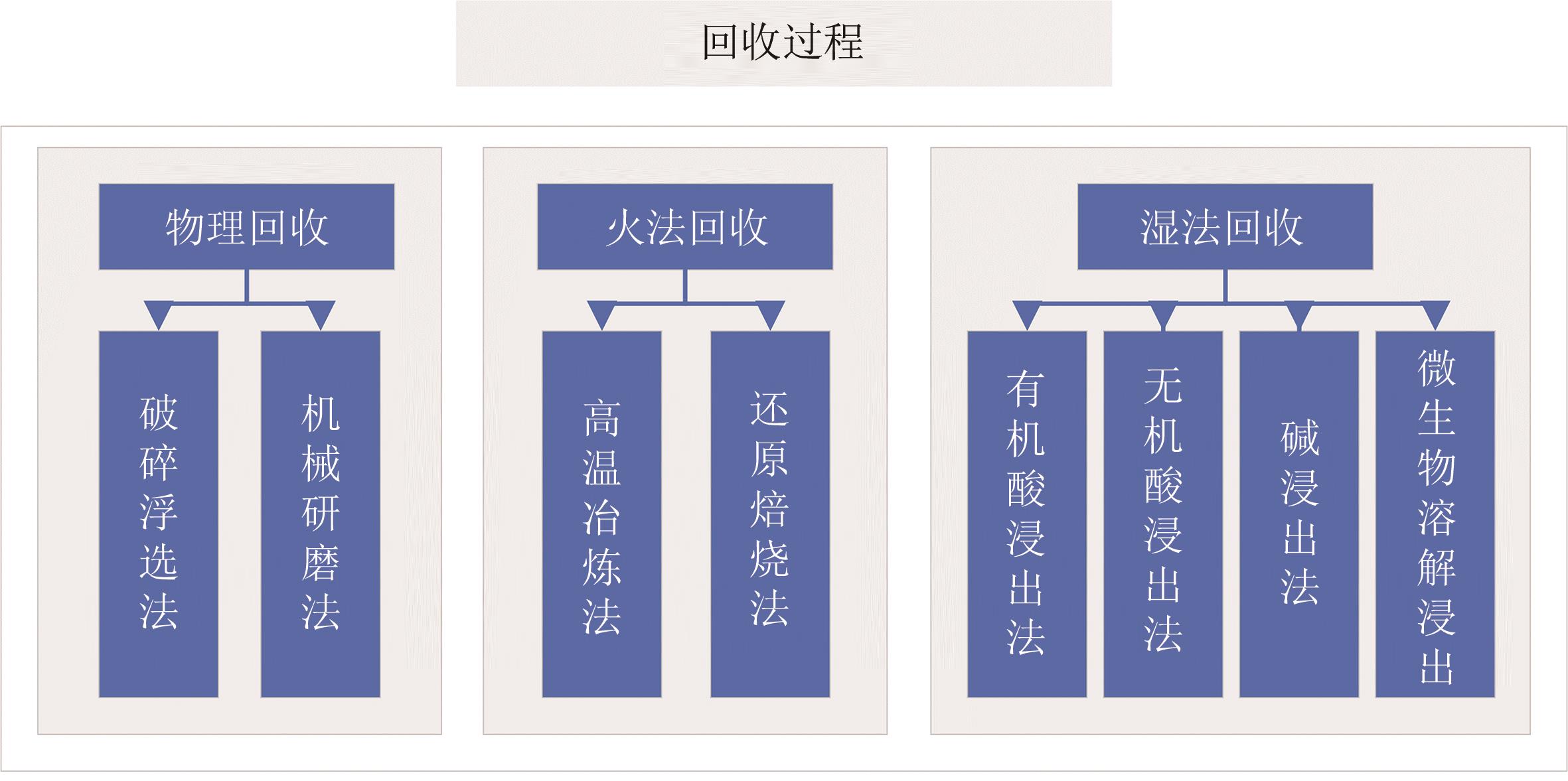
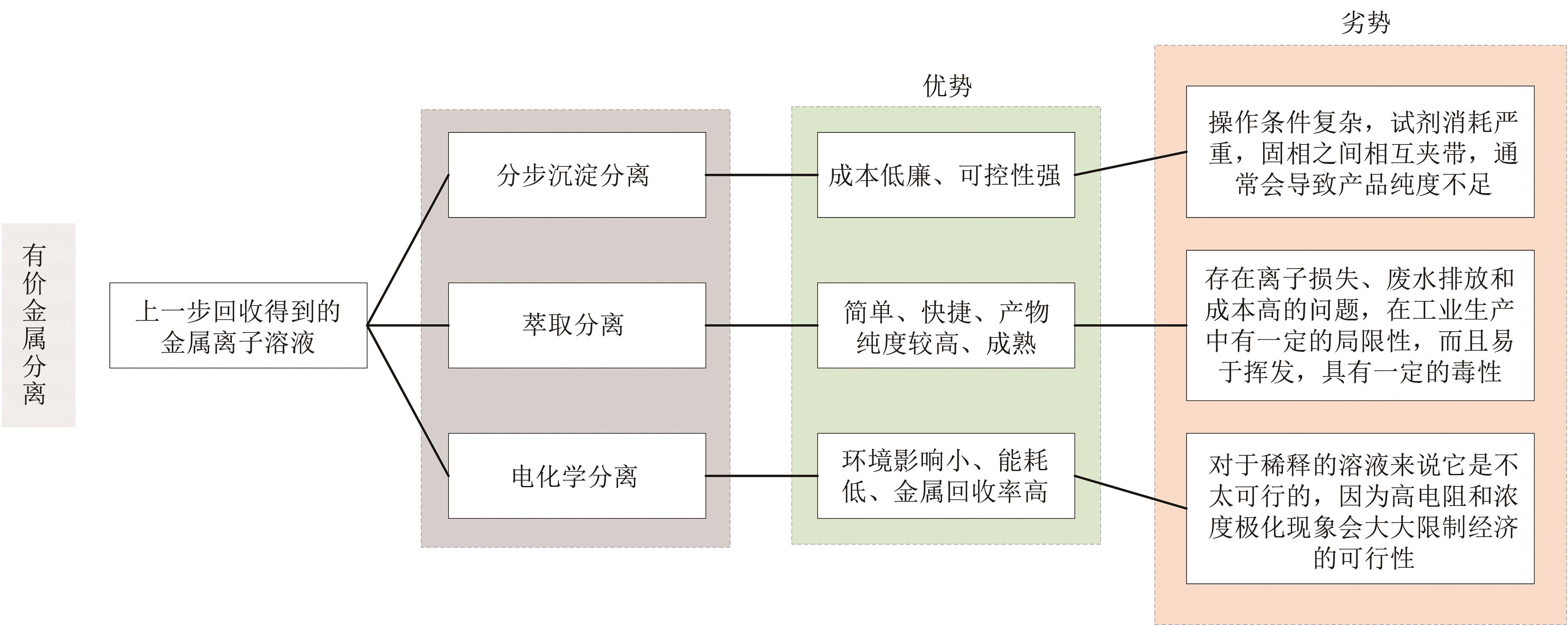
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (8): 4601-4613.DOI: 10.16085/j.issn.1000-6613.2023-1208
• Resources and environmental engineering • Previous Articles
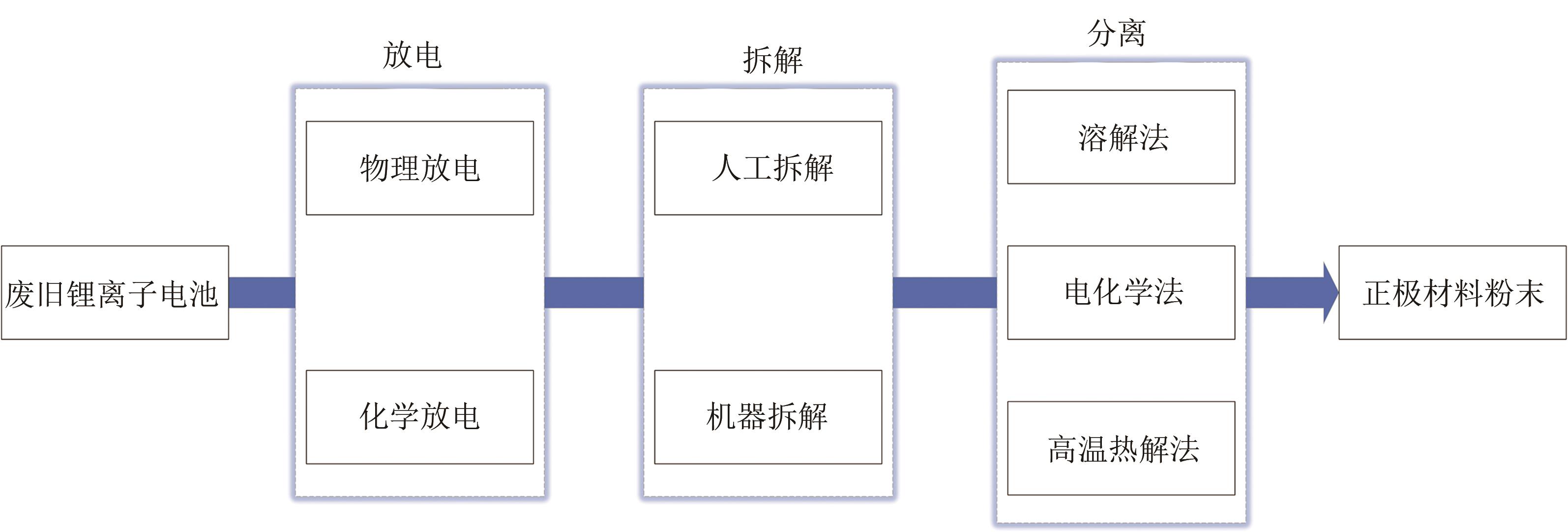
Lithium-ion battery disassembly and recycling technology and progress
LI Weijie1( ), LU Leilei1(
), LU Leilei1( ), LI Deke2, WANG Chunhang2, ZHANG Zuming2, TAN Qiang3
), LI Deke2, WANG Chunhang2, ZHANG Zuming2, TAN Qiang3
- 1.College of Science, Xi’an University of Technology, Xi’an 710048, Shaanxi, China
2.CEC Xi’an Qiyuan Mechanical and Electrical Equipment Co. , Xi’an 710200, Shaanxi, China
3.College of Materials Science and Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2023-07-16Revised:2023-10-20Online:2024-09-02Published:2024-08-15 -
Contact:LU Leilei
锂离子电池拆解回收技术及进展
黎伟杰1( ), 路蕾蕾1(
), 路蕾蕾1( ), 李得科2, 王春航2, 张祖铭2, 谭强3
), 李得科2, 王春航2, 张祖铭2, 谭强3
- 1.西安理工大学理学院,陕西 西安 710048
2.中节能西安启源机电装备有限公司,陕西 西安 710200
3.西安交通大学材料科学与工程学院,陕西 西安 710049
-
通讯作者:路蕾蕾 -
作者简介:黎伟杰(1999—),男,硕士研究生,研究方向为废旧锂离子电池正极材料回收。E-mail:weijie990701@163.com。 -
基金资助:西安交通大学基础科研项目(XJH012020027);陕西省教育厅科研计划(21JP082)
CLC Number:
Cite this article
LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613.
黎伟杰, 路蕾蕾, 李得科, 王春航, 张祖铭, 谭强. 锂离子电池拆解回收技术及进展[J]. 化工进展, 2024, 43(8): 4601-4613.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1208
| 序号 | 无机浸出剂 | 还原剂 | 固液比/mL·g-1 | 时间/min | 温度 /℃ | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 锂 | 镍 | 锰 | 钴 | |||||||
| 1 | 硫酸(2.0mol·L-1) | 双氧水(0.97mol·L-1) | 10 | 30 | 80 | 100 | 100 | 94 | 100 | [ |
| 2 | 硫酸(1.0mol·L-1) | 亚硫酸氢钠(0.075mol·L-1) | 50 | 240 | 95 | 96.7 | 96.4 | 87.9 | 91.6 | [ |
| 3 | 硫酸(1.0mol·L-1) | 双氧水(体积分数1%) | 25 | 60 | 40 | 99.7 | 99.7 | 99.7 | 99.7 | [ |
| 4 | 硫酸(1.0mol·L-1) | — | 10 | 60 | 90 | 100 | 100 | 93.0 | 100 | [ |
| 5 | 硫酸(2.0mol·L-1) | 双氧水(体积分数2%) | 10 | 60 | 75 | 99.1 | — | — | 70 | [ |
| 6 | 盐酸(3.0mol·L-1) | — | 50 | 90 | 80 | 99.4 | — | — | — | [ |
| 7 | 盐酸(2.0mol·L-1) | 双氧水(体积分数5%) | 33 | 150 | 60 | >99 | >99 | >99 | >99 | [ |
| 8 | 硝酸(1.0mol·L-1) | 双氧水(体积分数1.7%) | 50 | 60 | 75 | 85 | — | — | 85 | [ |
| 9 | 磷酸(0.7mol·L-1) | 双氧水(体积分数4.0%) | 20 | 60 | 40 | >99 | — | — | >99 | [ |
| 序号 | 无机浸出剂 | 还原剂 | 固液比/mL·g-1 | 时间/min | 温度 /℃ | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 锂 | 镍 | 锰 | 钴 | |||||||
| 1 | 硫酸(2.0mol·L-1) | 双氧水(0.97mol·L-1) | 10 | 30 | 80 | 100 | 100 | 94 | 100 | [ |
| 2 | 硫酸(1.0mol·L-1) | 亚硫酸氢钠(0.075mol·L-1) | 50 | 240 | 95 | 96.7 | 96.4 | 87.9 | 91.6 | [ |
| 3 | 硫酸(1.0mol·L-1) | 双氧水(体积分数1%) | 25 | 60 | 40 | 99.7 | 99.7 | 99.7 | 99.7 | [ |
| 4 | 硫酸(1.0mol·L-1) | — | 10 | 60 | 90 | 100 | 100 | 93.0 | 100 | [ |
| 5 | 硫酸(2.0mol·L-1) | 双氧水(体积分数2%) | 10 | 60 | 75 | 99.1 | — | — | 70 | [ |
| 6 | 盐酸(3.0mol·L-1) | — | 50 | 90 | 80 | 99.4 | — | — | — | [ |
| 7 | 盐酸(2.0mol·L-1) | 双氧水(体积分数5%) | 33 | 150 | 60 | >99 | >99 | >99 | >99 | [ |
| 8 | 硝酸(1.0mol·L-1) | 双氧水(体积分数1.7%) | 50 | 60 | 75 | 85 | — | — | 85 | [ |
| 9 | 磷酸(0.7mol·L-1) | 双氧水(体积分数4.0%) | 20 | 60 | 40 | >99 | — | — | >99 | [ |
| 序号 | 有机浸出剂 | 还原剂 | 固液比/mL·g-1 | 时间/min | 温度/℃ | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 锂 | 镍 | 锰 | 钴 | |||||||
| 1 | DL-苹果酸 (1.2mol·L-1) | 双氧水 (体积分数1.5%) | 25 | 30 | 80 | 98.9 | 95.1 | 96.4 | 94.3 | [ |
| 2 | DL-苹果酸 (1.0mol·L-1) | 双氧水 (体积分数4.0%) | 200 | 30 | 80 | 98 | 97.8 | 97.3 | 97.6 | [ |
| 3 | 柠檬酸 (1.2mol·L-1) | 硫代硫酸钠 (0.3 mol·L-1) | 50 | 30 | 70 | 99 | — | — | 96 | [ |
| 4 | 柠檬酸 (0.5mol·L-1) | 双氧水 (体积分数1.5%) | 50 | 60 | 90 | >95 | >95 | >95 | >95 | [ |
| 5 | 抗坏血酸 (1.24mol·L-1) | — | 31.30 | 59.79 | 69.26 | 92.75 | 56.83 | 89.91 | 96.78 | [ |
| 6 | 草酸 (1.0mol·L-1) | — | 67 | 150 | 95 | 98 | — | — | 97 | [ |
| 8 | 乙酸 (3.0mol·L-1) | 双氧水 (体积分数7.5%) | 50 | 40 | 70 | 99.9 | — | 99.5 | 98.7 | [ |
| 9 | 酒石酸 (4倍LiCoO2的物质的量) | — | 67 | 300 | 90 | 91.9 | — | — | 93.0 | [ |
| 序号 | 有机浸出剂 | 还原剂 | 固液比/mL·g-1 | 时间/min | 温度/℃ | 浸出效率/% | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 锂 | 镍 | 锰 | 钴 | |||||||
| 1 | DL-苹果酸 (1.2mol·L-1) | 双氧水 (体积分数1.5%) | 25 | 30 | 80 | 98.9 | 95.1 | 96.4 | 94.3 | [ |
| 2 | DL-苹果酸 (1.0mol·L-1) | 双氧水 (体积分数4.0%) | 200 | 30 | 80 | 98 | 97.8 | 97.3 | 97.6 | [ |
| 3 | 柠檬酸 (1.2mol·L-1) | 硫代硫酸钠 (0.3 mol·L-1) | 50 | 30 | 70 | 99 | — | — | 96 | [ |
| 4 | 柠檬酸 (0.5mol·L-1) | 双氧水 (体积分数1.5%) | 50 | 60 | 90 | >95 | >95 | >95 | >95 | [ |
| 5 | 抗坏血酸 (1.24mol·L-1) | — | 31.30 | 59.79 | 69.26 | 92.75 | 56.83 | 89.91 | 96.78 | [ |
| 6 | 草酸 (1.0mol·L-1) | — | 67 | 150 | 95 | 98 | — | — | 97 | [ |
| 8 | 乙酸 (3.0mol·L-1) | 双氧水 (体积分数7.5%) | 50 | 40 | 70 | 99.9 | — | 99.5 | 98.7 | [ |
| 9 | 酒石酸 (4倍LiCoO2的物质的量) | — | 67 | 300 | 90 | 91.9 | — | — | 93.0 | [ |
| 方法 | 优势 | 劣势 |
|---|---|---|
| 共沉淀法 | 获得正极材料颗粒细小、形态规则、元素分布均匀,有良好的电化学性能 | 设备要求高、工艺复杂、成本高 |
| 溶胶-凝胶法 | 可以在不添加锂源的情况下合成正极材料,高度可控性 | 制造时间长、成本高等不足大大限制了其工业化发展 |
| 固相合成法 | 流程简单、成本低 | 很难除去其中的杂质,杂质的含量及分布将会影响其电化学性能 |
| 水热法 | 无杂质引入,步骤简单 | 需注意废液处理,防止环境污染 |
| 方法 | 优势 | 劣势 |
|---|---|---|
| 共沉淀法 | 获得正极材料颗粒细小、形态规则、元素分布均匀,有良好的电化学性能 | 设备要求高、工艺复杂、成本高 |
| 溶胶-凝胶法 | 可以在不添加锂源的情况下合成正极材料,高度可控性 | 制造时间长、成本高等不足大大限制了其工业化发展 |
| 固相合成法 | 流程简单、成本低 | 很难除去其中的杂质,杂质的含量及分布将会影响其电化学性能 |
| 水热法 | 无杂质引入,步骤简单 | 需注意废液处理,防止环境污染 |
| 11 | ZHANG Qingsong, NIU Jianghao, YANG Juan, et al. In-situ explosion limit analysis and hazards research of vent gas from lithium-ion battery thermal runaway[J]. Journal of Energy Storage, 2022, 56: 106146. |
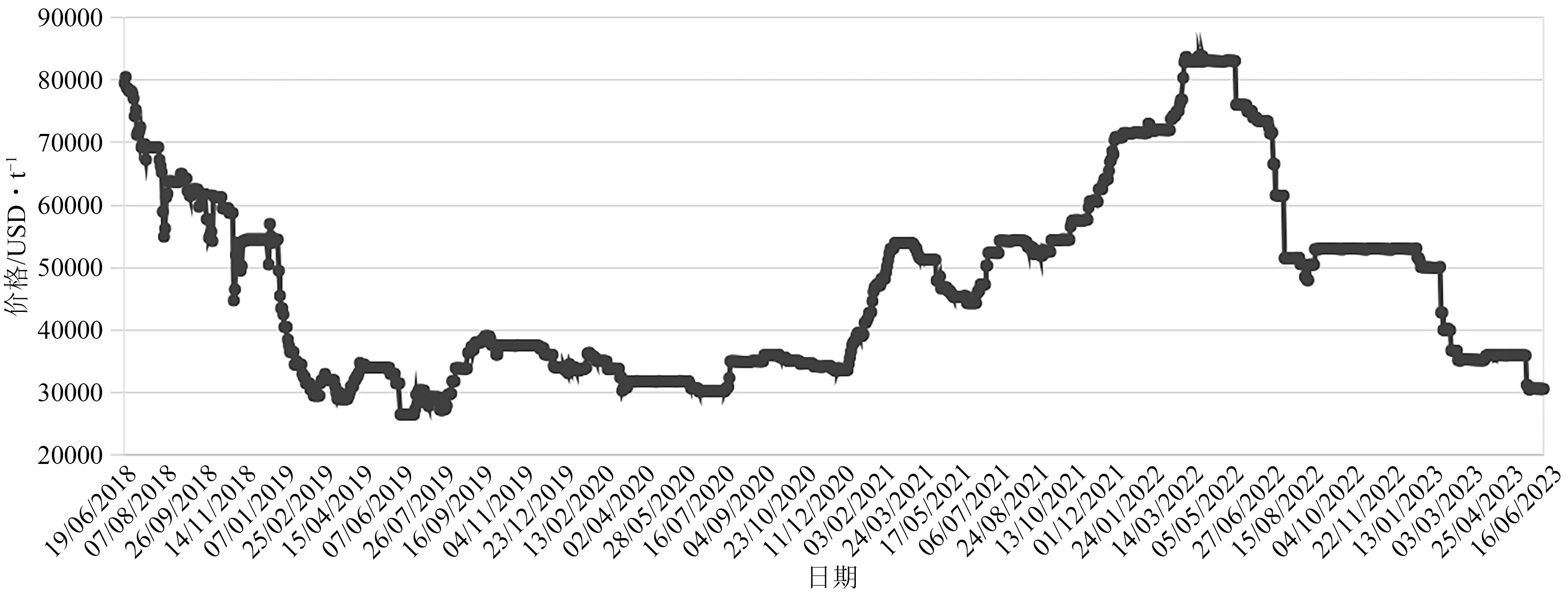
| 12 | MARTIN Gunther, RENTSCH Lars, Michael HÖCK, et al. Lithium market research-global supply, future demand and price development[J]. Energy Storage Materials, 2017, 6: 171-179. |
| 13 | ZENG Xianlai, LI Jinhui, LIU Lili. Solving spent lithium-ion battery problems in China: Opportunities and challenges[J]. Renewable and Sustainable Energy Reviews, 2015, 52: 1759-1767. |
| 14 | SPEIRS Jamie, CONTESTABILE Marcello, HOUARI Yassine, et al. The future of lithium availability for electric vehicle batteries[J]. Renewable and Sustainable Energy Reviews, 2014, 35: 183-193. |
| 15 | MUDD Gavin M. Global trends and environmental issues in nickel mining: Sulfides versus laterites[J]. Ore Geology Reviews, 2010, 38(1/2): 9-26. |
| 16 | MUDD G M, JOWITT S M. A detailed assessment of global nickel resource trends and endowments[J]. Economic Geology, 2014, 109(7): 1813-1841. |
| 17 | ZHANG Hongyan, LIU Guwang, LI Jianwu, et al. Modeling the impact of nickel recycling from batteries on nickel demand during vehicle electrification in China from 2010 to 2050[J]. Science of the Total Environment, 2023, 859(1): 159964. |
| 18 | KIM Youngjin, SEONG Won Mo, MANTHIRAM Arumugam. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives[J]. Energy Storage Materials, 2021, 34: 250-259. |
| 19 | DEHAINE Quentin, TIJSSELING Laurens T, GLASS Hylke J, et al. Geometallurgy of cobalt ores: A review[J]. Minerals Engineering, 2021, 160: 106656. |
| 20 | LUO Yuhong, WEI Hanxin, TANG Linbo, et al. Nickel-rich and cobalt-free layered oxide cathode materials for lithium ion batteries[J]. Energy Storage Materials, 2022, 50: 274-307. |
| 21 | London Metal Exchange. LME Cobalt Official Prices graph[DB/OL]. https://www.lme.com/Metals/EV/LME-Cobalt#Price+graphs, 2023-06-16. |
| 22 | LIU Bingbing, ZHANG Yuanbo, LU Manman, et al. Extraction and separation of manganese and iron from ferruginous manganese ores: A review[J]. Minerals Engineering, 2019, 131: 286-303. |
| 23 | 孙宏伟, 任军平, 王杰, 等. 南部非洲锰矿成矿规律与资源潜力[J]. 地质通报, 2022, 41(1): 60-71. |
| SUN Hongwei, REN Junping, WANG Jie, et al. Metallogenic regularity and resource potential of manganese deposits in southern Africa[J]. Geological Bulletin of China, 2022, 41(1): 60-71. | |
| 24 | WANG Fan, LONG Guangcheng, MA Kunlin, et al. Recyling manganese-rich electrolytic residues: A review[J]. Environmental Chemistry Letters, 2023, 21(4): 2251-2284. |
| 25 | SINGH Veerendra, CHAKRABORTY Tarun, TRIPATHY Sunil K. A review of low grade manganese ore upgradation processes[J]. Mineral Processing and Extractive Metallurgy Review, 2020, 41(6): 417-438. |
| 26 | LI Jia, WANG Guangxu, XU Zhenming. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries[J]. Waste Management, 2016, 52: 221-227. |
| 27 | HARPER Gavin, SOMMERVILLE Roberto, KENDRICK Emma, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86. |
| 28 | LARCHER D, J-M TARASCON. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. |
| 29 | FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063. |
| 30 | 王厚然,李德念,董楠航,等. 退役磷酸铁锂电池与三元锂电池正极材料直接修复研究进展[J]. 化工进展, 2024, 43(6): 3336-3346. |
| WANG Houran, LI Denian, DONG Nanhang, et al. Progress of direct repair of retired lithium iron phosphate and lithium ternary battery cathode materials[J]. Advances in Chemical Engineering, 2024, 43(6): 3336-3346. | |
| 31 | YAO Lin peng, ZENG Qi, QI Ting, et al. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries[J]. Journal of Cleaner Production, 2020, 245: 118820. |
| 32 | FANG Zheng, DUAN Qiangling, PENG Qingkui, et al. Comparative study of chemical discharge strategy to pretreat spent lithium-ion batteries for safe, efficient, and environmentally friendly recycling[J]. Journal of Cleaner Production, 2022, 359: 132116. |
| 33 | XIAO Jiefeng, GUO Jie, ZHAN Lu, et al. A cleaner approach to the discharge process of spent lithium ion batteries in different solutions[J]. Journal of Cleaner Production, 2020, 255: 120064. |
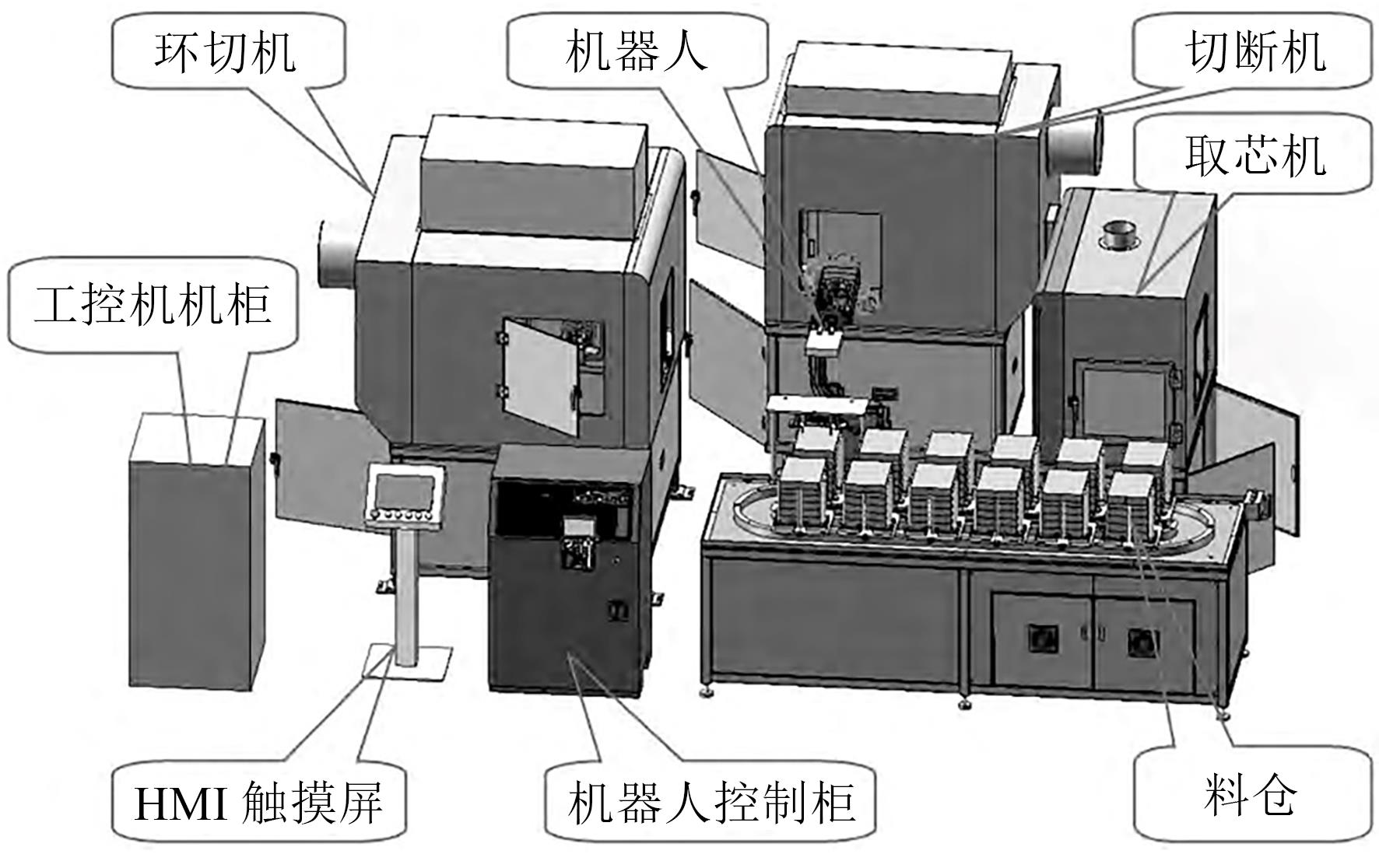
| 34 | 方国平, 季仲致. 基于PLC和机器人技术的锂电池拆解自动控制系统研究[J]. 工业控制计算机, 2019, 32(3): 81-82. |
| FANG Guoping, JI Zhongzhi. Lithium batteries dismantling automatic control system based on PLC and robot[J]. Industrial Control Computer, 2019, 32(3): 81-82. | |
| 35 | MURATA A. Ergonomics and cognitive engineering for robot-human cooperation[C]//Proceedings 9th IEEE International Workshop on Robot and Human Interactive Communication. IEEE RO-MAN 2000. September 27-29, 2000, Osaka, Japan. IEEE, 2002: 206-211. |
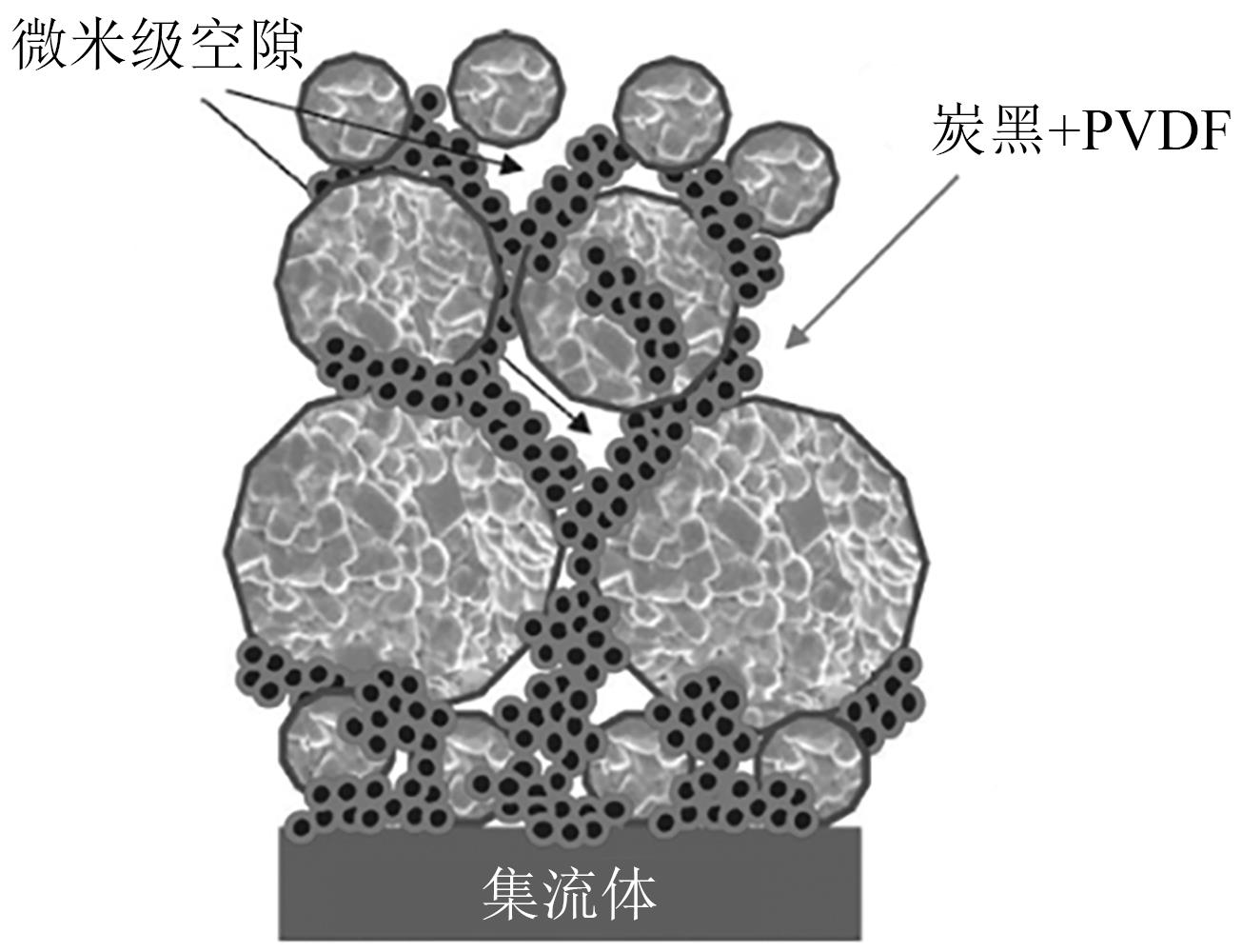
| 36 | ETIEMBLE A, BESNARD N, BONNIN A, et al. Multiscale morphological characterization of process induced heterogeneities in blended positive electrodes for lithium-ion batteries[J]. Journal of Materials Science, 2017, 52(7): 3576-3596. |
| 37 | ZHENG Rujuan, WANG Wenhui, DAI Yunkun, et al. A closed-loop process for recycling LiNi x Co y Mn(1– x– y)O2 from mixed cathode materials of lithium-ion batteries[J]. Green Energy & Environment, 2017, 2(1): 42-50. |
| 38 | CHU Wei, ZHANG Yali, CHEN Linlin, et al. Comprehensive recycling of Al foil and active materials from the spent lithium-ion battery[J]. Separation and Purification Technology, 2021, 269: 118704. |
| 39 | JIANG Siqi, NIE Chunchen, SHI Shunxiang, et al. Enhancements of dissociation of electrode materials in spent lithium-ion batteries by low-temperature heating pretreatment[J]. Process Safety and Environmental Protection, 2023, 170: 908-920. |
| 40 | LOU Ping, GUAN Minyuan, WU Guoqiang, et al. Recycle cathode materials from spent lithium-ion batteries by an innovative method[J]. Ionics, 2022, 28(5): 2135-2141. |
| 41 | CHEN Xiangping, LI Shuzhen, WANG Yi, et al. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation[J]. Waste Management, 2021, 136: 67-75. |
| 42 | ZHENG Xiaohong, ZHU Zewen, LIN Xiao, et al. A mini-review on metal recycling from spent lithium ion batteries[J]. Engineering, 2018, 4(3): 361-370. |
| 43 | WANG Mengmeng, TAN Quanyin, LIU Lili, et al. A facile, environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 12799-12806. |
| 1 | JIN Shan, MU Deying, LU Ziang, et al. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances[J]. Journal of Cleaner Production, 2022, 340: 130535. |
| 2 | XIAO Jinhua, JIANG Chengran, WANG Bo. A review on dynamic recycling of electric vehicle battery: Disassembly and echelon utilization[J]. Batteries, 2023, 9(1): 57. |
| 3 | SUN Jie, LI Jigang, ZHOU Tian, et al. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery[J]. Nano Energy, 2016, 27: 313-319. |
| 4 | NATARAJAN Subramanian, ARAVINDAN Vanchiappan. Burgeoning prospects of spent lithium-ion batteries in multifarious applications[J]. Advanced Energy Materials, 2018, 8(33): 1802303. |
| 5 | PALACÍN M R, DE GUIBERT A. Why do batteries fail?[J]. Science, 2016, 351(6273): 1253292. |
| 6 | YUE Liguo, GUO Hao, WANG Xiao, et al. Non-metallic element modified metal-organic frameworks as high-performance electrodes for all-solid-state asymmetric supercapacitors[J]. Journal of Colloid and Interface Science, 2019, 539: 370-378. |
| 7 | NORDBERG Gunnar. Assessment of risks in occupational cobalt exposures[J]. Science of the Total Environment, 1994, 150(1/2/3): 201-207. |
| 8 | ZAMBELLI Barbara, UVERSKY Vladimir N, CIURLI Stefano. Nickel impact on human health: An intrinsic disorder perspective[J]. Biochimica et Biophysica Acta, 2016, 1864(12): 1714-1731. |
| 9 | Hayder ALI, KHAN Hassan A, PECHT Michael G. Circular economy of Li Batteries: Technologies and trends[J]. Journal of Energy Storage, 2021, 40: 102690. |
| 10 | 张笑笑. 废旧锂离子电池的回收处理与资源化利用[D]. 北京: 北京理工大学, 2016. |
| ZHANG Xiaoxiao. Recycling and resource utilization of spent lithium-ion batteries[D].Beijing: Beijing Institute of Technology, 2016. | |
| 44 | 袁文辉, 邱定蕃, 王成彦. 还原熔炼失效锂离子电池的研究[J]. 有色金属(冶炼部分), 2007(4): 5-7, 26. |
| YUAN Wenhui, QIU Dingfan, WANG Chengyan. Research on recycling of spent lithium ion battery by reducing smelting process[J]. Nonferrous Metals (Extractive Metallurgy), 2007(4): 5-7, 26. | |
| 45 | 张英杰, 宁培超, 杨轩, 等. 废旧三元锂离子电池回收技术研究新进展[J]. 化工进展, 2020, 39(7): 2828-2840. |
| ZHANG Yingjie, NING Peichao, YANG Xuan, et al. Research progress on the recycling technology of spent ternary lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2828-2840. | |
| 46 | CHEN Yaodong, DOU Aichun, ZHANG Ye. A review of recycling status of decommissioned lithium batteries[J]. Frontiers in Materials, 2021, 8: 634667. |
| 47 | LIU Chao, QIU Xianyang, LIU Yong, et al. Research status and prospects of physical separation technology of spent lithium-ion batteries[J]. Chinese Journal of Rare Metals. 2021, 45(4): 493-501. |
| 48 | YU Jiadong, HE Yaqun, GE Zhenzhou, et al. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation[J]. Separation and Purification Technology, 2018, 190: 45-52. |
| 49 | SALCES Aliza Marie, BREMERSTEIN Irina, RUDOLPH Martin, et al. Joint recovery of graphite and lithium metal oxides from spent lithium-ion batteries using froth flotation and investigation on process water re-use[J]. Minerals Engineering, 2022, 184: 107670. |
| 50 | XIAO Jiefeng, NIU Bo, XU Zhenming. Highly efficient selective recovery of lithium from spent lithium-ion batteries by thermal reduction with cheap ammonia reagent[J]. Journal of Hazardous Materials, 2021, 418: 126319. |
| 51 | YANG Yue, LEI Shuya, SONG Shaole, et al. Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries[J]. Waste Management, 2020, 102: 131-138. |
| 52 | MESHRAM Pratima, PANDEY B D, MANKHAND T R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427. |
| 53 | HE Lipo, SUN Shuying, SONG Xingfu, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171-181. |
| 54 | GUIMARÃES L F, BOTELHO A B, ESPINOSA D C R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent[J]. Minerals Engineering, 2022, 183: 107597. |
| 55 | Manis Kumar JHA, KUMARI Anjan, Amrita Kumari JHA, et al. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone[J]. Waste Management, 2013, 33(9): 1890-1897. |
| 56 | GUO Yang, LI Feng, ZHU Haochen, et al. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl)[J]. Waste Management, 2016, 51: 227-233. |
| 57 | TUAN Tran Thanh, SEUNG Moon Hyun, SEUNG Lee Man. Recovery of valuable metals from the hydrochloric leaching solution of reduction smelted metallic alloys from spent lithium-ion batteries[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(5): 1247-1258. |
| 58 | LEE Churl Kyoung, RHEE Kang-In. Preparation of LiCoO2 from spent lithium-ion batteries[J]. Journal of Power Sources, 2002, 109(1): 17-21. |
| 59 | CHEN Xiangping, MA Hongrui, LUO Chuanbao, et al. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid[J]. Journal of Hazardous Materials, 2017, 326: 77-86. |
| 60 | MESHRAM Pratima, MISHRA Abhilash, ABHILASH, et al. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review[J]. Chemosphere, 2020, 242: 125291. |
| 61 | SUN Conghao, XU Liping, CHEN Xiangping, et al. Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: Leaching and kinetics aspect[J]. Waste Management & Research, 2018, 36(2): 113-120. |
| 62 | NING Peichao, MENG Qi, DONG Peng, et al. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system[J]. Waste Management, 2020, 103: 52-60. |
| 63 | GAO Guilan, HE Xin, LOU Xiaoyi, et al. A citric acid/Na2S2O3 system for the efficient leaching of valuable metals from spent lithium-ion batteries[J]. JOM, 2019, 71(10): 3673-3681. |
| 64 | LI Li, BIAN Yifan, ZHANG Xiaoxiao, et al. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching[J]. Waste Management, 2018, 71: 362-371. |
| 65 | 高桂兰, 范丹丹, 贺欣, 等. 采用响应面优化酸浸法回收报废三元电池中有价金属的研究[J]. 安全与环境学报, 2020, 20(1): 290-296. |
| GAO Guilan, FAN Dandan, HE Xin, et al. Acid leaching optimization by response surface to recover valuable metals from spent ternary battery[J]. Journal of Safety and Environment. 2020, 20(1): 290-296. | |
| 66 | ZENG Xianlai, LI Jinhui, SHEN Bingyu. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials, 2015, 295: 112-118. |
| 67 | NATARAJAN Subramanian, BORICHA Arvind B, BAJAJ Hari C. Recovery of value-added products from cathode and anode material of spent lithium-ion batteries[J]. Waste Management, 2018, 77: 455-465. |
| 68 | 郑莹, 罗涵璐, 李柏霖, 等. 酒石酸浸出钴酸锂动力学机理研究[J]. 环境工程, 2022, 40(2): 88-92, 99. |
| ZHENG Ying, LUO Hanlu, LI Bolin, et al. Kinetic mechanism of leaching lithium cobalt oxides using tartaric acid[J]. Environmental Engineering, 2022, 40(2): 88-92, 99. | |
| 69 | QI Yaping, MENG Fansong, YI Xiaoxia, et al. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries[J]. Journal of Cleaner Production, 2020, 251: 119665. |
| 70 | ZHENG Xiaohong, GAO Wenfang, ZHANG Xihua, et al. Spent lithium-ion battery recycling–Reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688. |
| 71 | DU Kaidi, Edison Huixiang ANG, WU Xinglong, et al. Progresses in sustainable recycling technology of spent lithium-ion batteries[J]. Energy & Environmental Materials, 2022, 5(4): 1012-1036. |
| 72 | TIAN Guangdong, YUAN Gang, ALEKSANDROV Anatoly, et al. Recycling of spent Lithium-ion Batteries: A comprehensive review for identification of main challenges and future research trends[J]. Sustainable Energy Technologies and Assessments, 2022, 53: 102447. |
| 73 | 张颢竞, 程洁红, 朱铖, 等. 用酸浸-生物浸出工艺从废锂离子电池电极材料中回收金属钴铜镍[J]. 湿法冶金, 2019, 38(1): 22-27. |
| ZHANG Haojing, CHENG Jiehong, ZHU Cheng, et al. Recovery of copper, cobalt and nickel from spent lithium ion batteries by a combined process of acid leaching and bioleaching[J]. Hydrometallurgy of China, 2019, 38(1): 22-27. | |
| 74 | BISWAL Basanta Kumar, JADHAV Umesh U, MADHAIYAN Munusamy, et al. Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12343-12352. |
| 75 | HUANG Bin, WANG Jiexi. Bio-hydrometallurgically treatment of spent lithium-ion batteries[M]//An L. Recycling of spent lithium-ion batteries. Cham: Springer, 2019: 85-92. |
| 76 | CHEN Xiangping, XU Bao, ZHOU Tao, et al. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries[J]. Separation and Purification Technology, 2015, 144: 197-205. |
| 77 | ZHANG Yingchao, WANG Wenqiang, HU Jiehui, et al. Stepwise recovery of valuable metals from spent lithium ion batteries by controllable reduction and selective leaching and precipitation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(41): 15496-15506. |
| 78 | Daniel QUINTERO-ALMANZA, Zeferino GAMIÑO-ARROYO, SÁNCHEZ-CADENA Lorena Eugenia, et al. Recovery of cobalt from spent lithium-ion mobile phone batteries using liquid–liquid extraction[J]. Batteries, 2019, 5(2): 44. |
| 79 | XUAN Wen, DE SOUZA BRAGA Antônio, CHAGNES Alexandre. Development of a novel solvent extraction process to recover cobalt, nickel, manganese, and lithium from cathodic materials of spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(1): 582-593. |
| 80 | LEI Shuya, SUN Wei, YANG Yue. Solvent extraction for recycling of spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2022, 424: 127654. |
| 81 | CHAN Ka Ho, MALIK Monu, AZIMI Gisele. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis[J]. Resources, Conservation and Recycling, 2022, 178: 106076. |
| 82 | CHEN Xiaoqing, YANG Chenfei, YANG Yubo, et al. Co-precipitation preparation of Ni-Co-Mn ternary cathode materials by using the sources extracting directly from spent lithium-ion batteries[J]. Journal of Alloys and Compounds, 2022, 909: 164691. |
| 83 | SHANG Miao, PENG Lixia. The regeneration and electrochemical performance study of NCM622 cathode materials[J]. Ionics, 2021, 27(2): 527-532. |
| 84 | ZHANG Zehui, YU Min, YANG Bin, et al. Regeneration of Al-doped LiNi1/3Co1/3Mn1/3O2 cathode material via a sustainable method from spent Li-ion batteries[J]. Materials Research Bulletin, 2020, 126: 110855. |
| 85 | LI Li, FAN Ersha, GUAN Yibiao, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233. |
| 86 | 梁力勃, 杨生龙, 罗茂枭, 等. 高温固相法再生废旧磷酸铁锂电池正极材料[J]. 矿冶工程, 2021, 41(3): 120-123, 128. |
| LIANG Libo, YANG Shenglong, LUO Maoxiao, et al. Regeneration of cathode materials in spent lithium iron phosphate batteries by using high temperature solid-phase method[J]. Mining and Metallurgical Engineering, 2021, 41(3): 120-123, 128. | |
| 87 | 李林林. 废旧镍钴锰酸锂电池的回收与再利用研究[D]. 广州: 广州大学, 2021. |
| LI Linlin. Study on recovery and reuse of waste nickel cobalt manganese lithium batteries[D]. Guangzhou: Guangzhou University, 2021. |
| [1] | GAO Xinyue, FAN Gaofeng, LIU Aiping, WANG Chang'an, HOU Yujie, ZHANG Jinming, XU Jie, CHE Defu. Research progress on waste heat recovery technology for flue gas and slurry after wet desulphurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4307-4319. |
| [2] | HU Tingxia, ZHAO Lixin, YAO Zonglu, HUO Lili, JIA Jixiu, XIE Teng. Research progress of bimetallic catalysts in catalytic steam reforming of biomass tar [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4354-4365. |
| [3] | HU Rui, LI Xianru, PIAO Weiling, FENG Pan, LUO Lei, LUO Gang, WEI Huangzhao, LIU Zhengang, ZHANG Shicheng. Progress on the hydrothermal conversion equipment and technology of organic waste [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3672-3691. |
| [4] | GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823. |
| [5] | ZHAO Peitao, FU Binbin, ZHAO Quan, ZUO Wu, ZHOU Haiyun, HAN Dongtai. Thermal decomposition of plastics via low pressure and superheated solvent steam and its characteristics [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3420-3429. |
| [6] | MA Jiahui, WANG Yibin, FENG Jingwu, TAN Houzhang, LIN Chi. Experimental of CO2 mineralization by industrial containing calcium solid wastes [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3440-3449. |
| [7] | MA Haifei, LIAO Yalong, WU Min, JIA Xiaobao, YANG Shuangyu. Extraction mechanism of sulfuric acid from copper leaching solution of hydrometallurgical process [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3410-3419. |
| [8] | YAO Xue, WU Shuhui, YANG Yang, WANG Xiao, FENG Lei, FENG Xuedong, MA Yanfei. Treatment of oily wastewater by oily sludge-based biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3398-3409. |
| [9] | WANG Houran, LI Denian, DONG Nanhang, YANG Jizhang, NI Xuanyuan, YE Jiahong, YUAN Haoran, CHEN Yong. Advances in direct repair of cathode materials from retired lithium iron phosphate battery and ternary lithium battery [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3336-3346. |
| [10] | MU Lianbo, WANG Suilin, LU Junhui, LIU Guichang, ZHAO Liqiu, LIU Jincheng, HAO Anfeng, ZHANG Tong. Analysis of flue gas deep waste heat recovery with cooperative flue gas pressure control for alkane dehydrogenation heating furnace [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3029-3041. |
| [11] | LI Si, TAO Yiyue, XIAO Zhenchong, ZHANG Liang, LI Jun, ZHU Xun, LIAO Qiang. Electrochemical characteristics of the coupled system of thermally regenerative battery stack and electrochemical CO2 reduction cell [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2568-2575. |
| [12] | LIU Shida, WANG Haiyan, HOU Shuandi, LIU Zhongsheng, LIAO Changjian, WANG Kuanling. Recent advances in safely efficient deep emission reduction, recovery and thermal oxidation of VOCs from petrochemical storage tanks in China [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2063-2076. |
| [13] | MA Wenjun, ZHANG Xu, LIU Mengshun, LIANG Zhiyuan. Research progress of novel hydrometallurgy in recycling cathode materials from spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2077-2090. |
| [14] | SUN Weiji, LIU Lang, FANG Zhiyu, ZHU Mengbo, XIE Geng, HE Wei, GAO Yuheng. Technique of wet carbonation of modified magnesium slag [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2161-2173. |
| [15] | ZHOU Mingxian, YE Xiaozhou. Optimization of preferential lithium extraction from waste ternary lithium ion batteries by carbothermal reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2174-2182. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||