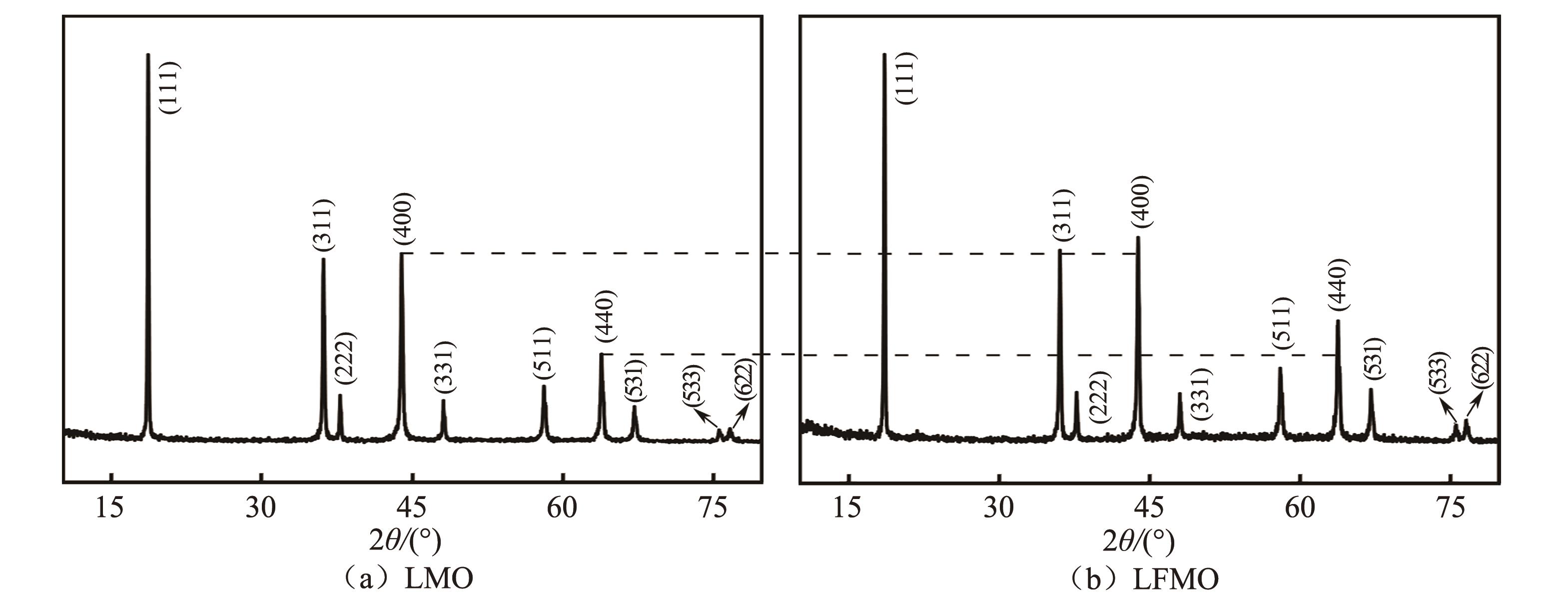
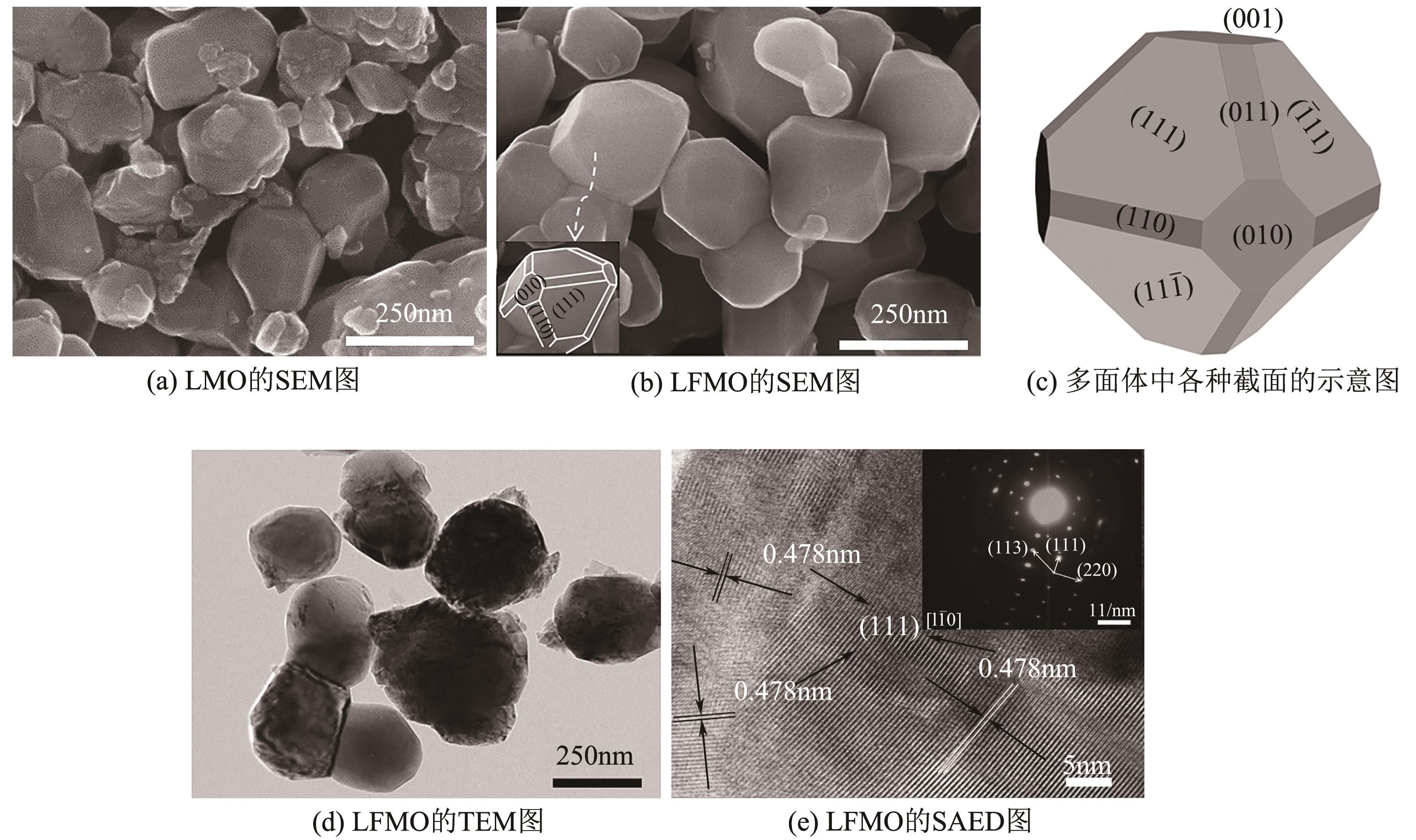
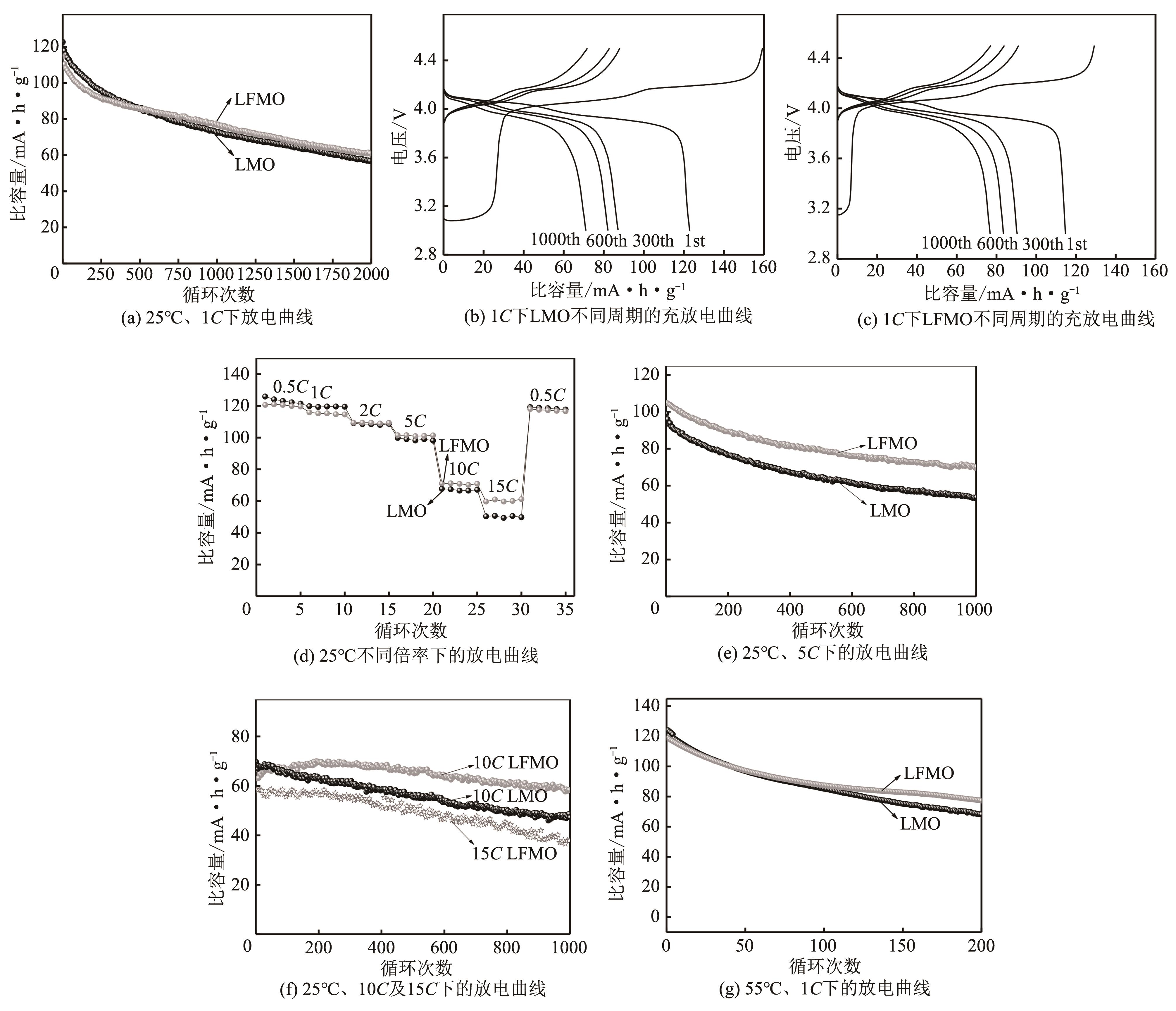
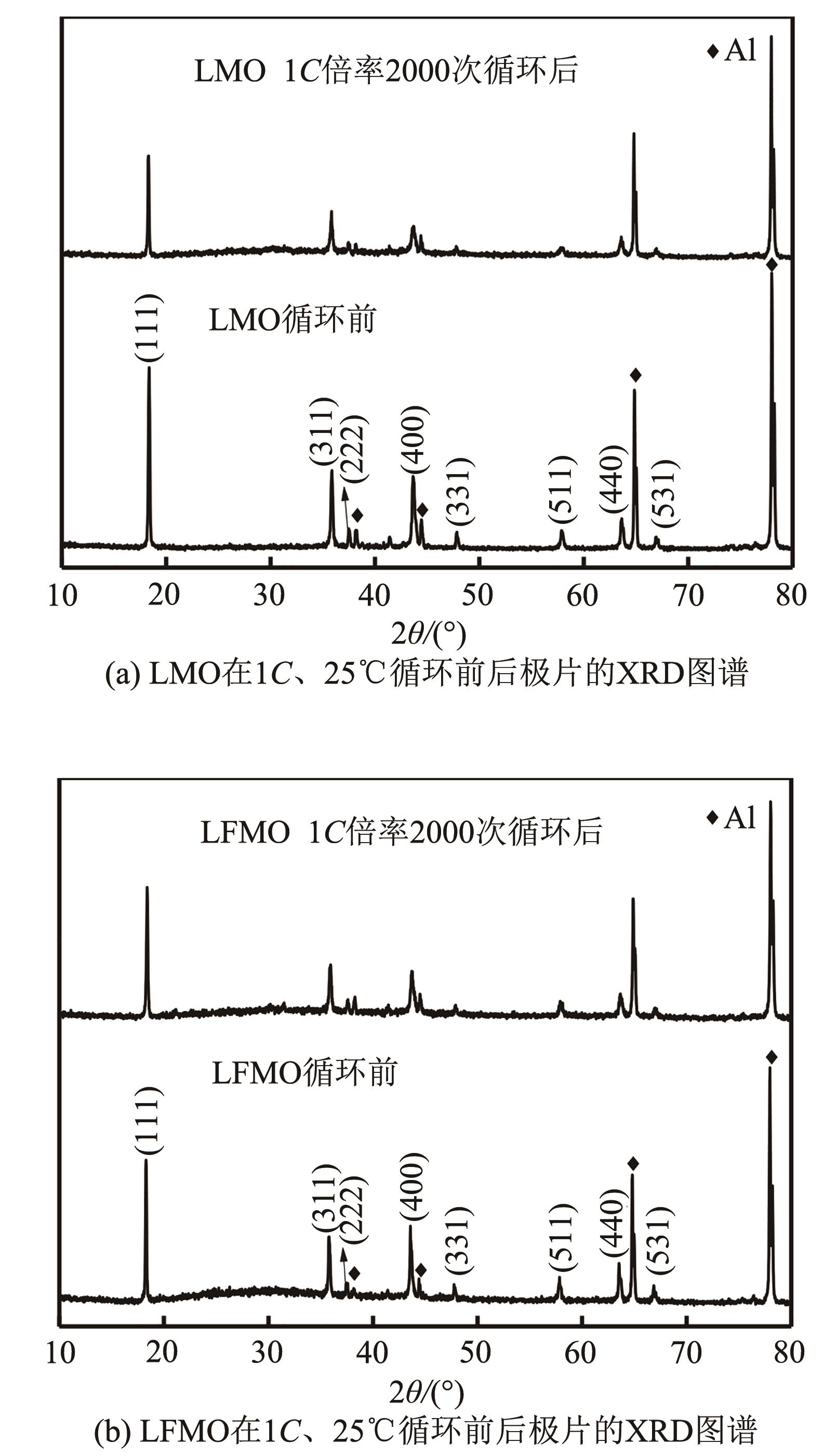
| 1 |
ZHANG W, SUN X, TANG Y, et al. Lowering charge transfer barrier of LiMn2O4via nickel surface doping to enhance Li+ intercalation kinetics at subzero temperatures[J]. Journal of the American Chemical Society, 2019, 141(36): 14038-14042.
|
| 2 |
张英杰, 宁培超, 杨轩, 等. 废旧三元锂离子电池回收技术研究新进展[J]. 化工进展, 2020, 39(7): 2828-2840.
|
|
ZHANG Yingjie, NING Peichao, YANG Xuan, et al. Research progress on the recycling technology of spent ternary lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2828-2840.
|
| 3 |
ZUO C, HU Z, QI R, et al. Double the capacity of manganese spinel for lithium-ion storage by suppression of cooperative Jahn-Teller distortion[J]. Advanced Energy Materials, 2020, 10(34): 2000363.
|
| 4 |
MAO F, GUO W, MA J. Research progress on design strategies, synthesis and performance of LiMn2O4-based cathodes[J]. RSC Advances, 2015, 5(127): 105248-105258.
|
| 5 |
ANGELOPOULOU P, PALOUKIS F, SŁOWIK G, et al. Combustion-synthesized LixMn2O4-based spinel nanorods as cathode materials for lithium-ion batteries[J]. Chemical Engineering Journal, 2017, 311: 191-202.
|
| 6 |
HIROSE S H, KODERA T, OGIHARA T. Synthesis and electrochemical properties of Li-rich spinel type LiMn2O4 powders by spray pyrolysis using aqueous solution of manganese carbonate[J]. Journal of Alloys and Compounds, 2010, 506(2): 883-887.
|
| 7 |
CHEN M, CHEN P, YANG F, et al. Ni,Mo co-doped lithium manganate with significantly enhanced discharge capacity and cycling stability[J]. Electrochimica Acta, 2016, 206: 356-365.
|
| 8 |
FANG D L, LI J C, LIU X, et al. Synthesis of a Co-Ni doped LiMn2O4 spinel cathode material for high-power Li-ion batteries by a sol-gel mediated solid-state route[J]. Journal of Alloys and Compounds, 2015, 640: 82-89.
|
| 9 |
LIU H, TIAN R, JIANG Y, et al. On the drastically improved performance of Fe-doped LiMn2O4 nanoparticles prepared by a facile solution-gelation route[J]. Electrochimica Acta, 2015, 180: 138-146.
|
| 10 |
CAO J, GUO S, YAN R, et al. Carbon-coated single-crystalline LiMn2O4 nanowires synthesized by high-temperature solid-state reaction with high capacity for Li-ion battery[J]. Journal of Alloys and Compounds, 2018, 741: 1-6.
|
| 11 |
KIM J S, KIM K, CHO W, et al. A truncated manganese spinel cathode for excellent power and lifetime in lithium-ion batteries[J]. Nano Letters, 2012, 12(12): 6358-6365.
|
| 12 |
杨茗佳, 陈猛, 张维维. LiFexMn2-xO4材料的制备与性能研究[J]. 电池工业, 2008, 13(6): 393-396.
|
|
YANG Mingjia, CHEN Meng, ZHANG Weiwei. Research on preparation and property of LiFexMn2-xO4[J]. Chinese Battery Industry, 2008, 13(6): 393-396.
|
| 13 |
GU H, WANG G, ZHU C, et al. Correlating cycle performance improvement and structural alleviation in LiMn2-xMxO4 spinel cathode materials: a systematic study on the effects of metal-ion doping[J]. Electrochimica Acta, 2019, 298: 806-817.
|
| 14 |
SINGH P, SIL A, NATH M, et al. Preparation and characterization of lithium manganese oxide cubic spinel Li1.03Mn1.97O4 doped with Mg and Fe[J]. Physica B: Condensed Matter, 2010, 405(2): 649-654.
|
| 15 |
DUAN Y Z, GUO J M, XIANG M W, et al. Single crystalline polyhedral LiNixMn2-xO4 as high-performance cathodes for ultralong cycling lithium-ion batteries[J]. Solid State Ionics, 2018, 326: 100-109.
|
| 16 |
YU Y, XIANG M W, GUO J M, et al. Enhancing high-rate and elevated-temperature properties of Ni-Mg co-doped LiMn2O4 cathodes for Li-ion batteries[J]. Journal of Colloid and Interface Science, 2019, 555: 64-71.
|
| 17 |
DENG Y, ZHOU Y, SHI Z, et al. Porous LiMn2O4 microspheres as durable high power cathode materials for lithium ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(28): 8170-8177.
|
| 18 |
HUANG S, WU H, CHEN P, et al. Facile pH-mediated synthesis of morphology-tunable MnCO3 and their transformation to truncated octahedral spinel LiMn2O4 cathode materials for superior lithium storage[J]. Journal of Materials Chemistry A, 2015, 3(7): 3633-3640.
|
| 19 |
HWANG B, KIM S, LEE Y, et al. Truncated octahedral LiMn2O4 cathode for high-performance lithium-ion batteries[J]. Materials Chemistry and Physics, 2015, 158: 138-143.
|
| 20 |
JIANG C, TANG Z, WANG S, et al. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries[J]. Journal of Power Sources, 2017, 357: 144-148.
|
| 21 |
Xia Y, WANG H, ZHANG Q, et al. Oxygen deficiency, a key factor in controlling the cycle performance of Mn-spinel cathode for lithium-ion batteries[J]. Journal of Power Sources, 2007, 166(2): 485-491.
|
| 22 |
YOSHIO M, NOGUCHI H, WANG H, et al. Correlation of oxygen deficiency with discharge capacity at 3.2 V for (LiMn)3O4-z[J]. Journal of Power Sources, 2006, 154(1): 273-275.
|
| 23 |
ZHU C, LIU J, YU X, et al. Boosting the stable Li storage performance in one-dimensional LiLaxMn2-xO4 nanorods at elevated temperature[J]. Ceramics International, 2019, 45(15): 19351-19359.
|
| 24 |
JIANG C, TANG Z, WANG S, et al. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries[J]. Journal of Power Sources, 2017, 357: 144-148.
|
| 25 |
ZHANG C, LIU X, SU Q, et al. Enhancing electrochemical performance of LiMn2O4 cathode material at elevated temperature by uniform nanosized TiO2 coating[J]. ACS Sustainable Chemistry & Engineering, 2016, 5(1): 640-647.
|
| 26 |
CHOU S, WANG J, LIU H, et al. Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery[J]. The Journal of Physical Chemistry C, 2011, 115(32): 16220-16227.
|
| 27 |
于月, 向明武, 白红丽, 等. 固相燃烧法快速合成LiNi0.03Mg0.10Mn1.87O4正极材料及电性能研究[J]. 稀有金属材料与工程, 2020, 49(4): 1437-1444.
|
|
YU Yue, XIANG Mingwu, BAI Hongli, et al. Rapid synthesis of LiNi0.03Mg0.10Mn1.87O4 cathode material by solid-state combustion method and its electrochemical properties[J]. Rare Metal Materials and Engineering, 2020, 49(4): 1437-1444.
|
 ), 郭俊明(
), 郭俊明( ), 向明武, 白玮, 白红丽, 刘晓芳, 段开娇
), 向明武, 白玮, 白红丽, 刘晓芳, 段开娇
 ), GUO Junming(
), GUO Junming( ), XIANG Mingwu, BAI Wei, BAI Hongli, LIU Xiaofang, DUAN Kaijiao
), XIANG Mingwu, BAI Wei, BAI Hongli, LIU Xiaofang, DUAN Kaijiao