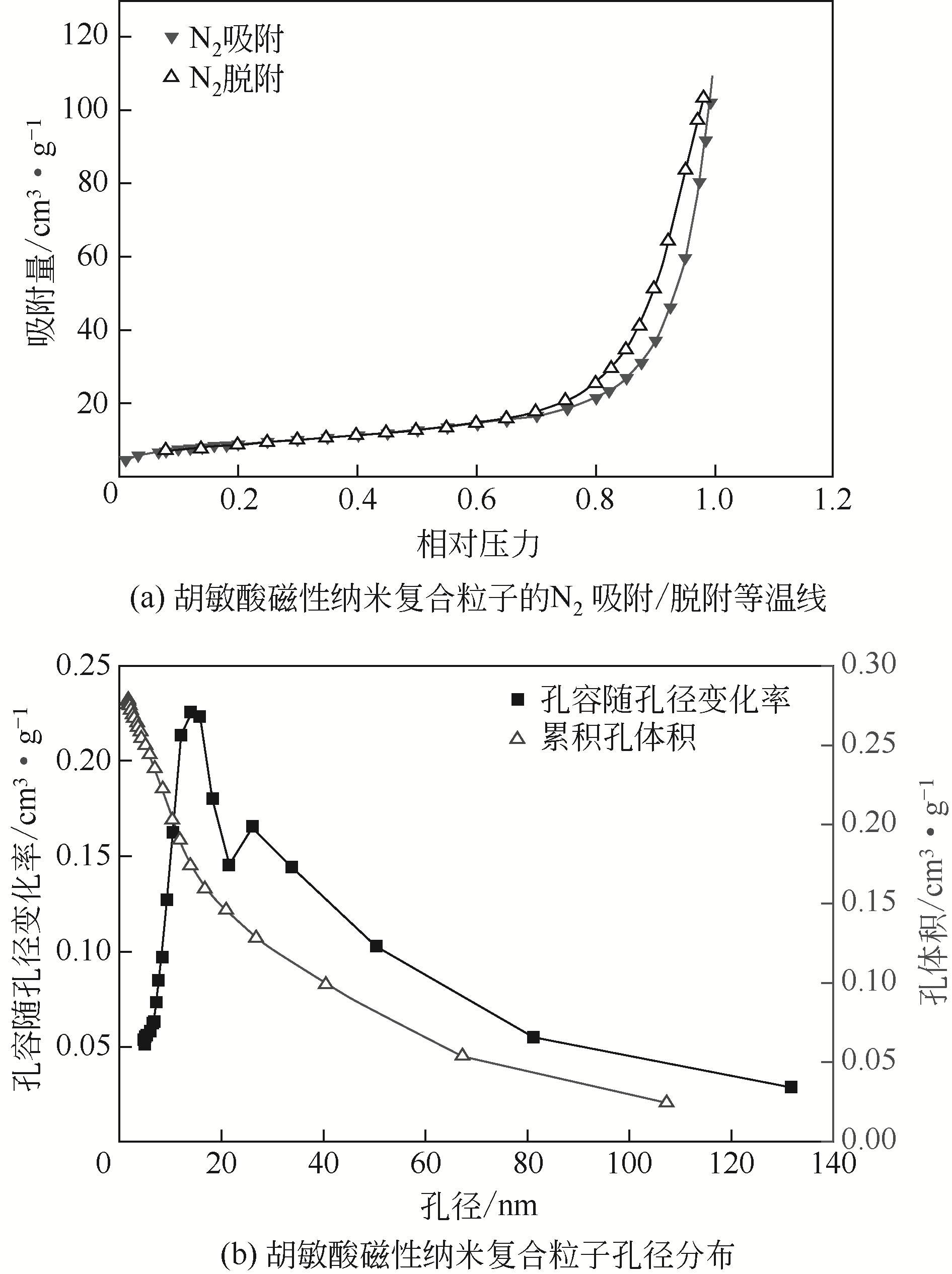
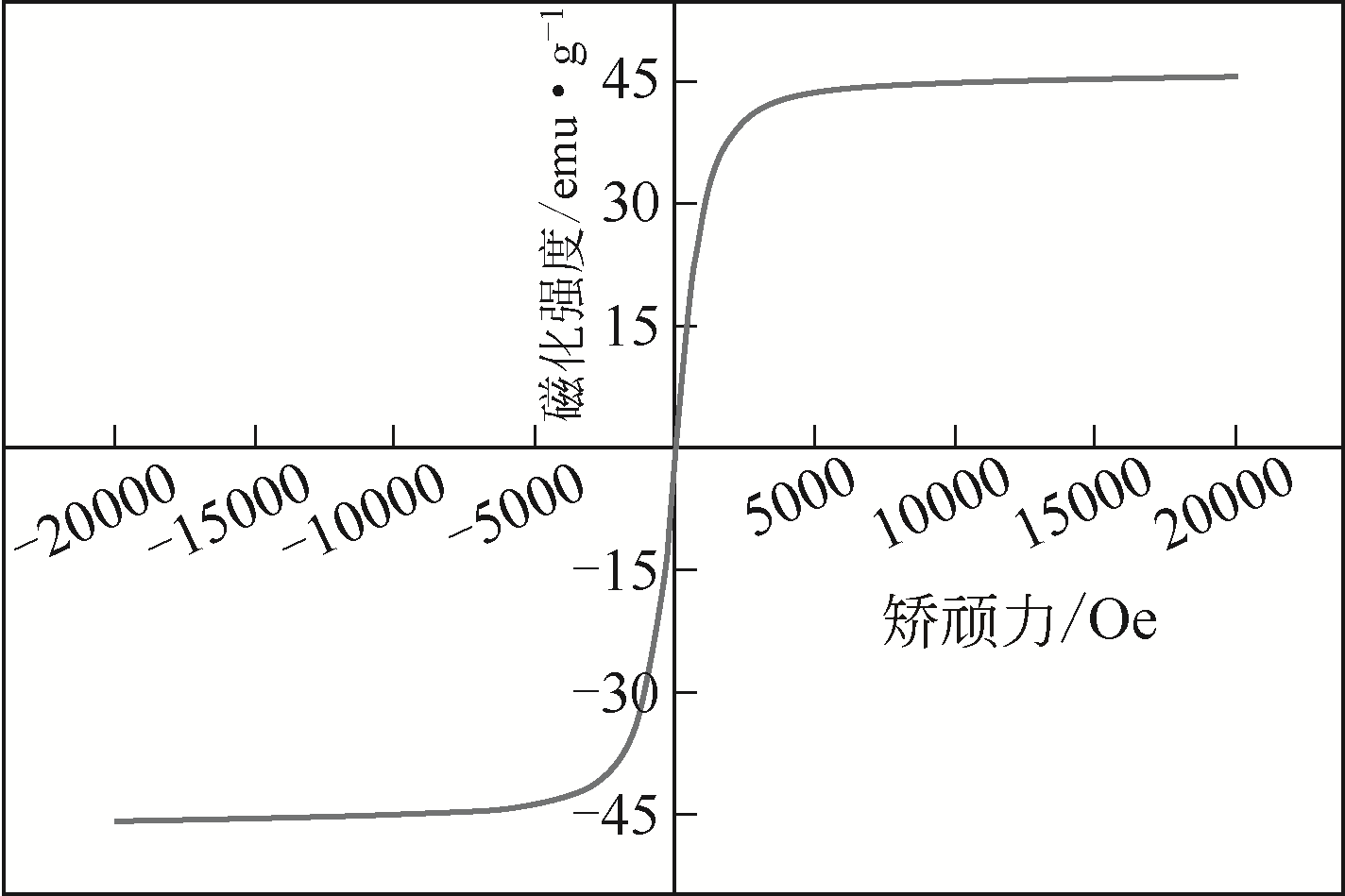
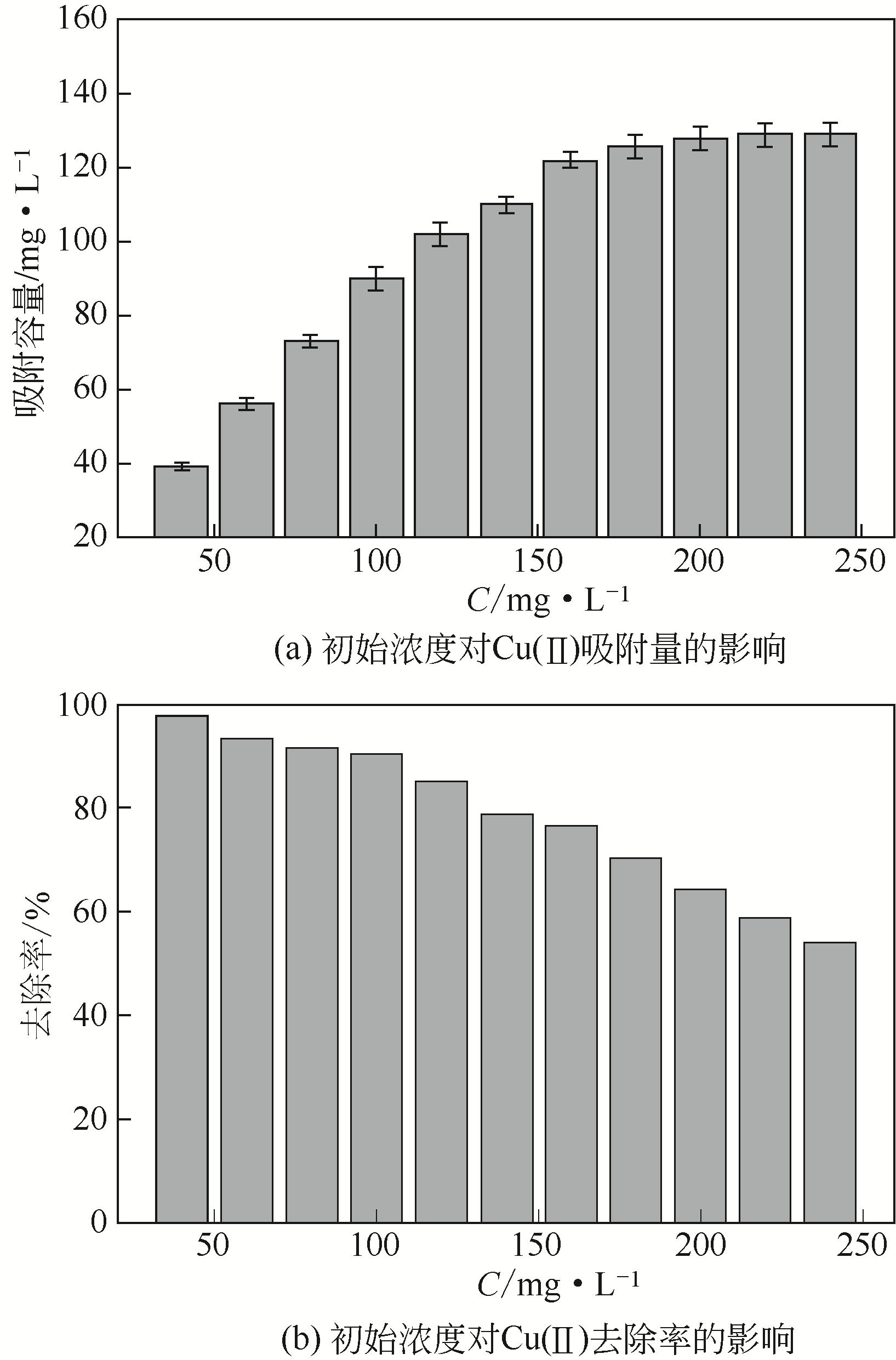
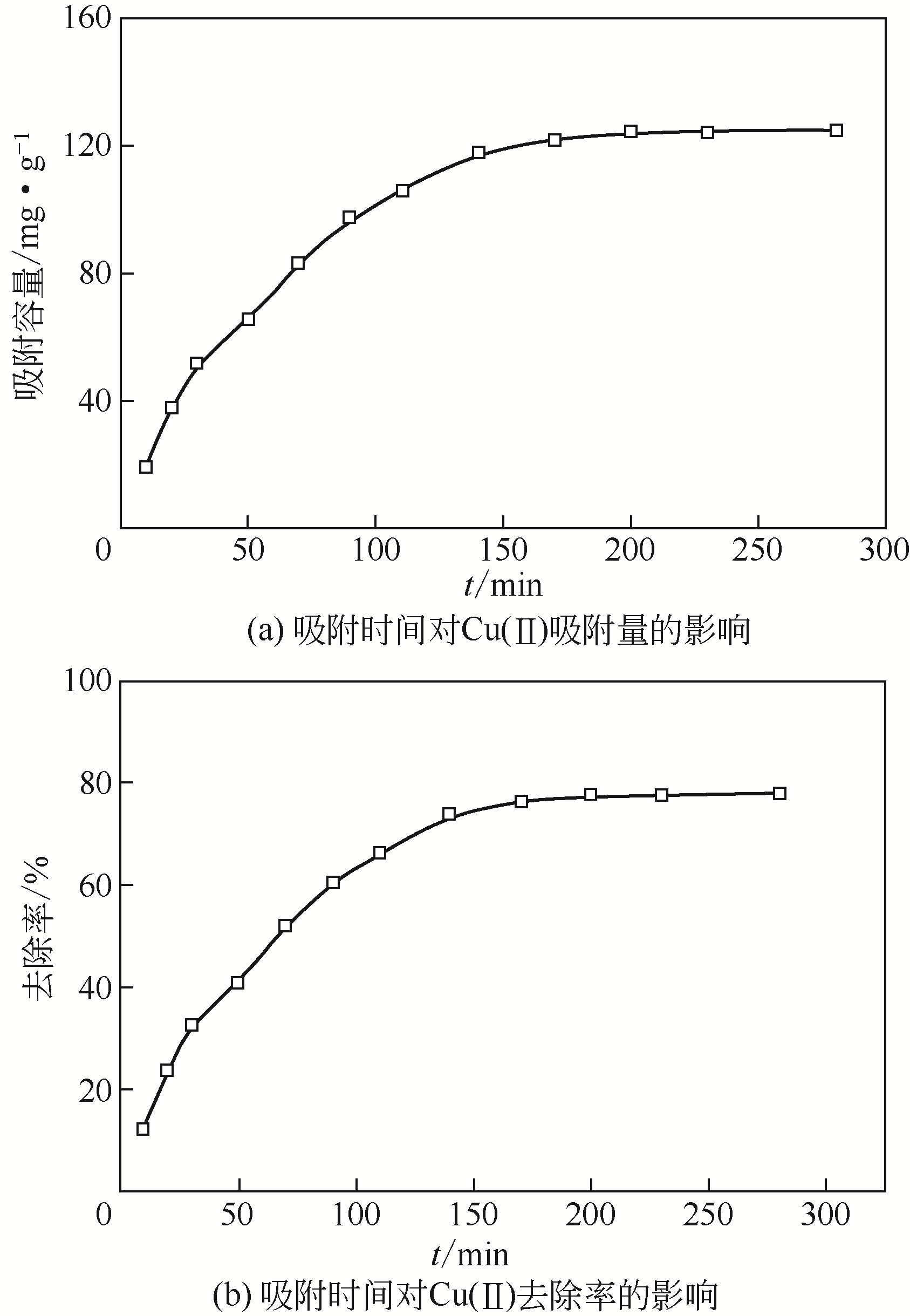
| 1 |
雷高伟,时春辉,甄卫军,等.改性腐植酸钠对Hg2+吸附性能的研究[J].腐植酸,2018,2:16-20.
|
|
LEI Gaowei,SHI Chunhui,ZHEN Weijun,et al.Study on adsorption performance of modified sodium humate on Hg2+[J].Humic acid.2018,2:16-20.
|
| 2 |
程亮,刘国际,刘伟,等.改性蒙脱土/纳米腐植酸复合材料制备及其对苯胺吸附性能的研究[J].化工矿物与加工,2016(4):22-27.
|
|
CHENG Liang,LIU Guoji,LIU Wei,et al.Preparation and adsorption properties of modified montmorillonite-nanoscale humic acid composites for aniline[J].Industrial Minerals and Processing,2016(4):22-27.
|
| 3 |
宋丽贤,卢忠远,刘德春,等.分解沉淀法制备磁性纳米Fe3O4的研究及表征[J].化工进展,2006,25(1):54-57.
|
|
SONG Lixian,LU Zhongyuan,LIU Dechun,et al.Preparation of magnetic nanometer Fe3O4 with the method of complex compound-hydrolyzation deposition[J].Chemical Industry and Engineering Progress,2006,25(1):54-57.
|
| 4 |
张鑫,李鑫钢,姜斌.四氧化三铁纳米粒子合成及表征[J].化学工业与工程,2006,23(1):45-48.
|
|
ZHANG Xin,LI Xingang,JIANG Bin.Preparation and characterization of nanometer magnetite[J].Chemical Industry and Engineering,2006,23(1):45-48.
|
| 5 |
XU C J,XU K M,GU H W,et al.Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles[J].Journal of American Chemical Society,2004,126(32):9938-9939.
|
| 6 |
KOMKIENE J,BALTRENAITE E.Biochar as adsorbent for removal of heavy metal ions [cadmium(Ⅱ), copper(Ⅱ), lead(Ⅱ), zinc(Ⅱ)] from aqueous phase[J].International Journal of Environmental Science and Technology,2015,13(2):471-482.
|
| 7 |
HOMEM V,SANTOS L.Degradation and removal methods of antibiotics from aqueous matrices—A review[J].J. Environ. Manage.,2011,92:2304-2347.
|
| 8 |
TAN X,LIU Y,ZENG G,et al.Application of biochar for the removal of pollutants from aqueous solutions[J].Chemosphere.2015,125:70-85.
|
| 9 |
LU H,ZHANG W,YANG Y,et al.Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar[J].Water Research,2012,46:854-862.
|
| 10 |
GUPTA V K,NAYAK A.Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles[J].Chemical Engineering Journal,2012,180:81-90.
|
| 11 |
王泽红,陶士杰,于福家,等.天然沸石的改性及其吸附Pb2+、Cu2+的研究[J].东北大学学报(自然科学版),2012,33(11):1637-1640.
|
|
WANG Zehong,TAO Shijie,YU Fujia,et al.Modification of natural zeolite and its adsorption of Pb2 +and Cu2 +[J].Journal of Northeastern University (Natural Science),2012,33(11):1637-1640.
|
| 12 |
BOPARAI H K,JOSEPH M,O’CARROLL D M.Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles[J].Hazardous Material,2011,186:458-465.
|
| 13 |
邱星屏.四氧化三铁磁性纳米粒子的合成及表征[J].厦门大学学报(自然版),1999,38(5):711-715.
|
|
QIU Xingping.Preparation and characterization of Fe3O4 magnetic nano-particles[J].Journal of Xiamen University (Natural Science),1999,38(5):711-715.
|
| 14 |
LI G Y,JIANG Y R,HUANG K L,et al.Kinetics of adsorption of saccharomyces cerevisiae mandelated dehydrogenase on magnetic Fe3O4-chitosan nanoparticles[J].Journal of Colloid and Interface Science,2008,320(1/2/3):11-18.
|
| 15 |
SUN Z H,WANG L F,LIU P P,et al.Magnetically motive porous sphere composite and its excellent properties for the removal of pollutants in water by adsorption and desorption cycles[J].Advanced Materials,2006,18(15):1968-1971.
|
| 16 |
FOLETTO E L,SIMOES J M,MAZUTTI M A,et al.Application of Zn2SnO4, photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight[J].Ceramics International,2013,39(4):4569-4574.
|
| 17 |
BALARK D,MOSTAFAPOUR F,AZARPIRA H,et al.Langmuir, Freundlich, Temkin and Dubinin-radushkevich isotherms studies of equilibrium sorption of ampicilin unto montmorillonite nanoparticles[J].Journal of Pharmaceutical Research International,2017,20(2):1-9.
|
| 18 |
KUNDU S,CHOWDHURY I H,NASKAR M K.Synthesis of hexagonal shaped nanoporous carbon for efficient adsorption of methyl orange dye[J].Journal of Molecular Liquids,2017,234:417-423.
|
| 19 |
MENG J,FENG X,DAI Z,et al.Adsorption characteristics of Cu(Ⅱ) from aqueous solution onto biochar derived from swine manure[J].Environmental Science and Pollution Research,2014,21(11):7035-7046.
|
| 20 |
余伟光,黎吉辉,王敦,等.香蕉茎秆生物炭的制备及其对铜离子的吸附特性[J].化工进展,2017,36(4):1499-1505.
|
|
YU Weiguang,LI Jihui,WANG Dun,et al.The preparation of biochar from pre-oxidation of banana stem and its adsorption of Cu2+[J].Chemical Industry and Engineering Progress,2017,36(4):1499-1505.
|
| 21 |
李云龙,曾安然,李大刚,等.壳聚糖-牡蛎壳粉复合微球的制备及对铜离子吸附性能[J].精细石油化工,2019,36(1):35-39.
|
|
LI Yunlong,ZENG Anran,LI Dagang,et al.Synthesis of chitosan-oyster shell power composite microspheres and absorption of cupricion[J].Speciality Petrochemicals,2019,36(1):35-39.
|
| 22 |
李超,王丽萍,郭昭华,等.粉煤灰酸溶渣合成13X分子筛及其对铜离子吸附性能[J].无机盐工业,2018,50(9):63-66.
|
|
LI Chao,WANG Liping,GUO Zhaohua,et al.Synthesis of 13X zeolite by fly ash acid residue and its adsorption performance to copper ions[J].Inorganic Chemical Industry,2018,50(9):63-66.
|
| 23 |
KENAWY I M,HAFEZ M A H,ISMAIL M A,et al.Adsorption of Cu(Ⅱ), Cd(Ⅱ), Hg(Ⅱ), Pb(Ⅱ) and Zn(Ⅱ) from aqueous single metal solutions by guanyl-modified cellulose[J].International Journal of Biological Macromolecules,2018,107 (B): 1538-1549.
|
| 24 |
MI F L,WU S J,LIN F M.Adsorption of copper(Ⅱ) ions by a chitosan-oxalate complex biosorbent[J].International Journal of Biological Macromolecules,2015,72:136-144.
|
| 25 |
LI Z,XIAO D,GE Y,et al.Surface-functionalized porous lignin for fast and efficient lead removal from aqueous solution[J].ACS Applied Materials & Interfaces,2015,7(27):15000-15009.
|
| 26 |
ASTHANA A,VERMA R,SINGH A K,et al.Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads: a promising adsorbent for removal of Cu(Ⅱ) ions[J].Journal of Environmental Chemical Engineering,2016,4(2):1985-1995.
|
 ),姚培1,2(
),姚培1,2( ),张启蒙1,2,李树白1,2,刘媛1,2,夏守鑫1
),张启蒙1,2,李树白1,2,刘媛1,2,夏守鑫1
 ),Pei YAO1,2(
),Pei YAO1,2( ),Qimeng ZHANG1,2,Shubai LI1,2,Yuan LIU1,2,Shouxin XIA1
),Qimeng ZHANG1,2,Shubai LI1,2,Yuan LIU1,2,Shouxin XIA1