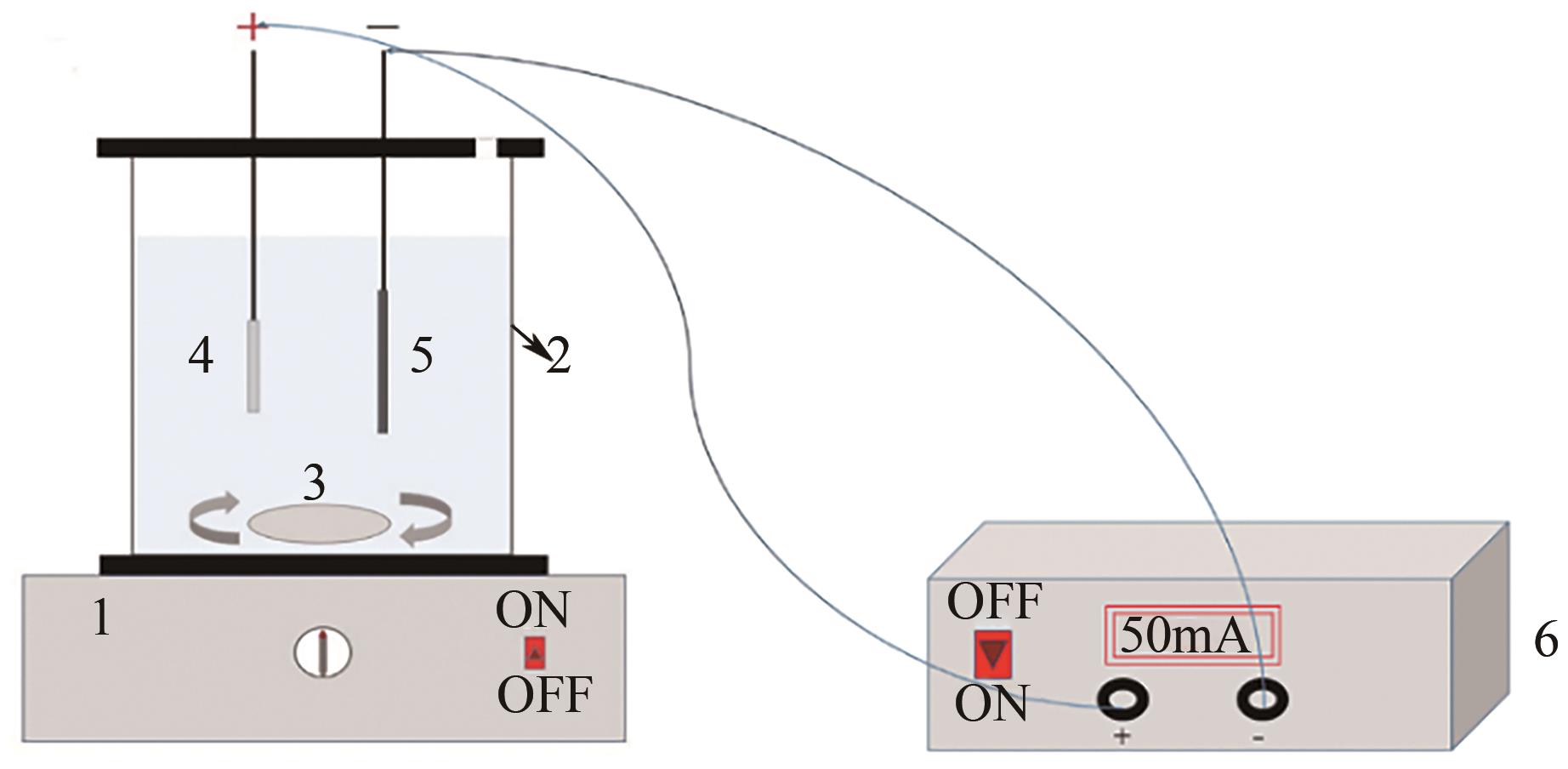
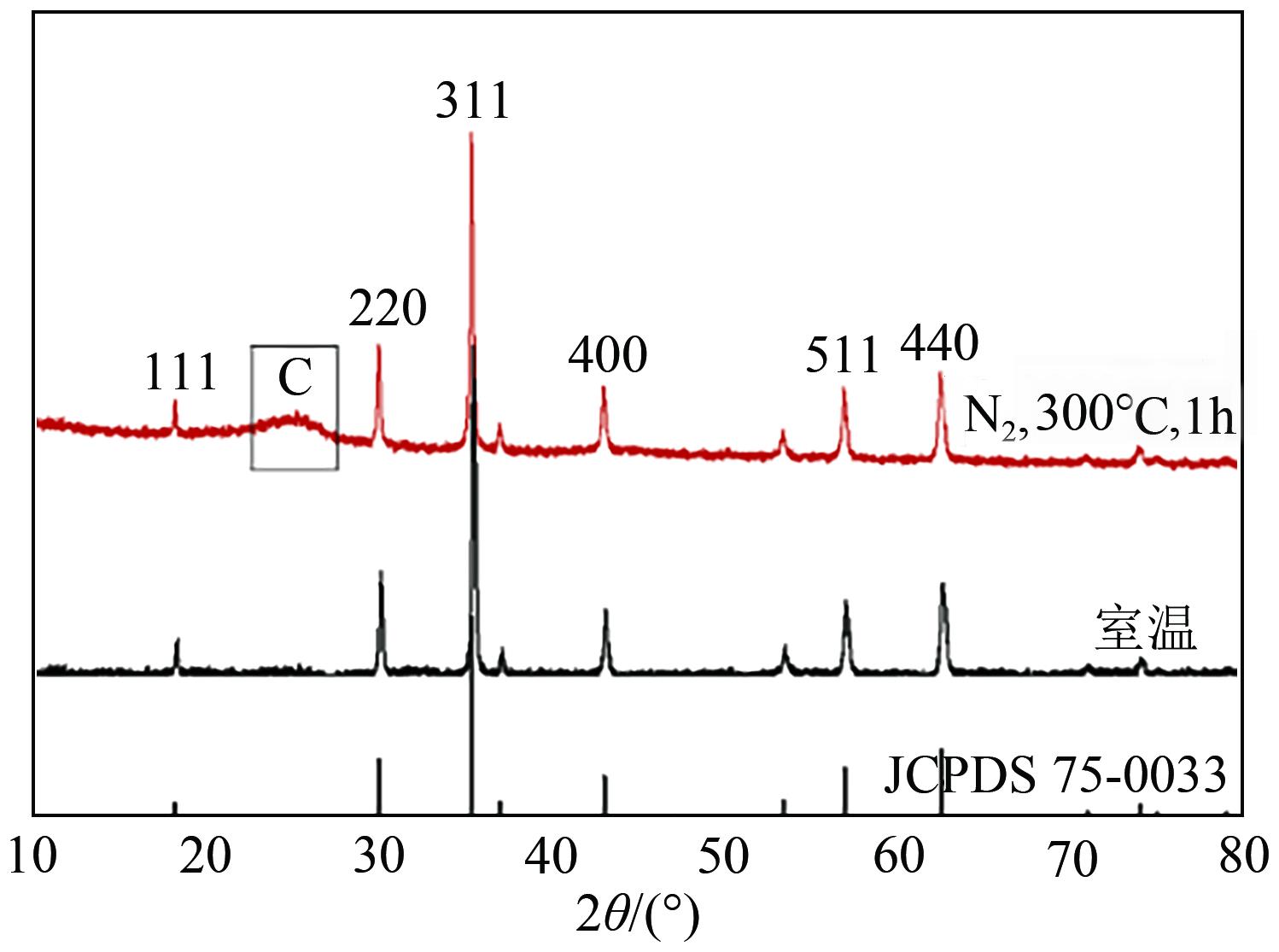
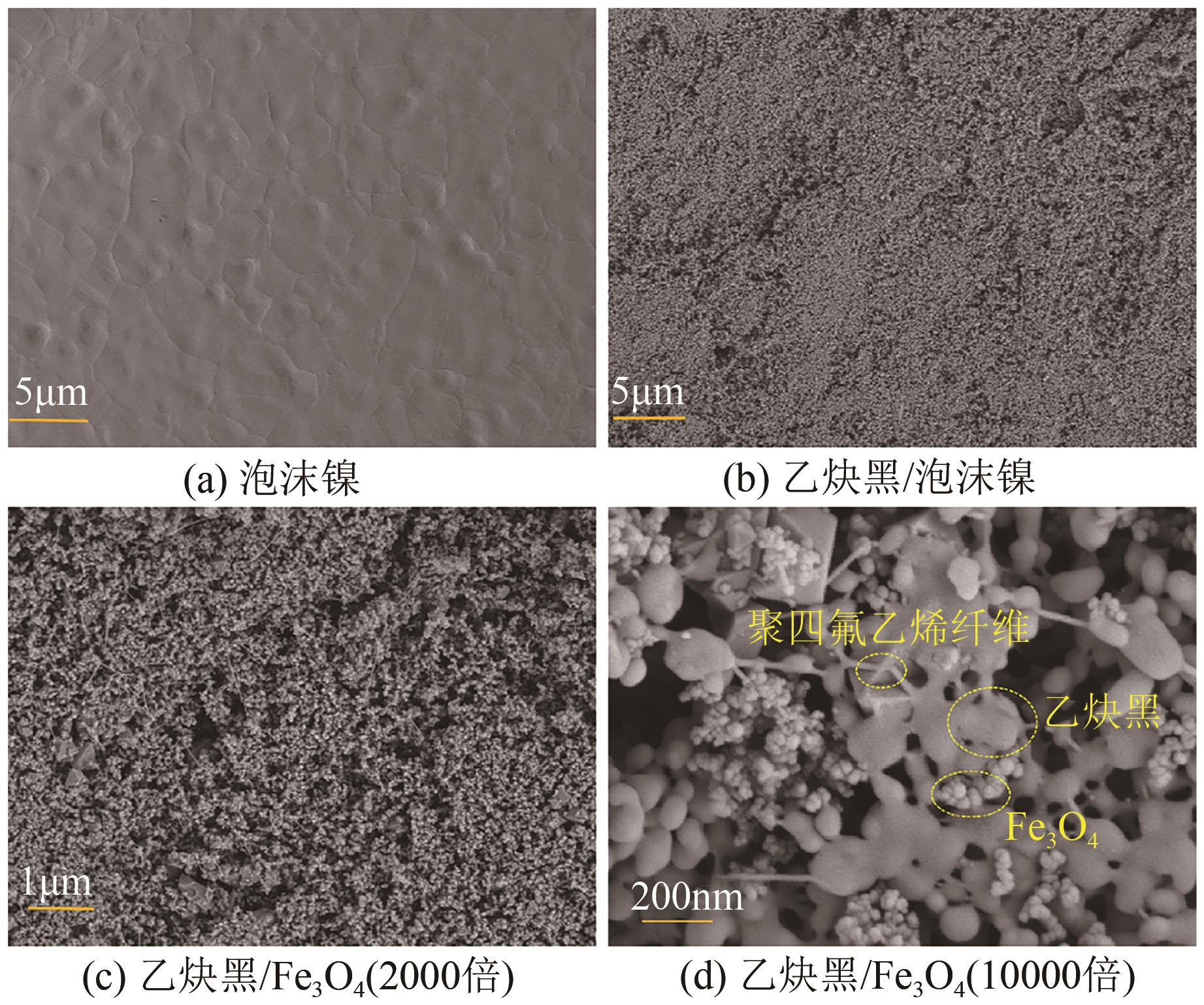
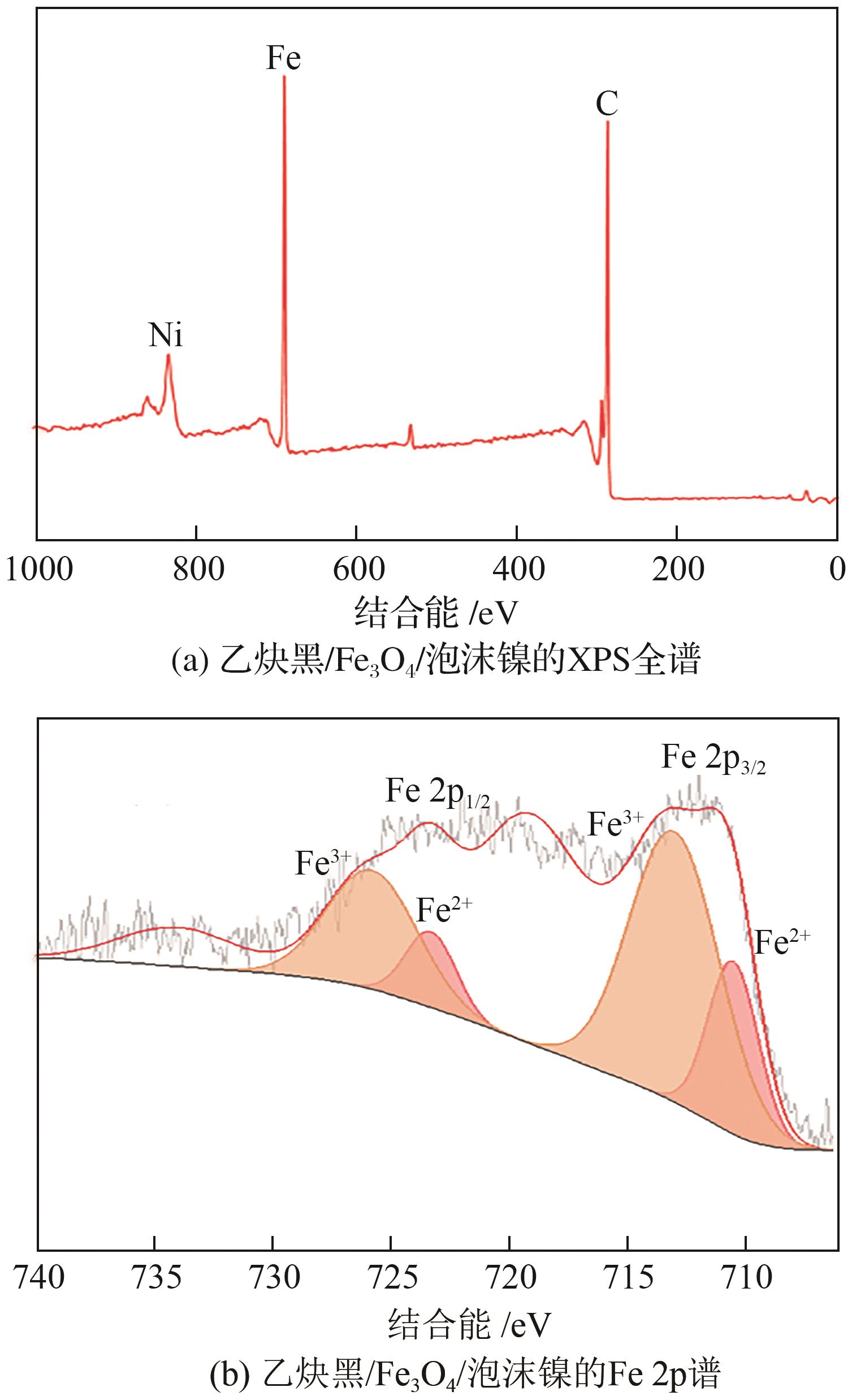
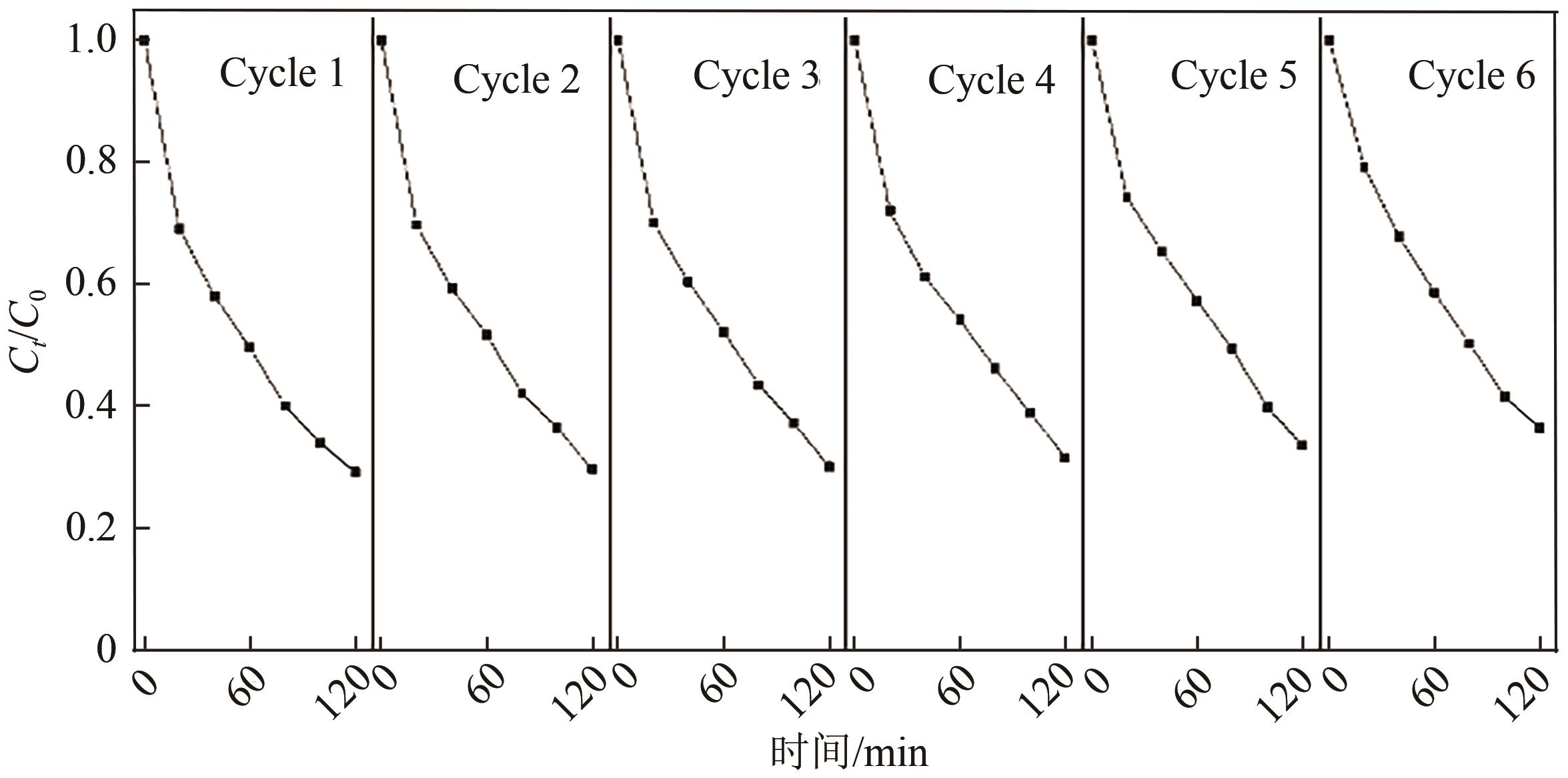
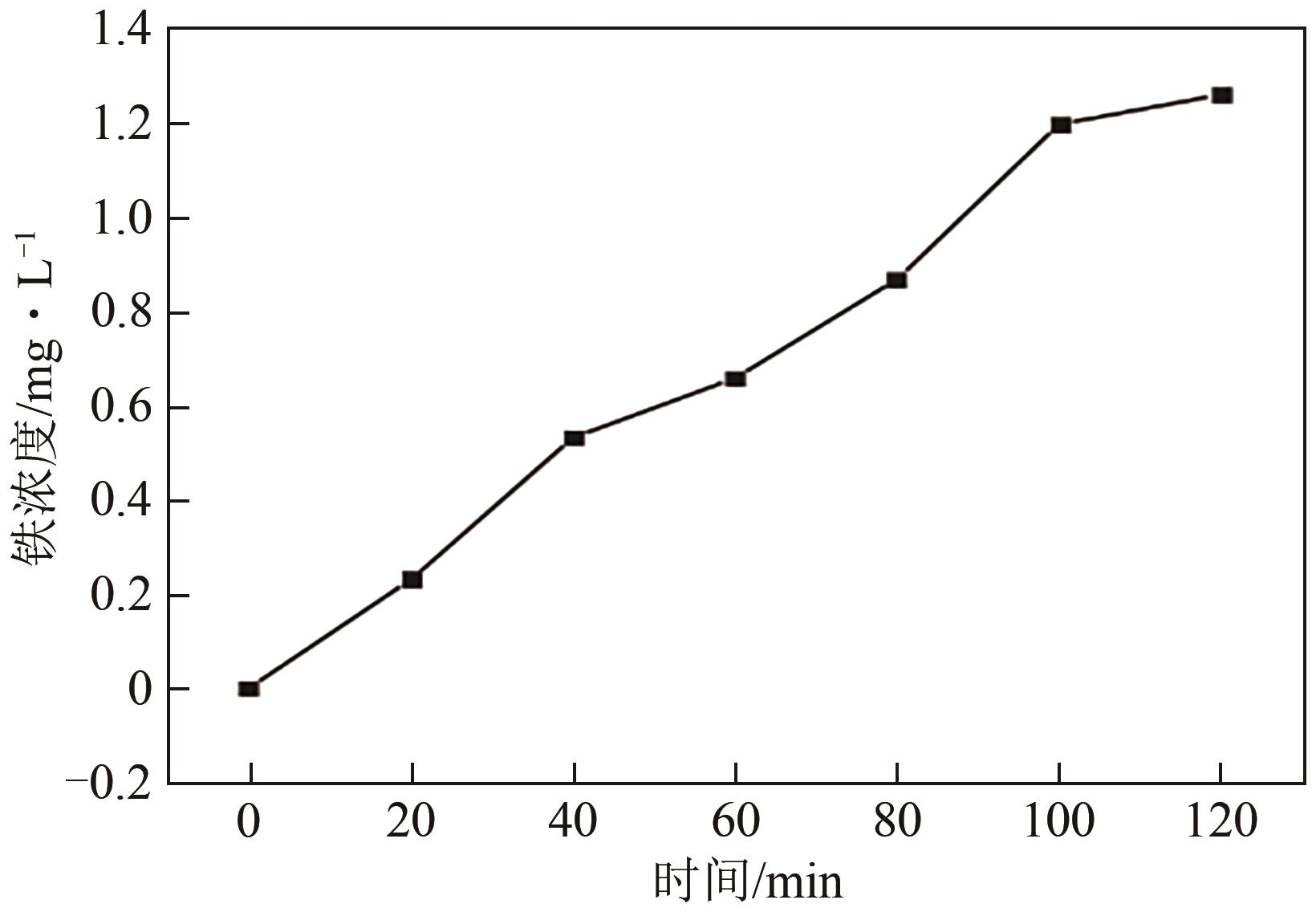
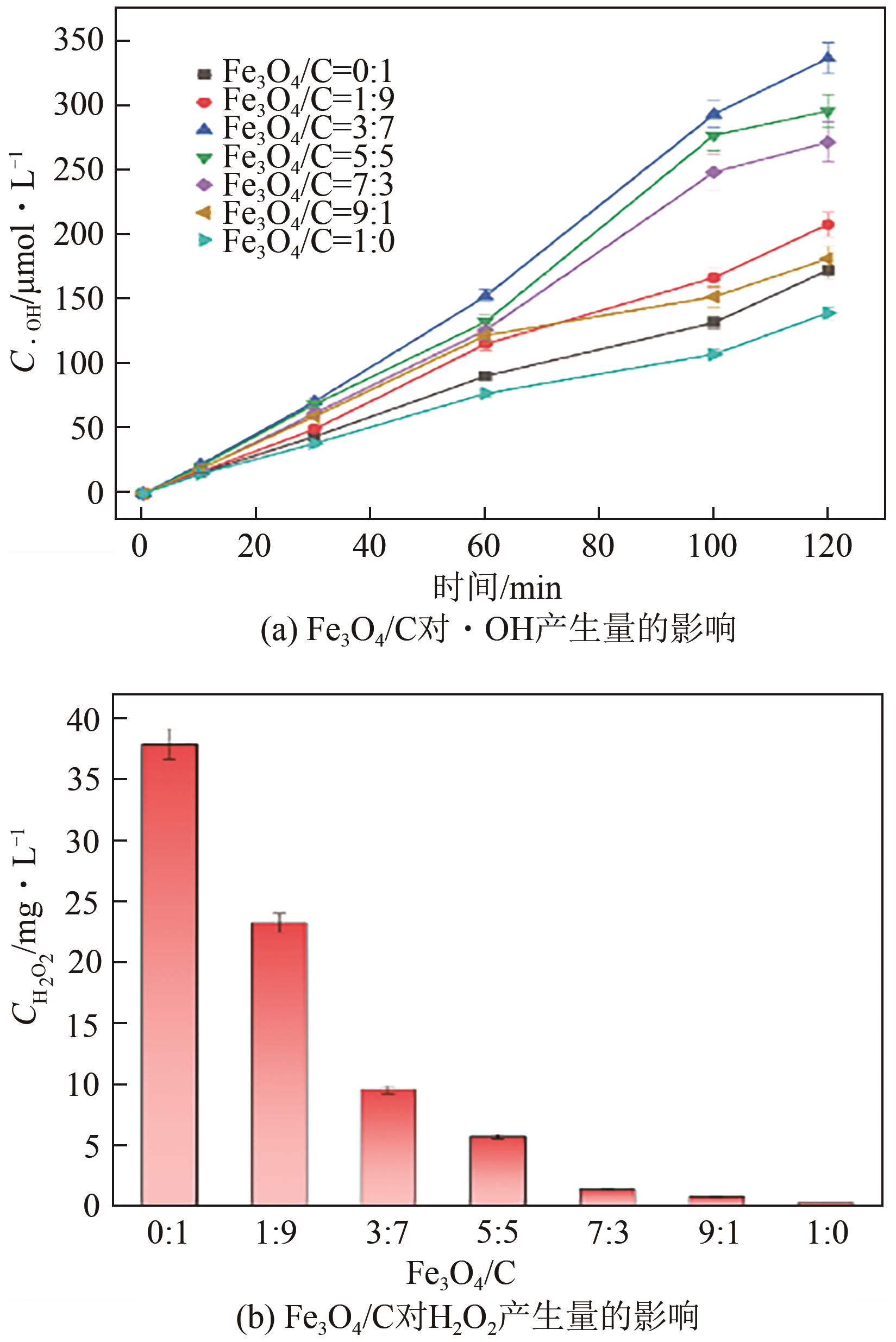
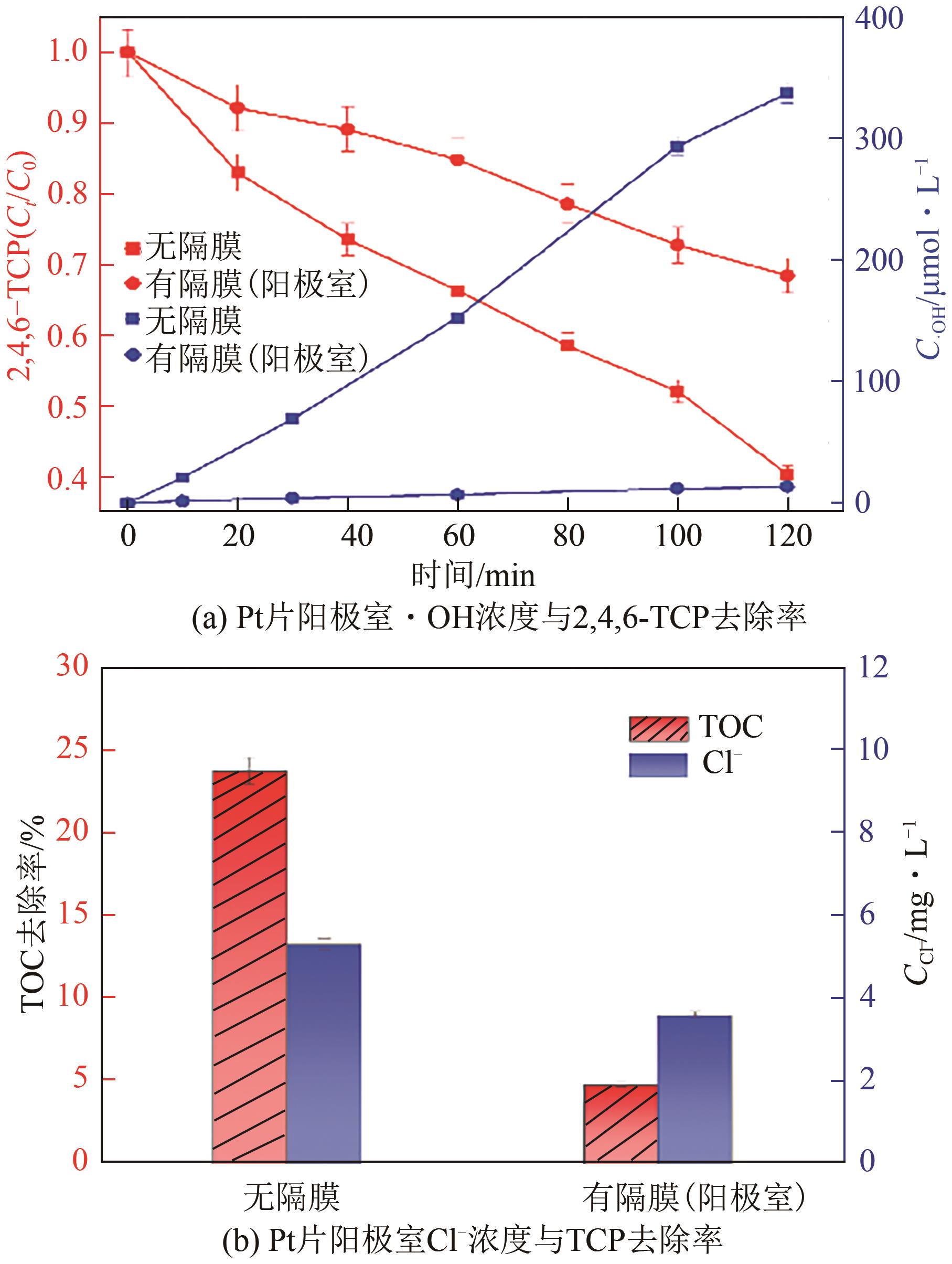
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (1): 572-582.DOI: 10.16085/j.issn.1000-6613.2024-0042
• Resources and environmental engineering • Previous Articles Next Articles
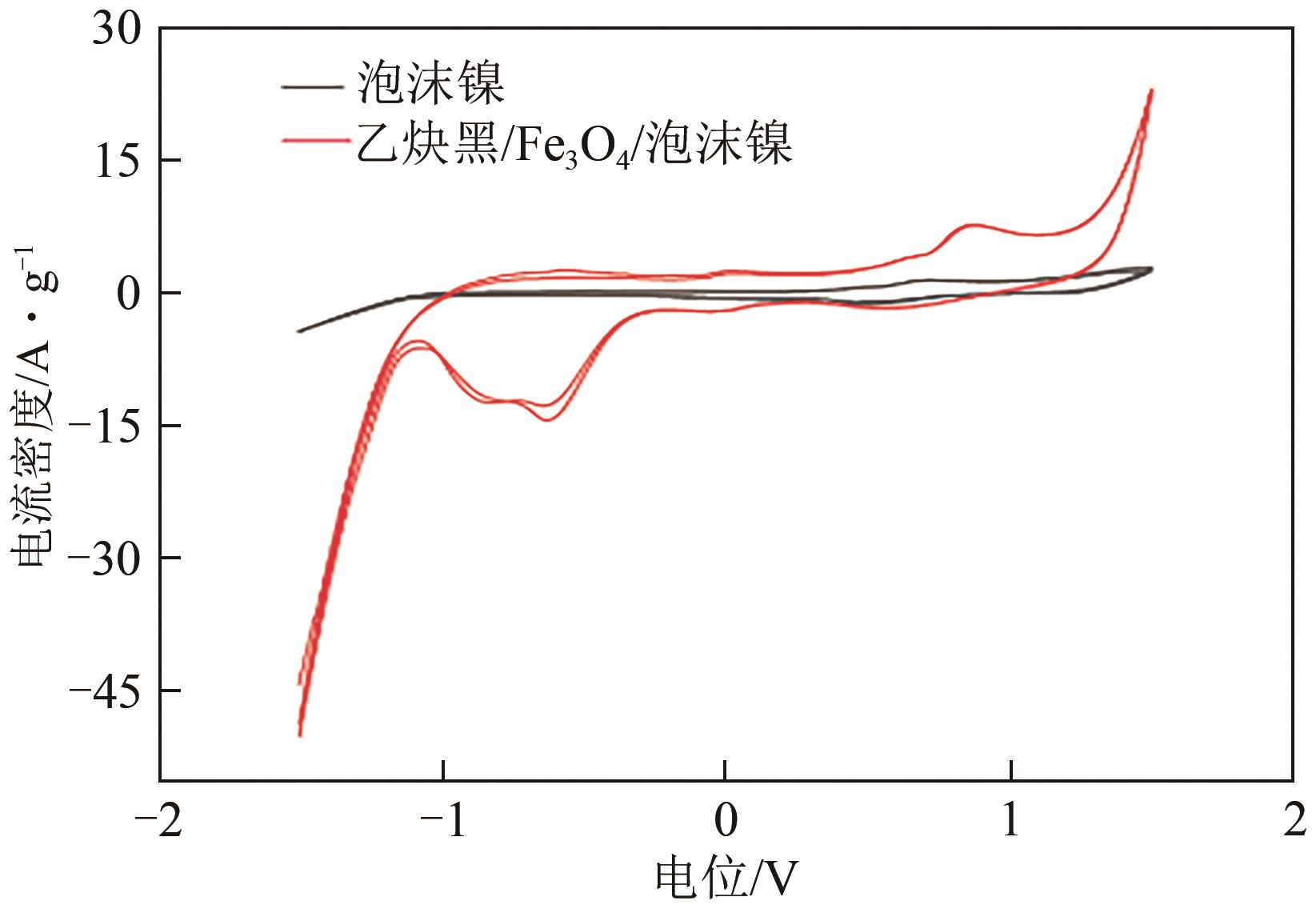
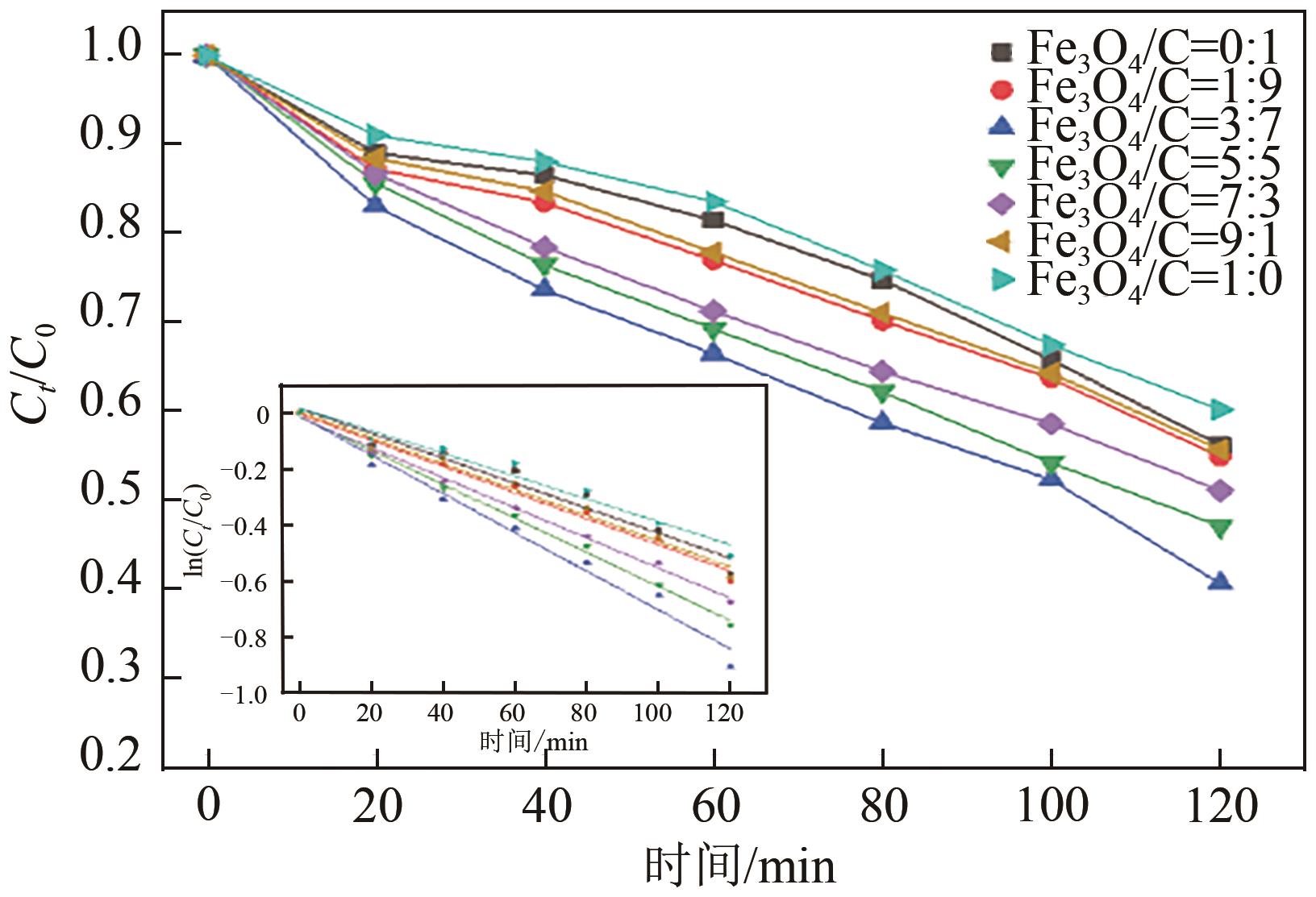
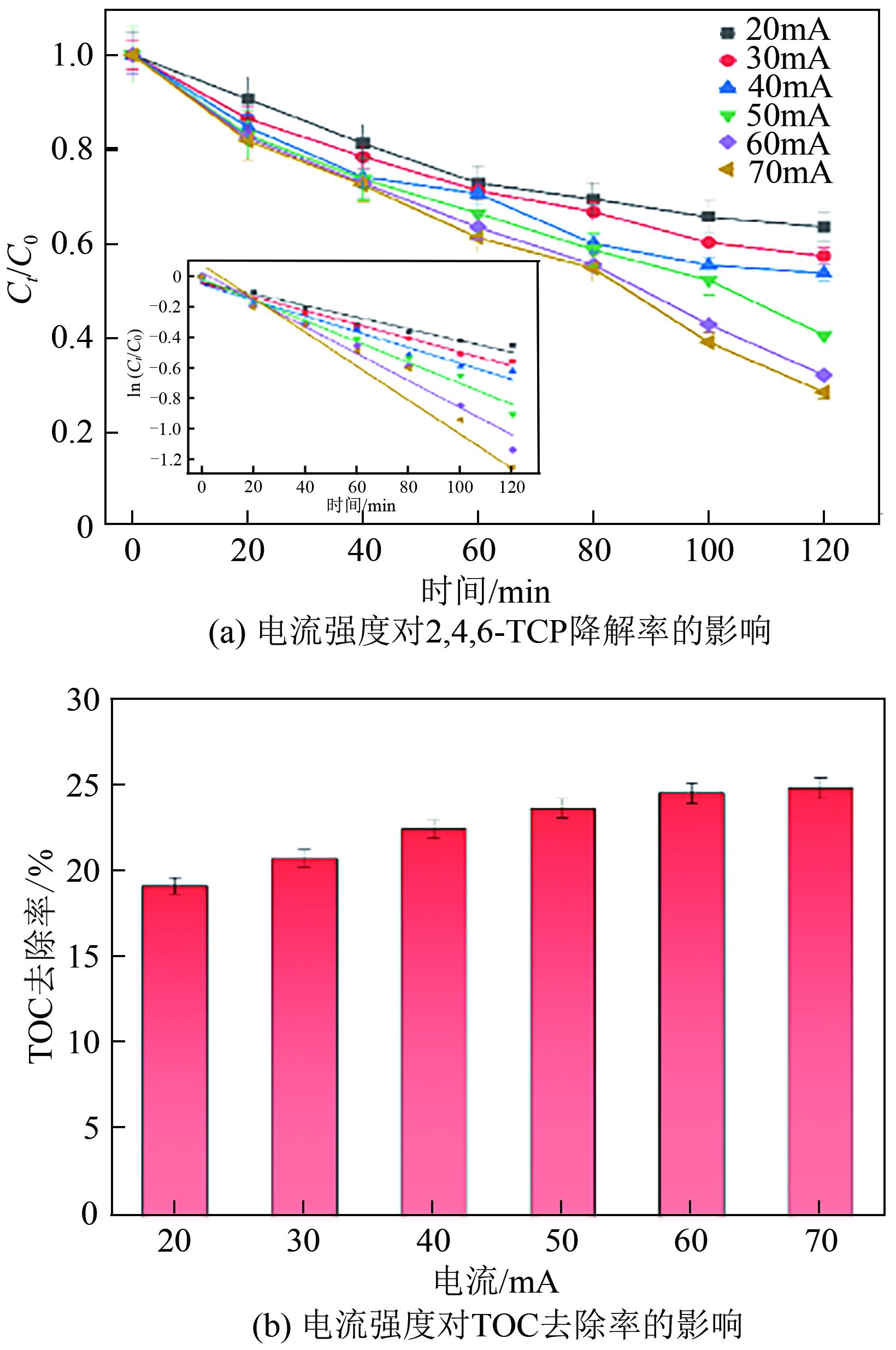
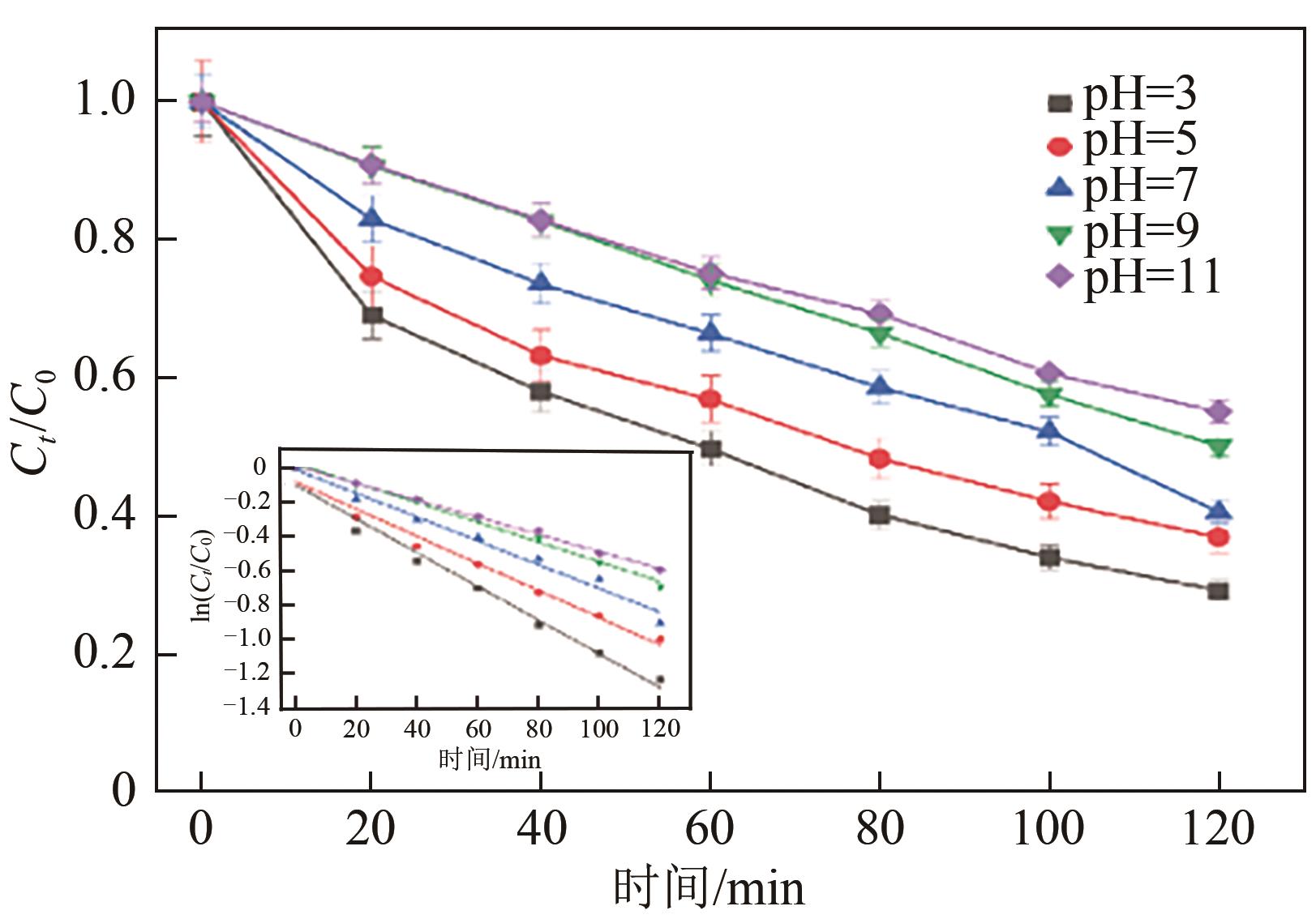
Preparation of acetylene black/Fe3O4 catalysed cathodic electrode and removal of 2,4,6-trichlorophenol by electro-Fenton oxidation
HE Ran( ), LIANG Hong(
), LIANG Hong( ), HUANG Hong, YANG Youli, ZHENG Qiang, LI Xi
), HUANG Hong, YANG Youli, ZHENG Qiang, LI Xi
- College of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 610500, Sichuan, China
-
Received:2024-01-08Revised:2024-05-30Online:2025-02-13Published:2025-01-15 -
Contact:LIANG Hong
乙炔黑/Fe3O4阴极制备及电Fenton氧化降解2,4,6-三氯苯酚
- 西南石油大学化学化工学院,四川 成都 610500
-
通讯作者:梁宏 -
作者简介:何然(1999—),男,硕士研究生,研究方向为电催化。E-mail:476947555@qq.com。 -
基金资助:国家油气重大专项(2016ZX05040-003);四川省重大科技专项(2018SZDZX0020)
CLC Number:
Cite this article
HE Ran, LIANG Hong, HUANG Hong, YANG Youli, ZHENG Qiang, LI Xi. Preparation of acetylene black/Fe3O4 catalysed cathodic electrode and removal of 2,4,6-trichlorophenol by electro-Fenton oxidation[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 572-582.
何然, 梁宏, 黄洪, 羊宥郦, 郑强, 李琋. 乙炔黑/Fe3O4阴极制备及电Fenton氧化降解2,4,6-三氯苯酚[J]. 化工进展, 2025, 44(1): 572-582.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0042
| Fe3O4/C | Fe(Ⅱ)摩尔浓度 /mmol·L-1 | H2O2摩尔浓度 /mmol·L-1 | 摩尔比 |
|---|---|---|---|
| 1∶9 | 0.12 | 0.68 | 1∶6 |
| 3∶7 | 0.37 | 0.28 | 5∶4 |
| 5∶5 | 0.61 | 0.17 | 10∶3 |
| 7∶3 | 0.87 | 0.04 | 20∶1 |
| 9∶1 | 1.12 | 0.02 | 55∶1 |
| Fe3O4/C | Fe(Ⅱ)摩尔浓度 /mmol·L-1 | H2O2摩尔浓度 /mmol·L-1 | 摩尔比 |
|---|---|---|---|
| 1∶9 | 0.12 | 0.68 | 1∶6 |
| 3∶7 | 0.37 | 0.28 | 5∶4 |
| 5∶5 | 0.61 | 0.17 | 10∶3 |
| 7∶3 | 0.87 | 0.04 | 20∶1 |
| 9∶1 | 1.12 | 0.02 | 55∶1 |
| 1 | ALONSO Francisco, BELETSKAYA Irina P, Miguel YUS. Metal-mediated reductive hydrodehalogenation of organic halides[J]. Chemical Reviews, 2002, 102(11): 4009-4092. |
| 2 | RICHARDSON S, PLEWA M, WAGNER E, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research[J]. Reviews in Mutation Research, 2007, 636(1/2/3): 178-242. |
| 3 | 肖川宝, 李林洋, 刘武锋, 等. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| XIAO Chuanbao, LI Linyang, LIU Wufeng, et al. Effective removal of 2,4,6-trichlorophenol by coupling photocatalysis with ion exchange adsorption[J]. CIESC Journal, 2023, 74(4): 1587-1597. | |
| 4 | LIANG Yinna, ZHAO Tianyu, XIAO Bing, et al. 2,4,6-Trichlorophenol degradation mechanism and microbial community analysis in an intimately coupled visible-light photocatalysis and biodegradation system[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(9): 2547-2556. |
| 5 | LIANG Peiying, RIVALLIN Matthieu, CERNEAUX Sophie, et al. Coupling cathodic electro-Fenton reaction to membrane filtration for AO7 dye degradation: A successful feasibility study[J]. Journal of Membrane Science, 2016, 510: 182-190. |
| 6 | ZHOU Wei, MENG Xiaoxiao, XIE Liang, et al. Simultaneous utilization of electro-generated O2 and H2 for H2O2 production: An upgrade of the Pd-catalytic electro-Fenton process for pollutants degradation[J]. Chinese Journal of Chemical Engineering, 2022, 44(4): 363-368 |
| 7 | 黄慧彬. 电芬顿中阴极材料的改性及降解邻苯二甲酸二甲酯的效能[D]. 哈尔滨: 哈尔滨工业大学, 2018. |
| HUANG Huibin. Modification of cathode materials in electro-Fenton and its performance in degradation of dimethyl phthalate[D]. Harbin: Harbin Institute of Technology, 2018. | |
| 8 | 孟建. 阴极类电芬顿体系降解有机污染物及机理研究[D]. 西安: 西安建筑科技大学, 2017. |
| MENG Jian. Degradation of the organic pollutants using electro-Fenton-like system[D]. Xi’an: Xi’an University of Architecture and Technology, 2017. | |
| 9 | 侯琳萌, 清华, 吉庆华. 类芬顿反应的催化剂、原理与机制研究进展[J]. 环境化学, 2022, 41(6): 1843-1855. |
| HOU Linmeng, QING Hua, JI Qinghua. Research progress on catalysts, principles and mechanisms of Fenton-like reactions[J]. Environmental Chemistry, 2022, 41(6): 1843-1855. | |
| 10 | 雷丽丹, 周正伟, 高雅, 等. 电化学氧化改性石墨毡电芬顿体系对三氯乙烯的降解研究[J]. 安全与环境工程, 2021, 28(3): 108-116. |
| LEI Lidan, ZHOU Zhengwei, GAO Ya, et al. TCE treatment in electro-Fenton system with electrochemical oxidation modified graphite felt electrode[J]. Safety and Environmental Engineering, 2021, 28(3): 108-116. | |
| 11 | 张冬梅, 刘蕾, 褚衍洋. 电-Fenton氧化降解2,4-二氯酚[J]. 环境科学研究, 2012, 25(9): 1041-1046. |
| ZHANG Dongmei, LIU Lei, CHU Yanyang. Degradation of 2,4-dichlorophenol by electro-Fenton oxidation process[J]. Research of Environmental Sciences, 2012, 25(9): 1041-1046. | |
| 12 | SI Yujun, PARK Moon Gyu, CANO Zachary Paul, et al. Heavily nitrogen-doped acetylene black as a high-performance catalyst for oxygen reduction reaction[J]. Carbon, 2017, 117: 12-19. |
| 13 | SHENG Yiping, SONG Shili, WANG Xiuli, et al. Electrogeneration of hydrogen peroxide on a novel highly effective acetylene black-PTFE cathode with PTFE film[J]. Electrochimica Acta, 2011, 56(24): 8651-8656. |
| 14 | SHENG Yiping, ZHAO Yue, WANG Xiuli, et al. Electrogeneration of H2O2 on a composite acetylene black-PTFE cathode consisting of a sheet active core and a dampproof coating[J]. Electrochimica Acta, 2014, 133: 414-421. |
| 15 | COSTA Regina C C, LELIS M F F, OLIVEIRA L C A, et al. Novel active heterogeneous Fenton system based on Fe3- x M x O4 (Fe, Co, Mn, Ni): The role of M2+ species on the reactivity towards H2O2 reactions[J]. Journal of Hazardous Materials, 2006, 129(1/2/3): 171-178. |
| 16 | LIU Wei, AI Zhihui, ZHANG Lizhi. Design of a neutral three-dimensional electro-Fenton system with foam nickel as particle electrodes for wastewater treatment[J]. Journal of Hazardous Materials, 2012, 243: 257-264. |
| 17 | XU Ranyun, REN Hang, CHI Tongtong, et al. Ozone oxidation of 2,4,6-TCP in the presence of halide ions: Kinetics, degradation pathways and toxicity evaluation[J]. Chemosphere, 2022, 288: 132343. |
| 18 | 姜成春, 庞素艳, 马军, 等. 钛盐光度法测定Fenton氧化中的过氧化氢[J]. 中国给水排水, 2006, 22(4): 88-91. |
| JIANG Chengchun, PANG Suyan, MA Jun, et al. Spectrophotometric determination of hydrogen peroxide in Fenton reaction with titanium oxalate[J]. China Water & Wastewater, 2006, 22(4): 88-91. | |
| 19 | 中国环境保护部。 水质游离氯和总氯的测定N,N-二乙基-1,4-苯二胺分光光度法: [S]. 北京: 中国环境科学出版社, 2010 |
| Ministry of Environmental Protection of China. Determination of free and total chlorine in water quality by spectrophotometric method ofN,N-diethyl-1,4-phenylenediamine: [S]. Beijing: China Environmental Science Press, 2010. | |
| 20 | TAI Chao, PENG Jinfeng, LIU Jingfu, et al. Determination of hydroxyl radicals in advanced oxidation processes with dimethyl sulfoxide trapping and liquid chromatography[J]. Analytica Chimica Acta, 2004, 527(1): 73-80. |
| 21 | ZENG Huiping, LIU Chengbo, XU He, et al. Preparation of Fe3O4@C with water treatment residuals and its potential in the magnetic coagulation process[J]. Journal of Cleaner Production, 2022, 362: 132327. |
| 22 | GHAEMI M, ATAHERIAN F, ZOLFAGHARI A, et al. Charge storage mechanism of sonochemically prepared MnO2 as supercapacitor electrode: Effects of physisorbed water and proton conduction[J]. Electrochimica Acta, 2008, 53(14): 4607-4614. |
| 23 | CUI Lele, HUANG Huihui, DING Peipei, et al. Cogeneration of H2O2 and OH via a novel Fe3O4/MWCNTs composite cathode in a dual-compartment electro-Fenton membrane reactor[J]. Separation and Purification Technology, 2020, 237: 116380. |
| 24 | HUANG Zhihui, LIU Jiaming, JI Zhiyong, et al. Effective and continuous degradation of levofloxacin via the graphite felt electrode loaded with Fe3O4 [J]. Separation and Purification Technology, 2022, 281: 119902. |
| 25 | 周珊, 陈传文. 电-Fenton法处理4-氯酚废水[J]. 环境污染治理技术与设备, 2004(10): 56-59. |
| ZHOU Shan, CHEN Chuanwen. Treatment of 4-chlorophenol wastewater by electro-Fenton method[J]. Techniques and Equipment for Environmental Pollution Control, 2004(10): 56-59. | |
| 26 | 赵龙飞, 万宁, 黄雨婷, 等. Fe3O4@CF电极在非均相电芬顿系统中降解四环素[J]. 吉林大学学报(理学版), 2023, 61(4): 982-990. |
| ZHAO Longfei, WAN Ning, HUANG Yuting, et al. Degradation of tetracycline by Fe3O4@CF electrode in non-homogeneous electro-Fenton system[J]. Journal of Jilin University (Science Edition), 2023, 61(4): 982-990. | |
| 27 | TAMURA Hiroki, GOTO Katsumi, YOTSUYANAGI Takao, et al. Spectrophotometric determination of iron(Ⅱ) with 1,10-phenanthroline in the presence of large amounts of iron(Ⅲ)[J]. Talanta, 1974, 21(4): 314-318. |
| 28 | LI Xianghui, GUO Weilin, LIU Zhonghua, et al. Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation[J]. Applied Surface Science, 2016, 369: 130-136. |
| 29 | LIU Wei, WANG Yueyao, AI Zhihui, et al. Hydrothermal synthesis of FeS2 as a high-efficiency Fenton reagent to degrade alachlor via superoxide-mediated Fe(Ⅱ)/Fe(Ⅲ) cycle[J]. ACS Applied Materials & Interfaces, 2015, 7(51): 28534-28544. |
| 30 | GEORGI Anett, KOPINKE Frank-Dieter. Interaction of adsorption and catalytic reactions in water decontamination processes Part Ⅰ. Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon[J]. Applied Catalysis B: Environmental, 2005, 58(1/2): 9-18. |
| 31 | 丁嘉, 李钰, 官宝红. 聚四氟乙烯改性掺硼金刚石电极强化降解阿特拉津[J]. 浙江大学学报(农业与生命科学版), 2022, 48(3): 369-376. |
| DING Jia, LI Yu, GUAN Baohong. Boron-doped diamond electrode modified by polytetrafluoroethylene to enhance the degradation of atrazine[J]. Journal of Zhejiang University (Agriculture and Life Sciences), 2022, 48(3): 369-376. | |
| 32 | 付永胜, 史鸿乐, 刘义青, 等. UV/H2O2光化学降解水中的三氯生[J]. 中国环境科学, 2018, 38(2): 616-626. |
| FU Yongsheng, SHI Hongle, LIU Yiqing, et al. Photochemical degradation of triclosan by UV/H2O2 in water[J]. China Environmental Science, 2018, 38(2): 616-626. | |
| 33 | 贾伟建, 朱化雨, 王德生, 等. BDD阳极去除水中阿特拉津的特性及机理研究[J]. 工业水处理, 2022, 42(6): 174-179. |
| JIA Weijian, ZHU Huayu, WANG Desheng, et al. Characteristics and mechanism of atrazine removal from water by BDD anode[J]. Industrial Water Treatment, 2022, 42(6): 174-179. | |
| 34 | 李灵香玉, 马香娟. 羟基自由基(·OH)的特性及其在光化学氧化中的反应机理[J]. 化工技术与开发, 2006, 35(8): 27-29. |
| LI Lingxiangyu, MA Xiangjuan. Characteristics of hydroxide radical and its reaction mechanism in photochemistry oxidation process[J]. Technology & Development of Chemical Industry, 2006, 35(8): 27-29. |
| [1] | JIN Yuyang, NIU Chuanfeng, LIU Yingshuo, DING Shi. Graphite powder/Nafion-Pb electrode for electrocatalytic reduction of oxalic acid to glycolic acid [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1003-1013. |
| [2] | ZHANG Tiantian, LIU Xia, ZHANG Hongfei, LI Qian, ZHOU Hongyu, LI Binglin. Green biosynthesis of docosahexaenoic acid-rich phosphatidylserine in solvent-free system [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1033-1041. |
| [3] | HONG Siqi, GU Fangwei, ZHENG Jinyu. Development status and prospect of low iridium catalysts for hydrogen production by PEM electrolysis [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 158-168. |
| [4] | DONG Jiatong, SHAN Mengqing, WANG Hua. Improved electrocatalytic CO2 reduction to ethanol by Au-CuO/Cu2O catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 277-285. |
| [5] | LI Jiayou, ZHANG Yuhan, JIANG Nan, JIANG Bolong. Preparation of transition metal sulfide NiS(x)@NFcatalyst by hydrothermal method and its hydrogen evolution performance [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 297-304. |
| [6] | LIU Wei, ZHANG Min, ZHU Zhaoqi, WANG Yi, LIANG Weidong, SUN Hanxue. Preparation and current applications of black titanium dioxide nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 341-353. |
| [7] | JIANG Liping, ZHANG Xueqiao, ZHONG Xiaojuan, WEI Yufan, XIAO Li, GUO Xujing, YANG Yijin. Optimization of acid leaching process of iron from vanadium slag and preparation of composite photocatalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 538-548. |
| [8] | LIN Meijie, MI Shuodong, BAO Cheng. Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 209-224. |
| [9] | LI Shuaizhe, NIE Yichen, PHIDJAVARD Keomeesay, GU Wen, ZHANG Wei, LIU Na, XU Gaoxiang, LIU Ying, LI Xingyong, CHEN Yubao. Research progress on non-precious metal-catalyzed hydrogenation and deoxygenation of biomass to produce hydrocarbon-based biofuels [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 225-242. |
| [10] | ZHANG Hao, LIU Shiyu, SHEN Weihua, FANG Yunjin. Dehydration of urea to cyanamide with Ca-ZSM-5 [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 365-373. |
| [11] | MA Guixuan, XU Zitong, XIAO Zhihua, Ning Guoqing, WEI Qiang, XU Chunming. O,S co-doped carbon nanotube aqueous conductive additive assisted construction of high-performance graphite/SiO anode [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 443-456. |
| [12] | WAN Zhen, WANG Shaoqing, LI Zhihe, ZHAO Tiansheng. Advances in HZSM-5 catalyzed pyrolysis of lignin to aromatic hydrocarbons [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 517-532. |
| [13] | CHEN Muhua, JI Zhen, WANG Fang, HUANG Kaijian, FU Bo, LIU Bo, LIU Shaozhong, ZHU Xinbao. Synthesis and properties of cellulose based copolymer polycarboxylate superplasticizer [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 564-570. |
| [14] | ZHU Hao, LIU Hanfei, GAO Yuan, HUANG Yiping, FEI Xiaocheng, HAN Weiqing. Effect of salt on electrocatalytic performance and mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 571-580. |
| [15] | LIANG Hongcheng, ZHAO Dongni, QUAN Yin, LI Jingni, HU Xinyi. Influence of SEI film morphology and structure on the performance of lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5049-5062. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||