Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5765-5777.DOI: 10.16085/j.issn.1000-6613.2023-1588
• Resources and environmental engineering • Previous Articles
Research advances of extraction separation of phenolic compounds by ionic liquids
- School of Chemistry and Chemical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
-
Received:2023-09-07Revised:2023-12-09Online:2024-10-29Published:2024-10-15 -
Contact:QI Yabing
离子液体萃取分离液相中酚类物质的研究进展
- 西安建筑科技大学化学与化工学院,陕西 西安 710055
-
通讯作者:齐亚兵 -
作者简介:齐亚兵(1983—),男,博士,讲师,研究方向为化工分离技术。E-mail: qiyabing123@163.com。 -
基金资助:西安市碑林区科技计划(GX2323);西安建筑科技大学人才科技基金(RC1714);西安建筑科技大学青年科技基金(QN1509)
CLC Number:
Cite this article
QI Yabing, LIU Ziyan. Research advances of extraction separation of phenolic compounds by ionic liquids[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5765-5777.
齐亚兵, 刘子炎. 离子液体萃取分离液相中酚类物质的研究进展[J]. 化工进展, 2024, 43(10): 5765-5777.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1588
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 参考 文献 |
|---|---|---|---|---|---|---|
| [Emim]SCN | 甲苯+正己烷+ 对甲酚 | 对甲酚 | [Emim]SCN的—SCN中的N与甲酚—OH中的H形成较强氢键,实现离子液体对甲酚的萃取。在间、对、邻甲酚3种甲酚异构体中,对甲酚与萃取剂形成的氢键最强 | m甲苯∶m正己烷∶m对甲酚=1∶7∶2,mIL∶m模拟油=0.2,T=298.15K, t=30min | E对甲酚=98.25% | [ |
| [Emim]Lac | 甲苯+己烷+苯酚 | 苯酚 | [Emim]Lac的—COO中的O与苯酚—OH中的H形成较强氢键,实现离子液体对苯酚的萃取 | m甲苯∶m己烷∶m苯酚=1∶7∶2,mIL∶ m模拟油=1,T=298.15K, t=30min | E苯酚=99.9% | [ |
| [Emim]OAc | 异丙基苯+正庚烷+间甲酚 | 间甲酚 | [Emim]OAc的—COO中的O与间甲酚—OH中的H形成较强氢键,实现对间甲酚的萃取 | m异丙基苯∶m正庚烷∶m间甲酚= 4∶2∶4,mIL∶ m模拟油=0.5,T=298.15K, t=60min | 对间甲酚萃取率排序为[Emim]OAc/[Bmim]OAc>[Bmim]Cl>[Bmim]BF4>[Bmim]PF6,E间甲酚=98.85% | [ |
| [Emim]Gly、[Emim]Ala(乙腈为稀释剂) | 己烷+α-生育酚+β-生育酚+γ-生育酚+δ-生育酚 | 生育酚 | 离子液体—COO中的O与生育酚—OH中的H形成较强氢键,离子液体与生育酚异构体形成氢键的强度排序为δ-生育酚>γ-生育酚>α-生育酚,实现对不同生育酚异构体的萃取 | T=303.15K, nIL∶n乙腈=2∶98,cδ-生育酚=1mg/cm3, cβ&γ-生育酚=0.98mg/cm3,cα-生育酚=0.2mg/cm3 | Dδ-生育酚=19.2~20.9, Dβ&γ-生育酚=9.31~10.5,Dα-生育酚=1.51~1.55;Sδ-生育酚/α-生育酚=12.7~13.5,Sβ&γ-生育酚/α-生育酚=6.2~6.8 | [ |
| [Bmim]Cl | 己烷+苯酚或煤液化油馏分 | 苯酚 | 离子液体阴离子与苯酚之间的氢键以及咪唑环与苯环之间的π-π共轭作用实现对苯酚的萃取 | c苯酚=5048mg/L,nIL∶n苯酚=2~3,T=30℃,t=30min | 对苯酚的萃取率排序为[Bmim]Cl>[Bmim]Br>[Bmim]BF4>[Bmim]PF6,E苯酚=99%或92.5% | [ |
| [Bmim]NTf2 | 生物油水相馏分 | 苯酚、愈创木酚、4-甲基愈创木酚 | [Bmim]NTf2的阴离子[NTf2]-中两个S原子上的O与酚类物质—OH中的H形成较强氢键,[Bmim]NTf2与酚类物质之间亦存在较弱的范德华力,实现对水相中酚类物质的萃取 | mIL∶mW =0.4,t=5min | E苯酚=95.41%,E愈创木酚=92.04%,E4-甲基愈创木酚=97.98% | [ |
| [Hemim]Ac、[Hemim]Pro、[Hemim]Lac、[Hemim]Gly | 甲苯+苯酚 | 苯酚 | 利用离子液体与苯酚之间的氢键作用以及离子液体咪唑环与苯酚苯环之间的π-π共轭作用实现对苯酚的萃取,氢键起主要作用 | c苯酚=0.2g/mL,nIL∶n苯酚=1,T=298.15K, t=60min | E苯酚=96.06%~96.98%,离子液体的阴离子对苯酚萃取率影响较小 | [ |
| [OHEmim]NO3 | 正己烷+ (苯酚,对甲酚,间甲酚,邻甲酚) | 苯酚、对甲酚、间甲酚、邻甲酚 | [OHEmim]NO3的[OHEmim]+阳离子—OH中的O、阴离子NO3-中的O与酚类物质—OH中的H均可形成氢键,实现对酚类物质的萃取;[OHEmim]NO3与酚类物质之间的氢键强于[HEmim]NO3与酚类物质之间的氢键 | T=298.15K, p=101.3kPa | S苯酚/正己烷=857~7390,S邻甲酚/正己烷=26~665, S间甲酚/正己烷=241~1289,S对甲酚/正己烷=135~895 | [ |
| DIL1、DIL2、DIL3 | 煤焦油 | 酚类 | 离子液体的一对阴离子Br-与酚类物质—OH中的H之间形成氢键,实现对酚类物质的萃取 | w酚类=33.5%,mIL∶m酚类=0.5 | 与单阳离子ILs相比,DILs在油相中的溶解度小得多,热稳定性也好得多,其对苯酚的萃取能力排序为DIL3>DIL2>DIL1;DIL3对煤焦油中酚类的萃取率为93.1% | [ |
| [ABZIM]Cl、[DBZIM]Cl、[BZVIM]Cl | 苯酚+2,4-二硝基苯酚+2,4-二氯苯酚+芴+荧蒽+ 己烷 | 苯酚、2,4-二硝基苯酚、2,4-二氯苯酚 | Cl-与酚类物质—OH中的H之间的氢键作用,咪唑苯环与酚类苯环之间的π-π共轭作用,实现对酚类物质的萃取 | — | 离子液体的阳离子结构对苯酚的萃取率有一定的影响,萃取率排序为烯丙基>苄基>乙烯基:[ABZIM]Cl为萃取剂时E苯酚=87.72%,E2,4-二硝基苯酚=64.77%,E2,4-二氯苯酚=63.47% | [ |
| [C2Mim]NTf2、 [C4Mim]NTf2、 [C8Mim]NTf2、 [C12Mim]NTf2 | 苯酚溶液 | 苯酚 | [NTf2]-的F与酚类物质—OH中的H之间形成强氢键,实现对酚类物质的萃取 | c苯酚=200mg/L,VIL∶VW=1∶3,pH=2,T=298.15K,t=3min | 给定烷基链长度的离子液体对苯酚的萃取率排序为吡咯烷类>咪唑类>铵盐类;增加咪唑的烷基链长度会降低对苯酚的萃取率,E苯酚=77%~80.9% | [ |
| [CeMim]NO3 | 己烷+苯+(苯酚、间甲酚、对甲酚或邻甲酚) | 苯酚、间甲酚、对甲酚或邻甲酚 | 酚类物质的—OH与离子液体NO3-之间形成氢键,离子液体的羧基与酚类物质通过酸碱配位强化了二者之间的氢键,两种作用共同促进了其对酚类物质的萃取 | m己烷∶m苯∶m酚类=7∶1∶2,mIL∶m模拟油=1∶5,T=298.15K,t=30min | E间甲酚=98.77%,E对甲酚= 98.03%,E邻甲酚=97.65%,E苯酚=98.14% | [ |
| [Bmim]PF6、 [Hmim]PF6、 [Omim]PF6 | 苯酚、对苯二酚、邻甲酚或邻硝基苯酚溶液 | 苯酚、对苯二酚、邻甲酚、邻硝基苯酚 | — | — | 亲水性酚类苯酚和对苯二酚,随咪唑类离子液体阳离子碳链长度增加,对酚类物质的萃取率降低;对于疏水性酚类邻甲酚和邻硝基苯酚,情况恰好相反 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 参考 文献 |
|---|---|---|---|---|---|---|
| [Emim]SCN | 甲苯+正己烷+ 对甲酚 | 对甲酚 | [Emim]SCN的—SCN中的N与甲酚—OH中的H形成较强氢键,实现离子液体对甲酚的萃取。在间、对、邻甲酚3种甲酚异构体中,对甲酚与萃取剂形成的氢键最强 | m甲苯∶m正己烷∶m对甲酚=1∶7∶2,mIL∶m模拟油=0.2,T=298.15K, t=30min | E对甲酚=98.25% | [ |
| [Emim]Lac | 甲苯+己烷+苯酚 | 苯酚 | [Emim]Lac的—COO中的O与苯酚—OH中的H形成较强氢键,实现离子液体对苯酚的萃取 | m甲苯∶m己烷∶m苯酚=1∶7∶2,mIL∶ m模拟油=1,T=298.15K, t=30min | E苯酚=99.9% | [ |
| [Emim]OAc | 异丙基苯+正庚烷+间甲酚 | 间甲酚 | [Emim]OAc的—COO中的O与间甲酚—OH中的H形成较强氢键,实现对间甲酚的萃取 | m异丙基苯∶m正庚烷∶m间甲酚= 4∶2∶4,mIL∶ m模拟油=0.5,T=298.15K, t=60min | 对间甲酚萃取率排序为[Emim]OAc/[Bmim]OAc>[Bmim]Cl>[Bmim]BF4>[Bmim]PF6,E间甲酚=98.85% | [ |
| [Emim]Gly、[Emim]Ala(乙腈为稀释剂) | 己烷+α-生育酚+β-生育酚+γ-生育酚+δ-生育酚 | 生育酚 | 离子液体—COO中的O与生育酚—OH中的H形成较强氢键,离子液体与生育酚异构体形成氢键的强度排序为δ-生育酚>γ-生育酚>α-生育酚,实现对不同生育酚异构体的萃取 | T=303.15K, nIL∶n乙腈=2∶98,cδ-生育酚=1mg/cm3, cβ&γ-生育酚=0.98mg/cm3,cα-生育酚=0.2mg/cm3 | Dδ-生育酚=19.2~20.9, Dβ&γ-生育酚=9.31~10.5,Dα-生育酚=1.51~1.55;Sδ-生育酚/α-生育酚=12.7~13.5,Sβ&γ-生育酚/α-生育酚=6.2~6.8 | [ |
| [Bmim]Cl | 己烷+苯酚或煤液化油馏分 | 苯酚 | 离子液体阴离子与苯酚之间的氢键以及咪唑环与苯环之间的π-π共轭作用实现对苯酚的萃取 | c苯酚=5048mg/L,nIL∶n苯酚=2~3,T=30℃,t=30min | 对苯酚的萃取率排序为[Bmim]Cl>[Bmim]Br>[Bmim]BF4>[Bmim]PF6,E苯酚=99%或92.5% | [ |
| [Bmim]NTf2 | 生物油水相馏分 | 苯酚、愈创木酚、4-甲基愈创木酚 | [Bmim]NTf2的阴离子[NTf2]-中两个S原子上的O与酚类物质—OH中的H形成较强氢键,[Bmim]NTf2与酚类物质之间亦存在较弱的范德华力,实现对水相中酚类物质的萃取 | mIL∶mW =0.4,t=5min | E苯酚=95.41%,E愈创木酚=92.04%,E4-甲基愈创木酚=97.98% | [ |
| [Hemim]Ac、[Hemim]Pro、[Hemim]Lac、[Hemim]Gly | 甲苯+苯酚 | 苯酚 | 利用离子液体与苯酚之间的氢键作用以及离子液体咪唑环与苯酚苯环之间的π-π共轭作用实现对苯酚的萃取,氢键起主要作用 | c苯酚=0.2g/mL,nIL∶n苯酚=1,T=298.15K, t=60min | E苯酚=96.06%~96.98%,离子液体的阴离子对苯酚萃取率影响较小 | [ |
| [OHEmim]NO3 | 正己烷+ (苯酚,对甲酚,间甲酚,邻甲酚) | 苯酚、对甲酚、间甲酚、邻甲酚 | [OHEmim]NO3的[OHEmim]+阳离子—OH中的O、阴离子NO3-中的O与酚类物质—OH中的H均可形成氢键,实现对酚类物质的萃取;[OHEmim]NO3与酚类物质之间的氢键强于[HEmim]NO3与酚类物质之间的氢键 | T=298.15K, p=101.3kPa | S苯酚/正己烷=857~7390,S邻甲酚/正己烷=26~665, S间甲酚/正己烷=241~1289,S对甲酚/正己烷=135~895 | [ |
| DIL1、DIL2、DIL3 | 煤焦油 | 酚类 | 离子液体的一对阴离子Br-与酚类物质—OH中的H之间形成氢键,实现对酚类物质的萃取 | w酚类=33.5%,mIL∶m酚类=0.5 | 与单阳离子ILs相比,DILs在油相中的溶解度小得多,热稳定性也好得多,其对苯酚的萃取能力排序为DIL3>DIL2>DIL1;DIL3对煤焦油中酚类的萃取率为93.1% | [ |
| [ABZIM]Cl、[DBZIM]Cl、[BZVIM]Cl | 苯酚+2,4-二硝基苯酚+2,4-二氯苯酚+芴+荧蒽+ 己烷 | 苯酚、2,4-二硝基苯酚、2,4-二氯苯酚 | Cl-与酚类物质—OH中的H之间的氢键作用,咪唑苯环与酚类苯环之间的π-π共轭作用,实现对酚类物质的萃取 | — | 离子液体的阳离子结构对苯酚的萃取率有一定的影响,萃取率排序为烯丙基>苄基>乙烯基:[ABZIM]Cl为萃取剂时E苯酚=87.72%,E2,4-二硝基苯酚=64.77%,E2,4-二氯苯酚=63.47% | [ |
| [C2Mim]NTf2、 [C4Mim]NTf2、 [C8Mim]NTf2、 [C12Mim]NTf2 | 苯酚溶液 | 苯酚 | [NTf2]-的F与酚类物质—OH中的H之间形成强氢键,实现对酚类物质的萃取 | c苯酚=200mg/L,VIL∶VW=1∶3,pH=2,T=298.15K,t=3min | 给定烷基链长度的离子液体对苯酚的萃取率排序为吡咯烷类>咪唑类>铵盐类;增加咪唑的烷基链长度会降低对苯酚的萃取率,E苯酚=77%~80.9% | [ |
| [CeMim]NO3 | 己烷+苯+(苯酚、间甲酚、对甲酚或邻甲酚) | 苯酚、间甲酚、对甲酚或邻甲酚 | 酚类物质的—OH与离子液体NO3-之间形成氢键,离子液体的羧基与酚类物质通过酸碱配位强化了二者之间的氢键,两种作用共同促进了其对酚类物质的萃取 | m己烷∶m苯∶m酚类=7∶1∶2,mIL∶m模拟油=1∶5,T=298.15K,t=30min | E间甲酚=98.77%,E对甲酚= 98.03%,E邻甲酚=97.65%,E苯酚=98.14% | [ |
| [Bmim]PF6、 [Hmim]PF6、 [Omim]PF6 | 苯酚、对苯二酚、邻甲酚或邻硝基苯酚溶液 | 苯酚、对苯二酚、邻甲酚、邻硝基苯酚 | — | — | 亲水性酚类苯酚和对苯二酚,随咪唑类离子液体阳离子碳链长度增加,对酚类物质的萃取率降低;对于疏水性酚类邻甲酚和邻硝基苯酚,情况恰好相反 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [Ch]Pro、[Ch]Lac、 [Ch]Gly、[Ch]Ac | 甲苯+苯酚 | 苯酚 | 离子液体与酚类之间的氢键实现对酚类的萃取 | c苯酚=0.2g/mL,nIL∶n苯酚=1,T=298.15K, t=60min | 在阴离子相同时,不同类型阳离子对苯酚的萃取率排序为[Ch]>[Hemim],[Ch]Pro对苯酚的萃取率最高,为99.49% | [ |
| [Ch]C2OO、[Ch]C8OO、 [Ch]C12OO、[Ch]C14OO (二甲基亚砜为稀释剂) | 间甲酚+对甲酚+ 2,6-二甲酚+ 正己烷 | 间甲酚、 对甲酚、 2,6-二甲酚 | 离子液体与酚类之间的氢键实现对酚类的萃取 | T=25℃,V原料液∶V萃取剂= 1∶1,c酚=5mg/mL,t=2h | 随阴离子碳链的增长,离子液体对亲水性较强的间甲酚、对甲酚的萃取分配系数减小;离子液体对亲水性较弱的2,6-二甲酚萃取分配系数增大 | [ |
| [Ch]NTf2 | 苯酚(愈创木酚、丁香酚、邻苯二酚)溶液 | 苯酚、愈创木酚、丁香酚、邻苯二酚 | — | T=295.15K,p=0.1MPa,t=48h | D苯酚=18.59~26.13,D愈创木酚=59.55~166,D丁香酚=92~218,D邻苯二酚=2.07~12;S苯酚=27.8~41.3,S愈创木酚=175.1~232.4,S丁香酚=165.1~309.4,S邻苯二酚=2.6~16.8 | [ |
| 19种羧酸胆碱类离子液体 | 苯酚+甲苯 | 苯酚 | 离子液体阴阳离子与酚类物质—OH之间形成氢键实现对酚类物质的萃取,阴离子的羧基与酚羟基之间的氢键起主要作用 | c苯酚=0.2g/mL,t=30min | 阴离子的结构和烷基碳链长度对苯酚的萃取有重要影响;二元羧酸胆碱对苯酚的萃取率高于一元羧酸胆碱;苯酚的萃取率随阴离子烷基碳链长度增加而降低,随离子液体亲水性增加而提高;E苯酚=86%~99% | [ |
| [Ch]Lac、[Ch]Alic、 [Ch]HOCH2COO、 [Ch]BrCOO、[Ch]Pro、 [Ch]2C4H6(COO)2 | 苯酚+甲苯 | 苯酚 | — | c苯酚=0.2g/mL,t=30min | 苯甲酸类胆碱取代基的种类和位置均会对苯酚的萃取产生影响,离子液体对苯酚的萃取分配系数排序为[Ch]2C4H6(COO)2>[Ch]HOCH2COO>[Ch]Lac>[Ch]Pro>[Ch]Alic>[Ch]BrCOO | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [Ch]Pro、[Ch]Lac、 [Ch]Gly、[Ch]Ac | 甲苯+苯酚 | 苯酚 | 离子液体与酚类之间的氢键实现对酚类的萃取 | c苯酚=0.2g/mL,nIL∶n苯酚=1,T=298.15K, t=60min | 在阴离子相同时,不同类型阳离子对苯酚的萃取率排序为[Ch]>[Hemim],[Ch]Pro对苯酚的萃取率最高,为99.49% | [ |
| [Ch]C2OO、[Ch]C8OO、 [Ch]C12OO、[Ch]C14OO (二甲基亚砜为稀释剂) | 间甲酚+对甲酚+ 2,6-二甲酚+ 正己烷 | 间甲酚、 对甲酚、 2,6-二甲酚 | 离子液体与酚类之间的氢键实现对酚类的萃取 | T=25℃,V原料液∶V萃取剂= 1∶1,c酚=5mg/mL,t=2h | 随阴离子碳链的增长,离子液体对亲水性较强的间甲酚、对甲酚的萃取分配系数减小;离子液体对亲水性较弱的2,6-二甲酚萃取分配系数增大 | [ |
| [Ch]NTf2 | 苯酚(愈创木酚、丁香酚、邻苯二酚)溶液 | 苯酚、愈创木酚、丁香酚、邻苯二酚 | — | T=295.15K,p=0.1MPa,t=48h | D苯酚=18.59~26.13,D愈创木酚=59.55~166,D丁香酚=92~218,D邻苯二酚=2.07~12;S苯酚=27.8~41.3,S愈创木酚=175.1~232.4,S丁香酚=165.1~309.4,S邻苯二酚=2.6~16.8 | [ |
| 19种羧酸胆碱类离子液体 | 苯酚+甲苯 | 苯酚 | 离子液体阴阳离子与酚类物质—OH之间形成氢键实现对酚类物质的萃取,阴离子的羧基与酚羟基之间的氢键起主要作用 | c苯酚=0.2g/mL,t=30min | 阴离子的结构和烷基碳链长度对苯酚的萃取有重要影响;二元羧酸胆碱对苯酚的萃取率高于一元羧酸胆碱;苯酚的萃取率随阴离子烷基碳链长度增加而降低,随离子液体亲水性增加而提高;E苯酚=86%~99% | [ |
| [Ch]Lac、[Ch]Alic、 [Ch]HOCH2COO、 [Ch]BrCOO、[Ch]Pro、 [Ch]2C4H6(COO)2 | 苯酚+甲苯 | 苯酚 | — | c苯酚=0.2g/mL,t=30min | 苯甲酸类胆碱取代基的种类和位置均会对苯酚的萃取产生影响,离子液体对苯酚的萃取分配系数排序为[Ch]2C4H6(COO)2>[Ch]HOCH2COO>[Ch]Lac>[Ch]Pro>[Ch]Alic>[Ch]BrCOO | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| TPAC | 间甲酚+异丙苯 | 间甲酚 | Cl-与间甲酚之间形成较强氢键实现对间甲酚的萃取 | 常压,T=25℃ | 阴离子不变时,随阳离子碳链长度的增加,季铵盐类离子液体对间甲酚的萃取效果呈先上升后下降的趋势;离子液体对间甲酚的分离性能排序为[Emim]OAc>[Bmim]OAc>TPAC>[Bmim]Cl | [ |
| [HMEA]L | 甲苯+正己烷+ 邻甲酚(间甲酚、对甲酚) | 邻甲酚、间甲酚、对甲酚 | 离子液体与甲酚之间的氢键、酸碱配位和静电相互作用实现对甲酚的萃取 | m甲酚∶m甲苯∶m正己烷=2∶1∶7,mIL∶m模拟油=1∶5,T=298.15K, t=30min | E邻甲酚=84.53%,E间甲酚=94.44%,E对甲酚=96.83%;D邻甲酚=5.46,D间甲酚=16.98,D对甲酚=30.5 | [ |
| [TOA]3,4-DMB、 [TOA]4-TBB、 [TOA]4-PB | 苯酚溶液 | 苯酚 | 离子液体羧酸阴离子中的O与苯酚羟基之间形成氢键实现对苯酚的萃取 | VIL∶VW=1∶5,c苯酚=3000mg/L,pH=5,T=298K,t=5min | [TOA]4-PB对苯酚的萃取性能最佳;E苯酚>95% | [ |
| HEDBr、HPDBr、 HBDBr | 煤焦油 | 苯酚 | 离子液体与苯酚之间形成氢键实现对苯酚的萃取 | nIL∶n苯酚=0.3,t=5min | HPDBr对苯酚的萃取率最高,E苯酚=92.7%;双阳离子型离子液体的热稳定性更高,在原料液中的溶解度更小 | [ |
| [PA]FA、[PA]AC | 间甲酚+己烷 | 间甲酚 | 在离子液体的氨基、羧基与间甲酚之间的氢键以及与间甲酚间的静电相互作用下实现对间甲酚的萃取 | m间甲酚∶m己烷=2∶8,mIL∶m模拟油=0.2,T=298.15K | [PA]FA的萃取率更高;E间甲酚=97.8% | [ |
| [HMEA]FA、[HDEA]FA、[HTEA]FA、[HMEA]AC、[HDEA]AC、[HTEA]AC | 苯酚+正己烷+甲苯 | 苯酚 | 醇胺类离子液体与苯酚之间形成氢键实现对苯酚的萃取 | m苯酚∶m正己烷∶m甲苯=2∶7∶1,mIL∶m模拟油=1∶10,T=25℃,t=20min | 6种离子液体对苯酚的萃取率均大于95%;醇胺类离子液体对苯酚的萃取率大于多元醇 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| TPAC | 间甲酚+异丙苯 | 间甲酚 | Cl-与间甲酚之间形成较强氢键实现对间甲酚的萃取 | 常压,T=25℃ | 阴离子不变时,随阳离子碳链长度的增加,季铵盐类离子液体对间甲酚的萃取效果呈先上升后下降的趋势;离子液体对间甲酚的分离性能排序为[Emim]OAc>[Bmim]OAc>TPAC>[Bmim]Cl | [ |
| [HMEA]L | 甲苯+正己烷+ 邻甲酚(间甲酚、对甲酚) | 邻甲酚、间甲酚、对甲酚 | 离子液体与甲酚之间的氢键、酸碱配位和静电相互作用实现对甲酚的萃取 | m甲酚∶m甲苯∶m正己烷=2∶1∶7,mIL∶m模拟油=1∶5,T=298.15K, t=30min | E邻甲酚=84.53%,E间甲酚=94.44%,E对甲酚=96.83%;D邻甲酚=5.46,D间甲酚=16.98,D对甲酚=30.5 | [ |
| [TOA]3,4-DMB、 [TOA]4-TBB、 [TOA]4-PB | 苯酚溶液 | 苯酚 | 离子液体羧酸阴离子中的O与苯酚羟基之间形成氢键实现对苯酚的萃取 | VIL∶VW=1∶5,c苯酚=3000mg/L,pH=5,T=298K,t=5min | [TOA]4-PB对苯酚的萃取性能最佳;E苯酚>95% | [ |
| HEDBr、HPDBr、 HBDBr | 煤焦油 | 苯酚 | 离子液体与苯酚之间形成氢键实现对苯酚的萃取 | nIL∶n苯酚=0.3,t=5min | HPDBr对苯酚的萃取率最高,E苯酚=92.7%;双阳离子型离子液体的热稳定性更高,在原料液中的溶解度更小 | [ |
| [PA]FA、[PA]AC | 间甲酚+己烷 | 间甲酚 | 在离子液体的氨基、羧基与间甲酚之间的氢键以及与间甲酚间的静电相互作用下实现对间甲酚的萃取 | m间甲酚∶m己烷=2∶8,mIL∶m模拟油=0.2,T=298.15K | [PA]FA的萃取率更高;E间甲酚=97.8% | [ |
| [HMEA]FA、[HDEA]FA、[HTEA]FA、[HMEA]AC、[HDEA]AC、[HTEA]AC | 苯酚+正己烷+甲苯 | 苯酚 | 醇胺类离子液体与苯酚之间形成氢键实现对苯酚的萃取 | m苯酚∶m正己烷∶m甲苯=2∶7∶1,mIL∶m模拟油=1∶10,T=25℃,t=20min | 6种离子液体对苯酚的萃取率均大于95%;醇胺类离子液体对苯酚的萃取率大于多元醇 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [P66614]Cl | 腰果、大米工业废水 | 酚类物质 | — | c酚类=96或32mg/L,pH=7, t=10min | E酚类=100% | [ |
| [P66614]Br(乙酸 乙酯为稀释剂) | 苯酚(4-氯苯酚、间苯三酚或2,5-二硝基苯酚)溶液 | 苯酚、4-氯苯酚、间苯 三酚、2,5-二硝基苯酚 | 离子液体与酚类物质之间形成较强氢键,实现对酚类物质的萃取 | nIL∶n乙酸乙酯=1∶4,mW∶m萃取剂=3∶1,T=25℃,t=2h | D苯酚=345,D4-氯苯酚=1492,D2,5-二硝基苯酚>450,D间苯三酚=100 | [ |
| [P66614]C7H15COO | 苯酚(对甲酚)溶液 | 苯酚、对甲酚 | 离子液体与酚类物质之间形成较强氢键,此氢键具有较强碱性,实现对酚类物质的萃取 | — | D苯酚=4818,D对甲酚=338 | [ |
| [P66614](i-C8)POO、[P66614]C9H19COO | 苯酚(2,4-二氯苯酚)溶液 | 苯酚、2,4-二氯苯酚 | — | c苯酚=c2,4-二氯苯酚=1000mg/L,VW∶VIL=135,t=10min | E2,4-二氯苯酚=99%,E苯酚=89% | [ |
| [THTDP]FeCl4 | 苯酚+4-硝基苯酚+2-氯酚+ 4-氯酚+2,4-二氯酚+3,5-二氯酚+五氯苯酚+2-苯甲基-4-氯苯酚+甲醇 | 苯酚、4-硝基苯酚、2-氯酚、4-氯酚、2,4-二氯酚、3,5-二氯酚、五氯苯酚、2-苯甲基-4-氯苯酚 | — | — | 在酸性条件下酚类物质的分配系数较高;含氯或硝基取代基数目多的酚类物质分配系数更高 | [ |
[THTDP]BF4、[TBMP]C9H19COO、[TBHP]C9H19COO等96种季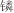 类离子液体 类离子液体 | 邻甲酚(间甲酚、对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | 季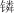 类离子液体对酚类物质的萃取选择性与离子液体阳离子有关,排序为[THTDP]+>[TBMP]+>[TMTDP]+>[TBHP]+,对3种甲酚选择性排序为间甲酚>对甲酚>邻甲酚,[THTDP]BF4对3种甲酚的选择性最高 类离子液体对酚类物质的萃取选择性与离子液体阳离子有关,排序为[THTDP]+>[TBMP]+>[TMTDP]+>[TBHP]+,对3种甲酚选择性排序为间甲酚>对甲酚>邻甲酚,[THTDP]BF4对3种甲酚的选择性最高 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [P66614]Cl | 腰果、大米工业废水 | 酚类物质 | — | c酚类=96或32mg/L,pH=7, t=10min | E酚类=100% | [ |
| [P66614]Br(乙酸 乙酯为稀释剂) | 苯酚(4-氯苯酚、间苯三酚或2,5-二硝基苯酚)溶液 | 苯酚、4-氯苯酚、间苯 三酚、2,5-二硝基苯酚 | 离子液体与酚类物质之间形成较强氢键,实现对酚类物质的萃取 | nIL∶n乙酸乙酯=1∶4,mW∶m萃取剂=3∶1,T=25℃,t=2h | D苯酚=345,D4-氯苯酚=1492,D2,5-二硝基苯酚>450,D间苯三酚=100 | [ |
| [P66614]C7H15COO | 苯酚(对甲酚)溶液 | 苯酚、对甲酚 | 离子液体与酚类物质之间形成较强氢键,此氢键具有较强碱性,实现对酚类物质的萃取 | — | D苯酚=4818,D对甲酚=338 | [ |
| [P66614](i-C8)POO、[P66614]C9H19COO | 苯酚(2,4-二氯苯酚)溶液 | 苯酚、2,4-二氯苯酚 | — | c苯酚=c2,4-二氯苯酚=1000mg/L,VW∶VIL=135,t=10min | E2,4-二氯苯酚=99%,E苯酚=89% | [ |
| [THTDP]FeCl4 | 苯酚+4-硝基苯酚+2-氯酚+ 4-氯酚+2,4-二氯酚+3,5-二氯酚+五氯苯酚+2-苯甲基-4-氯苯酚+甲醇 | 苯酚、4-硝基苯酚、2-氯酚、4-氯酚、2,4-二氯酚、3,5-二氯酚、五氯苯酚、2-苯甲基-4-氯苯酚 | — | — | 在酸性条件下酚类物质的分配系数较高;含氯或硝基取代基数目多的酚类物质分配系数更高 | [ |
[THTDP]BF4、[TBMP]C9H19COO、[TBHP]C9H19COO等96种季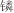 类离子液体 类离子液体 | 邻甲酚(间甲酚、对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | 季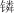 类离子液体对酚类物质的萃取选择性与离子液体阳离子有关,排序为[THTDP]+>[TBMP]+>[TMTDP]+>[TBHP]+,对3种甲酚选择性排序为间甲酚>对甲酚>邻甲酚,[THTDP]BF4对3种甲酚的选择性最高 类离子液体对酚类物质的萃取选择性与离子液体阳离子有关,排序为[THTDP]+>[TBMP]+>[TMTDP]+>[TBHP]+,对3种甲酚选择性排序为间甲酚>对甲酚>邻甲酚,[THTDP]BF4对3种甲酚的选择性最高 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [EPY]Cl、 [OPY]C9H19COO等 96种吡啶类离子液体 | 邻甲酚(间甲酚、 对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | [EPY]Cl和[OPY]C9H19COO对间甲酚的萃取选择性较高,[OPY]C9H19COO对邻甲酚和对甲酚的萃取选择性最高 | [ |
| [C4Py]NTf2、 [C4C1Py]NTf2 | 苯酚、邻甲酚、 2-氯酚溶液 | 苯酚、邻甲酚、2-氯酚 | — | c酚类=15000mg/L,VIL∶VW=1∶2,T=298.15K,pH=6,t=120min | 两种离子液体对3种酚的萃取率均大于94%,对酚类的萃取率排序为2-氯酚>邻甲酚>苯酚 | [ |
| [C2Py]N(CN)2 | 低温煤焦油馏出油 | 酚类物质 | 离子液体与酚类物质之间形成氢键实现对酚类物质的萃取 | mIL∶m馏出油=1∶1,w酚类=28%,T=25℃,t=1h | E酚类=99.99% | [ |
| [C2OH-4-pic]NO3 | 间甲酚(邻甲酚、 对甲酚或苯酚)+ 苯+己烷 | 间甲酚、邻甲酚、对甲酚、苯酚 | 在离子液体与间甲酚间的范德华力、静电作用、氢键共同作用下,实现对间甲酚的萃取,氢键起主要作用 | m酚类∶m苯∶m己烷=2∶1∶7,mIL∶m模拟油=1∶5,T=298.15K,t=30min | E间甲酚=98.99%,E邻甲酚=98.39%,E对甲酚=98.65%,E苯酚=99.99% | [ |
| [Et2NEmpic]Cl2 | 苯酚+甲苯 | 苯酚 | Cl-与酚羟基之间形成氢键实现对苯酚的萃取 | c苯酚=200g/dm3,nIL∶n苯酚=0.6,T=25℃,t=15min | 与[Bmin]Cl相比,达到相同的苯酚萃取率,[Et2NEmpic]Cl2所需的萃取剂更少,E苯酚=96% | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [EPY]Cl、 [OPY]C9H19COO等 96种吡啶类离子液体 | 邻甲酚(间甲酚、 对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | [EPY]Cl和[OPY]C9H19COO对间甲酚的萃取选择性较高,[OPY]C9H19COO对邻甲酚和对甲酚的萃取选择性最高 | [ |
| [C4Py]NTf2、 [C4C1Py]NTf2 | 苯酚、邻甲酚、 2-氯酚溶液 | 苯酚、邻甲酚、2-氯酚 | — | c酚类=15000mg/L,VIL∶VW=1∶2,T=298.15K,pH=6,t=120min | 两种离子液体对3种酚的萃取率均大于94%,对酚类的萃取率排序为2-氯酚>邻甲酚>苯酚 | [ |
| [C2Py]N(CN)2 | 低温煤焦油馏出油 | 酚类物质 | 离子液体与酚类物质之间形成氢键实现对酚类物质的萃取 | mIL∶m馏出油=1∶1,w酚类=28%,T=25℃,t=1h | E酚类=99.99% | [ |
| [C2OH-4-pic]NO3 | 间甲酚(邻甲酚、 对甲酚或苯酚)+ 苯+己烷 | 间甲酚、邻甲酚、对甲酚、苯酚 | 在离子液体与间甲酚间的范德华力、静电作用、氢键共同作用下,实现对间甲酚的萃取,氢键起主要作用 | m酚类∶m苯∶m己烷=2∶1∶7,mIL∶m模拟油=1∶5,T=298.15K,t=30min | E间甲酚=98.99%,E邻甲酚=98.39%,E对甲酚=98.65%,E苯酚=99.99% | [ |
| [Et2NEmpic]Cl2 | 苯酚+甲苯 | 苯酚 | Cl-与酚羟基之间形成氢键实现对苯酚的萃取 | c苯酚=200g/dm3,nIL∶n苯酚=0.6,T=25℃,t=15min | 与[Bmin]Cl相比,达到相同的苯酚萃取率,[Et2NEmpic]Cl2所需的萃取剂更少,E苯酚=96% | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [HMPL]Cl、 [EMPL]C9H19COO等 | 邻甲酚(间甲酚、对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | 阳离子类型对吡咯烷类离子液体萃取酚类物质选择性排序为[HMPL]+>[EMPL]+,阳离子为[HMPL]+时,阴离子为Cl-时对酚类物质的萃取选择性最高;对3种甲酚萃取选择性排序为间甲酚>邻甲酚>对甲酚。 | [ |
| [C4C1Pyr]NTf2、 [C4C1Pyr]Nf2 | 苯酚、邻甲酚、2-氯酚溶液 | 苯酚、邻甲酚、2-氯酚 | — | c酚类=15000mg/L,VIL∶VW=1∶2,T=298.15K,pH=6,t=120min | 两种离子液体对3种酚的萃取率均大于94%,对酚类的萃取率排序为2-氯酚>邻甲酚>苯酚 | [ |
| [Et2NEmpyr]Cl2 | 苯酚+甲苯 | 苯酚 | Cl-与酚羟基之间形成氢键实现对苯酚的萃取 | c苯酚=200g/dm3,nIL∶n苯酚=0.6,T=25℃,t=15min | E苯酚=97.05%,与含1个Cl-的离子液体相比,实现相同的苯酚萃取率,[Et2NEmpyr]Cl2的用量更少 | [ |
| [HMPyr]NTf2 | 苯酚、邻甲酚、间苯二酚溶液 | 苯酚、邻甲酚、间苯二酚 | 离子液体与酚类物质之间形成氢键,实现对酚类物质的萃取 | — | 吡咯烷类离子液体对酚类物质萃取率排序为邻甲酚>苯酚>间苯二酚 | [ |
| 离子液体 | 原料液 | 目标组分 | 萃取机制 | 萃取工艺条件 | 分离性能 | 文献 |
|---|---|---|---|---|---|---|
| [HMPL]Cl、 [EMPL]C9H19COO等 | 邻甲酚(间甲酚、对甲酚)溶液 | 邻甲酚、间甲酚、对甲酚 | — | T=298.15K | 阳离子类型对吡咯烷类离子液体萃取酚类物质选择性排序为[HMPL]+>[EMPL]+,阳离子为[HMPL]+时,阴离子为Cl-时对酚类物质的萃取选择性最高;对3种甲酚萃取选择性排序为间甲酚>邻甲酚>对甲酚。 | [ |
| [C4C1Pyr]NTf2、 [C4C1Pyr]Nf2 | 苯酚、邻甲酚、2-氯酚溶液 | 苯酚、邻甲酚、2-氯酚 | — | c酚类=15000mg/L,VIL∶VW=1∶2,T=298.15K,pH=6,t=120min | 两种离子液体对3种酚的萃取率均大于94%,对酚类的萃取率排序为2-氯酚>邻甲酚>苯酚 | [ |
| [Et2NEmpyr]Cl2 | 苯酚+甲苯 | 苯酚 | Cl-与酚羟基之间形成氢键实现对苯酚的萃取 | c苯酚=200g/dm3,nIL∶n苯酚=0.6,T=25℃,t=15min | E苯酚=97.05%,与含1个Cl-的离子液体相比,实现相同的苯酚萃取率,[Et2NEmpyr]Cl2的用量更少 | [ |
| [HMPyr]NTf2 | 苯酚、邻甲酚、间苯二酚溶液 | 苯酚、邻甲酚、间苯二酚 | 离子液体与酚类物质之间形成氢键,实现对酚类物质的萃取 | — | 吡咯烷类离子液体对酚类物质萃取率排序为邻甲酚>苯酚>间苯二酚 | [ |
| 1 | 中华人民共和国国家环境保护总局. 污水综合排放标准: [S]. 北京: 中国标准出版社, 1998. |
| State Environmental Protection Administration of the People’s Republic of China. Integrated wastewater discharge standard: [S]. Beijing: Standards Press of China, 1998. | |
| 2 | 齐亚兵, 杨清翠. 煤化工废水脱酚技术研究进展[J]. 应用化工, 2021, 50(5): 1414-1419. |
| QI Yabing, YANG Qingcui. Research progress on removal of phenols from coal chemical wastewater[J]. Applied Chemical Industry, 2021, 50(5): 1414-1419. | |
| 3 | 齐亚兵, 罗橙, 张思敬. 含酚废水液膜脱酚技术研究进展[J]. 化工进展, 2021, 40(S2): 348-355. |
| QI Yabing, LUO Cheng, ZHANG Sijing. Research progress on dephenolization technologies of wastewater containing phenolic compounds by liquid membrane[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 348-355. | |
| 4 | QI Yabing, ZHANG Sijing. Efficient recovery of phenol from phenolic wastewater by emulsion liquid membrane[J]. The Canadian Journal of Chemical Engineering, 2023, 101(2): 1027-1042. |
| 5 | 齐亚兵. 活化过硫酸盐氧化法降解酚类污染物的研究进展[J]. 化工进展, 2022, 41(11): 6068-6079. |
| QI Yabing. Research progress on degradation of phenolic pollutants by activated persulfate oxidation[J]. Chemical Industry | |
| and Engineering Progress, 2022, 41(11): 6068-6079. | |
| 6 | LIU Zonghao, LAN Hao, WANG Yuan, et al. Highly efficient degradation of bisphenol A with persulfate activated by vacuum-ultraviolet/ultraviolet light (VUV/UV): Experiments and theoretical calculations[J]. Chemical Engineering Journal, 2022, 429: 132485. |
| 7 | 齐亚兵, 何佳玮, 冉佳城, 等. 萃取法处理含酚废水的工艺[J]. 应用化工, 2021, 50(4): 961-964. |
| QI Yabing, HE Jiawei, RAN Jiacheng, et al. Research on treating wastewater containing phenols by extraction[J]. Applied | |
| Industry Chemical, 2021, 50(4): 961-964. | |
| 8 | 刘潜, 张香兰, 李巍. 基于 COSMO-RS 模型的分离油酚混合物的离子液体萃取剂筛[J]. 化工学报, 2018, 69(12): 5100-5111. |
| LIU Qian, ZHANG Xianglan, LI Wei. Screening ionic liquids solvent for separation of oil and hydroxybenzene mixtures based on COSMO-RS model[J]. CIESC Journal, 2018, 69(12): 5100-5111. | |
| 9 | QI Yabing. Oxidative degradation of phenol in aqueous solution by using heat, ZVI, AC, heat/ZVI, or heat/AC activated persulfate[J]. Indian Journal of Chemical Technology, 2023, 30(6): 805-811. |
| 10 | 张红, 余肇誉, 苏远海. 微反应器耦合离子液体强化萃取过程的研究进展[J]. 化工进展, 2020, 39(12): 4908-4918. |
| ZHANG Hong, YU Zhaoyu, SU Yuanhai. Research progress of extraction enhancement with the use of microreactors coupled with ionic liquids[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4908-4918. | |
| 11 | 杨启炜, 鲍宗必, 邢华斌, 等. 离子液体萃取分离结构相似化合物研究进展[J]. 化工进展, 2019, 38(1): 91-99. |
| YANG Qiwei, BAO Zongbi, XING Huabin, et al. Research progress on the extractive separation of structurally-related compounds by ionic liquids[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 91-99. | |
| 12 | 张香平, 白银鸽, 闫瑞一, 等. 离子液体萃取分离有机物研究进展[J]. 化工进展,2016, 35(6): 1587-1605. |
| ZHANG Xiangping, BAI Yinge, YAN Ruiyi, et al. Progress in ionic liquids for extraction of organic compounds[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1587-1605. | |
| 13 | YAN Weiwei, WEI Xianyong, WANG Mengxiao, et al. Overview: Effective separation of oxygen-, nitrogen-, and sulfur-containing aromatics in high-temperature coal tar by ionic liquids and deep eutectic solvents: Experimental and computational[J]. Industrial & Engineering Chemistry Research, 2022, 61(13): 4481-4492. |
| 14 | 易兰, 李文英, 冯杰. 离子液体/低共熔溶剂在煤基液体分离中的应用[J]. 化工进展, 2020, 39(6): 2066-2078. |
| YI Lan, LI Wenying, FENG Jie. Application of ionic liquids and deep eutectic solvents in the separation of coal-based liquids[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2066-2078. | |
| 15 | 张淼. 有机溶质在离子液体中的热力学性质及油酚混合物的分离研究[D]. 北京: 北京石油化工学院, 2019. |
| ZHANG Miao. Thermodynamic properties of organic solutes in ionic liquids and separation of oil-phenol mixture[D]. Beijing: Beijing Institute of Petrochemical Technology, 2019. | |
| 16 | KHAN Amir Sada, IBRAHIM Taleb Hassan, RASHID Zeeshan, et al. COSMO-RS based screening of ionic liquids for extraction of phenolic compounds from aqueous media[J]. Journal of Molecular Liquids, 2021, 328: 115387. |
| 17 | LI Ao, XU Xin, ZHANG Lianzheng, et al. Separation of cresol from coal tar by imidazolium-based ionic liquid [Emim] [SCN]: Interaction exploration and extraction experiment[J]. Fuel, 2020, 264: 116908. |
| 18 | XU Xin, LI Ao, ZHANG Tao, et al. Efficient extraction of phenol from low-temperature coal tar model oil via imidazolium-based ionic liquid and mechanism analysis[J]. Journal of Molecular Liquids, 2020, 306: 112911. |
| 19 | LIU Qian, ZHANG Xianglan, LI Wei. Separation of m-cresol from aromatic hydrocarbon and alkane using ionic liquids via hydrogen bond interaction[J]. Chinese Journal of Chemical Engineering, 2019, 27(11): 2675-2686. |
| 20 | YANG Qiwei, XING Huabin, SU Baogen, et al. The essential role of hydrogen-bonding interaction in the extractive separation of phenolic compounds by ionic liquid[J]. AIChE Journal, 2013, 59(5): 1657-1667. |
| 21 | HOU Yucui, REN Yuehong, PENG Wei, et al. Separation of phenols from oil using imidazolium-based ionic liquids[J]. Industrial & Engineering Chemistry Research, 2013, 52(50): 18071-18075. |
| 22 | 邓晶晶, 罗泽军, 王储, 等. 疏水性离子液体对生物油水相馏分中酚类物质的萃取研究[J]. 燃料化学学报, 2021, 49(12): 1832-1838. |
| DENG Jingjing, LUO Zejun, WANG Chu, et al. Extraction of phenols from bio-oil aqueous fraction by hydrophobic ionic liquids[J]. Journal of Fuel Chemistry and Technology, 2021, 49(12): 1832-1838. | |
| 23 | ZHAO Yue, TONG Jing. Investigation of the extraction ability and mechanism of environmentally friendly ionic liquids for phenol in the model oil[J]. Fuel, 2023, 341: 127673. |
| 24 | ZHU Chen, LI Fangfang, ZHANG Jun, et al. Performance of functionalized ionic liquid with double chemical sites for separating phenolic compounds: Mechanism and liquid-liquid behavior studies[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106790. |
| 25 | JI Youan, HOU Yucui, REN Shuhang, et al. Highly efficient separation of phenolic compounds from oil mixtures by imidazolium-based dicationic ionic liquids via forming deep eutectic solvents[J]. Energy & Fuels, 2017, 31(9): 10274-10282. |
| 26 | SIDEK Nadiah, MANAN Ninie S A, MOHAMAD Sharifah. Efficient removal of phenolic compounds from model oil using benzyl Imidazolium-based ionic liquids[J]. Journal of Molecular Liquids, 2017, 240: 794-802. |
| 27 | KHAN Amir Sada, IBRAHIM Taleb Hassan, KHAMIS Mustafa I, et al. Role of cation and alkyl chain length on the extraction of phenol from aqueous solution using NTf2-based ionic liquids: Experimental and computational analysis[J]. Journal of Molecular Liquids, 2021, 326: 115305. |
| 28 | XU Dongmei, ZHONG Pei, PENG Lijie, et al. Multiscale evaluation of the efficiently separation of phenols using a designed cationic functionalized ionic liquid based on Brønsted/Lewis coordination[J]. Journal of Molecular Liquids, 2022, 345: 117901. |
| 29 | 陈碧. 离子液体用于含酚废水处理的研究[J]. 化工新型材料, 2015, 43(6): 231-234. |
| CHEN Bi. Extraction of phenols from wastewater with ionic liquid[J]. New Chemical Materials, 2015, 43(6): 231-234. | |
| 30 | 王菊芳. 离子型材料选择性分离甲酚异构体及同系物[D]. 杭州: 浙江大学, 2020. |
| WANG Jufang. Selective separation of cresol isomers and homologues with ionic materials[D]. Hangzhou: Zhejiang University, 2020. | |
| 31 | CESARI Laëtitia, Laetitia CANABADY-ROCHELLE, MUTELET Fabrice. Extraction of phenolic compounds from aqueous solution using choline bis(trifluoromethylsulfonyl) imide[J]. Fluid Phase Equilibria, 2017, 446: 28-35. |
| 32 | LI Zhiyong, LI Ruipeng, YUAN Xiaoqing, et al. Anionic structural effect in liquid-liquid separation of phenol from model oil by choline carboxylate ionic liquids[J]. Green Energy & Environment, 2019, 4(2): 131-138. |
| 33 | 李瑞鹏. 胆碱类离子液体对苯酚和碘的萃取与捕集性能研究[D]. 新乡: 河南师范大学, 2018. |
| LI Ruipeng. The extraction and capture performance of phenol and iodine by choline-based ionic liquids[D]. Xinxiang: Henan Normal University, 2018. | |
| 34 | BING Xiaobin, MENG Xianglin, LI Ao, et al. Extraction and mechanism exploration for separating cresols from coal tar by ionic liquid ethanolamine lactate[J]. Journal of Molecular Liquids, 2020, 305: 112845. |
| 35 | SADA KHAN Amir, IBRAHIM Taleb, AKBAR Noor, et al. Application of protic ammonium-based ionic liquids with carboxylate anions for phenol extraction from aqueous solution and their cytotoxicity on human cells[J]. Journal of Molecular Liquids, 2021, 342: 117447. |
| 36 | JI Youan, HOU Yucui, REN Shuhang, et al. Highly efficient extraction of phenolic compounds from oil mixtures by trimethylamine-based dicationic ionic liquids via forming deep eutectic solvents[J]. Fuel Processing Technology, 2018(171): 183-191. |
| 37 | BING Xiaobin, WANG Zenghui, WEI Feng, et al. Separation of m-cresol from coal tar model oil using propylamine-based ionic liquids: Extraction and interaction mechanism exploration[J]. ACS Omega, 2020, 5(36): 23090-23098. |
| 38 | 葛长涛. 醇胺离子液体萃取分离中低温煤焦油酚类化合物的研究[D]. 北京: 北京化工大学, 2013. |
| GE Changtao. Study on extracting phenolic compounds from low temperature coal tar using hydroxyl amine ionic liquids[D]. Beijing: Beijing University of Chemical Technology, 2013. | |
| 39 | THASNEEMA K K, DIPIN T, Shahin THAYYIL M, et al. Removal of toxic heavy metals, phenolic compounds and textile dyes from industrial waste water using phosphonium based ionic liquids[J]. Journal of Molecular Liquids, 2021, 323: 114645. |
| 40 | 郭少聪, 杨启炜, 邢华斌, 等. 离子液体-分子溶剂复合萃取剂脱除水中酚类化合物[J]. 化工学报, 2016, 67(7): 2851-2856. |
| GUO Shaocong, YANG Qiwei, XING Huabin, et al. Removal of phenols from aqueous solution by ionic liquid-molecular solvent composite extractant[J]. CIESC Journal, 2016, 67(7): 2851-2856. | |
| 41 | YANG Qiwei, XU Dan, ZHANG Jingzhu, et al. Long-chain fatty acid-based phosphonium ionic liquids with strong hydrogen-bond basicity and good lipophilicity: Synthesis, characterization, and application in extraction[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(2): 309-316. |
| 42 | SKORONSKI Everton, FERNANDES Mylena, MALARET Francisco J, et al. Use of phosphonium ionic liquids for highly efficient extraction of phenolic compounds from water[J]. Separation and Purification Technology, 2020, 248: 117069. |
| 43 | DENG Ning, LI Min, ZHAO Lijie, et al. Highly efficient extraction of phenolic compounds by use of magnetic room temperature ionic liquids for environmental remediation[J]. Journal of Hazardous Materials, 2011, 192(3): 1350-1357. |
| 44 | KUMAR Lalan, BANERJEE Tamal, MOHANTY Kaustubha. Prediction of selective extraction of cresols from aqueous solutions by ionic liquids using theoretical approach[J]. Separation Science and Technology, 2011, 46(13): 2075-2087. |
| 45 | Olalla G SAS, SÁNCHEZ Pablo B, Begoña GONZÁLEZ, et al. Removal of phenolic pollutants from wastewater streams using ionic liquids[J]. Separation and Purification Technology, 2020, 236: 116310. |
| 46 | LIU Qian, LIU Qin, ZHANG Xianglan. Separation of m-cresol from model oil by pyridinium-based ionic liquids: COSMO-RS screening, experimental study, and process simulation[J]. Journal of Environmental Chemical Engineering, 2022, 10: 108874. |
| 47 | XU Dongmei, WANG Suxu, ZHANG Tao, et al. Extraction and interaction insights for enhanced separation of phenolic compounds from model coal tar using a hydroxyl-functionalized ionic liquid[J]. Chemical Engineering Research and Design, 2022, 178: 567-574. |
| 48 | YAO Congfei, HOU Yucui, REN Shuhang, et al. Efficient separation of phenolic compounds from model oils by dual-functionalized ionic liquids[J]. Chemical Engineering & Processing: Process Intensification, 2018, 128: 216-222. |
| 49 | GONZÁLEZ Emilio J, Ismael DÍAZ, Maria GONZALEZ-MIQUEL, et al. On the behavior of imidazolium versus pyrrolidinium ionic liquids as extractants of phenolic compounds from water: Experimental and computational analysis[J]. Separation and Purification Technology, 2018, 201: 214-222. |
| 50 | GAI Hengjun, QIAO Lin, ZHONG Caiyun, et al. Designing ionic liquids with dual Lewis basic sites to efficiently separate phenolic compounds from low-temperature coal tar[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 10841-10850. |
| 51 | YAO Tian, LI Hongmei, REN Yuanhang, et al. Extraction and recovery of phenolic compounds from aqueous solution by thermo-separating magnetic ionic liquid aqueous two-phase system[J]. Separation and Purification Technology, 2022, 282: 120034. |
| 52 | 吴雅睿. 离子液体[Omim]PF6的合成及其深度处理焦化含酚废水的研究[J]. 化工新型材料, 2014, 42(6): 206-209. |
| WU Yarui. Synthesis of ionic liquid [Omim]PF6 and application on the extraction of coking wastewater contained toxic phenols[J]. New Chemical Materials, 2014, 42(6): 206-209. | |
| 53 | SAIEN Javad, ASRAMI Mahdieh Razi, SALEHZADEH Sadegh. Phase equilibrium measurements and thermodynamic modelling of {water + phenol + [Hmim][NTf 2]} ionic liquid system at several temperatures[J]. The Journal of Chemical Thermodynamics, 2018, 119: 76-83. |
| 54 | MENG Hong, GE Changtao, REN Nannan, et al. Complex extraction of phenol and cresol from model coal tar with polyols, ethanol amines, and ionic liquids thereof[J]. Industrial & Engineering Chemistry Research, 2014, 53(1): 355-362. |
| 55 | FAN Yunchang, LI Yun, DONG Xing, et al. Extraction of phenols from water with functionalized ionic liquids[J]. Industrial & Engineering Chemistry Research, 2014, 53(51): 20024-20031. |
| 56 | KATSUTA Shoichi, NAKAMURA Ko-ichi, KUDO Yoshihiro, et al. Partition behavior of chlorophenols and nitrophenols between hydrophobic ionic liquids and water[J]. Journal of Chemical & Engineering Data, 2011, 56(11): 4083-4089. |
| 57 | 付宏权, 刘培元, 陈盈, 等. 离子液体[Bmim]PF6萃取处理硝基酚废水[J]. 华侨大学学报(自然科学版), 2013, 34(3): 303-307. |
| FU Hongquan, LIU Peiyuan, CHEN Ying, et al. Extraction of nitrophenol from wastewater with [Bmim]PF6 ionic liquid[J]. Journal of Huaqiao University (Natural Science), 2013, 34(3): 303-307. | |
| 58 | Olalla G SAS, Irene DOMÍNGUEZ, Ángeles DOMÍNGUEZ, et al. Using bis(trifluoromethylsulfonyl) imide based ionic liquids to extract phenolic compounds[J]. The Journal of Chemical Thermodynamics, 2019, 131: 159-167. |
| 59 | ASRAMI Mahdieh Razi, SAIEN Javad. Salting-out effect on extraction of phenol from aqueous solutions by [Hmim] [NTf 2] ionic liquid: Experimental investigations and modeling[J]. Separation and Purification Technology, 2018, 204: 175-184. |
| [1] | LIAO Xu, ZHOU Jun, LUO Jie, ZENG Ruilin, WANG Zeyu, LI Zunhua, LIN Jinqing. Research progress on CO2 cycloaddition reaction catalyzed by porous ionic polymers [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4925-4940. |
| [2] | GENG Xiumei, ZHANG Feng, ZHANG Xiang, SHAN Meixia, ZHANG Yatao. Research progress on the stability of Pebax-based mixed matrix membranes for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4996-5012. |
| [3] | LI Zhenwu, PU Di, XIONG Yachun, WU Dingying, JIN Cheng, GUO Yongjun. Research progress of nanomaterials for oil displacement in enhancing oil recovery [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5035-5048. |
| [4] | ZHANG Yufeng, PANG Yuqian, PEI Haonan, FAN Xiaoqing. Three-way rod metal-organic frameworks for purifying of C2—C3 from natural gas [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5185-5192. |
| [5] | YANG Xinheng, JI Zhiyong, GUO Zhiyuan, LIU Qi, ZHANG Panpan, WANG Jing, LIU Jie, BI Jingtao, ZHAO Yingying, YUAN Junsheng. Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5262-5274. |
| [6] | MA Hongzhou, DANG Yubo, WANG Yaoning, ZENG Jinyang, ZHAO Xiaojun, SHI Jianwei. Zinc-based desulfurizer scrap and copper-zinc-based catalyst synergistic vacuum carbothermal extraction of zinc [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5275-5281. |
| [7] | SONG Jiakai, KONG Lingzhen, CHEN Jiaqing, SUN Huan, LI Qi, LI Changhe, WANG Sicheng, KONG Biao. Liquid film flow and separation characteristics in the swirl separation section of a tubular deliquidiser [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4297-4306. |
| [8] | GAO Xinyue, FAN Gaofeng, LIU Aiping, WANG Chang'an, HOU Yujie, ZHANG Jinming, XU Jie, CHE Defu. Research progress on waste heat recovery technology for flue gas and slurry after wet desulphurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4307-4319. |
| [9] | SUN Yan, FENG Qianying, XIE Xiaoyang, HE Jiaojie, YANG Liwei, BAI Bo. Research progress on the cyclodextrin-based thin film composite membranes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4464-4476. |
| [10] | ZHANG Rui, JIANG Jing, XU Hongfei, YANG Shengkai, LI Yahong, ZHOU Jingyuan, ZENG Jianxian, HUANG Xiaoping, LIU Pengfei, ZHANG Mingming, LI Zhiqiang. Progress of ceramic membrane separation technology and its application in bio-manufacturing field [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4550-4561. |
| [11] | LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613. |
| [12] | SHI Jiabo, ZHANG Yuxuan, CHEN Xuefeng, TAN Jiaojun. Preparation and oil-water separation property of tannic acid-nanoclay synergistically modified collagen fiber-based porous materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4624-4629. |
| [13] | JIAO Wenlei, LIU Zhen, CHEN Junxian, ZHANG Tianyu, JI Zhongli. Structure and performance influencing factors of vane separation components: The reviews [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4187-4202. |
| [14] | HU Rui, LI Xianru, PIAO Weiling, FENG Pan, LUO Lei, LUO Gang, WEI Huangzhao, LIU Zhengang, ZHANG Shicheng. Progress on the hydrothermal conversion equipment and technology of organic waste [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3672-3691. |
| [15] | TANG Anqi, WEI Xin, DING Liming, WANG Yujie, XU Yixiao, LIU Yiqun. Discussing physical aging phenomenon of polyimide gas separation membranes [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3923-3933. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
