化工进展 ›› 2019, Vol. 38 ›› Issue (02): 876-884.DOI: 10.16085/j.issn.1000-6613.2018-0188
氯化氢氧化反应催化剂研究进展
石坚( ),杨建明(
),杨建明( ),惠丰,袁俊,梅苏宁,余秦伟,张前,李亚妮,王为强,赵锋伟,吕剑(
),惠丰,袁俊,梅苏宁,余秦伟,张前,李亚妮,王为强,赵锋伟,吕剑( )
)
- 1. 西安近代化学研究所,氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065
-
收稿日期:2018-01-22修回日期:2018-08-24出版日期:2019-02-05发布日期:2019-02-05 -
通讯作者:杨建明,吕剑 -
作者简介:<named-content content-type="corresp-name">石坚</named-content>(1994—),男,硕士研究生,研究方向为工业催化。E-mail:<email>a38860075@126.com</email>。|杨建明,研究员,研究方向为工业催化。E-mail:<email>yangjm204@163.com</email>|吕剑,研究员,研究方向为工业催化。E-mail:<email>Lujian204@263.net</email> -
基金资助:陕西省科技创新统筹创新工程计划(2017ZDXM-GY-073)
Progress on catalysts for catalytic oxidation of hydrogen chloride
Jian SHI( ),Jianming YANG(
),Jianming YANG( ),Feng HUI,Jun YUAN,Suning MEI,Qinwei YU,Qian ZHANG,Yani LI,Weiqiang WANG,Fengwei ZHAO,Jian LÜ(
),Feng HUI,Jun YUAN,Suning MEI,Qinwei YU,Qian ZHANG,Yani LI,Weiqiang WANG,Fengwei ZHAO,Jian LÜ( )
)
- 1. State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi’an Modern Chemistry Research Institute, Xi’an 710065, Shaanxi, China
-
Received:2018-01-22Revised:2018-08-24Online:2019-02-05Published:2019-02-05 -
Contact:Jianming YANG,Jian Lü
摘要:
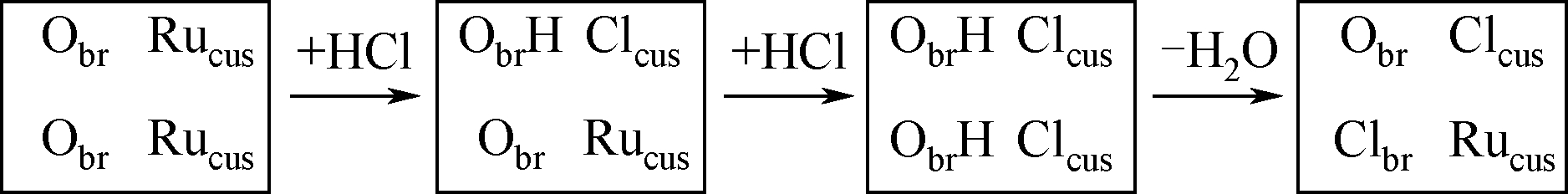
大量副产氯化氢的资源化高效利用是涉氯行业亟需解决的共性难题。氯化氢催化氧化循环制氯气是一个低能耗、可持续发展的有效途径,而催化剂的设计与制备是该过程的核心。本文重点介绍了铜基、钌基和铈基等催化剂,并对各类催化剂的作用机理及其活性、稳定性等性能进行了归纳。不同于铜基催化剂,钌基和铈基等催化剂主要按照Langmuir-Hinshelwood路径催化反应,具有更佳的反应活性及稳定性。基于该反应为放热过程,指出降低反应温度是增强氯化氢转化的关键。此外,活性组分烧结导致分散性变差是钌基和铈基催化剂的主要失活因素。高低温活性、高稳定性的复合氧化物催化剂将是未来本领域重点研究的方向。
中图分类号:
引用本文
石坚, 杨建明, 惠丰, 袁俊, 梅苏宁, 余秦伟, 张前, 李亚妮, 王为强, 赵锋伟, 吕剑. 氯化氢氧化反应催化剂研究进展[J]. 化工进展, 2019, 38(02): 876-884.
Jian SHI, Jianming YANG, Feng HUI, Jun YUAN, Suning MEI, Qinwei YU, Qian ZHANG, Yani LI, Weiqiang WANG, Fengwei ZHAO, Jian LÜ. Progress on catalysts for catalytic oxidation of hydrogen chloride[J]. Chemical Industry and Engineering Progress, 2019, 38(02): 876-884.
| 催化剂 | 质量/g | O2/HCl 摩尔比 | 反应温度 /℃ | 转化率 /% | 氯气产率 /g·gcat -1·h-1 |
|---|---|---|---|---|---|
| CuO | 0.5 | 1 | 450 | 5 | 0.15 |
| 2 | |||||
| 4 | |||||
| RuO2 | 0.25 | 1 | 300 | 59 | 7.2 |
| 2 | 62 | 7.6 | |||
| 4 | 65 | 8 |
表1 铜基与钌基催化剂反应活性[11]
| 催化剂 | 质量/g | O2/HCl 摩尔比 | 反应温度 /℃ | 转化率 /% | 氯气产率 /g·gcat -1·h-1 |
|---|---|---|---|---|---|
| CuO | 0.5 | 1 | 450 | 5 | 0.15 |
| 2 | |||||
| 4 | |||||
| RuO2 | 0.25 | 1 | 300 | 59 | 7.2 |
| 2 | 62 | 7.6 | |||
| 4 | 65 | 8 |
| 催化剂 | E/kJ·mol–1 | 催化剂 | E/kJ·mol–1 |
|---|---|---|---|
| Cu/Ce 复合氧化物 CuAlO2 CeO2 | 82 100 90 | Cr2O3 RuO2/SnO2 | 97 69 |
表2 各催化剂HCl氧化的活化能[50]
| 催化剂 | E/kJ·mol–1 | 催化剂 | E/kJ·mol–1 |
|---|---|---|---|
| Cu/Ce 复合氧化物 CuAlO2 CeO2 | 82 100 90 | Cr2O3 RuO2/SnO2 | 97 69 |
| 催化剂 | 反应条件 (摩尔比、温度) | X HCl /% | STY / | 参考文献 |
|---|---|---|---|---|
| RuO2/SnO2-Al2O3 | HCl∶O2 = 1∶2,T = 320℃ | 34 | 2.65 | [ |
| CeO2 | HCl∶O2 = 1∶2,T = 430℃ | 29 | 0.92 | [ |
| CeO2/ZrO2 | HCl∶O2 = 1∶4,T = 430℃ | 19.4 | 1.23 | [ |
| Ce0.9Zr0.1O x | HCl∶O2 = 1∶9,T = 430℃ | 47.5 | 3.0 | [ |
| Ce0.9Hf0.1O x | HCl∶O2 = 1∶9,T = 430℃ | 49.1 | 3.1 | [ |
| γ-UO3 | HCl∶O2 = 1∶2,T = 500℃ | 22.8 | 0.72 | [ |
| U3O8/ZrO2 | HCl∶O2 = 1∶2,T = 500℃ | 29.3 | 1.85 | [ |
| Cu-K-La/ | HCl∶O2 = 2∶1,T = 360℃ | 83.9 | 0.66 | [ |
| CuCrO2 | HCl∶O2 = 1∶2,T = 380℃ | 6.3 | 0.2 | [ |
| CuCrO2-CeO2 | HCl∶O2 = 1∶2,T = 380℃ | 22.2 | 0.7 | [ |
| CuO-CeO2/Y | HCl∶O2 = 1∶1, T = 410℃ | 73 | 4.45 | [ |
表3 各催化剂HCl催化氧化活性
| 催化剂 | 反应条件 (摩尔比、温度) | X HCl /% | STY / | 参考文献 |
|---|---|---|---|---|
| RuO2/SnO2-Al2O3 | HCl∶O2 = 1∶2,T = 320℃ | 34 | 2.65 | [ |
| CeO2 | HCl∶O2 = 1∶2,T = 430℃ | 29 | 0.92 | [ |
| CeO2/ZrO2 | HCl∶O2 = 1∶4,T = 430℃ | 19.4 | 1.23 | [ |
| Ce0.9Zr0.1O x | HCl∶O2 = 1∶9,T = 430℃ | 47.5 | 3.0 | [ |
| Ce0.9Hf0.1O x | HCl∶O2 = 1∶9,T = 430℃ | 49.1 | 3.1 | [ |
| γ-UO3 | HCl∶O2 = 1∶2,T = 500℃ | 22.8 | 0.72 | [ |
| U3O8/ZrO2 | HCl∶O2 = 1∶2,T = 500℃ | 29.3 | 1.85 | [ |
| Cu-K-La/ | HCl∶O2 = 2∶1,T = 360℃ | 83.9 | 0.66 | [ |
| CuCrO2 | HCl∶O2 = 1∶2,T = 380℃ | 6.3 | 0.2 | [ |
| CuCrO2-CeO2 | HCl∶O2 = 1∶2,T = 380℃ | 22.2 | 0.7 | [ |
| CuO-CeO2/Y | HCl∶O2 = 1∶1, T = 410℃ | 73 | 4.45 | [ |
| 1 | TILL Z , VARGA T , RÉTI J , et al . Optimization strategies in a fixed-bed reactor for HCl oxidation[J].Industrial & Engineering Chemistry Research, 2017, 56(18): 5352-5359. |
| 2 | 毕荣山, 胡明明, 谭心舜, 等 . 光气化反应技术生产异氰酸酯的研究进展[J]. 化工进展, 2017, 36(5): 1565-1572. |
| BI R S , HU M M , TAN X S , et al . Research progress on development of phosgenation reaction technology in isocyanate industry[J].Chemical Industry and Engineering Progress, 2017, 36(5): 1565-1572. | |
| 3 | SEKI K . Development of RuO2/rutile-TiO2 catalyst for industrial HCl oxidation process[J]. Catalysis Surveys from Asia, 2010, 14(3/4): |
| 168-175. | |
| 4 | LI C , SUN Y , DJERDJ I , et al .Shape-controlled CeO2 nanoparticles:stability and activity in the catalyzed HCl oxidation reaction[J]. ACS Catalysis, 2017, 7(10): 6453-6463. |
| 5 | HAMMES M , VALTCHEV M , ROTH M B , et al .A search for alternative Deacon catalysts[J].Applied Catalysis B: Environmental, 2013, 132/133: 389-400. |
| 6 | OVER H , SCHOMÄCKER R .What makes a good catalyst for the Deacon process?[J].ACS Catalysis, 2013, 3(5): 1034-1046. |
| 7 | AMRUTE A P , MONDELLI C , PÉREZ-RAMÍREZ J .Kinetic aspects and deactivation behaviour of chromia-based catalysts in hydrogen chloride oxidation[J].Catalysis Science & Technology, 2012, 2(1): 257-265. |
| 8 | HESS F , OVER H .Rate-determining step or rate-determining configuration? The Deacon reaction over RuO2(110) studied by DFT-based KMC simulations[J].ACS Catalysis, 2017, 7(1): 128-138. |
| 9 | MÖLLER M , OVER H , SMARSLY B , et al .Electrospun ceria-based nanofibers for the facile assessment of catalyst morphological stability under harsh HCl oxidation reaction conditions[J].Catalysis Today, 2015, 253:207-218. |
| 10 | STUDT F , ABILD-PEDERSEN F , HANSEN H A , et al .Volcano relation for the Deacon process over transition-metal oxides[J].ChemCatChem, 2010, 2(1): 98-102. |
| 11 | AMRUTE A P , MONDELLI C , HEVIA M A G , et al .Temporal analysis of productsstudy of HCl oxidation on copper- and ruthenium-based catalysts[J].The Journal of Physical Chemistry C, 2011, 115(4): 1056-1063. |
| 12 | HAMMES M , SOERIJANTO H , SCHOMÄCKER R , et al .Niobium:activator and stabilizer for a copper-based Deacon catalyst[J].ChemCatChem, 2014, 6(1): 245-254. |
| 13 | SUN Y , LI C , GUO Y , et al .Catalytic oxidation of hydrogen chloride to chlorine over Cu-K-Sm/γ-Al2O3 catalyst with excellent catalytic performance[J]. Catalysis Today, 2017.doi.org/10.1016/j.cattod.. 04.014. |
| 14 | FENG K K , LI C W , GUO Y L , et al .Effect of KCl on the performance of Cu-K-La/γ-Al2O3 catalyst for HCl oxidation[J]. Chinese Journal of Catalysis, 2014(8): 1359-1363. |
| 15 | MONDELLI C , AMRUTE A P , SCHMIDT T , et al .A delafossite-based copper catalyst for sustainable Cl2 production by HCl oxidation[J].Chemical Communications, 2011, 47(25): 7173. |
| 16 | AMRUTE A P , LARRAZÁBAL G O , MONDELLI C , et al . CuCrO2delafossite:astable copper catalyst for chlorine production[J].Angewandte Chemie International Edition, 2013, 52(37): 9772-9775. |
| 17 | OVER H . Atomic-scale understanding of the HCl oxidation over RuO2, anovel Deacon process[J]. The Journal of Physical Chemistry C, 2012, 116(12): 6779-6792. |
| 18 | MONDELLI C , AMRUTE A P , KRUMEICH F , et al .Shaped RuO2/SnO2 -Al2O3 catalyst for large-scale stable Cl2 production by HCl oxidation[J].ChemCatChem, 2011, 3(4): 657-660. |
| 19 | GESTERMANN F , OTTAVIANI A . Chlorine production with oxygen-depolarised cathodes on an industrial scale[J]. Modern Chlor-alkali Technology, 2001, 8:49-56. |
| 20 | KIM I H, JEONG M , HAN S W , et al . CO oxidation catalyzed by RuO2 nanoparticles supported on mesoporous Al2O3 prepared via atomic layer deposition[J]. Current Applied Physics, 2016, 16(10): 1407-1412. |
| 21 | HESS F , SACK C , LANGSDORF D , et al .Probing the activity of different oxygen species in the CO oxidation over RuO2(110) by combining transient reflection-absorption infrared spectroscopy with kinetic Monte Carlo simulations[J]. ACS Catalysis, 2017, 7(12): 8420-8428. |
| 22 | ERDTMAN E , ANDERSSON M , LLOYD SPETZ A , et al .Simulations of the thermodynamics and kinetics of NH3 at the RuO2(110) surface[J]. Surface Science, 2017, 656:77-85. |
| 23 | LATIMER A A , ABILD-PEDERSEN F , NØRSKOV J K .A theoretical study of methanol oxidation on RuO2(110):bridging the pressure gap[J]. ACS Catalysis, 2017, 7(7): 4527-4534. |
| 24 | LIU Z , LI C , SRIRAM V , et al .XANES study of elemental mercury oxidation over RuO2/TiO2 and selective catalytic reduction catalysts for mercury emissions control[J]. Fuel Processing Technology, 2016, 153:156-162. |
| 25 | LIU Z , SRIRAM V , LEE J .Heterogeneous oxidation of elemental mercury vapor over RuO2/rutile TiO2 catalyst for mercury emissions control[J]. Applied Catalysis B: Environmental, 2017, 207:143-152. |
| 26 | LÓPEZ N , GÓMEZ-SEGURA J , MARÍN R P , et al .Mechanism of HCl oxidation(Deacon process) over RuO2 [J]. Journal of Catalysis, 2008, 255(1): 29-39. |
| 27 | CRIHAN D , KNAPP M , ZWEIDINGER S , et al .Stable Deacon process for HCl oxidation over RuO2 [J]. Angewandte Chemie International Edition, 2008, 47(11): 2131-2134. |
| 28 | HEVIA M A G , AMRUTE A P , SCHMIDT T , et al . Transient mechanistic study of the gas-phase HCl oxidation to Cl2 on bulk and supported RuO2 catalysts[J]. Journal of Catalysis, 2010, 276(1): 141-151. |
| 29 | KIM Y D, SEITSONEN A P , WENDT S , et al .Characterization of various oxygen species on an oxide surface:RuO2(110)[J]. The Journal of Physical Chemistry B, 2001, 105(18): 3752-3758. |
| 30 | ZWEIDINGER S , CRIHAN D , KNAPP M , et al . Reaction mechanism of the oxidation of HCl over RuO2(110)[J].The Journal of Physical Chemistry C, 2008, 112(27): 9966-9969. |
| 31 | HIBI T , NISHIDA H , ABEKAWA H .Process for producing chlorine:US5871707[P]. 1999-02-16. |
| 32 | IWANAGA K , SEKI K , HIBI T , et al . The development of improved hydrogen chloride oxidation process[J].Sumitomo Kagaku, 2004, 1(1): 4-12. |
| 33 | MOSER M , MONDELLI C , AMRUTE A P , et al .HCl oxidation on IrO2 -based catalysts:from fundamentals to scale-up[J]. ACS Catalysis, 2013, 3(12): 2813-2822. |
| 34 | AMRUTE A P , MONDELLI C , SCHMIDT T , et al . Industrial RuO2-based Deacon catalysts:carrier stabilization and active phase content optimization[J]. ChemCatChem, 2013, 5(3): 748-756. |
| 35 | ESCH F , FABRIS S , ZHOU L , et al . Electron localization determines defect formation on ceria substrates[J].Science, 2005, 309(5735): 752-755. |
| 36 | 陈然, 高晓亚, 王晶, 等 .Ce改性Fe2O3催化剂对CO催化氧化的影响[J].化工进展, 2017, 36(10):3737-3742. |
| CHEN R , GAO X Y , WANG J , et al .Effect of Ce addition on Fe2O3 catalyst towards CO catalytic oxidation[J].Chemical Industry and Engineering Progress, 2017, 36(10):3737-3742. | |
| 37 | CAI W , ZHAO Y , CHEN M , et al .The formation of 3D spherical Cr-Ce mixed oxides with roughness surface and their enhanced low-temperature NO oxidation[J]. Chemical Engineering Journal, 2018, 333:414-422. |
| 38 | LORICERA C V , ALVAREZ-GALVAN M C , CAMPOS C H , et al . Sulfated Ce x Zr1- x O2 oxides.Surface properties and performance for methane oxidation under fuel-rich conditions[J]. Materials Chemistry and Physics, 2017, 200:223-232. |
| 39 | YU H , ZHONG S , ZHU B , et al .Synthesis and CO oxidation activity of 1D mixed binary oxide CeO2-LaO x supported gold catalysts[J].Nanoscale Research Letters, 2017, 12(1): 579. |
| 40 | 侯扶林, 李红欣, 杨阳, 等 . 特定形貌和多孔纳米CeO2的制备及其CO催化氧化研究进展[J]. 化工进展, 2017, 36(7): 2481-2487. |
| HOU F L , LI H X , YANG Y , et al .Preparation and catalytic oxidation of CO with specific morphology andporous nano CeO2 [J]. Chemical Industry and Engineering Progress, 2017, 36(7): 2481-2487. | |
| 41 | AMRUTE A P , MONDELLI C , MOSER M , et al .Performance, structure, and mechanism of CeO2 in HCl oxidation to Cl2 [J].Journal of Catalysis, 2012, 286:287-297. |
| 42 | 谢兴星, 费兆阳, 戴勇, 等 .铈基复合氧化物的结构及其对HCl催化氧化性能的影响[J]. 分子催化, 2014(6): 507-514. |
| XIE X X , FEI Z Y , DAI Y , et al . Structure of ceria-based mixed oxides and its influence on HCl catalytic oxidation performance[J]. Journal of Molecular Catalysis(China), 2014(6): 507-514. | |
| 43 | KANZLER C H , URBAN S , ZALEWSKA-WIERZBICKA K , et al .Electrospun metal oxide nanofibres for the assessment of catalyst morphological stability under harsh reaction conditions[J].ChemCatChem, 2013, 5(9): 2621-2626. |
| 44 | FARRA R , GIRGSDIES F , FRANDSEN W , et al . Synthesis and catalytic performance of CeOCl in Deacon reaction[J]. Catalysis Letters, 2013, 143(10): 1012-1017. |
| 45 | MOSER M , MONDELLI C , SCHMIDT T , et al . Supported CeO2 catalysts in technical form for sustainable chlorine production[J].Applied Catalysis B:Environmental, 2013, 132/133:123-131. |
| 46 | CHEN X , XU X , FEI Z , et al . CeO2 nanodots embedded in a porous silica matrix as an active yet durable catalyst for HCl oxidation[J]. Catalysis Science & Technology, 2016, 6(13): 5116-5123. |
| 47 | 徐希化, 楼家伟, 谢兴星, 等 . 非晶态ZrO2镶嵌的超细CeO2催化HCl氧化[J]. 无机化学学报, 2017, 33(3): 421-428. |
| XU X H , LOU J W , XIE X X , et al .Superfine CeO2 embedded in a porous ZrO2 matrix for catalytic HCl oxidation[J].Chinese Journal of Inorganic Chemistry, 2017, 33(3): 421-428. | |
| 48 | CHEN X , LV G M , TANG J H , et al .Research on preparation of nano complex Ce-Cu-K catalyst loaded in the Y-type zeolite and its performance[J]. J. Chem. Eng. Chin. Univ., 2011, 25(1): 109-113. |
| 49 | CHEN X , DAI Y , FEI Z , et al .HCl oxidation to recycle Cl2 over a Cu/Ce composite oxide catalyst.Part 2. Single-tube-reactor simulation[J]. Industrial & Engineering Chemistry Research, 2015, 54(41): 9931-9937. |
| 50 | TANG J , CHEN X , FEI Z , et al . HCl oxidation to recycle Cl2 over a Cu/Ce composite oxide catalyst.Part 1.Intrinsic kinetic study[J].Industrial & Engineering Chemistry Research, 2013, 52(34): 11897-11903. |
| 51 | DAI Y , FEI Z , XU X , et al .Oxygen consumption rate model in HCl oxidation over a supported CuO-CeO2 composite oxide catalyst under lean oxygen condition[J]. The Canadian Journal of Chemical Engineering, 2016, 94(6): 1140-1147. |
| 52 | 杨成武, 曹烁, 左宜赞, 等 . 氯化氢催化氧化制氯气催化剂的失活与再生[J]. 过程工程学报, 2012(2): 349-352. |
| YANG C W , CAO S , ZUO Y Z , et al .Regeneration of a copper based catalyst for sustainable Cl2 production by HCl oxidation[J]. The Chinese Journal of Process Engineering, 2012(2): 349-352. | |
| 53 | 谢兴星, 费兆阳, 邹冲, 等 .稀土掺杂对氯化氢氧化制氯气CuO-CeO2-SiO2催化剂结构和性能的影响[J].物理化学学报, 2015(6): 1153-1161. |
| XIE X X , FEI Z Y , ZOU C , et al .Effects of rare-earth additives on structures and performances of CuO-CeO2-SiO2 catalysts for recycling Cl2 from HCl oxidation[J]. Acta Physico-Chimica Sinica, 2015(6): 1153-1161. | |
| 54 | 楼家伟, 费兆阳, 刘清, 等 . 氧化铈基HCl氧化循环制Cl2催化剂研究进展[J].化工进展, 2018, 37(5): 1804-1814. |
| LOU J W , FEI Z Y , LIU Q , et al . Research progress in recycle oxidation of HCl to Cl2 over ceria basedcatalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(5): 1804-1814. | |
| 55 | FARRA R , GARCÍA-MELCHOR M , EICHELBAUM M , et al .Promoted ceria:astructural, catalytic, and computational study[J].ACS Catalysis, 2013, 3(10): 2256-2268. |
| 56 | AMRUTE A P , KRUMEICH F , MONDELLI C , et al . Depleted uranium catalysts for chlorine production[J]. Chemical Science, 2013, 4(5): 2209. |
| 57 | FEI Z , LIU H , DAI Y , et al .Efficient catalytic oxidation of HCl to recycle Cl2 over the CuO-CeO2 composite oxide supported on Y type zeolite[J]. Chemical Engineering Journal, 2014, 257: 273-280. |
| 58 | MOSER M , VILE G , COLUSSI S , et al . Structure and reactivity of ceria zirconia catalysts for bromine and chlorine production via the oxidation of hydrogen halides[J]. Journal of Catalysis, 2015, 331: 128-137. |
| 59 | MOSER M , AMRUTE A P , PÉREZ-RAMÍREZ J . Impact of feed impurities on catalysts for chlorine recycling[J]. Applied Catalysis B: Environmental, 2015, 162: 602-609. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [10] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||