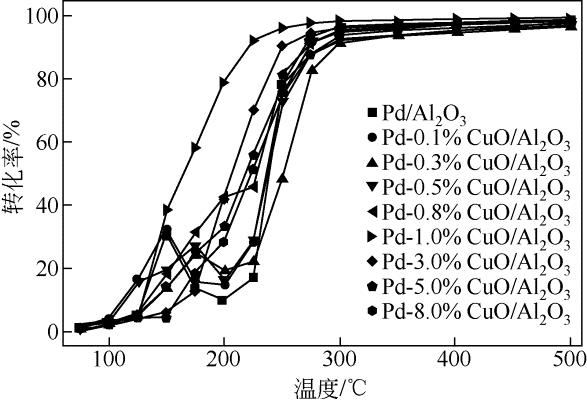
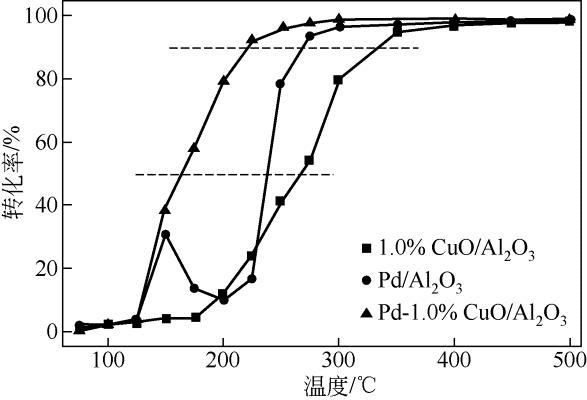
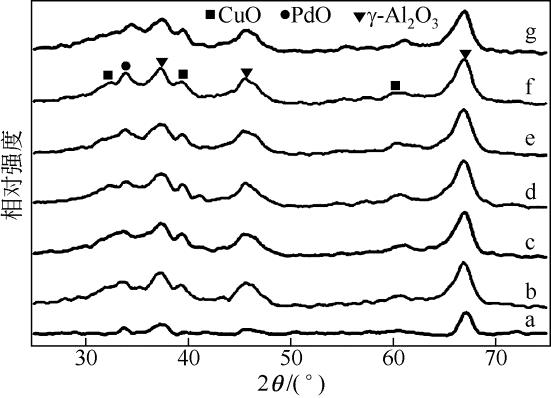
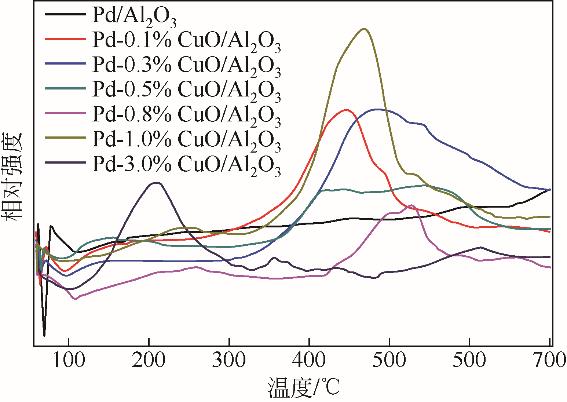
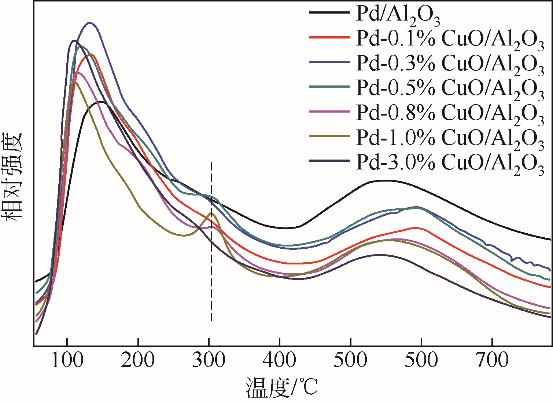
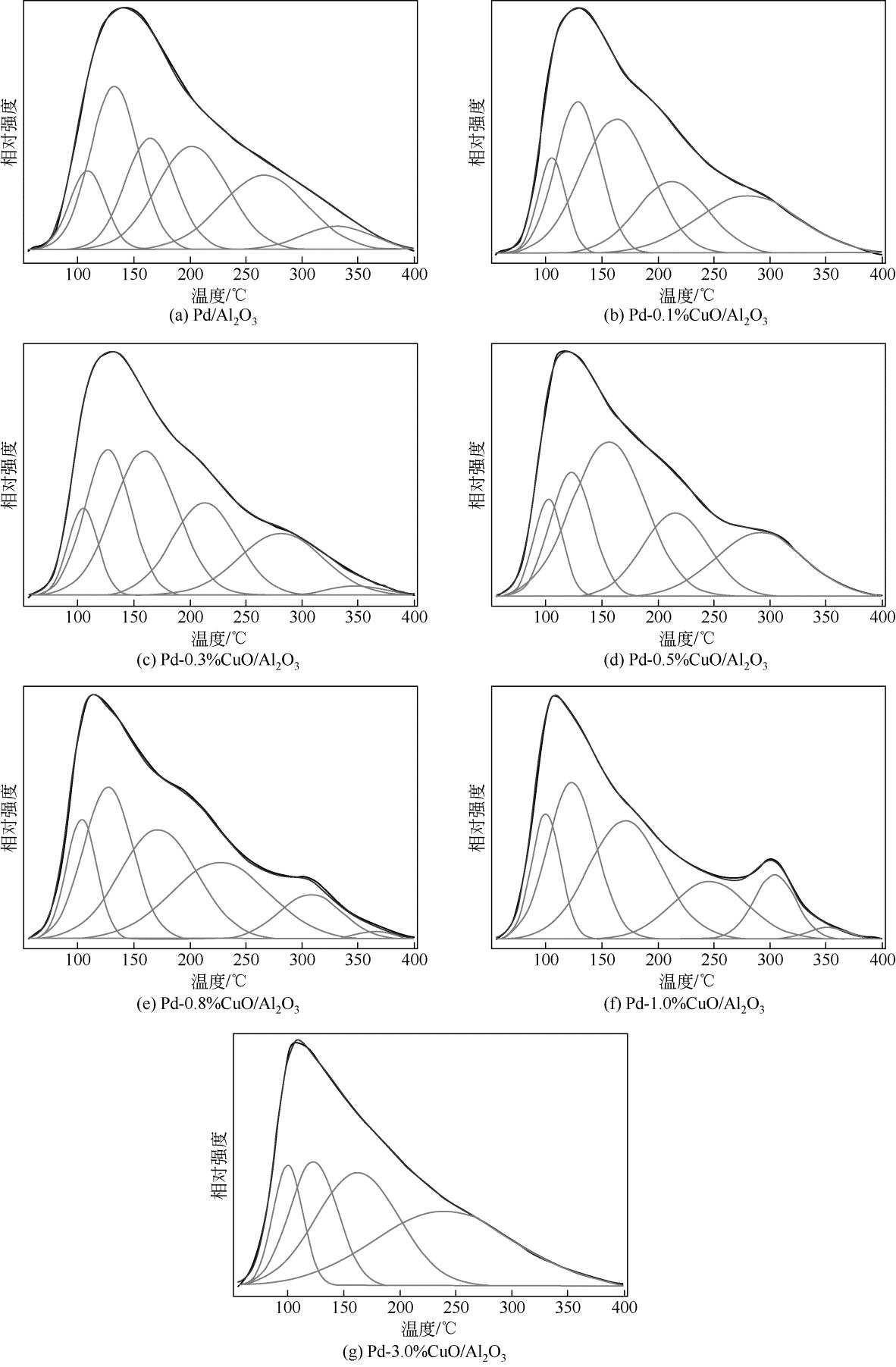
| 1 |
马超, 薛志钢, 李树文, 等 .VOCs排放、污染以及控制对策[J].环境工程技术学报, 2012, 2(2): 103-109.
|
|
Chao MA , XUE Zhigang , LI Shuwen , et al . VOCs emission, pollution and control measures[J]. Journal of Environmental Engineering Technology, 2012, 2(2): 103-109.
|
| 2 |
张桂芹, 姜德超, 李曼, 等 . 城市大气挥发性有机物排放源及来源解析[J]. 环境科学与技术, 2014, 37(120): 195-200.
|
|
ZHANG Guiqin , JIANG Dechao , LI Man , et al . Emission sources and analytical sources of volatile organic compounds in urban atmospheric[J]. Environmental Science & Technology, 2014, 37(120): 195-200.
|
| 3 |
MIRZAEI A , LEONARDI S G , NERI G . Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors:a review[J]. Ceramics International, 2016, 42(14): 15119-15141.
|
| 4 |
李振宇, 李顶杰, 黄格省, 等 . 燃料乙醇发展现状及思考[J]. 化工进展, 2013, 32(7): 1457-1467.
|
|
LI Zhenyu , LI Dingjie , HUANG Gesheng , et al . Insights on current development of fuel ethanol[J]. Chemical Industry and Engineering Progress, 2013, 32(7): 1457-1467.
|
| 5 |
RINTRAMEE K , KARIN F , RUPPRECHTER G , et al . Ethanol adsorption and oxidation on bimetallic catalysts containing platinum and base metal oxide supported on MCM-41[J]. Applied Catalysis B:Environmental, 2012, 115/116:225-235.
|
| 6 |
LI H J , QI G S , TANA, et al . Low-temperature oxidation of ethanol over a Mn0.6Ce0.4O2 mixed oxide[J]. Applied Catalysis B:Environmental, 2011, 103(1/2): 54-61.
|
| 7 |
彭雨程, 王恒, 冯俊小, 等 . 催化燃烧技术处理VOCs的研究进展[J]. 环境与可持续发展, 2015, 40(3): 97-100.
|
|
PENG Yucheng , WANG Heng , FENG Junxiao , et al . Latest researches of catalytic combustion of removing VOCs[J]. Environment and Sustainable Development, 2015, 40(3): 97-100.
|
| 8 |
TSOU J , MAGNOUX P , GUISNET M , et al . Catalytic oxidation of volatile organic compounds:oxidation of methyl-isobutyl-ketone over Pt/zeolite catalysts[J]. Applied Catalysis B:Environmental, 2005, 57(2): 117-123.
|
| 9 |
GARCIA T , AGOURAM S , SÁNCHEZ-ROYO J F , et al . Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route[J]. Applied Catalysis A:General, 2010, 386(1/2): 16-27.
|
| 10 |
ZHENG G , HOHN K L . Catalytic oxidation of methanol on nanoscale copper oxide and nickel oxide[J]. Industrial & Engineering Chemistry Research, 2004, 43(1): 30-35.
|
| 11 |
MERGLER Y J , AALST A V , DELFT J V , et al . CO oxidation over promoted Pt catalysts[J]. Applied Catalysis B:Environmental, 1996, 10(4): 245-261.
|
| 12 |
FERNÁNDEZ G M , MARTI A A , BELVER C , et al . Behavior of palladium-copper catalysts for CO and NO elimination[J]. Journal of Catalysis, 2000, 190(2): 387-395.
|
| 13 |
TIDAHY H L , SIFFERT S , WYRWALSKI F , et al . Catalytic activity of copper and palladium based catalysts for toluene total oxidation[J]. Catalysis Today, 2007, 119(1-4): 317-320.
|
| 14 |
GÜNTER M M , RESSLER T , JENTOFT R E , et al . Redox behavior of copper oxide/zinc oxide catalysts in the steam reforming of methanol studied by in situ X-ray diffraction and absorption spectroscopy[J]. Journal of Catalysis, 2001, 203(1): 133-149.
|
| 15 |
RAJESH H , OZKAN U S . Complete oxidation of ethanol, acetaldehyde and ethanol/methanol mixtures over copper oxide and copper-chromium oxide catalysts[J]. Industrial & Engineering Chemistry Research, 1993, 32(8): 1622-1630.
|
| 16 |
LI W W , QIANG Z M , ZHANG T , et al . Efficient degradation of pyruvic acid in water by catalytic ozonation with PdO/CeO2 [J]. Journal of Molecular Catalysis A:Chemical, 2011, 348(1/2): 70-76.
|
| 17 |
HUANG S Y , ZHANG C B , HE H . Complete oxidation of o-xylene over Pd/Al2O3 catalyst at low temperature[J]. Catalysis Today, 2008, 139(1/2): 15-23.
|
| 18 |
UMAR A , ALSHAHRANI A A , ALGARNI H , et al . CuO nanosheets as potential scaffolds for gas sensing applications[J]. Sensors & Actuators B: Chemical, 2017, 250: 24-31.
|
| 19 |
LOU X R , LIU P F , LI J , et al . Effects of calcination temperature on Mn species and catalytic activities of Mn/ZSM-5 catalyst for selective catalytic reduction of NO with ammonia[J]. Applied Surface Science, 2014, 307:382-387.
|
| 20 |
SANJA P , VLADIMIR S , TAMÁS V , et al . Diversity of Pd-Cu active sites supported on pristine carbon nanotubes in terms of water denitration structure sensitivity[J]. Applied Catalysis A:General, 2018, 559: 187-194.
|
| 21 |
LIU W W , FENG Y S , WANG G Y , et al . Characterization and reactivity of γ-Al2O3 supported Pd-Cu bimetallic nanocatalysts for the selective oxygenization of cyclopentene[J]. Chinese Chemical Letters, 2016, 27:905-909.
|
| 22 |
ZHOU R X , ZHAO B , YUE B H . Effects of CeO2-ZrO2 present in Pd/Al2O3 catalysts on the redox behavior of PdO x and their combustion activity[J]. Applied Surface Science, 2008, 254(15): 4701-4707.
|
| 23 |
MOLENBROEK A M , HAUKKA S , CLAUSEN B S . Alloying in Cu/Pd nanoparticle catalysts[J]. Journal of Physical Chemistry B, 1998, 102(52): 10680-10689.
|
| 24 |
BATISTA J , PINTAR A , MANDRINO D , et al . XPS and TPR examinations of γ alumina-supported Pd-Cu catalysts[J]. Applied Catalysis A:General, 2001, 206(1): 113-124.
|
| 25 |
ZHANG Z X , JIANG Z , SHANGGUAN W F . Low-temperature catalysis for VOCs removal in technology and application:a state-of-the-art review[J]. Catalysis Today, 2016, 264:270-278.
|
| 26 |
LIOTTA L F . Catalytic oxidation of volatile organic compounds on supported noble metals[J]. Applied Catalysis B:Environmental, 2010, 100(3/4): 403-412.
|
| 27 |
DAVIS J L , BARTEAU M A . The influence of oxygen on the selectivity of alcohol conversion on the Pd(111) surface[J]. Surface Science, 1988, 197(1): 123-152.
|
 ),Yuyu GUO,Xingying LI,Zhe LI(
),Yuyu GUO,Xingying LI,Zhe LI( )
)