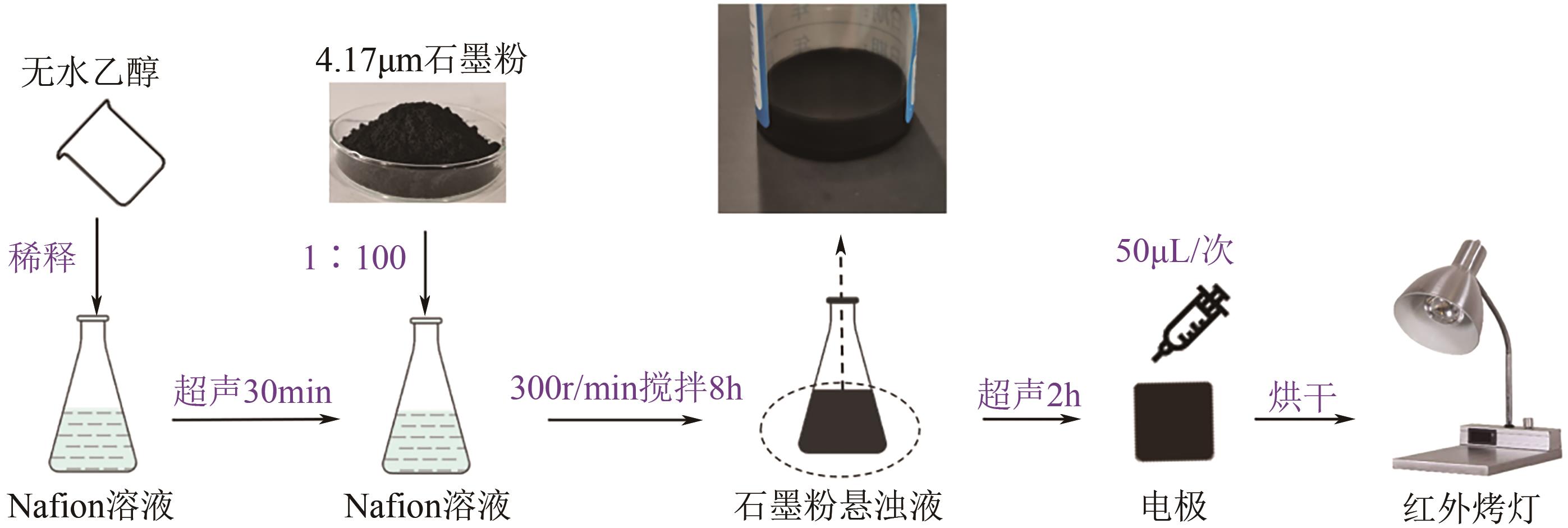
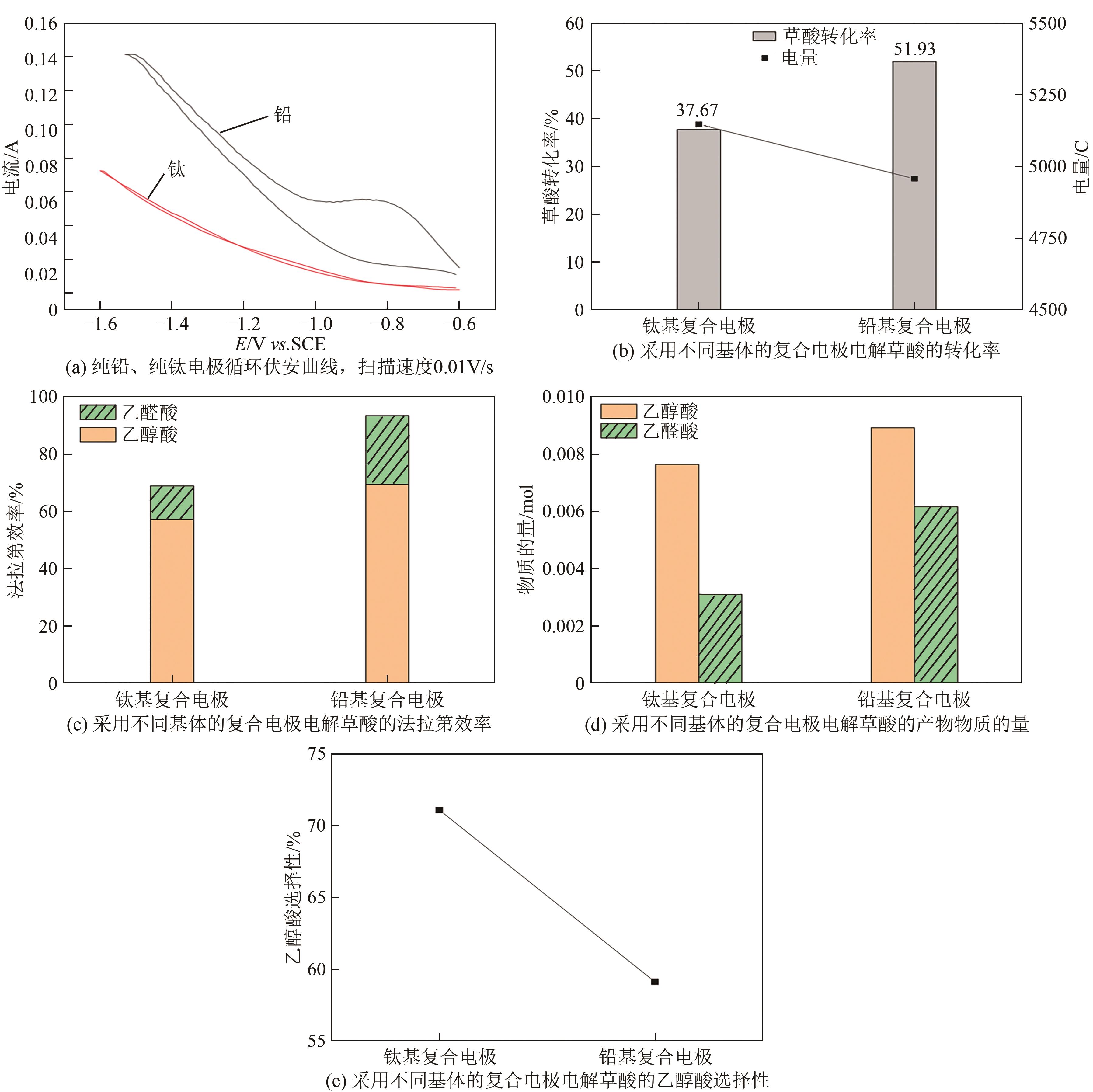
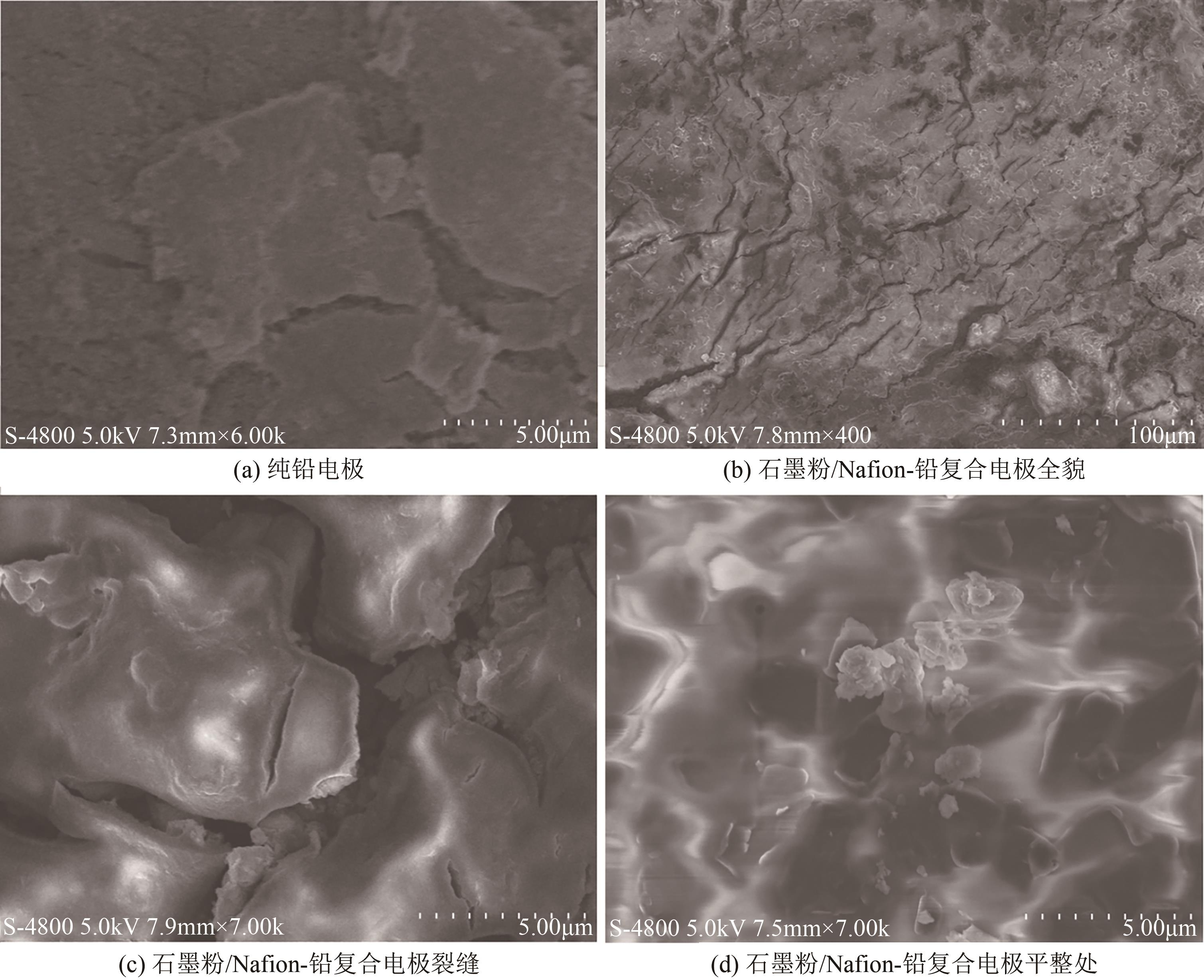
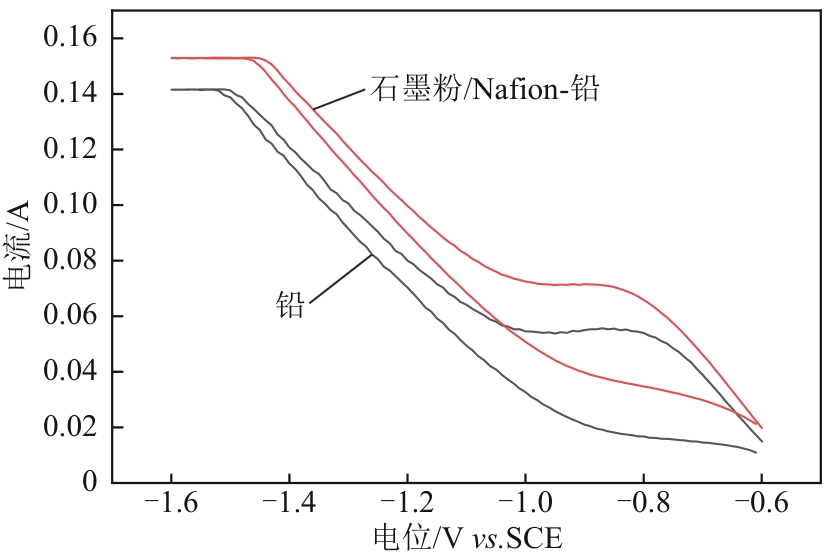
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (2): 1003-1013.DOI: 10.16085/j.issn.1000-6613.2024-0242
• Materials science and technology •
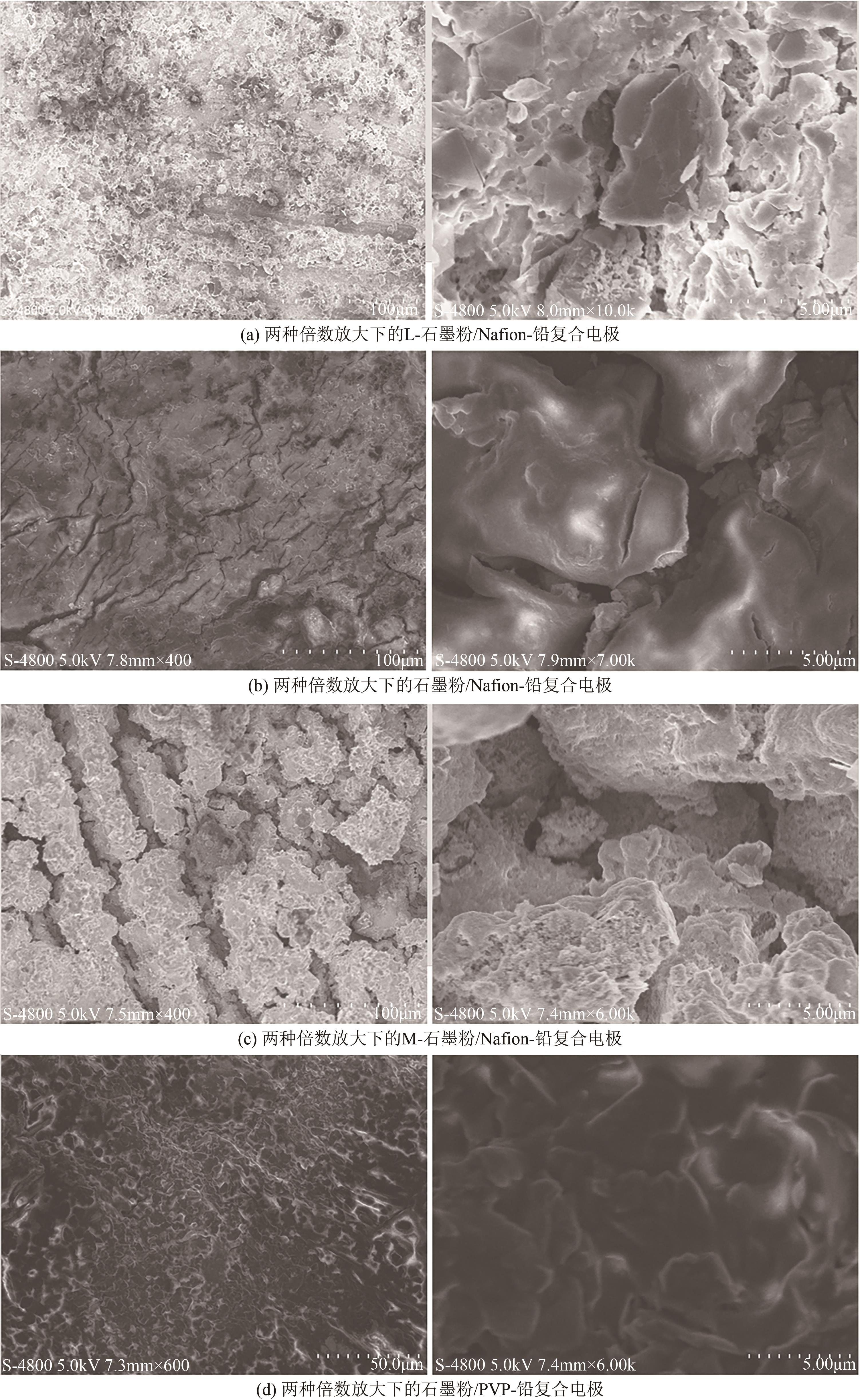
Graphite powder/Nafion-Pb electrode for electrocatalytic reduction of oxalic acid to glycolic acid
JIN Yuyang( ), NIU Chuanfeng(
), NIU Chuanfeng( ), LIU Yingshuo, DING Shi
), LIU Yingshuo, DING Shi
- Sinopec Research Institute of Petroleum Processing Co. , Ltd. , Beijing 100083, China
-
Received:2024-02-01Revised:2024-06-07Online:2025-03-10Published:2025-02-25 -
Contact:NIU Chuanfeng
石墨粉/Nafion-铅复合电极电还原草酸制乙醇酸
- 中石化石油化工科学研究院有限公司,北京 100083
-
通讯作者:牛传峰 -
作者简介:靳钰阳(1998—),男,硕士研究生,研究方向为电化学加氢。E-mail:jyy1272067805@163.com。
CLC Number:
Cite this article
JIN Yuyang, NIU Chuanfeng, LIU Yingshuo, DING Shi. Graphite powder/Nafion-Pb electrode for electrocatalytic reduction of oxalic acid to glycolic acid[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1003-1013.
靳钰阳, 牛传峰, 刘英硕, 丁石. 石墨粉/Nafion-铅复合电极电还原草酸制乙醇酸[J]. 化工进展, 2025, 44(2): 1003-1013.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0242
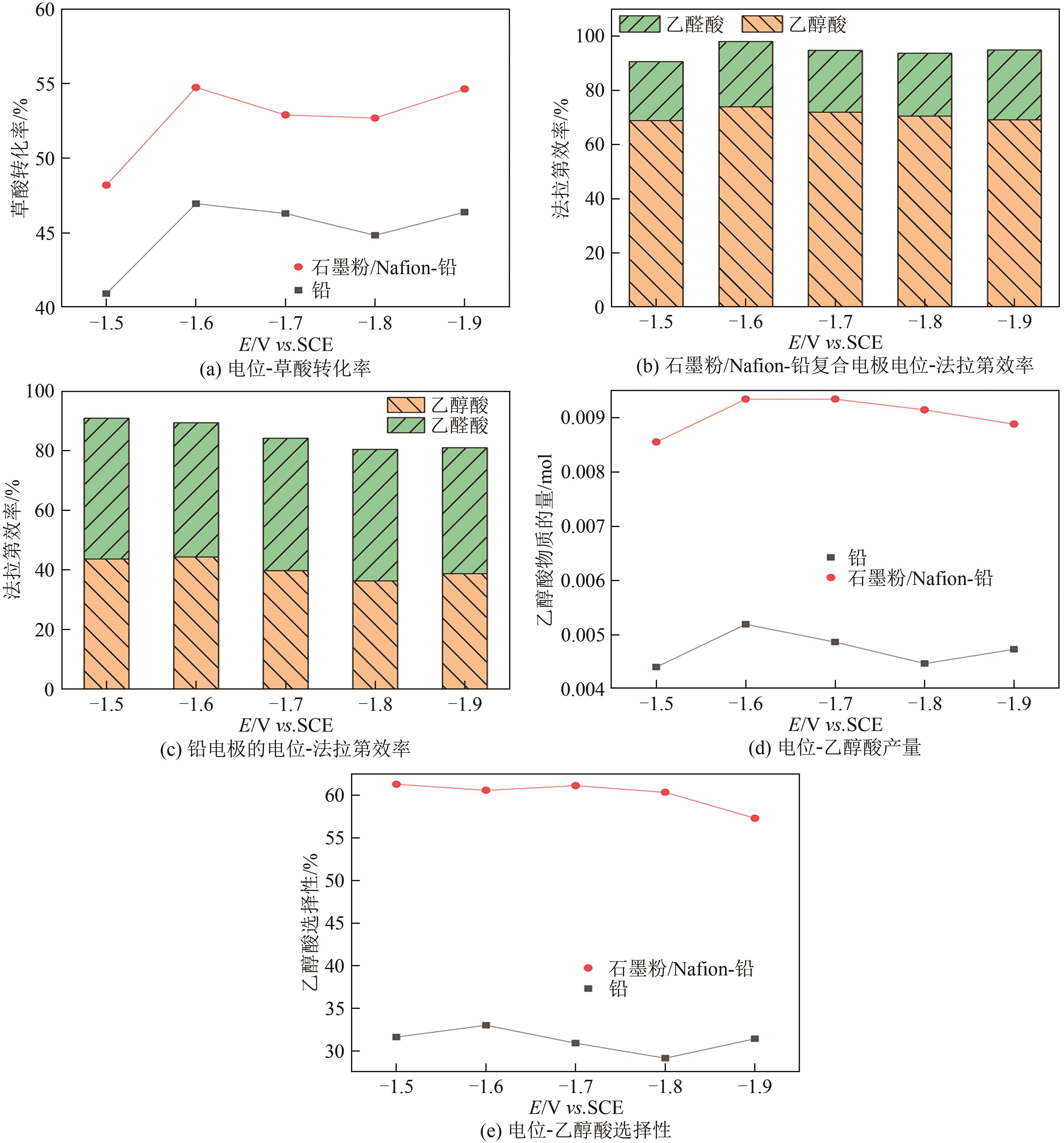
| 恒定电位/V | 铅电极 | 石墨粉/Nafion-铅复合电极 | ||
|---|---|---|---|---|
| 平均电流密度/mA·cm-2 | 耗电量/C | 平均电流密度/mA·cm-2 | 耗电量/C | |
| -1.5 | 33.86 | 3901 | 41.63 | 4796 |
| -1.6 | 39.31 | 4528 | 42.34 | 4878 |
| -1.7 | 41.09 | 4734 | 43.54 | 5016 |
| -1.8 | 41.35 | 4763 | 43.46 | 5007 |
| -1.9 | 41.05 | 4729 | 43.06 | 4960 |
| 恒定电位/V | 铅电极 | 石墨粉/Nafion-铅复合电极 | ||
|---|---|---|---|---|
| 平均电流密度/mA·cm-2 | 耗电量/C | 平均电流密度/mA·cm-2 | 耗电量/C | |
| -1.5 | 33.86 | 3901 | 41.63 | 4796 |
| -1.6 | 39.31 | 4528 | 42.34 | 4878 |
| -1.7 | 41.09 | 4734 | 43.54 | 5016 |
| -1.8 | 41.35 | 4763 | 43.46 | 5007 |
| -1.9 | 41.05 | 4729 | 43.06 | 4960 |
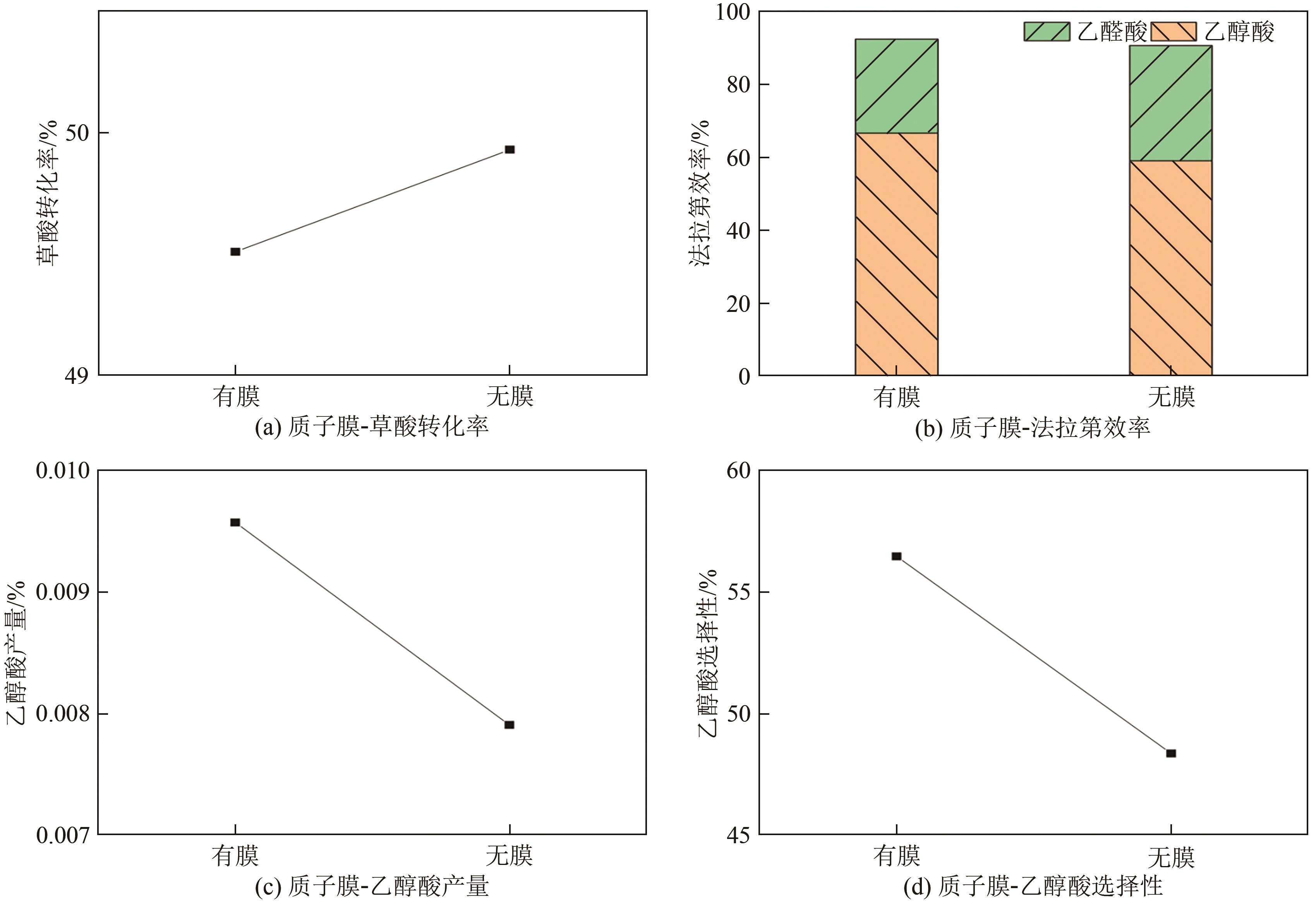
| 参数 | 有膜 | 无膜 |
|---|---|---|
| 平均电流密度/mA·cm-2 | 48.13 | 89.73 |
| 耗电量/C | 5544 | 10337 |
| 参数 | 有膜 | 无膜 |
|---|---|---|
| 平均电流密度/mA·cm-2 | 48.13 | 89.73 |
| 耗电量/C | 5544 | 10337 |
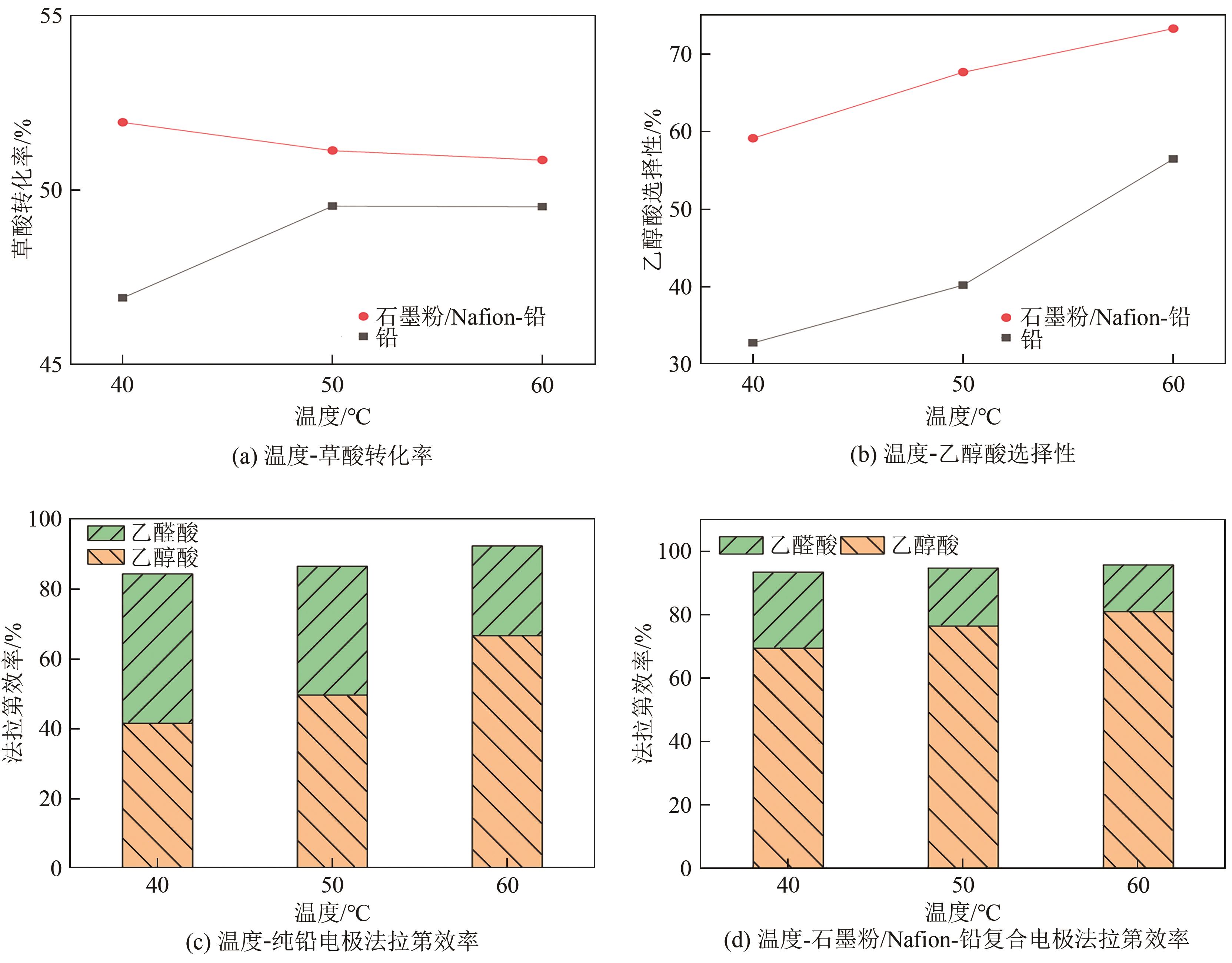
| 温度/℃ | 铅电极 | 石墨粉/Nafion-铅复合电极 | ||
|---|---|---|---|---|
| 平均电流密度/mA·cm-2 | 耗电量/C | 平均电流密度/mA·cm-2 | 耗电量/C | |
| 40 | 40.20 | 4631 | 43.03 | 4957 |
| 50 | 43.64 | 5028 | 43.61 | 5024 |
| 60 | 48.12 | 5544 | 45.67 | 5261 |
| 温度/℃ | 铅电极 | 石墨粉/Nafion-铅复合电极 | ||
|---|---|---|---|---|
| 平均电流密度/mA·cm-2 | 耗电量/C | 平均电流密度/mA·cm-2 | 耗电量/C | |
| 40 | 40.20 | 4631 | 43.03 | 4957 |
| 50 | 43.64 | 5028 | 43.61 | 5024 |
| 60 | 48.12 | 5544 | 45.67 | 5261 |
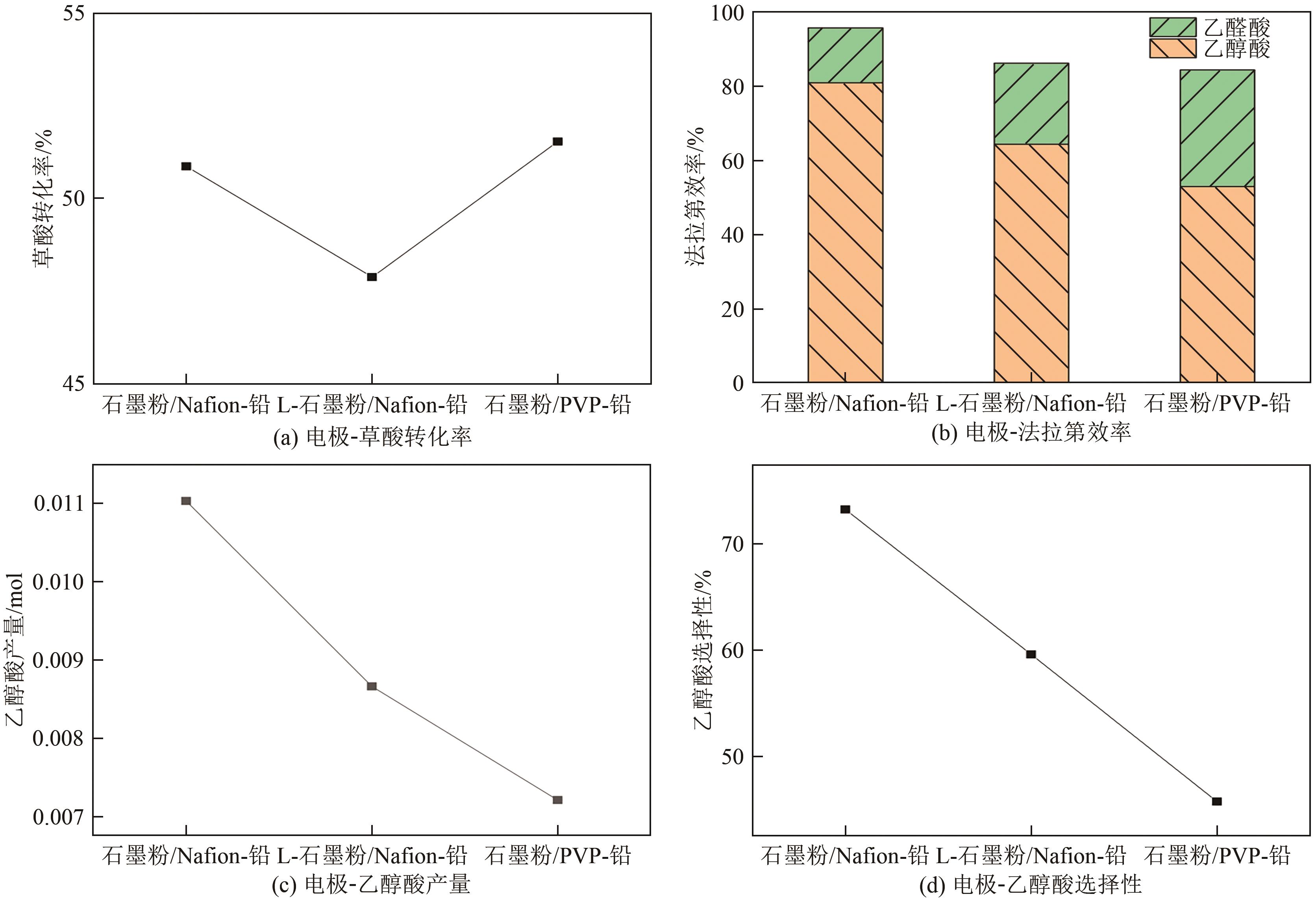
| 参数 | 石墨粉/Nafion-铅复合电极 | L-石墨粉/Nafion-铅复合电极 | 石墨粉/PVP-铅复合电极 |
|---|---|---|---|
| 平均电流密度/mA·cm-2 | 45.67 | 45.09 | 45.62 |
| 耗电量/C | 5261 | 5194 | 5255 |
| 参数 | 石墨粉/Nafion-铅复合电极 | L-石墨粉/Nafion-铅复合电极 | 石墨粉/PVP-铅复合电极 |
|---|---|---|---|
| 平均电流密度/mA·cm-2 | 45.67 | 45.09 | 45.62 |
| 耗电量/C | 5261 | 5194 | 5255 |
| 1 | 田克胜, 王保伟, 许根慧. 乙醇酸的合成及应用[J]. 天然气化工, 2006, 31(6): 60-63, 71. |
| TIAN Kesheng, WANG Baowei, XU Genhui. Synthesis and application of glycolic acid[J]. Natural Gas Chemical Industry, 2006, 31(6): 60-63, 71. | |
| 2 | 黄燕, 梁朝林. 乙醇酸生产技术的研究进展[J]. 广东石油化工学院学报, 2013, 23(3): 8-11. |
| HUANG Yan, LIANG Chaolin. The research progress of glycolic acid production technology[J]. Journal of Guangdong University of Petrochemical Technology, 2013, 23(3): 8-11. | |
| 3 | 钟维民, 刘立鹏, 魏志勇. 生物可降解塑料聚乙醇酸的合成及其工业化研究进展[J]. 合成树脂及塑料, 2021, 38(2): 80-84. |
| ZHONG Weimin, LIU Lipeng, WEI Zhiyong. Synthesis and industrialization of biodegradable plastics PGA[J]. China Synthetic Resin and Plastics, 2021, 38(2): 80-84. | |
| 4 | 商宽祥, 张大洲, 卢文新, 等. 乙醇酸市场前景及技术进展分析[J]. 化肥设计, 2022, 60(4): 5-7, 35. |
| SHANG Kuanxiang, ZHANG Dazhou, LU Wenxin, et al. Analysis of glycolic acid market prospects and technology progress[J]. Chemical Fertilizer Design, 2022, 60(4): 5-7, 35. | |
| 5 | 王淑敏, 商宽祥, 谢鸿洲, 等. 聚乙醇酸产业现状及发展前景[J]. 化肥设计, 2021, 59(4): 1-4, 11. |
| WANG Shumin, SHANG Kuanxiang, XIE Hongzhou, et al. Present situation and development prospect of polyglycolic acid industry[J]. Chemical Fertilizer Design, 2021, 59(4): 1-4, 11. | |
| 6 | 高姣, 杨帅龙. 聚乙醇酸的合成及工业化进展[J]. 河南化工, 2022, 39(4): 8-10. |
| GAO Jiao, YANG Shuailong. Synthesis and industrialization progress of polyglycolic acid[J]. Henan Chemical Industry, 2022, 39(4): 8-10. | |
| 7 | BUDAK Kamil, SOGUT Oguz, AYDEMIR SEZER Umran. A review on synthesis and biomedical applications of polyglycolic acid[J]. Journal of Polymer Research, 2020, 27(8): 208. |
| 8 | 魏景东, 赵增海, 郭雁珩, 等. 2021年中国光伏发电发展现状与展望[J]. 水力发电, 2022, 48(10): 4-8. |
| WEI Jingdong, ZHAO Zenghai, GUO Yanheng, et al. Status and prospect of China’s solar PV generation development in 2021[J]. Water Power, 2022, 48(10): 4-8. | |
| 9 | PICKETT D J, YAP K S. A study of the production of glyoxylic acid by the electrochemical reduction of oxalic acid solutions[J]. Journal of Applied Electrochemistry, 1974, 4(1): 17-23. |
| 10 | SADAKIYO Masaaki, HATA Shinichi, FUKUSHIMA Takashi, et al. Electrochemical hydrogenation of non-aromatic carboxylic acid derivatives as a sustainable synthesis process: From catalyst design to device construction[J]. Physical Chemistry Chemical Physics, 2019, 21(11): 5882-5889. |
| 11 | POZDNIAKOV M A, ZHUK I V, LYAPUNOVA M V, et al. Glyoxylic acid: Synthesis, isolation, and crystallization[J]. Russian Chemical Bulletin, 2019, 68(3): 472-479. |
| 12 | ZHOU Yangliu, ZHANG Xinsheng, DAI Yingchun, et al. Studies on chemical activators for electrode I: Electrochemical activation of deactivating cathode for oxalic acid reduction[J]. Chemical Engineering Science, 2003, 58(3/4/5/6): 1021-1027. |
| 13 | ZHAO Fengming, YAN Fei, QIAN Yang, et al. Roughened TiO2 film electrodes for electrocatalytic reduction of oxalic acid to glyoxylic acid[J]. Journal of Electroanalytical Chemistry, 2013, 698: 31-38. |
| 14 | YANG Jun, CHENG Junfang, TAO Jie, et al. Wrapping multiwalled carbon nanotubes with anatase titanium oxide for the electrosynthesis of glycolic acid[J]. ACS Applied Nano Materials, 2019, 2(10): 6360-6367. |
| 15 | ABRAMO Francesco Pio, DE LUCA Federica, PASSALACQUA Rosalba, et al. Electrocatalytic production of glycolic acid via oxalic acid reduction on titania debris supported on a TiO2 nanotube array[J]. Journal of Energy Chemistry, 2022, 68: 669-678. |
| 16 | Sunmi IM, SAAD Sarwar, PARK Yiseul. Facilitated series electrochemical hydrogenation of oxalic acid to glycolic acid using TiO2 nanotubes[J]. Electrochemistry Communications, 2022, 135: 107204. |
| 17 | SCOTT K. The role of temperature in oxalic acid electroreduction[J]. Electrochimica Acta, 1992, 37(8): 1381-1388. |
| 18 | 张苏洪, 陈昌国, 黄晓军. 草酸电解还原生成乙醛酸的影响因素[J]. 化工科技, 2001, 9(4): 35-37. |
| ZHANG Suhong, CHEN Changguo, HUANG Xiaojun. The influence factors of the production of glyoxylic acid by electrochemical reduction of aqueous oxalic acid soplution[J]. Science & Technology in Chemical Industry, 2001, 9(4): 35-37. | |
| 19 | SU Yingshi, CHENG Yonghui, LI Zhen, et al. Exploring the impact of Nafion modifier on electrocatalytic CO2 reduction over Cu catalyst[J]. Journal of Energy Chemistry, 2024, 88: 543-551. |
| [1] | HONG Siqi, GU Fangwei, ZHENG Jinyu. Development status and prospect of low iridium catalysts for hydrogen production by PEM electrolysis [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 158-168. |
| [2] | LI Jiayou, ZHANG Yuhan, JIANG Nan, JIANG Bolong. Preparation of transition metal sulfide NiS(x)@NFcatalyst by hydrothermal method and its hydrogen evolution performance [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 297-304. |
| [3] | JIANG Liping, ZHANG Xueqiao, ZHONG Xiaojuan, WEI Yufan, XIAO Li, GUO Xujing, YANG Yijin. Optimization of acid leaching process of iron from vanadium slag and preparation of composite photocatalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 538-548. |
| [4] | HE Ran, LIANG Hong, HUANG Hong, YANG Youli, ZHENG Qiang, LI Xi. Preparation of acetylene black/Fe3O4 catalysed cathodic electrode and removal of 2,4,6-trichlorophenol by electro-Fenton oxidation [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 572-582. |
| [5] | LIN Meijie, MI Shuodong, BAO Cheng. Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 209-224. |
| [6] | XIE Yulin, RAU Jui-yeh, HUANG Jian, HAO Jiayi, WANG Youyi, HUANG Qi. Preparation of continuous ZIF-8 membrane and its progress in hydrogen separation [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 403-418. |
| [7] | MA Guixuan, XU Zitong, XIAO Zhihua, Ning Guoqing, WEI Qiang, XU Chunming. O,S co-doped carbon nanotube aqueous conductive additive assisted construction of high-performance graphite/SiO anode [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 443-456. |
| [8] | ZHU Hao, LIU Hanfei, GAO Yuan, HUANG Yiping, FEI Xiaocheng, HAN Weiqing. Effect of salt on electrocatalytic performance and mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 571-580. |
| [9] | LIANG Hongcheng, ZHAO Dongni, QUAN Yin, LI Jingni, HU Xinyi. Influence of SEI film morphology and structure on the performance of lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5049-5062. |
| [10] | WU Jianyang, WANG Runa, CHEN Yao, SHEN Lanyao, YU Yongli, JIANG Ning, QIU Jingyi, ZHOU Henghui. Preparation process of high nickel cathode precursor for lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5079-5085. |
| [11] | LI Meixuan, CHENG Jianfeng, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Junlian, WANG Chunxia, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Synthesis and electrochemical mechanism of high voltage lithium nickel manganate cathode materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5086-5094. |
| [12] | LI Ying, WANG Qizhao, BAI Bo, ZHANG Qian, SUN Wenjing, ZHANG Linxuan. Unidirectional water transportation and fog collection performance of Janus surface-patterned structures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5133-5141. |
| [13] | WU Yuqi, LI Jiangtao, DING Jianzhi, SONG Xiulan, SU Bingqin. Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5250-5261. |
| [14] | SUN Yan, XIE Xiaoyang, FENG Qianying, ZHENG Lu, HE Jiaojie, YANG Liwei, BAI Bo. Preparation of forward osmosis membrane modified by tannic acid-iron (Ⅲ) and its antifouling performance [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5309-5319. |
| [15] | SONG Jiakai, KONG Lingzhen, CHEN Jiaqing, SUN Huan, LI Qi, LI Changhe, WANG Sicheng, KONG Biao. Liquid film flow and separation characteristics in the swirl separation section of a tubular deliquidiser [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4297-4306. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||