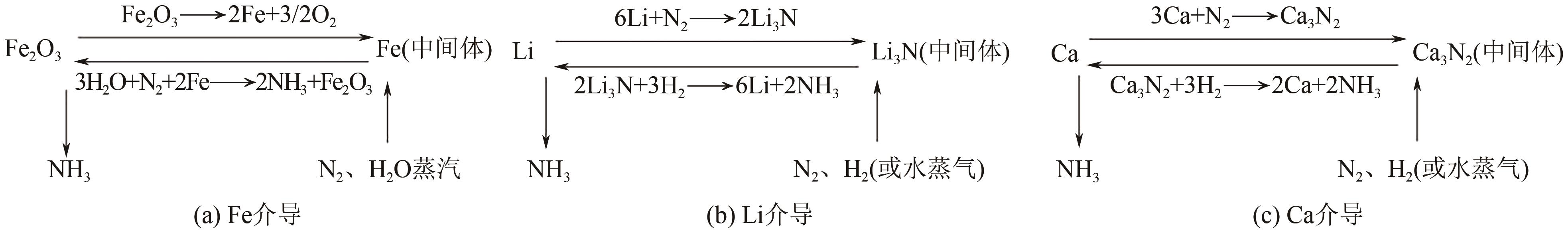
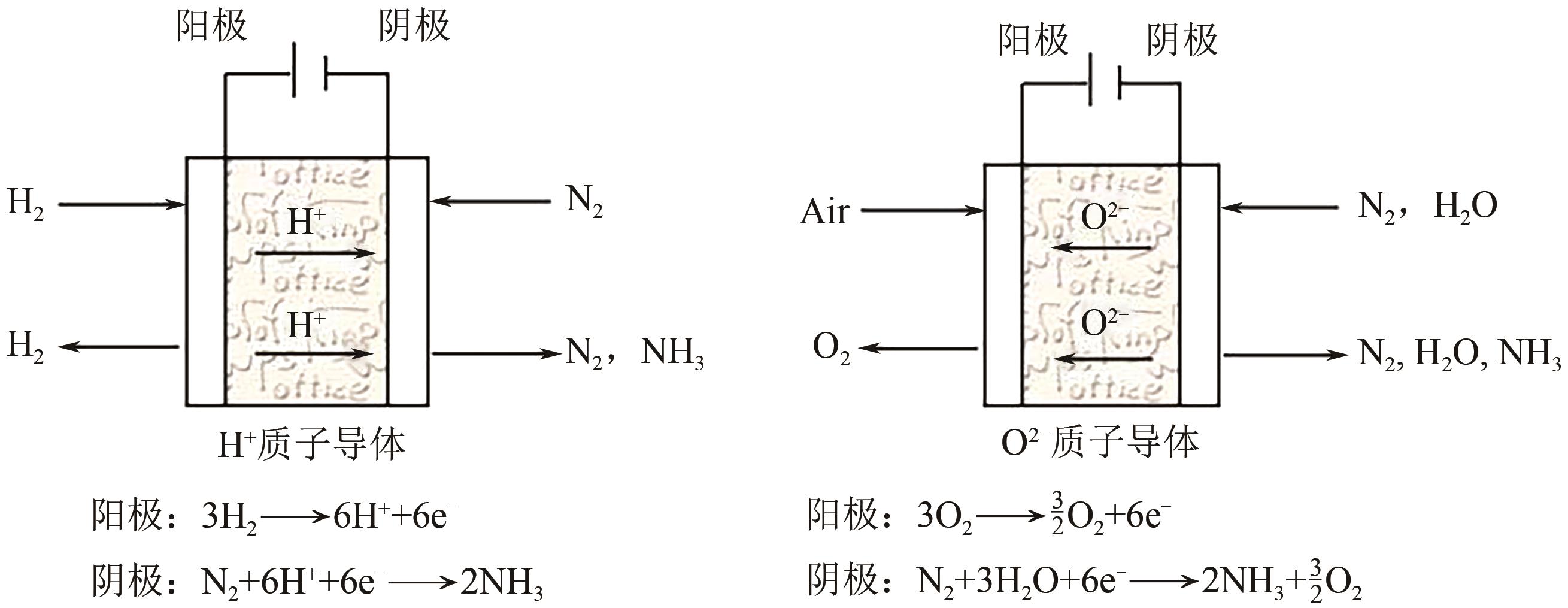
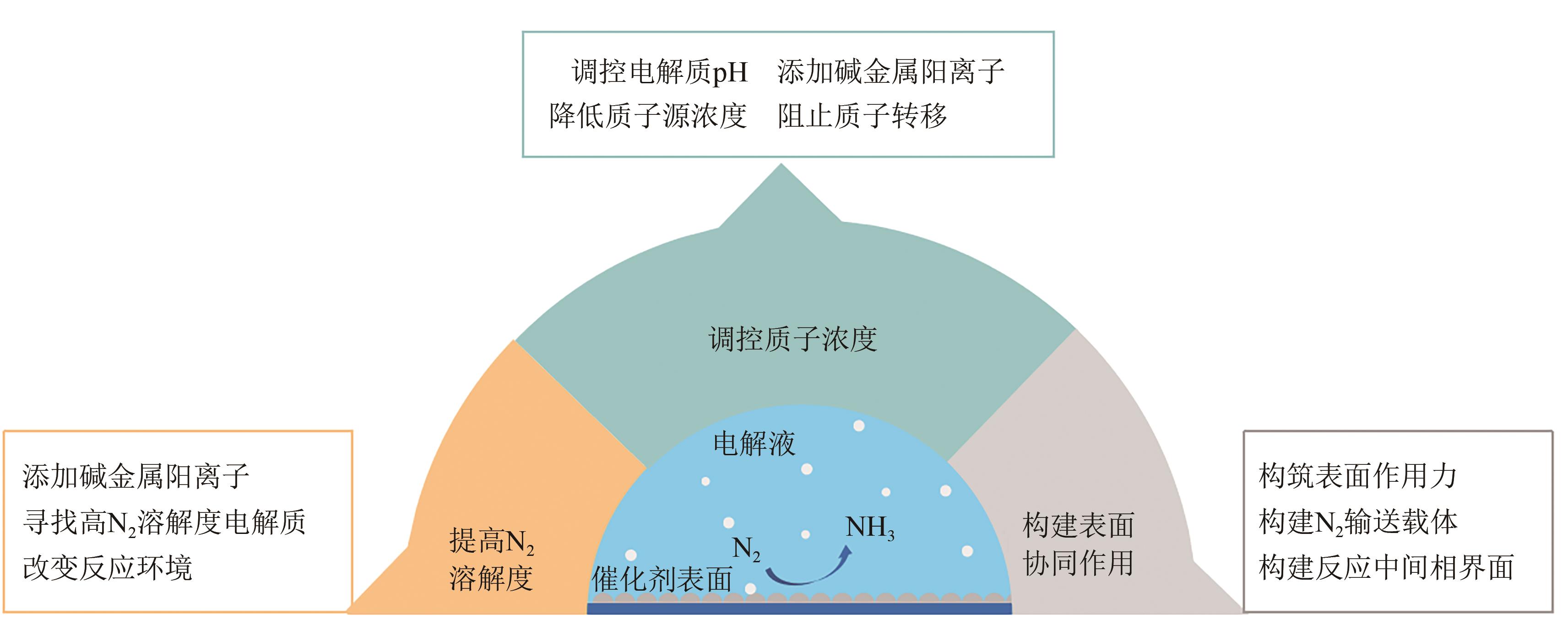
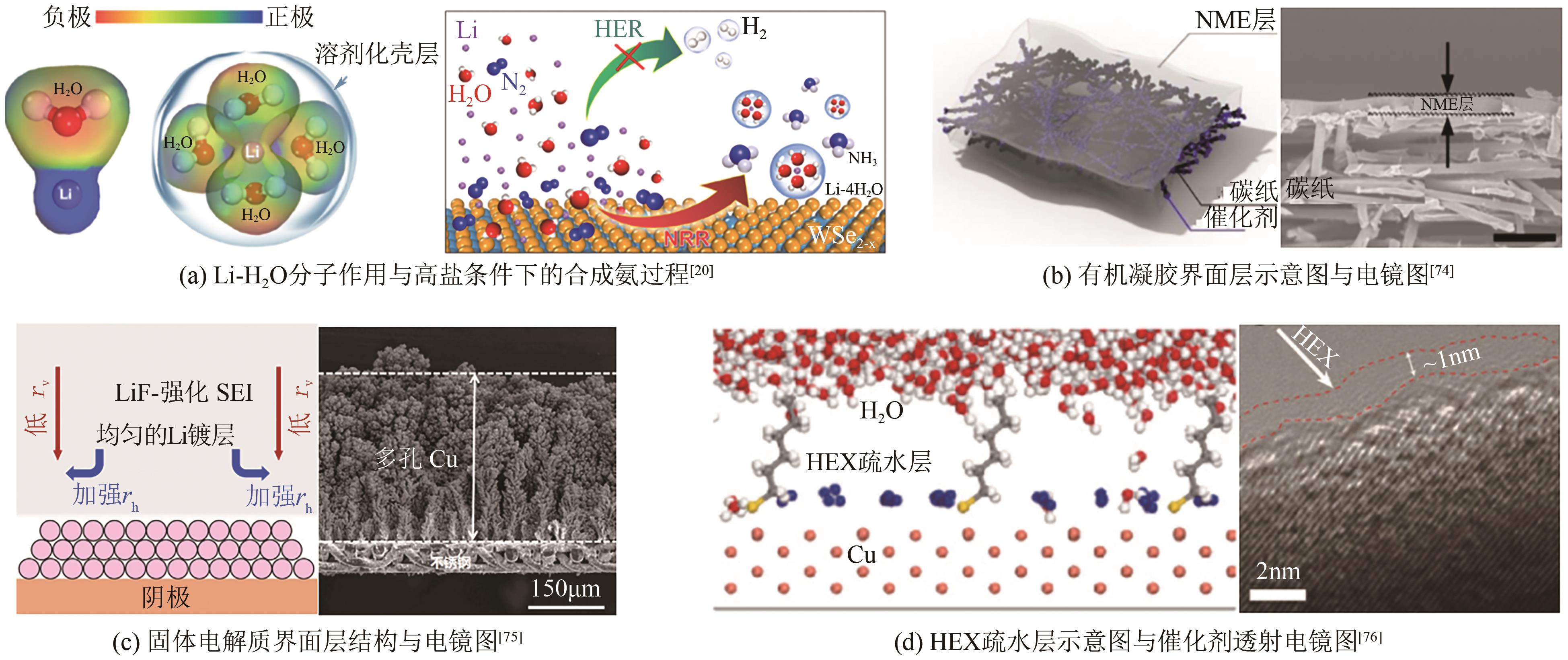
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (2): 809-819.DOI: 10.16085/j.issn.1000-6613.2024-0198
• Industrial catalysis • Previous Articles Next Articles
Present situation and strategy of electrolytes for electrochemical nitrogen reduction to ammonia
ZHANG Xiaofang( ), GAN Wen, JI Zhijiao, XU Ming, LI Chufu, HE Guangli
), GAN Wen, JI Zhijiao, XU Ming, LI Chufu, HE Guangli
- Beijing Low Carbon and Clean Energy Institute, Beijing 102200, China
-
Received:2024-01-26Revised:2024-03-26Online:2025-03-10Published:2025-02-25 -
Contact:ZHANG Xiaofang
电化学氮还原合成氨电解质利用现状与调控策略
- 北京低碳清洁能源研究院,北京 102200
-
通讯作者:张晓方 -
作者简介:张晓方(1983—),女,硕士,高级工程师,研究方向为清洁能源利用、电化学合成氨。E-mail:xiaofang.zhang.h@chnenergy.com.cn。
CLC Number:
Cite this article
ZHANG Xiaofang, GAN Wen, JI Zhijiao, XU Ming, LI Chufu, HE Guangli. Present situation and strategy of electrolytes for electrochemical nitrogen reduction to ammonia[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 809-819.
张晓方, 甘汶, 纪之骄, 许明, 李初福, 何广利. 电化学氮还原合成氨电解质利用现状与调控策略[J]. 化工进展, 2025, 44(2): 809-819.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0198
| 电解质种类 | 反应器类型 | 工作温度 | 氨产率 | 最高法拉第效率 |
|---|---|---|---|---|
| 水相 | H型电解槽 | 室温 | 1.4×10-8mol/(s·cm2) | 66%[ |
| 有机相 | H型电解槽 | 室温 | 262.5μg/(h·mgcat.) | 75.9%[ |
| 离子液体 | 膜电极电解槽 | 室温 | 1.5 10-7mol/(s·cm2) | 约100%[ |
| 熔融盐电解槽 | 中温,200~500℃ | 1.34×10-8mol/(s·cm2) | 79.8%[ | |
| 固体 | 固体氧化物电解槽 | 高温,500~800℃ | 10-9~10-10mol/(s·cm2) | 10% |
| 聚合物膜电极电解槽或H型电解槽 | <100℃ | 10-8mol/(s·cm2) | 10%[ |
| 电解质种类 | 反应器类型 | 工作温度 | 氨产率 | 最高法拉第效率 |
|---|---|---|---|---|
| 水相 | H型电解槽 | 室温 | 1.4×10-8mol/(s·cm2) | 66%[ |
| 有机相 | H型电解槽 | 室温 | 262.5μg/(h·mgcat.) | 75.9%[ |
| 离子液体 | 膜电极电解槽 | 室温 | 1.5 10-7mol/(s·cm2) | 约100%[ |
| 熔融盐电解槽 | 中温,200~500℃ | 1.34×10-8mol/(s·cm2) | 79.8%[ | |
| 固体 | 固体氧化物电解槽 | 高温,500~800℃ | 10-9~10-10mol/(s·cm2) | 10% |
| 聚合物膜电极电解槽或H型电解槽 | <100℃ | 10-8mol/(s·cm2) | 10%[ |
| 1 | 李金翰, 程方益. 惰性小分子电催化还原反应的电解液调控[J]. 电化学, 2020, 26(4): 474-485. |
| LI Jinhan, CHENG Fangyi. Electrolyte tailoring for electrocatalytic reduction of stable molecules[J]. Journal of Electrochemistry, 2020, 26(4): 474-485. | |
| 2 | PALIT Santi R. Electrochemical reduction and oxidation of nitrogen through low-current electrolysis[J]. Proceedings of the Indian Academy of Sciences-Section A, 1977, 85(2):68-70. |
| 3 | 于丽平. 电化学绿色合成氨技术的探索与研究[D]. 济南: 山东师范大学, 2021. |
| YU Liping. Exploration and research on electrochemical green synthetic ammonia technology[D]. Jinan: Shandong Normal University, 2021. | |
| 4 | LEECH Matthew C, Kevin LAM. A practical guide to electrosynthesis[J]. Nature Reviews Chemistry, 2022, 6: 275-286. |
| 5 | 吕增祥. 关于电催化合成氨体系中的问题研究以及非金属基掺杂在电催化合成氨中的应用[D]. 北京: 北京化工大学, 2022. |
| Zengxiang LYU. Research on problems in electrocatalytic ammonia synthesis systems and the application of metal-free based doping in electrocatalytic ammonia synthesis[D]. Beijing: Beijing University of Chemical and Technology, 2022. | |
| 6 | CHEN Shiming, PERATHONER Siglinda, AMPELLI Claudio, et al. Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas-liquid-solid three-phase reactor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 7393-7400. |
| 7 | Xianwei LYU, WANG Lu, WANG Guichang, et al. ZIF-supported AuCu nanoalloy for ammonia electrosynthesis from nitrogen and thin air[J]. Journal of Materials Chemistry A, 2020, 8(18): 8868-8874. |
| 8 | HU Lin, KHANIYA Asim, WANG Jun, et al. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe-oxide catalyst[J]. ACS Catalysis, 2018, 8(10): 9312-9319. |
| 9 | YAN Yu, QU Hongjiao, ZHENG Xiaonan, et al. Amorphous core/shell Ti-doped SnO2 with synergistically improved N2 adsorption/activation and electrical conductivity for electrochemical N2 reduction[J]. Chinese Chemical Letters, 2022, 33(10): 4655-4658. |
| 10 | CLARK Ezra L, RESASCO Joaquin, LANDERS Alan, et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide[J]. ACS Catalysis, 2018, 8(7): 6560-6570. |
| 11 | GAO Feiyue, HU Shaojin, ZHANG Xiaolong, et al. High-curvature transition-metal chalcogenide nanostructures with a pronounced proximity effect enable fast and selective CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 59(22): 8706-8712. |
| 12 | SONG Yang, JOHNSON Daniel, PENG Rui, et al. A physical catalyst for the electrolysis of nitrogen to ammonia[J]. Science Advances, 2018, 4(4): e1700336. |
| 13 | HAO Yuchen, GUO Yu, CHEN Liwei, et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water[J]. Nature Catalysis, 2019, 2: 448-456. |
| 14 | ZHANG Qikun, LIU Baoliang, YU Liping, et al. Synergistic promotion of the electrochemical reduction of nitrogen to ammonia by phosphorus and potassium[J]. ChemCatChem, 2020, 12(1): 334-341. |
| 15 | BU Tongan, HAO Yuchen, GAO Wenyan, et al. Promoting photocatalytic nitrogen fixation with alkali metal cations and plasmonic nanocrystals[J]. Nanoscale, 2019, 11(20): 10072-10079. |
| 16 | Fatih KÖLELI, KAYAN Didem Balun. Low overpotential reduction of dinitrogen to ammonia in aqueous media[J]. Journal of Electroanalytical Chemistry, 2010, 638(1):119-122. |
| 17 | WANG Weikang, ZHANG Haimin, ZHANG Shengbo, et al. Potassium-ion-assisted regeneration of active cyano groups in carbon nitride nanoribbons: Visible-light-driven photocatalytic nitrogen reduction[J]. Angewandte Chemie International Edition, 2019, 58(46): 16644-16650. |
| 18 | ZHOU Li, BOYD Claude E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture[J]. Aquaculture, 2016, 450: 187-193. |
| 19 | WANG Mengfan, LIU Sisi, JI Haoqing, et al. Salting-out effect promoting highly efficient ambient ammonia synthesis[J]. Nature Communications, 2021, 12(1): 3198. |
| 20 | SHEN Peng, LI Xingchuan, LUO Yaojing, et al. High-efficiency N2 electroreduction enabled by Se-vacancy-rich WSe2- x in water-in-salt electrolytes[J]. ACS Nano, 2022, 16(5): 7915-7925. |
| 21 | KILBURN Duncan, Joon Ho ROH, Guo Liang, et al. Molecular crowding stabilizes folded RNA structure by the excluded volume effect[J]. Journal of the American Chemical Society, 2010, 132(25): 8690-8696. |
| 22 | GUO Ying, GU Jinxing, ZHANG Rong, et al. Molecular crowding effect in aqueous electrolytes to suppress hydrogen reduction reaction and enhance electrochemical nitrogen reduction[J]. Advanced Energy Materials, 2021, 11(36): 2101699. |
| 23 | LEE Hiang Kwee, Charlynn Sher Lin KOH, LEE Yih Hong, et al. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach[J]. Science Advances, 2018, 4(3): eaar3208. |
| 24 | LAZOUSKI Nikifar, SCHIFFER Zachary J, WILLIAMS Kindle, et al. Understanding continuous lithium-mediated electrochemical nitrogen reduction[J]. Joule, 2019, 3(4): 1127-1139. |
| 25 | BECKER James Y, AVRAHAM TSARFATY Shlomit, POSIN Barry. Nitrogen fixation: Part Ⅰ. Electrochemical reduction of titanium compounds in the presence of catechol and N2 in MeOH or THF[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1987, 230(1/2): 143-153. |
| 26 | NIELANDER Adam C, MCENANEY Joshua M, SCHWALBE Jay A, et al. A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques[J]. ACS Catalysis, 2019, 9(7): 5797-5802. |
| 27 | KIM Kwiyong, LEE Nara, YOO Chung-Yul, et al. Communication—Electrochemical reduction of nitrogen to ammonia in 2-propanol under ambient temperature and pressure[J]. Journal of the Electrochemical Society, 2016, 163(7): F610-F612. |
| 28 | KIM Kwiyong, YOO Chung-Yul, KIM Jong-Nan, et al. Electrochemical synthesis of ammonia from water and nitrogen in ethylenediamine under ambient temperature and pressure[J]. Journal of the Electrochemical Society, 2016, 163(14): F1523-F1526. |
| 29 | 丁良鑫, 任诗雨, 王海辉.一种电催化合成氨的有机电解液及其制备方法与应用: CN110284144B[P]. 2021-05-14. |
| DING Liangxin, REN Shiyu, WANG Haihui. Preparation method and application of organic electrolyte for electrocatalytic synthesis of ammonia: CN110284144B[P]. 2021-05-14. | |
| 30 | 邱介山, 任勇文, 于畅. 一种电催化氮气还原合成氨用醇-水混合电解液及其应用: CN115896819A[P]. 2023-04-04. |
| QIU Jieshan, REN Yongwen, YU Chang. Alcohol-water mixed electrolyte for synthesizing ammonia through electrocatalytic nitrogen reduction and application of alcohol-water mixed electrolyte: CN115896819A[P]. 2023-04-04. | |
| 31 | REN Yongwen, YU Chang, HAN Xiaotong, et al. Methanol-mediated electrosynthesis of ammonia[J]. ACS Energy Letters, 2021, 6(11): 3844-3850. |
| 32 | 任勇文. 电化学合成氨体系关键组成优化及改进策略[D]. 大连: 大连理工大学, 2022. |
| REN Yongwen. Strategies to optimize and improve the key components of ammonia electrosynthesis system[D]. Dalian: Dalian University of Technology, 2022. | |
| 33 | LAZOUSKI Nikifar, CHUNG Minju, WILLIAMS Kindle, et al. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen[J]. Nature Catalysis, 2020, 3: 463-469. |
| 34 | ANDERSEN Suzanne Z, STATT Michael J, BUKAS Vanessa J, et al. Increasing stability, efficiency, and fundamental understanding of lithium-mediated electrochemical nitrogen reduction[J]. Energy & Environmental Science, 2020, 13(11): 4291-4300. |
| 35 | CAI Xiyang, FU Cehuang, IRIAWAN Haldrian, et al. Lithium-mediated electrochemical nitrogen reduction: Mechanistic insights to enhance performance[J]. iScience, 2021, 24(10): 103105. |
| 36 | HUANG Hao, TU Wenguang, FANG Liping, et al. Lithium-mediated photoelectrochemical ammonia synthesis with 95% selectivity on silicon photocathode[J]. ACS Energy Letters, 2023, 8(10): 4235-4241. |
| 37 | PAPPENFUS Ted M, LEE Kyung-mee, THOMA Laura M, et al. Wind to ammonia: Electrochemical processes in room temperature ionic liquids[J]. Electrochemical Society Transactions, 2009, 16(49): 89-93. |
| 38 | SURYANTO Bryan, KANG Colin S M, WANG Dabin, et al. Rational electrode-electrolyte design for efficient ammonia electrosynthesis under ambient conditions[J]. ACS Energy Letters, 2018, 3(6): 1219-1224. |
| 39 | SIDDIQUI Osamah, DINCER Ibrahim. A review and comparative assessment of direct ammonia fuel cells[J]. Thermal Science and Engineering Progress, 2018, 5: 568-578. |
| 40 | FENG Chuanping, SUGIURA Norio, SHIMADA Satoru, et al. Development of a high performance electrochemical wastewater treatment system[J]. Journal of Hazardous Materials, 2003, 103(1/2): 65-78. |
| 41 | José-María SANSIÑENA, CHLISTUNOFF Jerzy, TOMSON Neil C, et al. Ionic liquids for ammonia electrosynthesis and energy storage[J]. ECS Meeting Abstracts, 2013(45): 2608. |
| 42 | YANG Jiarong, WENG Wei, XIAO Wei. Electrochemical synthesis of ammonia in molten salts[J]. Journal of Energy Chemistry, 2020, 43: 195-207. |
| 43 | TSUNETO Akira, KUDO Akihiko, SAKATA Tadayoshi. Lithium-mediated electrochemical reduction of high pressure N2 to NH3 [J]. Journal of Electroanalytical Chemistry, 1994, 367(1/2): 183-188. |
| 44 | MURAKAMI Tsuyoshi, NISHIKIORI Tokujiro, NOHIRA Toshiyuki, et al. Electrolytic synthesis of ammonia in molten salts under atmospheric pressure[J]. Journal of the American Chemical Society, 2003, 125(2): 334-335. |
| 45 | LIU Xinye, LI Fangfang, PENG Ping, et al. Efficient electrocatalytic synthesis of ammonia from water and air in a membrane-free cell: Confining the iron oxide catalyst to the cathode[J]. European Journal of Inorganic Chemistry, 2020, 2020(15/16): 1428-1436. |
| 46 | CUI Baochen, YU Zhongjun, LIU Shuzhi, et al. Highly selective and efficient ammonia synthesis from N2 and H2O via an iron-based electrolytic-chemical cycle[J]. International Journal of Hydrogen Energy, 2020, 45(1): 94-102. |
| 47 | SURYANTO Bryan H-R, MATUSZEK Karolina, CHOI Jaecheol, et al. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle[J]. Science, 2021, 372(6547): 1187-1191. |
| 48 | DU Hoang-Long, CHATTI Manjunath, HODGETTS Rebecca Y, et al. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency[J]. Nature, 2022, 609: 722-727. |
| 49 | FU Xianbiao, PEDERSEN Jakob B, ZHOU Yuanyuan, et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation[J]. Science, 2023, 379(6633): 707-712. |
| 50 | FU Xianbiao, NIEMANN Valerie A, ZHOU Yuanyuan, et al. Calcium-mediated nitrogen reduction for electrochemical ammonia synthesis[J]. Nature Materials, 2024, 23: 101-107. |
| 51 | LI Ya, WANG Zhenkang, JI Haoqing, et al. Extending ring-chain coupling empirical law to lithium-mediated electrochemical ammonia synthesis[J], Angewandte Chemie International Edition, 2024, 63(2): e202311413. |
| 52 | 李林哲. 固体电解池合成氨电解质研究进展[J]. 山东化工, 2022, 51(20):83-86. |
| LI Linzhe. Research progress of electrolyte of solid electrolytic cell for ammonia synthesis[J]. Shandong Chemical Industry, 2022, 51(20):83-86. | |
| 53 | 刘民, 任宇梅, 于庆河, 等. 中高温质子导体电解质陶瓷研究进展[J]. 稀有金属, 2022, 46(9): 1244-1253. |
| LIU Min, REN Yumei, YU Qinghe, et al. Research progress of middle and high temperature proton conductor electrolyte ceramics[J]. Chinese Journal of Rare Metals, 2022, 46(9): 1244-1253. | |
| 54 | PANAGOS E, VOUDOURIS I, STOUKIDES M. Modelling of equilibrium limited hydrogenation reactions carried out in H+ conducting solid oxide membrane reactors[J]. Chemical Engineering Science, 1996, 51(11): 3175-3180. |
| 55 | 宿新泰,刘瑞泉,王吉德. SrCe0.95Y 0.05O3-δ在中温区的电化学性质及其在常压合成氨中的应用[J]. 化学学报, 2003, 61(4): 505-509, 446. |
| SU Xintai, LIU Ruiquan, WANG Jide. Electrochemical properties of SrCe0.95Y0.05O3- δ at intermediate temperature and its application to ammonia synthesis at atmospheric pressure[J]. Acta Chimica Sinica, 2003, 61(4): 505-509, 446. | |
| 56 | 张峰. LaGaO3基陶瓷的合成及其电性能研究[D]. 苏州: 苏州大学, 2008. |
| ZHANG Feng. Synthesis and electrical conductivity of LaGaO3 based ceramics[D]. Suzhou: Soochow University, 2008. | |
| 57 | 刘宝信. LaYO3基陶瓷的合成及其电性能研究[D]. 苏州: 苏州大学, 2009. |
| LIU Baoxin. Synthesis and electrical conductivity of LaYO3 based ceramics[D]. Suzhou: Soochow University, 2009. | |
| 58 | 杨青. LaErO3基陶瓷的合成及其电性能研究[D]. 苏州: 苏州大学, 2009. |
| YANG Qing. Synthesis and electrical conductivity of LaErO3 based ceramics[D]. Suzhou: Soochow University, 2009. | |
| 59 | NIGARA Yasmin, MIZUSAKI Junichiro, KAWAMURA Kazunori, et al. Hydrogen permeability in (CeO2)0.9(CaO)0.1 at high temperatures[J].Solid State Ionics, 1998, 113: 347-354. |
| 60 | SKODRA Alexandra, STOUKIDES M. Electrocatalytic synthesis of ammonia from steam and nitrogen at atmospheric pressure[J]. Solid State Ionics, 2009, 180(23/24/25): 1332-1336. |
| 61 | MULMI Suresh, THANGADURAI Venkataraman. Editors’ choice—Review—Solid-state electrochemical carbon dioxide sensors: Fundamentals, materials and applications[J]. Journal of Electrochemical Society, 2020, 167(3): 037567 |
| 62 | 魏光华, 蔡熙阳, 章俊良, 等. 一种用于锂介导合成氨的膜电极及其制备方法和应用: CN116791118A[P]. 2023-09-22. |
| WEI Guanghua, CAI Xiyang, ZHANG Junliang, et al. Membrane electrode for lithium-mediated ammonia synthesis as well as preparation method and application of membrane electrode: CN116791118A[P]. 2023-09-22. | |
| 63 | MA Guilin, ZHANG Feng, ZHU Jianli, et al. Proton conduction in La0.9Sr0.1Ga0.8Mg0.2O3- α [J]. Chemistry of Materials, 2006, 18(25): 6006-6011. |
| 64 | GIDDEY Sarbjit, BADWAL Sukhvinder P S, KULKARNI A. Review of electrochemical ammonia production technologies and materials[J].International Journal of Hydrogen Energy, 2013, 38(34):14576-14594. |
| 65 | 练文超, 雷励斌, 梁波, 等. 质子导体固体氧化物电化学装置中氨的利用与合成[J].储能科学与技术, 2021, 10(6): 1998-2007. |
| LIAN Wenchao, LEI Libin, LIANG Bo, et al. Utilization and synthesis of ammonia in proton-conducting solid oxide electrochemical devices[J]. Energy Storage Science and Technology, 2021, 10(6): 1998-2007. | |
| 66 | 勾匀婕, 李广东, 王振华, 等. 固体氧化物电解池技术的应用前景与挑战[J]. 石油化工高等学校学报, 2022, 35(6): 28-37. |
| GOU Yunjie, LI Guangdong, WANG Zhenhua, et al. Application prospects and challenges of solid oxide electrolysis cell technology[J]. Journal of Petrochemical Universities, 2022, 35(6): 28-37. | |
| 67 | SOLOVEICHIK G. Renewable energy to fuels through utilization of energy-dense liquids(FEFUEL)[EB/OL]. ARPA-E. . |
| 68 | 徐高超, 刘瑞泉, 王进. 利用Nafion膜和SFCN在低温常压下电化学合成氨[J]. 中国科学, 2009, 39(5): 447-451. |
| XU Gaochao, LIU Ruiquan, WANG Jin. Using the nafion membrane and SFCN to synthesize ammonia at atmospheric pressure and lower temperature[J]. Science in China, 2009, 39(5): 447-451. | |
| 69 | LIU Ruiquan, XU Gaochao. Comparison of electrochemical synthesis of ammonia by using sulfonated polysulfone and nafion membrane with Sm1.5Sr0.5NiO4 [J]. Chinese Journal of Chemistry, 2010, 28(2): 139-142. |
| 70 | 王忱, 陈成. 基于陶瓷电极的质子交换膜在低温常压合成氨中的应用[J]. 科技创新导报, 2014, 11(15): 75-76. |
| WANG Chen, CHEN Cheng. Application of proton exchange membrane based on ceramic electrode in ammonia synthesis at low temperature and atmospheric pressure[J]. Science and Technology Innovation Herald, 2014, 11(15): 75-76. | |
| 71 | SINGH Aayush R, ROHR Brian A, SCHWALBE Jay A, et al. Electrochemical ammonia synthesis-The selectivity challenge [J]. ACS Catalysis, 2017, 7(1): 706-709. |
| 72 | LIU Yanyan, HAN Miaomiao, XIONG Qizhong, et al. Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li-S interactions on MoS2 electrocatalyst[J]. Advanced Energy Materials, 2019, 9(14): 1803935. |
| 73 | 刘洋. 策略性提升常温常压下电催化合成氨效率的研究[D]. 南宁: 广西大学, 2020. |
| LIU Yang. Strategically increasing the efficiency of electrocatalytic ammonia synthesis under ambient conditions[D]. Nanning: Guangxi University, 2020. | |
| 74 | SHEN Xiaowei, LIU Sisi, XIA Xinyao, et al. Interfacial microextraction boosting nitrogen feed for efficient ambient ammonia synthesis in aqueous electrolyte[J]. Advanced Functional Materials, 2022, 32(17): 2109422. |
| 75 | LI Shaofeng, ZHOU Yuanyuan, LI Katja, et al. Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid-electrolyte interphase[J]. Joule, 2022, 6(9): 2083-2101. |
| 76 | DU Cheng, QIU Chenglong, FANG Zhongying, et al. Interface hydrophobic tunnel engineering: A general strategy to boost electrochemical conversion of N2 to NH3 [J]. Nano Energy, 2022, 92: 106784. |
| [1] | CHEN Kexin, LI Xi, CHANG Fucheng, WU Xiaoyi, LOU Jiacheng, LI Huixiong. Investigation on pressure drop and characteristics of flow-pattern transition of steam-water two-phase flows in helically coiled tubes [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 613-624. |
| [2] | JIN Yuyang, NIU Chuanfeng, LIU Yingshuo, DING Shi. Graphite powder/Nafion-Pb electrode for electrocatalytic reduction of oxalic acid to glycolic acid [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1003-1013. |
| [3] | HONG Siqi, GU Fangwei, ZHENG Jinyu. Development status and prospect of low iridium catalysts for hydrogen production by PEM electrolysis [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 158-168. |
| [4] | LI Jiayou, ZHANG Yuhan, JIANG Nan, JIANG Bolong. Preparation of transition metal sulfide NiS(x)@NFcatalyst by hydrothermal method and its hydrogen evolution performance [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 297-304. |
| [5] | HE Ran, LIANG Hong, HUANG Hong, YANG Youli, ZHENG Qiang, LI Xi. Preparation of acetylene black/Fe3O4 catalysed cathodic electrode and removal of 2,4,6-trichlorophenol by electro-Fenton oxidation [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 572-582. |
| [6] | LIN Meijie, MI Shuodong, BAO Cheng. Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 209-224. |
| [7] | MA Guixuan, XU Zitong, XIAO Zhihua, Ning Guoqing, WEI Qiang, XU Chunming. O,S co-doped carbon nanotube aqueous conductive additive assisted construction of high-performance graphite/SiO anode [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 443-456. |
| [8] | ZHU Hao, LIU Hanfei, GAO Yuan, HUANG Yiping, FEI Xiaocheng, HAN Weiqing. Effect of salt on electrocatalytic performance and mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 571-580. |
| [9] | GAO Yuli, WANG Hongqiu, HUANG Gexing, XIAN Nanying, SHI Xiaoyu. Research progress and the industrialization of all-solid-state battery [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4767-4778. |
| [10] | LIANG Hongcheng, ZHAO Dongni, QUAN Yin, LI Jingni, HU Xinyi. Influence of SEI film morphology and structure on the performance of lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5049-5062. |
| [11] | WU Jianyang, WANG Runa, CHEN Yao, SHEN Lanyao, YU Yongli, JIANG Ning, QIU Jingyi, ZHOU Henghui. Preparation process of high nickel cathode precursor for lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5079-5085. |
| [12] | LI Meixuan, CHENG Jianfeng, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Junlian, WANG Chunxia, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Synthesis and electrochemical mechanism of high voltage lithium nickel manganate cathode materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5086-5094. |
| [13] | LI Haoran, WANG Yan, ZHANG Tao, LYU Li, TANG Wenxiang, TANG Shengwei. Controllable regulation of microdroplets size in W/O microemulsion with Cu(Ac)2-Zn(Ac)2 solution as aqueous phase [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5168-5176. |
| [14] | LI Zihan, SHU Jiancheng, CAO Wenxing, YANG Huimin, CHEN Mengjun. Physicochemical properties of rhodochrosite before and after leaching and the changing law of interface hydration behavior [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5320-5328. |
| [15] | XIE Juan, HE Wen, ZHAO Xucheng, LI Shuaihui, LU Zhenzhen, DING Zheyu. Research progress on the application of molecular dynamics simulation in asphalt systems [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4432-4449. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||