde from air using mixed-metal oxides [J]. Industrial & Engineering Chemistry Research, 2018, 57(38): 12916-12925. |
| 37 | SAEUNG S, BOONAMNUAYVITAYA V. Adsorption of formaldehyde vapor by amine-functionalized mesoporous silica materials[J]. Journal of Environmental Science, 2008, 20(3): 379-384. |
| 38 | CHEN D, QU Z, SUN Y, et al. Adsorption-desorption behavior of gaseous formaldehyde on different porous Al2O3 materials[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 441: 433-440. |
| 39 | LE Y, GUO D, CHENG B, et al. Bio-template-assisted synthesis of hierarchically hollow SiO2 microtubes and their enhanced formaldehyde adsorption performance[J]. Applied Surface Science, 2013, 274: 110-116. |
| 40 | YE J W, ZHU X F, CHENG B, et al. Few-layered graphene-like boron nitride: a highly efficient adsorbent for indoor formaldehyde removal[J]. Environmental Science & Technology Letters, 2017, 4(1): 20-25. |
| 41 | PAN L, CHEN Q, ZHU J H, et al. Hypercrosslinked porous polycarbazoles via one-step oxidative coupling reaction and Friedel-Crafts alkylation[J]. Polymer Chemistry, 2015, 6(13): 2478-2487. |
| 42 | YANG Z, MIAO H, RUI Z, et al. Enhanced formaldehyde removal from air using fully biodegradable chitosan grafted beta-cyclodextrin adsorbent with weak chemical interaction[J]. Polymers, 2019, 11(2): 276 |
| 43 | WANG B, GAO B, FANG J. Recent advances in engineered biochar productions and applications[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(22): 2158-2207. |
| 44 | 刘宝成, 赵晓明. 活性炭吸附去除室内甲醛的研究进展[J]. 成都纺织高等专科学校学报, 2017, 34(1): 224-229. |
| LIU Baocheng, ZHAO Xiaoming. Research progress in abatement of indoor formaldehyde via adsorption by activated carbon[J]. Journal of Chengdu Textile College, 2017, 34(1): 224-229. | |
| 45 | 林莉莉, 邱兆富, 韩晓琳, 等. 吸附气相甲醛活性炭的选型研究[J]. 环境污染与防治, 2013, 35(12): 19-25. |
| LIN Lili, QIU Zhaofu, HAN Xiaolin, et al. Selecting activated carbon for removing formaldehyde from air[J]. Environmental Pollution & Control, 2013, 35(12): 19-25. | |
| 46 | REZAEE A, RANGKOOY H, JONIDI-JAFARI A, et al. Surface modification of bone char for removal of formaldehyde from air[J]. Applied Surface Science, 2013, 286: 235-239. |
| 47 | 马俊. 表面化学改性气相吸附用活性炭的研究进展[J]. 资源节约与环保, 2015(9): 44-46. |
| MA Jun. Research progress in surface chemical modification of activated carbon for gaseous phase adsorption[J]. Resources Economization & Environmental Protection, 2015(9): 44-46. | |
| 48 | MA C J, LI X H, ZHU T L. Removal of low-concentration formaldehyde in air by adsorption on activated carbon modified by hexamethylene diamine[J]. Carbon, 2011, 49(8): 2873-2875. |
| 49 | LU Y W, WANG D H, MA C F, et al. The effect of activated carbon adsorption on the photocatalytic removal of formaldehyde[J]. Building and Environment, 2010, 45(3): 615-21. |
| 50 | 王明贤, 赵圣, 支恒学. 白炭黑吸附甲醛实验研究[J]. 硅酸盐通报, 2013, 32(10): 2030-2036. |
| WANG Mingxian, ZHAO Sheng, ZHI Hengxue. Experimental study on white carbon black adsorption of formaldehyde[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(10): 2030-2036. | |
| 51 | ZHANG G, LIU Y, ZHENG S, et al. Adsorption of volatile organic compounds onto natural porous minerals[J]. Journal of Hazardous Materials, 2019, 364: 317-324. |
| 52 | 李铭哲, 刘阳钰, 郑水林, 等. 改性硅藻土的甲醛吸附性能与吸附机理[J]. 非金属矿, 2019, 42(3): 83-86. |
| LI Mingzhe, LIU Yangyu, ZHENG Shuilin, et al. Formaldehyde adsorption performance and mechanism of modified diatomite[J]. Non-Metallic Mines, 2019, 42(3): 83-86. | |
| 53 | 薛梦婷, 李勇. VOCs在分子筛上吸附性能的研究进展[J]. 无机盐工业, 2019(5): 12-16. |
| XUE Mengting, LI Yong. Research progress on adsorption properties of volatile organic compounds on molecular sieves[J]. Inorganic Chemicals Industry, 2019(5): 12-16. | |
| 54 | VEERAPANDIAN S K P, DE GEYTER N, GIRAUDON J M, et al. The use of aeolites for VOCs abatement by combining non-thermal plasma, adsorption, and/or catalysis: a review[J]. Catalysts, 2019, 9(1): 98 |
| 55 | WEN M, LI G, LIU H, et al. Metal-organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: recent progress and challenges[J]. Environmental Science: Nano, 2019, 6(4): 1006-1025. |
| 56 | 胡刘平, 莫开林, 杨凌, 等. 活性炭对甲醛吸附的研究[J]. 四川林业科技, 2007(4): 52-54, 15. |
| HU Liuping, MO Kailin, YANG Ling, et al. Research on activated carbon to adsorption of formaldehyde[J]. Journal of Sichuan Forestry Science and Technology, 2007(4): 52-54, 15. | |
| 57 | 刘露, 杨永强, 张增峰, 等. 市售净化产品对空气中甲醛去除效果的研究[J]. 广州化学, 2016, 41(3): 57-61. |
| LIU Lu, YANG Yongqiang, ZHANG Zengfeng, et al. Study on the removal efficiency of formaldehyde in air by the commercially available decontamination products[J]. Guangzhou Chemistry, 2016, 41(3): 57-61. | |
| 58 | 张蓓, 陈畅, 胡朋举, 等. 空气净化用活性炭甲醛净化性能研究[J]. 环境科技, 2018, 31(3): 28-31. |
| ZHANG B, CHEN C, HU P J, et al. Study on the purification of formaldehyde from air purified activated carbon[J]. Environmental Science and Technology, 2018, 31(3): 28-31. | |
| 59 | RONG H Q, RYU Z Y, ZHENG J T, et al. Influence of heat treatment of rayon-based activated carbon fibers on the adsorption of formaldehyde[J]. Journal of Colloid and Interface Science, 2003, 261(2): 207-212. |
| 60 | LEE K J, MIYAWAKI J, SHIRATORI N, et al. Toward an effective adsorbent for polar pollutants: formaldehyde adsorption by activated carbon[J]. Journal of Hazardous Materials, 2013, 260: 82-88. |
| 61 | SONG Y, QIAO W, YOON S H, et al. Removal of formaldehyde at low concentration using various activated carbon fibers[J]. Journal of Applied Polymer Science, 2007, 106(4): 2151-2157. |
| 62 | CHEN H, MO J, XIAO R, et al. Gaseous formaldehyde removal: a laminated plate fabricated with activated carbon, polyimide, and copper foil with adjustable surface temperature and capable of in situ thermal regeneration[J]. Indoor Air, 2019, 29(3): 469-476. |
| 63 | XIAO R, MO J, ZHANG Y, et al. An in-situ thermally regenerated air purifier for indoor formaldehyde removal[J]. Indoor Air, 2018, 28(2): 266-275. |
| 64 | AXET M R, DECHY-CABARET O, DURAND J, et al. Coordination chemistry on carbon surfaces[J]. Coordination Chemistry Reviews, 2016, 308: 236-345. |
| 65 | FIGUEIREDO J L, PEREIRA M F R. The role of surface chemistry in catalysis with carbons[J]. Catalysis Today, 2010, 150(1/2): 2-7. |
| 66 | KICIŃSKI W, SZALA M, BYSTRZEJEWSKI M. Sulfur-doped porous carbons: synthesis and applications[J]. Carbon, 2014, 68: 1-32. |
| 67 | 唐国旗, 张春富, 孙长山, 等. 活性氧化铝载体的研究进展[J]. 化工进展, 2011, 30(8): 1756-1765. |
| TANG Guoqi, ZHANG Chunfu, SUN Changshan, et al. Research progress of γ-alumina support[J]. Chemical Industry and Engineering Progress, 2011, 30(8): 1756-1765. | |
| 68 | KOOHSARYAN E, ANBIA M. 纳米多级孔分子筛:简短的综述(英文)[J]. 催化学报, 2016, 37(4): 447-467. |
| KOOHSARYAN E, ANBIA M. Nanosized and hierarchical zeolites: a short review[J]. Chinese Journal of Catalysis, 2016, 37(4): 447-467. | |
| 69 | 操强, 陈琦, 韩宝航. 有机多孔聚咔唑的制备及性能研究进展[J]. 化学学报, 2015, 73(6): 541-556. |
| CAO Qiang, CHEN Qi, HAN Baohang. Recent advance in organic porous polycarbazoles: preparation and properties[J]. Acta Chimica Sinica, 2015, 73(6): 541-556. | |
| 70 | BUSCA G, LAMOTTE J, LAVALLEY J C, et al. FT-IR study of the adsorption and transformation of formaldehyde on oxide surfaces[J]. Journal of American Chemical Society, 1987, 109(17): 5197-5202. |
| 71 | LI J, ZHANG P, WANG J, et al. Birnessite-type manganese oxide on granular activated carbon for formaldehyde removal at room temperature[J]. The Journal of Physical Chemistry C, 2016, 120(42): 24121-24129. |
| 72 | FANG R, HUANG H, JI J, et al. Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature[J]. Chemical Engineering Journal, 2018, 334: 2050-2057. |
| 73 | OH K J, PARK D W, KIM S S, et al. Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon[J]. Korean Journal of Chemical Engineering, 2010, 27(2): 632-638. |
| 74 | 汤进华, 梁晓怿, 龙东辉, 等. 活性炭孔结构和表面官能团对吸附甲醛性能影响[J]. 炭素技术, 2007(3): 21-25. |
| TANG Jinhua, LIANG Xiaoyi, LONG Donghui, et al. Effects of micropore and functional groups of activated carbon on adsorption behavior of formaldehyde[J]. Carbon Techniques, 2007(3): 21-25. | |
| 75 | CARTER E M, KATZ L E, SPEITEL G E, et al. Gas-phase formaldehyde adsorption isotherm studies on activated carbon: correlations of adsorption capacity to surface functional group density[J]. Environmental Science & Technology, 2011, 45(15): 6498-6503. |
| 76 | BOONAMNUAYVITAYA V, SAE-UNG S, TANTHAPANICHAKOON W. Preparation of activated carbons from coffee residue for the adsorption of formaldehyde[J]. Separation and Purification Technology, 2005, 42(2): 159-168. |
| 77 | 梅凡民, 傅成诚, 杨青莉, 等. 活性炭表面酸性含氧官能团对吸附甲醛的影响[J]. 环境污染与防治, 2010, 32(3): 18-22. |
| MEI Fanming, FU Chengcheng, YANG Qingli, et al. The effect of acid functional groups of modified activated carbon on formaldehyde absorption[J]. Environmental Pollution & Control, 2010, 32(3): 18-22. | |
| 78 | KIM D I, PARK J H, KIM S D, et al. Comparison of removal ability of indoor formaldehyde over different materials functionalized with various amine groups[J]. Journal of Industrial and Engineering Chemistry, 2011, 17(1): 1-5. |
| 79 | PEI J J, ZHANG J S S. On the performance and mechanisms of formaldehyde removal by chemi-sorbents[J]. Chemical Engineering Journal, 2011, 167(1): 59-66. |
| 80 | LI J, LI Z, LIU B, et al. Effect of relative humidity on adsorption of formaldehyde on modified activated carbons[J]. Chinese Journal of Chemical Engineering, 2008, 16(6): 871-875. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [3] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [4] | 杨子育, 朱玲, 王文龙, 于超凡, 桑义敏. 阴燃法处理含油污泥的研究及应用进展[J]. 化工进展, 2023, 42(7): 3760-3769. |
| [5] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [6] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [7] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [8] | 朱紫旋, 陈俊江, 张星星, 李祥, 刘文如, 吴鹏. 基于短程反硝化厌氧氨氧化新型污水生物脱氮工艺的研究进展[J]. 化工进展, 2023, 42(4): 2091-2100. |
| [9] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [10] | 郭帅帅, 陈锦路, 金梁程龙, 陶醉, 陈小丽, 彭国文. 基于海水提铀的多孔芳香框架材料研究进展[J]. 化工进展, 2023, 42(3): 1426-1436. |
| [11] | 苏景振, 詹健. 生物炭对水环境中微塑料的去除研究进展[J]. 化工进展, 2023, 42(10): 5445-5458. |
| [12] | 王胜楠, 郑旭. 空气取水用活性炭纤维复合吸附剂的研究[J]. 化工进展, 2023, 42(10): 5567-5573. |
| [13] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [14] | 曹正凯, 米晓斌, 吴子明, 孙士可, 曹均丰, 彭德强, 梁相程. 煤合成气净化除尘装置压降问题分析及应用优化[J]. 化工进展, 2022, 41(S1): 15-21. |
| [15] | 王子航, 梁瑞升, 邓超和, 王佳韵. 离子凝胶复合吸附剂的制备及空气取水性能[J]. 化工进展, 2022, 41(S1): 389-396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||