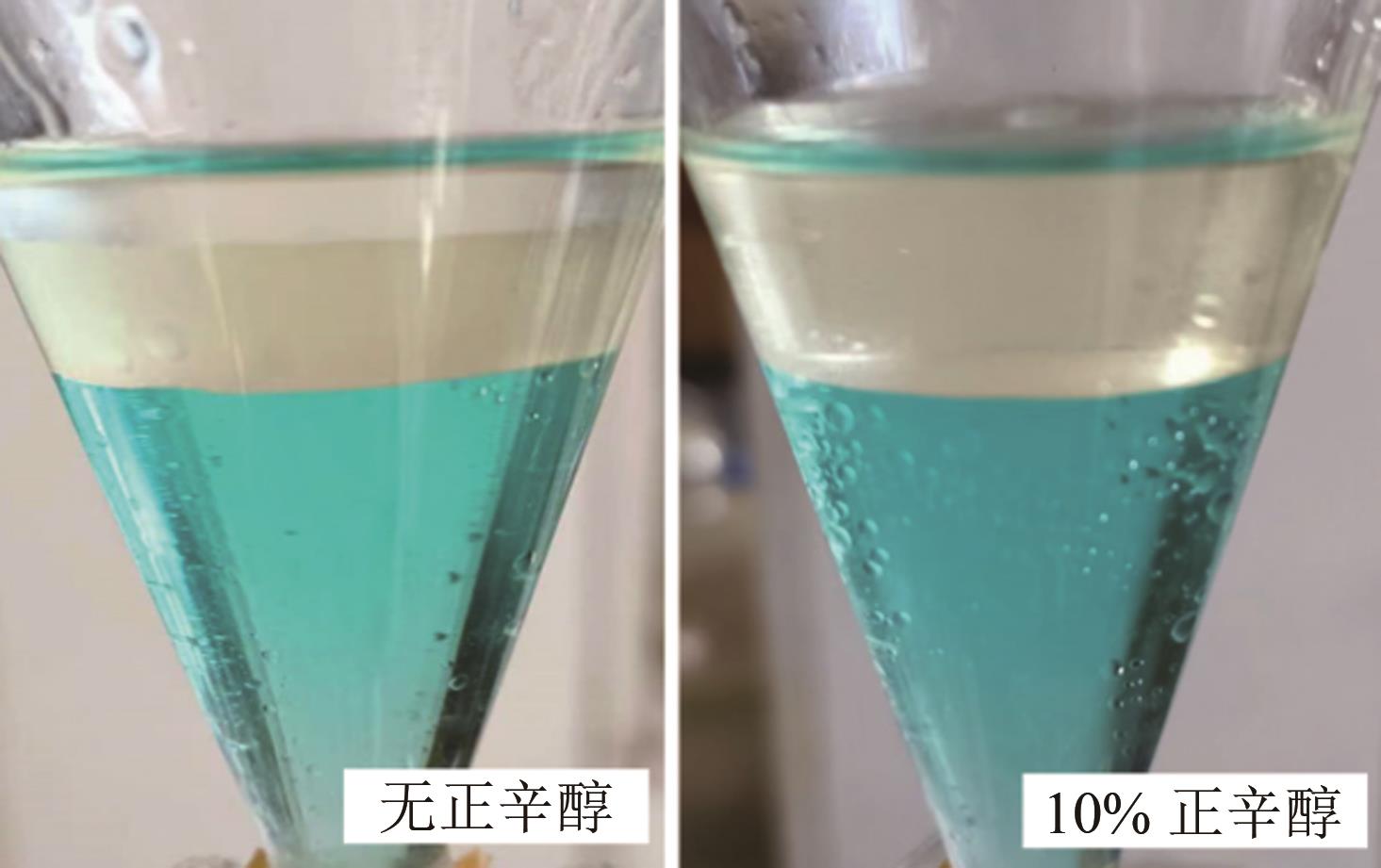
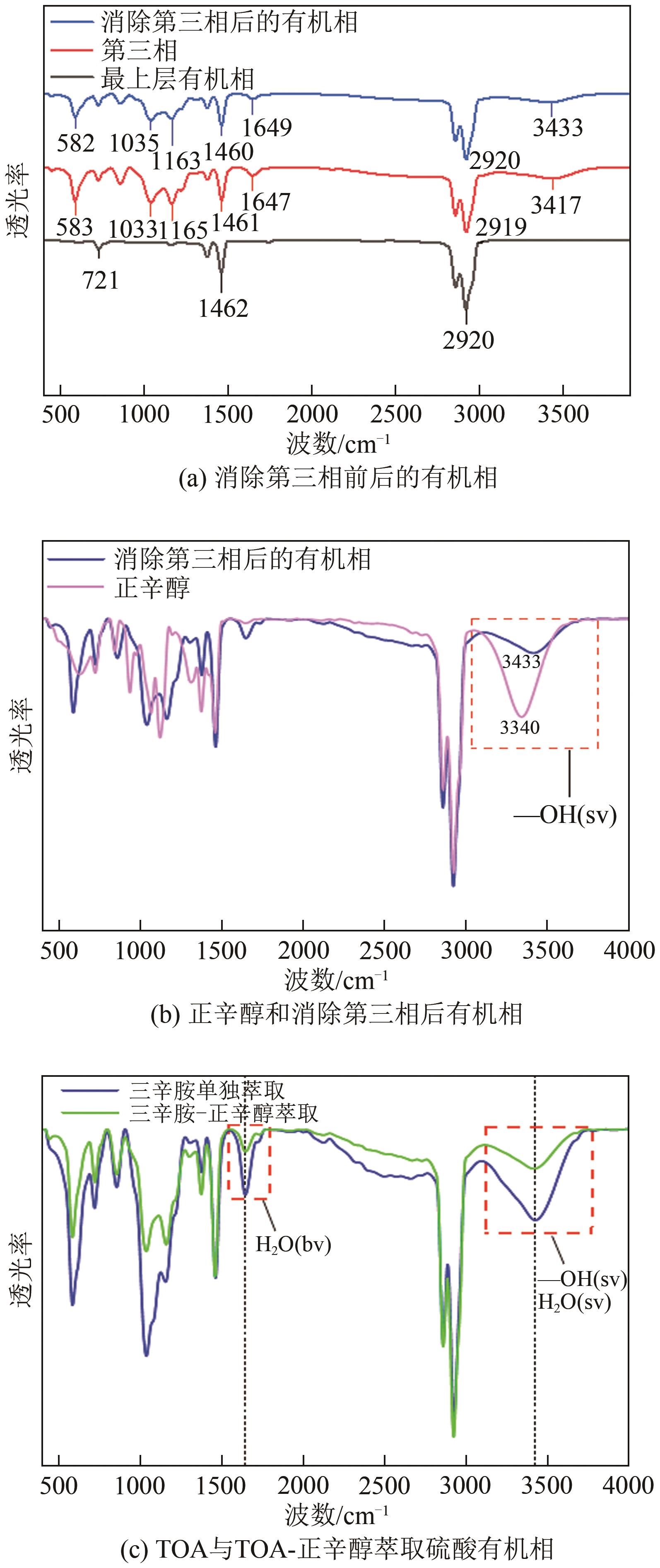
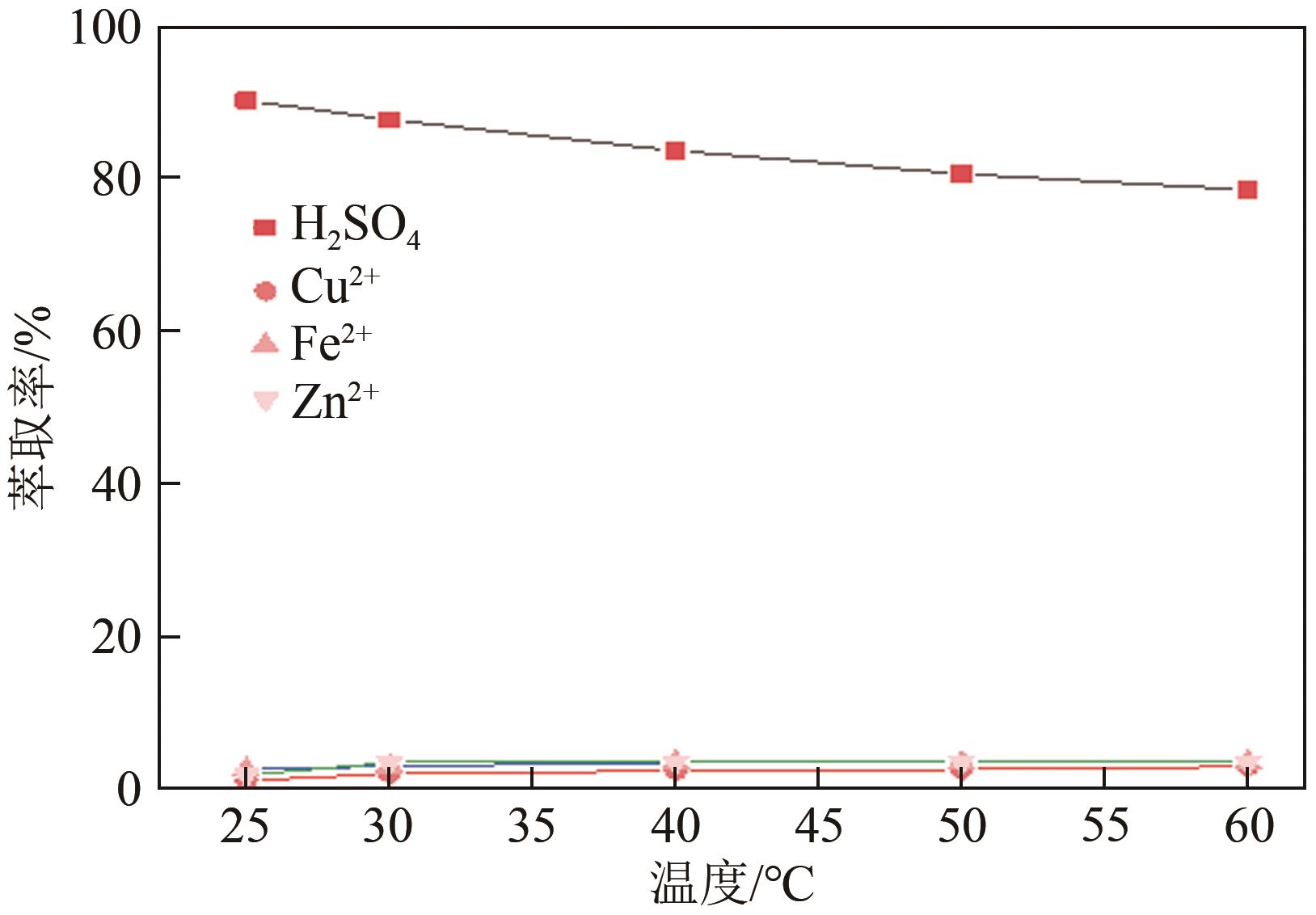
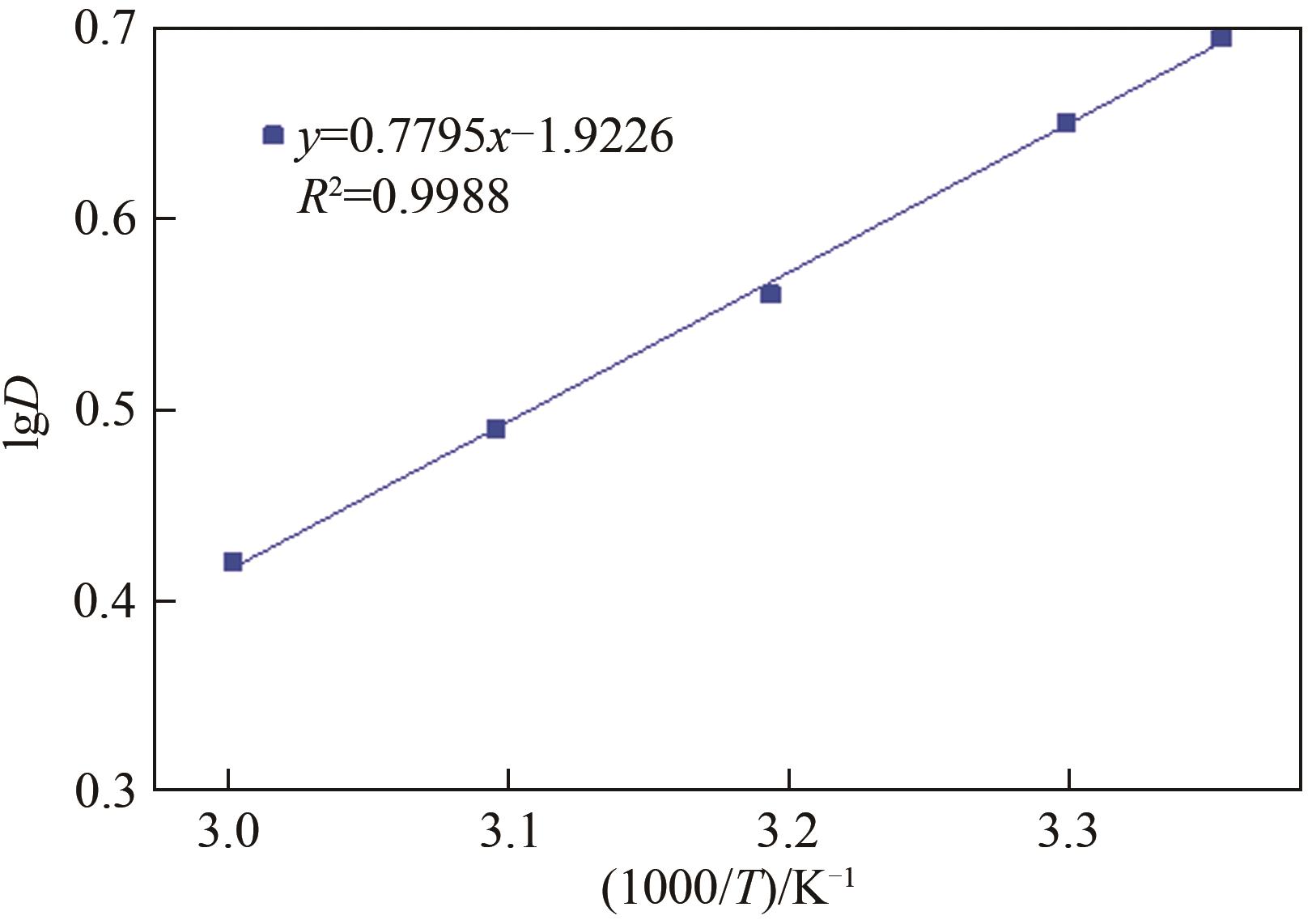
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (6): 3410-3419.DOI: 10.16085/j.issn.1000-6613.2023-0756
• Resources and environmental engineering • Previous Articles
Extraction mechanism of sulfuric acid from copper leaching solution of hydrometallurgical process
MA Haifei( ), LIAO Yalong(
), LIAO Yalong( ), WU Min, JIA Xiaobao, YANG Shuangyu
), WU Min, JIA Xiaobao, YANG Shuangyu
- Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
-
Received:2023-05-08Revised:2023-06-19Online:2024-07-02Published:2024-06-15 -
Contact:LIAO Yalong
湿法炼铜浸出液萃取分离硫酸机理
- 昆明理工大学冶金与能源工程学院,云南 昆明 650093
-
通讯作者:廖亚龙 -
作者简介:马海飞(1997—),男,硕士研究生,研究方向为资源综合回收利用。E-mail:1697185352@qq.com。 -
基金资助:国家自然科学基金(21978122)
CLC Number:
Cite this article
MA Haifei, LIAO Yalong, WU Min, JIA Xiaobao, YANG Shuangyu. Extraction mechanism of sulfuric acid from copper leaching solution of hydrometallurgical process[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3410-3419.
马海飞, 廖亚龙, 武敏, 贾小宝, 杨双宇. 湿法炼铜浸出液萃取分离硫酸机理[J]. 化工进展, 2024, 43(6): 3410-3419.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0756
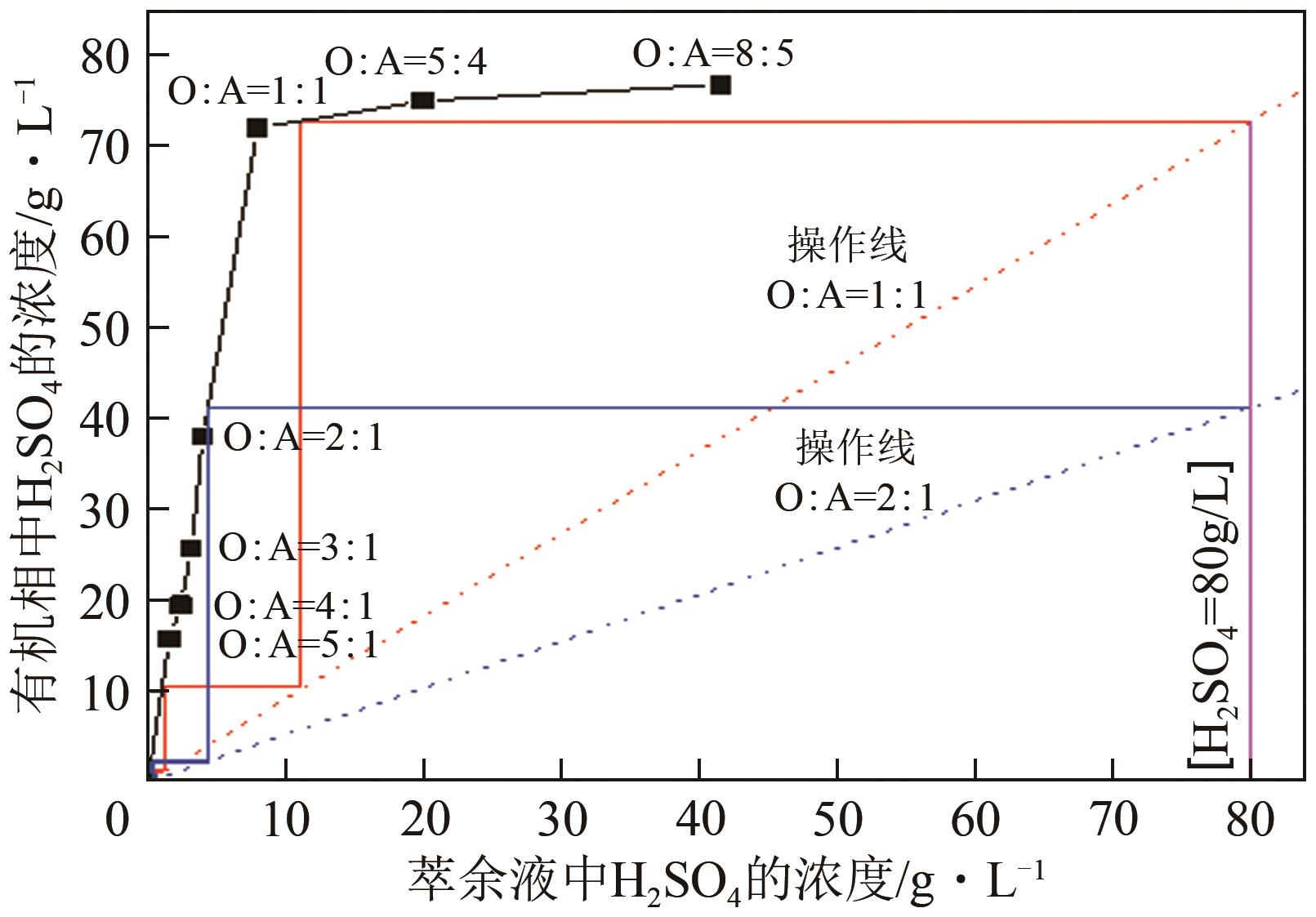
| 成分 | 含量/g·L-1 |
|---|---|
| H2SO4 | 80 |
| Cu2+ | 10 |
| Fe2+ | 4 |
| Zn2+ | 2 |
| 成分 | 含量/g·L-1 |
|---|---|
| H2SO4 | 80 |
| Cu2+ | 10 |
| Fe2+ | 4 |
| Zn2+ | 2 |
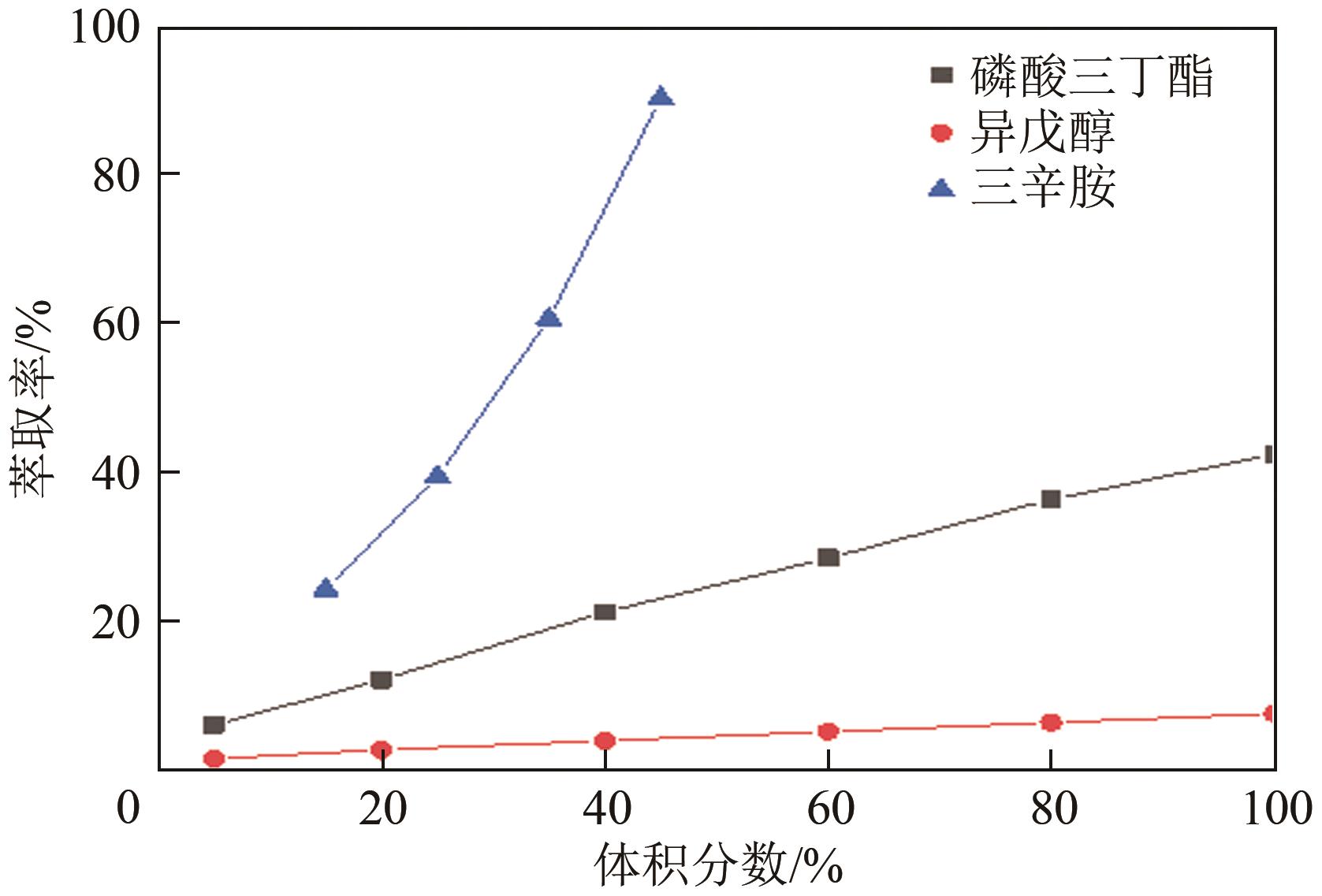
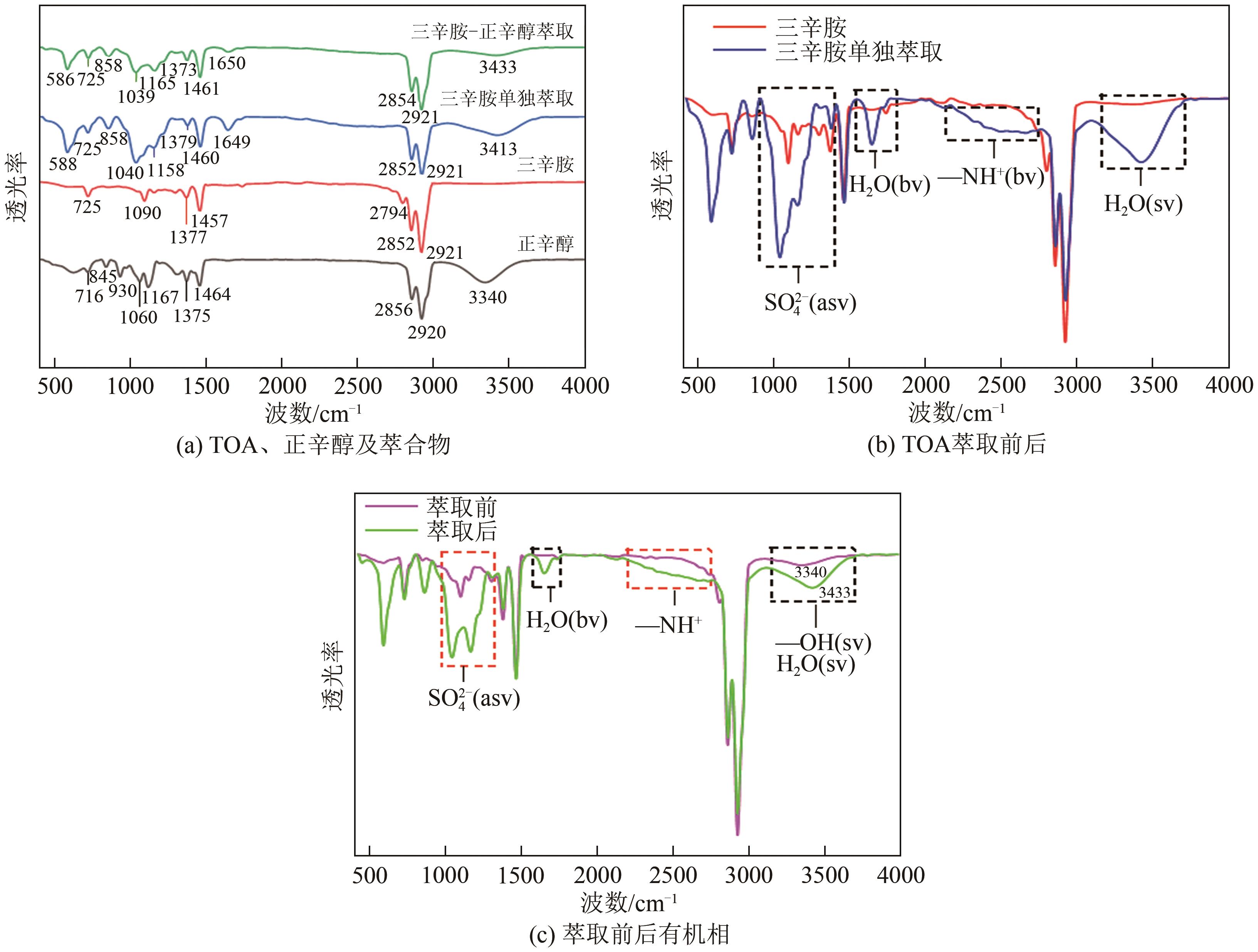
| 有机相构成体积分数/% | 稀释剂 | 萃取率/% | ||
|---|---|---|---|---|
| 三辛胺 | 正辛醇 | 正丁醇 | ||
| 45 | 55 | — | — | 86.04 |
| 45 | 10 | — | 45%甲苯 | 87.76 |
| 45 | 10 | — | 45% 260#溶剂油 | 90.21 |
| 45 | — | 55 | — | 85.04 |
| 45 | — | 10 | 45%甲苯 | 85.48 |
| 45 | — | 10 | 45% 260#溶剂油 | 86.48 |
| 有机相构成体积分数/% | 稀释剂 | 萃取率/% | ||
|---|---|---|---|---|
| 三辛胺 | 正辛醇 | 正丁醇 | ||
| 45 | 55 | — | — | 86.04 |
| 45 | 10 | — | 45%甲苯 | 87.76 |
| 45 | 10 | — | 45% 260#溶剂油 | 90.21 |
| 45 | — | 55 | — | 85.04 |
| 45 | — | 10 | 45%甲苯 | 85.48 |
| 45 | — | 10 | 45% 260#溶剂油 | 86.48 |
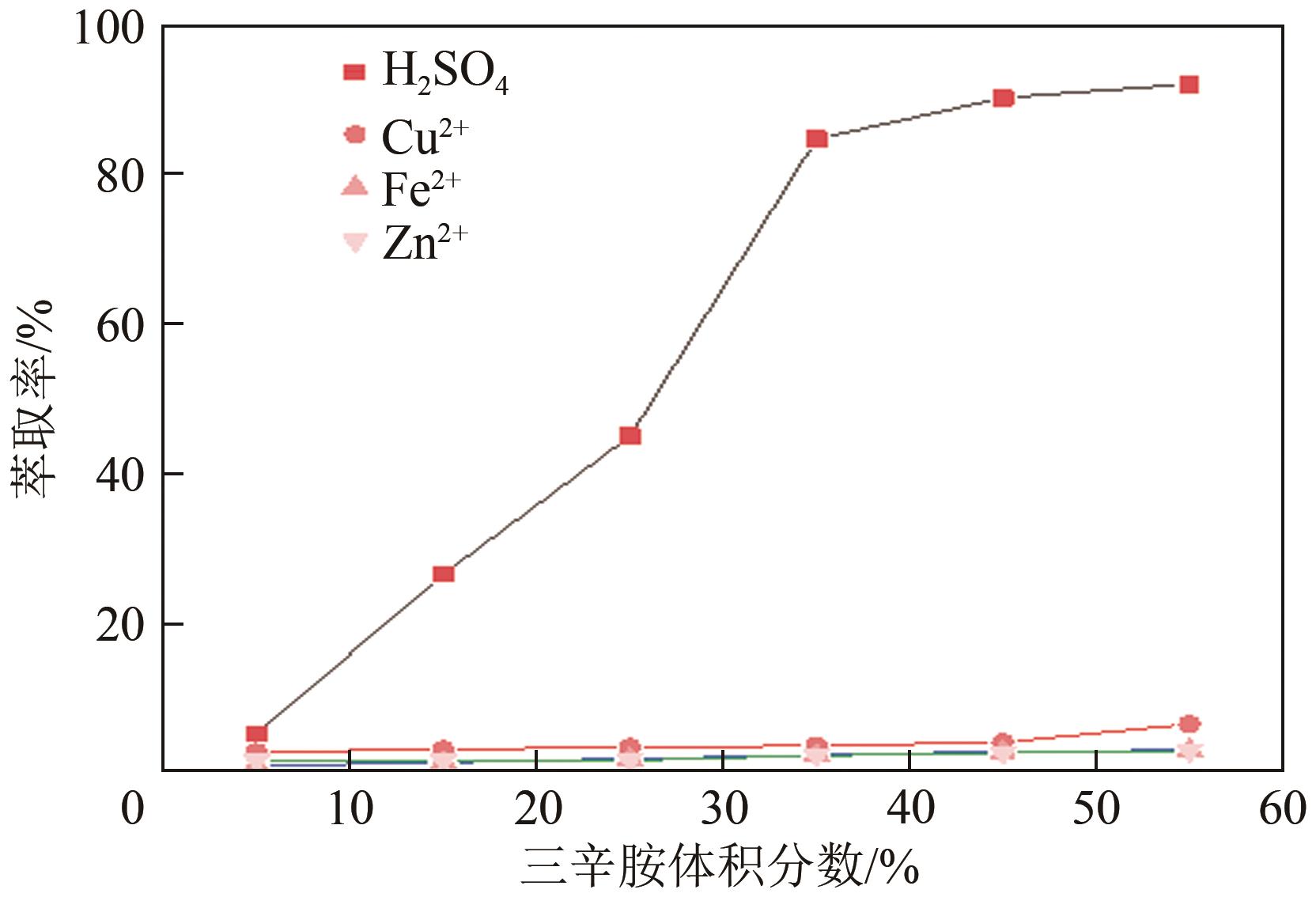
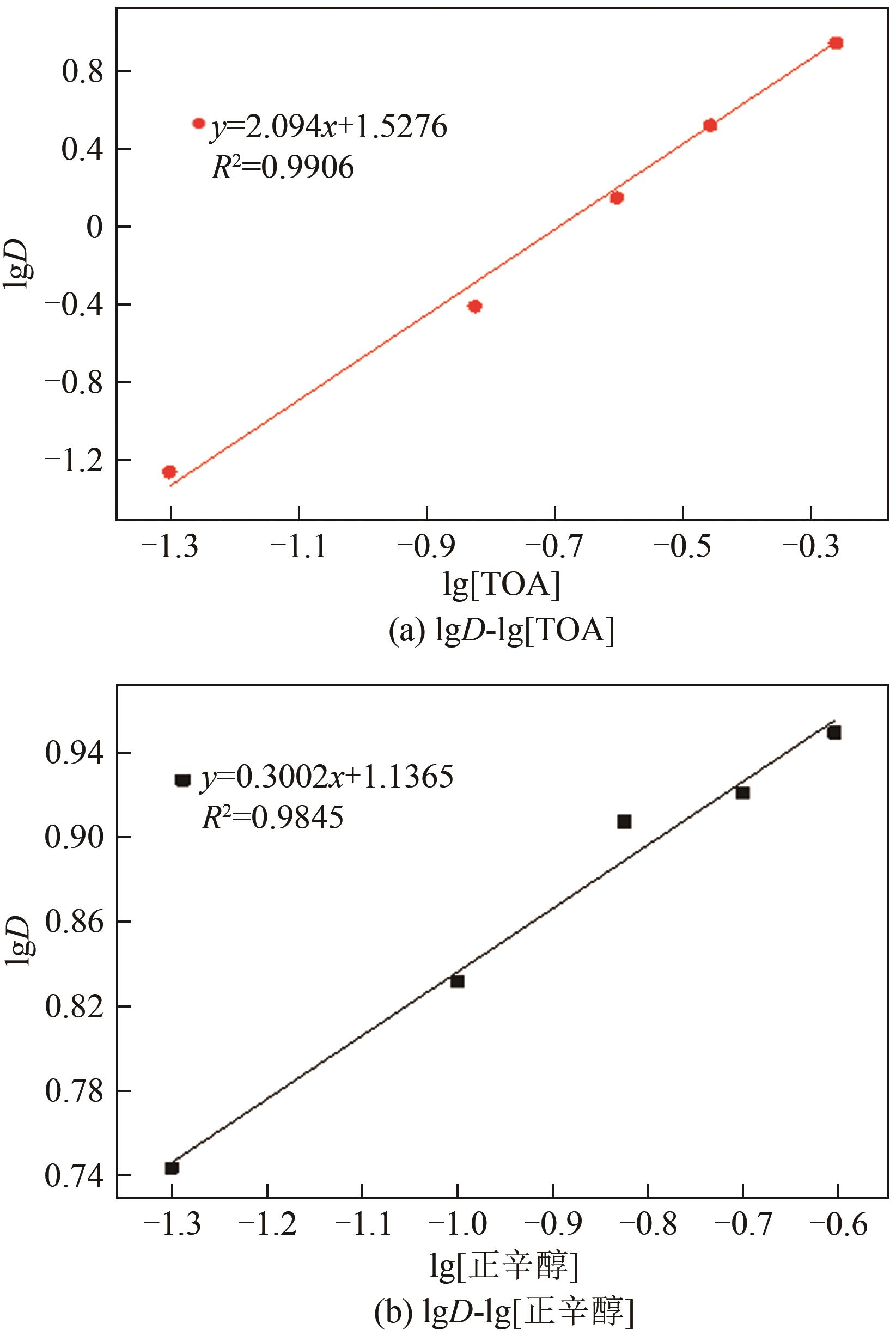
| 斜率分析 | 三辛胺体积 分数/% | 260#溶剂油体积 分数/% | 正辛醇体积 分数/% |
|---|---|---|---|
| 三辛胺作用 | 45 | 45 | 10 |
| 35 | 55 | 10 | |
| 25 | 65 | 10 | |
| 15 | 75 | 10 | |
| 5 | 85 | 10 | |
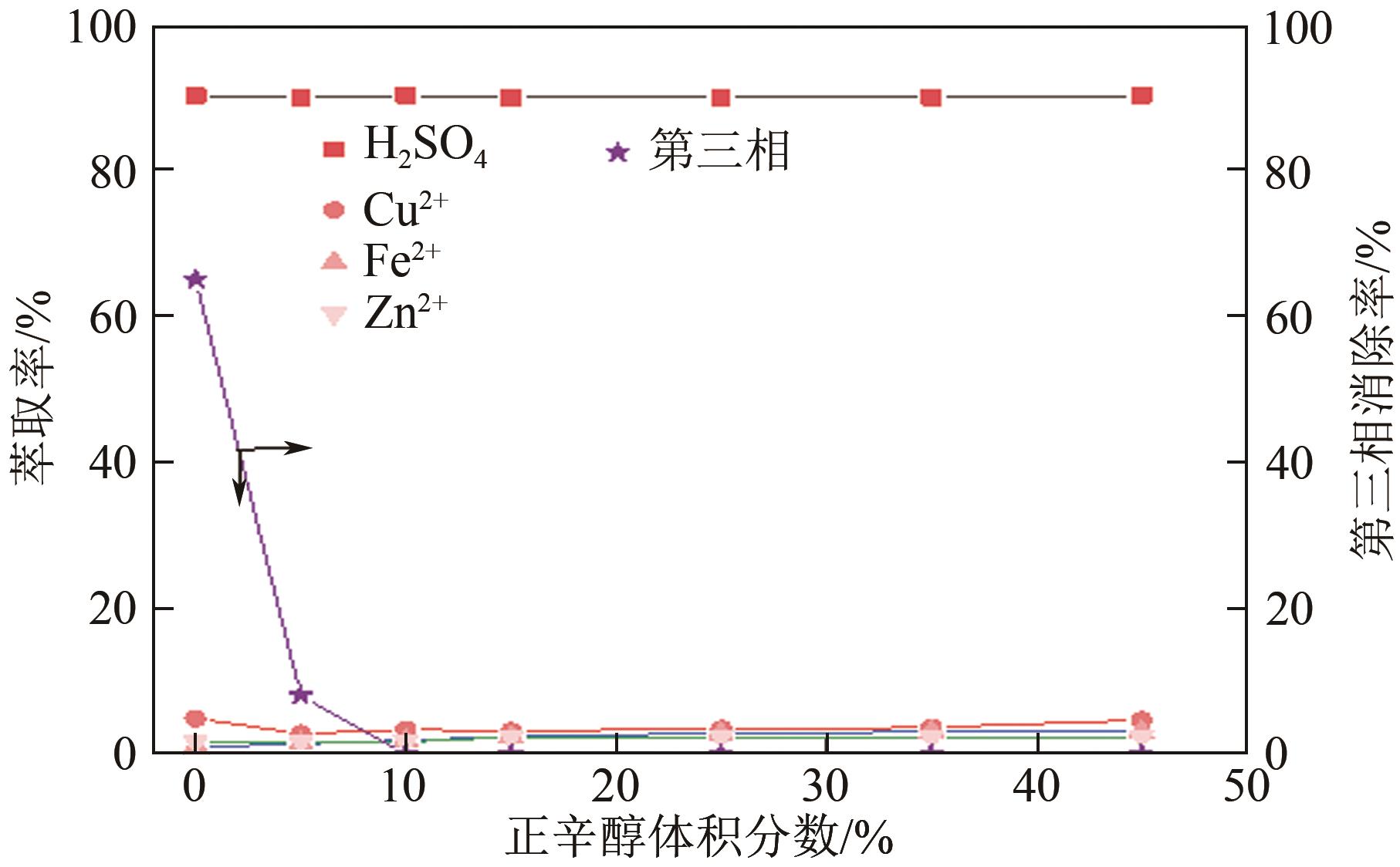
| 正辛醇作用 | 45 | 30 | 25 |
| 45 | 35 | 20 | |
| 45 | 40 | 15 | |
| 45 | 45 | 10 | |
| 45 | 50 | 5 |
| 斜率分析 | 三辛胺体积 分数/% | 260#溶剂油体积 分数/% | 正辛醇体积 分数/% |
|---|---|---|---|
| 三辛胺作用 | 45 | 45 | 10 |
| 35 | 55 | 10 | |
| 25 | 65 | 10 | |
| 15 | 75 | 10 | |
| 5 | 85 | 10 | |
| 正辛醇作用 | 45 | 30 | 25 |
| 45 | 35 | 20 | |
| 45 | 40 | 15 | |
| 45 | 45 | 10 | |
| 45 | 50 | 5 |
| 1 | 周娟, 廖亚龙, 李冰洁, 等. 多金属复杂硫化铜矿中有价金属的分离研究现状与进展[J]. 化工进展, 2015, 34(1): 252-257. |
| ZHOU Juan, LIAO Yalong, LI Bingjie, et al. Valuable metals extraction from complex multi-metal copper sulfide ore[J]. Chemical Industry and Engineering Progress, 2015, 34(1): 252-257. | |
| 2 | 刘庆丰, 廖亚龙, 吴越, 等. 黄铜矿强化浸出研究进展[J]. 化工进展, 2022, 41(11): 6099-6110. |
| LIU Qingfeng, LIAO Yalong, WU Yue, et al. Research progress on enhancing leaching efficiency of chalcopyrite[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6099-6110. | |
| 3 | 闫江梅, 董庆, 张之翔. 多组分废催化剂中钯的分离工艺研究进展[J]. 化工进展, 2014, 33(9): 2478-2483. |
| YAN Jiangmei, DONG Qing, ZHANG Zhixiang. Research development on the separation of Palladium from multicomponent waste catalyst[J]. Chemical Industry and Engineering Progress, 2014, 33(9): 2478-2483. | |
| 4 | 段亨攀, 刘红盼, 杨项军. 湿法冶金提取分离铜镍技术研究进展[J]. 稀有金属, 2020, 44(5): 547-554. |
| DUAN Hengpan, LIU Hongpan, YANG Xiangjun. Research progress of extraction and separation of copper and nickel in hydrometallurgy[J]. Chinese Journal of Rare Metals, 2020, 44(5): 547-554. | |
| 5 | 韩光辉, 黄玉代, 王省超, 等. 高砷酸性浸出液中回收铜的试验研究[J]. 新疆大学学报(自然科学版), 2021, 38(1): 29-36. |
| HAN Guanghui, HUANG Yudai, WANG Xingchao, et al. Experimental study on recycle of copper from high arsenic acid leaching solution[J]. Journal of Xinjiang University (Natural Science Edition), 2021, 38(1): 29-36. | |
| 6 | AKCIL Ata, KOLDAS Soner. Acid Mine Drainage (AMD): Causes, treatment and case studies[J]. Journal of Cleaner Production, 2006, 14(12/13): 1139-1145. |
| 7 | Magdalena REGEL-ROSOCKA. A review on methods of regeneration of spent pickling solutions from steel processing[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 57-69. |
| 8 | 孙启, 黄松涛, 杨丽梅, 等. 工业废酸中硫酸的萃取分离与回收研究现状[J]. 稀有金属, 2022, 46(5): 652-664. |
| SUN Qi, HUANG Songtao, YANG Limei, et al. Separation and recovery of sulfuric acid from industrial spent acid by solvent extraction: A review[J]. Chinese Journal of Rare Metals, 2022, 46(5): 652-664. | |
| 9 | MARTÍ-CALATAYUD M C, BUZZI D C, GARCÍA-GABALDÓN M, et al. Sulfuric acid recovery from acid mine drainage by means of electrodialysis[J]. Desalination, 2014, 343: 120-127. |
| 10 | BLANC Claire-Line, LEMAIRE Julien, DUVAL Fanny, et al. Purification of pentoses from hemicellulosic hydrolysates without neutralization for sulfuric acid recovery[J]. Separation and Purification Technology, 2017, 174: 513-519. |
| 11 | 李伟, 纪仲光, 王巍, 等. 冶炼污酸管式气隙膜蒸馏过程研究[J]. 稀有金属, 2020, 44(6): 639-646. |
| LI Wei, JI Zhongguang, WANG Wei, et al. Distillation process of smelting waste acid by tube-type air-gap membrane distillation[J]. Chinese Journal of Rare Metals, 2020, 44(6): 639-646. | |
| 12 | GROMOV P B, KASIKOV A G, SHCHELOKOVA E A, et al. Regeneration of sulfuric acid from electrolyte waste of the copper-smelting plant using solvent extraction[J]. Hydrometallurgy, 2018, 175: 187-192. |
| 13 | AYDIN M I, YUZER B, HASANCEBI B, et al. Application of electrodialysis membrane process to recovery sulfuric acid and wastewater in the chalcopyrite mining industry[J]. Desalination and Water Treatment, 2019, 172: 206-211. |
| 14 | 葛倩倩, 葛亮, 汪耀明, 等. 离子交换膜的发展态势与应用展望[J]. 化工进展, 2016, 35(6): 1774-1785. |
| GE Qianqian, GE Liang, WANG Yaoming, et al. Perspective in ion exchange membranes[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1774-1785. | |
| 15 | FENG X, WU P, JIANG L Y. Titanium white waste acid concentration by DCMD: Wetting, crystallization, and fouling[J]. Desalination, 2018, 440: 161-174. |
| 16 | KESIEME Uchenna, CHRYSANTHOU Andreas, CATULLI Maurizio, et al. The optimal use of tris-2-ethylhexylamine to recover hydrochloric acid and metals from leach solutions and comparison with other extractants[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 3177-3184. |
| 17 | KESIEME Uchenna K, ARAL Hal, DUKE Mikel, et al. Recovery of sulphuric acid from waste and process solutions using solvent extraction[J]. Hydrometallurgy, 2013, 138: 14-20. |
| 18 | AGRAWAL Archana, KUMARI S, RAY B C, et al. Extraction of acid and iron values from sulphate waste pickle liquor of a steel industry by solvent extraction route[J]. Hydrometallurgy, 2007, 88(1/2/3/4): 58-66. |
| 19 | MANAA El-Sayed A, LASHEEN Taysser A. Liquid-liquid extraction of sulfuric acid from aqueous sulfate waste solution using alamine 336/kerosene/TBP solvent[J]. Journal of Dispersion Science and Technology, 2016, 37(2): 137-143. |
| 20 | GOTTLIEBSEN Ken, GRINBAUM Baruch, CHEN Dehong, et al. Recovery of sulfuric acid from copper tank house electrolyte bleeds[J]. Hydrometallurgy, 2000, 56(3): 293-307. |
| 21 | Fatmehsari HAGHSHENAS D, DARVISHI D, RAFIEIPOUR H, et al. A comparison between TEHA and Cyanex 923 on the separation and the recovery of sulfuric acid from aqueous solutions[J]. Hydrometallurgy, 2009, 97(3/4): 173-179. |
| 22 | Ján MARTÁK, L’ubica KUBIŠOVÁ, Štefan SCHLOSSER. Liquid-liquid equilibria of 5-methyl-2-pyrazinecarboxylic acid in systems (aqueous solution with increased ionic strength) + (solvent with trioctylamine)[J]. Journal of Chemical & Engineering Data, 2011, 56(2): 171-184. |
| 23 | YANG Xiao, ZHANG Yimin, BAO Shenxu. Separation and recovery of sulfuric acid from acidic vanadium leaching solution of stone coal via solvent extraction[J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 1399-1405. |
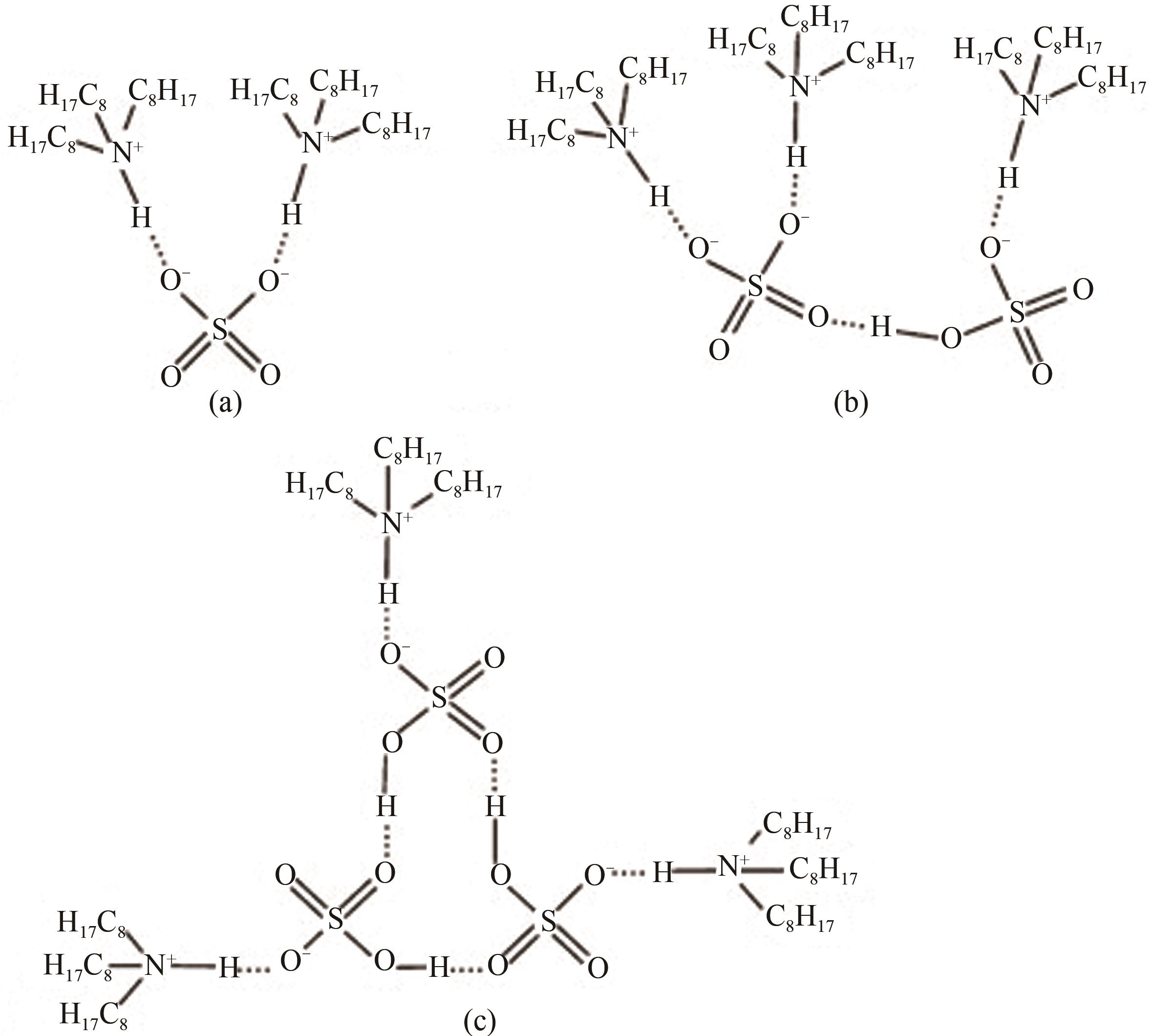
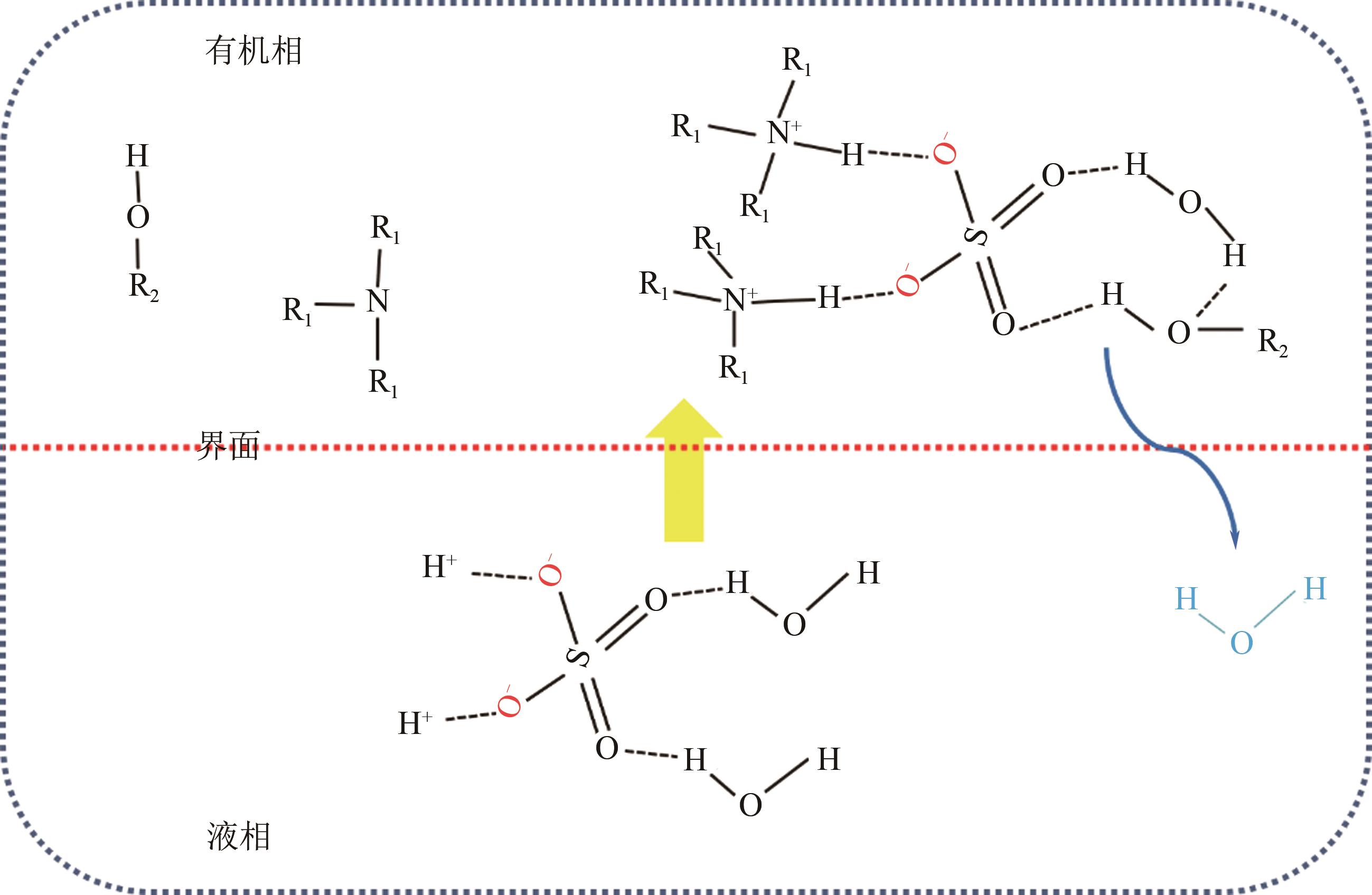
| 24 | QIU Xinrong, CHANG Zhidong, ZHOU Hualei, et al. Effect of hydroxyl on formation of viscoelastic scum during solvent extraction of sulfuric acid with trioctylamine[J]. Separation and Purification Technology, 2012, 86: 137-142. |
| 25 | 王娟. 萃取法回收含酸废水中盐酸的研究[D]. 天津: 天津大学, 2014. |
| WANG Juan. Study on recovery of hydrochloric acid from acid-containing wastewater by extraction[D]. Tianjin: Tianjin University, 2014. | |
| 26 | XU Tongwen, YANG Weihua. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs[J]. Journal of Membrane Science, 2001, 183(2): 193-200. |
| 27 | Ján MARTÁK, L’ubica KUBIŠOVÁ, Štefan SCHLOSSER. Liquid-liquid equilibria of 5-methyl-2-pyrazinecarboxylic and sulfuric acids for solvents with trioctylamine[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3578-3589. |
| 28 | SONG Xinyu, SUN Sixiu, YIN Zhilei, et al. The study on formation of the reversed micelle in extraction system of primary amine N1923 sulfate[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 209(1): 57-63. |
| 29 | 宋新宇, 印志磊, 张为民 等. 伯胺N1923萃取体系反胺束的形式研究[J]. 高等学校化学学报, 2002, 23(6):1177-1179. |
| SONG Xingu, YIN Zhilei, ZHANG Weimin, et al. The formation of the reversed micelle in the extraction system of primary amine N1923[J]. Chemical Journal of Chinese Universities, 2002, 23(6): 1177-1179. | |
| 30 | QIU Xinrong, CHANG Zhidong, LI Wenjun, et al. Structural analysis of NH—O in viscoelastic scum formation during solvent extraction of sulfuric acid with trioctylamine[J]. Separation and Purification Technology, 2012, 95: 196-201. |
| 31 | KESIEME Uchenna, CHRYSANTHOU Andreas, CATULLI Maurizio, et al. A review of acid recovery from acidic mining waste solutions using solvent extraction[J]. Journal of Chemical Technology & Biotechnology, 2018, 93(12): 3374-3385. |
| 32 | 谢琦莹, 陈景, 杨项军. 叔胺N235萃取盐酸时酸度对产生第三相的影响[J]. 无机化学学报, 2008, 24(6): 897-901. |
| XIE Qiying, CHEN Jing, YANG Xiangjun. Effect of HCl concentration on formation of third-phase in N235-C12H26-HCl solvent extraction system[J]. Chinese Journal of Inorganic Chemistry, 2008, 24(6): 897-901. | |
| 33 | LIANG Jie, FAN Lijun, XU Kai. Study on extraction of germanium with tri-n-octylamine[C]//Proceedings of 2011 AASRI Conference on Artificial Intelligence and Industry Application, 2011: 67-72. |
| 34 | 刘月英, 陈景, 谢琦莹. N235萃取Pt体系产生第三相的影响因素[J]. 中国有色金属学报, 2009, 19(7): 1316-1321. |
| LIU Yueying, CHEN Jing, XIE Qiying. Effect factors of third phase produced by N235 extracting Pt system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1316-1321. | |
| 35 | 顾智辉, 李存兄, 李倡纹, 等. 湿法炼锌溶液中锗萃取机理及选择性分离行为[J]. 中国有色金属学报, 2023,30(10):3476-3487. |
| GU Zhihui, LI Cunxiong, LI Changwen, et al. Extraction mechanism and selective separation behavior of germanium in hydrometallurgical zinc solution[J]. The Chinese Journal of Nonferrous Metals, 2023,30(10):3476-3487. | |
| 36 | 冯冬, 惠俊峰, 董婷婷, 等. 膜分散萃取工艺中三辛胺与丙酸结合方式的红外光谱研究[J]. 光谱学与光谱分析, 2018, 38(S1): 17-18. |
| FENG Dong, HUI Junfeng, DONG Tingting, et al. Study on the combination of tri-octylamine and propionic acid in membrane dispersion extraction with FTIR[J]. Spectroscopy and Spectral Analysis, 2018, 38(S1): 17-18. | |
| 37 | 白会华, 段慕华, 张凌, 等. 磷酸三丁酯(TBP)-正辛醇络合萃取邻氯苯酚的平衡特性[J]. 河南大学学报(自然科学版), 2015, 45(6): 655-659, 665. |
| BAI Huihua, DUAN Muhua, ZHANG Ling, et al. Reactive extraction of o-chlorophenol with TBP-n-octanol[J]. Journal of Henan University (Natural Science), 2015, 45(6): 655-659, 665. | |
| 38 | WEI Qifeng, REN Xiulian, GUO Jingjing, et al. Recovery and separation of sulfuric acid and iron from dilute acidic sulfate effluent and waste sulfuric acid by solvent extraction and stripping[J]. Journal of Hazardous Materials, 2016, 304: 1-9. |
| [1] | LI Yan, WU Qin, CHEN Kangcheng, ZHANG Yaoyuan, SHI Daxin, LI Hansheng. Modified polyimide pervaporation membranes for dehydration of organic solvent [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2915-2927. |
| [2] | WANG Tao, GAO Xiang, GAO Jifeng, DENG Haiquan, YU Xianyong, ZHOU Zhenhua, TANG Ling, LYU Hang. Application of modified Cu-BTC-based mixed matrix membrane in CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3240-3246. |
| [3] | LIU Xianzhe, HU Zhenzhong, HU Dawei, LI Xian, HE Shize, ZHAO Chunliang, XIA Ciliang, WU Bo, ZHANG Xiaoyong, LUO Guangqian, YAO Hong. Coking performance of extracts from degradative solvent extraction of low-rank coals for coal blending and coke making [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2420-2427. |
| [4] | FENG Feifei, TIAN Bin, MA Pengfei, WEI Jianxin, XU Long, TIAN Yuanyu, MA Xiaoxun. Research progress on mechanism and methods of lignin separation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2512-2525. |
| [5] | LIU Miao, JIAO Yingying, DING Ling, LI Chengcheng, HE Ying, SUN Liangliang, HAO Qingqing, CHEN Huiyong, LUO Qunxing. Acid-catalyzed dehydration of hexoses to 5-hydroxymethylfurfural: Reaction, separation and process coupling [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2526-2543. |
| [6] | FAN Wenxuan, XU Shuangping, JIA Hongge, ZHANG Mingyu, QU Yanqing. Research progress on polymeric membranes containing fluorenyl, imide and naphthyl groups for gas separation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1897-1911. |
| [7] | ZHOU Mingxian, YE Xiaozhou. Optimization of preferential lithium extraction from waste ternary lithium ion batteries by carbothermal reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2174-2182. |
| [8] | HE Lin, HE Changqing, SUI Hong. Prospects for the creation of novel interfacial separation materials driven by artificial intelligence [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1649-1654. |
| [9] | DU Yongliang, LIANG Zhuobin, GONG Yaoxu, BI Haojie, XU Zhiyuan, YUAN Hongying. Air gap membrane distillation research status and applications [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1655-1666. |
| [10] | JIN Binhao, ZHU Xiaoqian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, SU Baogen, YANG Qiwei. Advances in adsorbents for aromatics/cycloalkanes separation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1863-1881. |
| [11] | YANG Dongxiao, XIONG Qizhao, WANG Yi, CHEN Yang, LI Libo, LI Jinping. Progress in the preparation of hierarchically porous MOF and applications in adsorption and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1882-1896. |
| [12] | ZHAO Guoke, ZHANG Yang, LIU Yiqun. Membrane technologies for monovalent/divalent cation separation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1363-1373. |
| [13] | XU Zewen, WANG Ming, WANG Qiang, HOU Yingfei. Recent advances in amine-rich membrane for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1374-1386. |
| [14] | QIAN Junming, GUO Meng, REN Xiuxiu, YU Liang, ZHONG Jing, XU Rong. Fabrication of aromatic functionalized organosilica membranes and gas separation performance [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1428-1435. |
| [15] | HE Lan, GAO Zhuwei, QI Xinyu, LI Chengxin, WANG Shihao, LIU Zhongxin. Research progress in hydrophobic modification of melamine sponge and its application in oil-water separation field [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 984-1000. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||