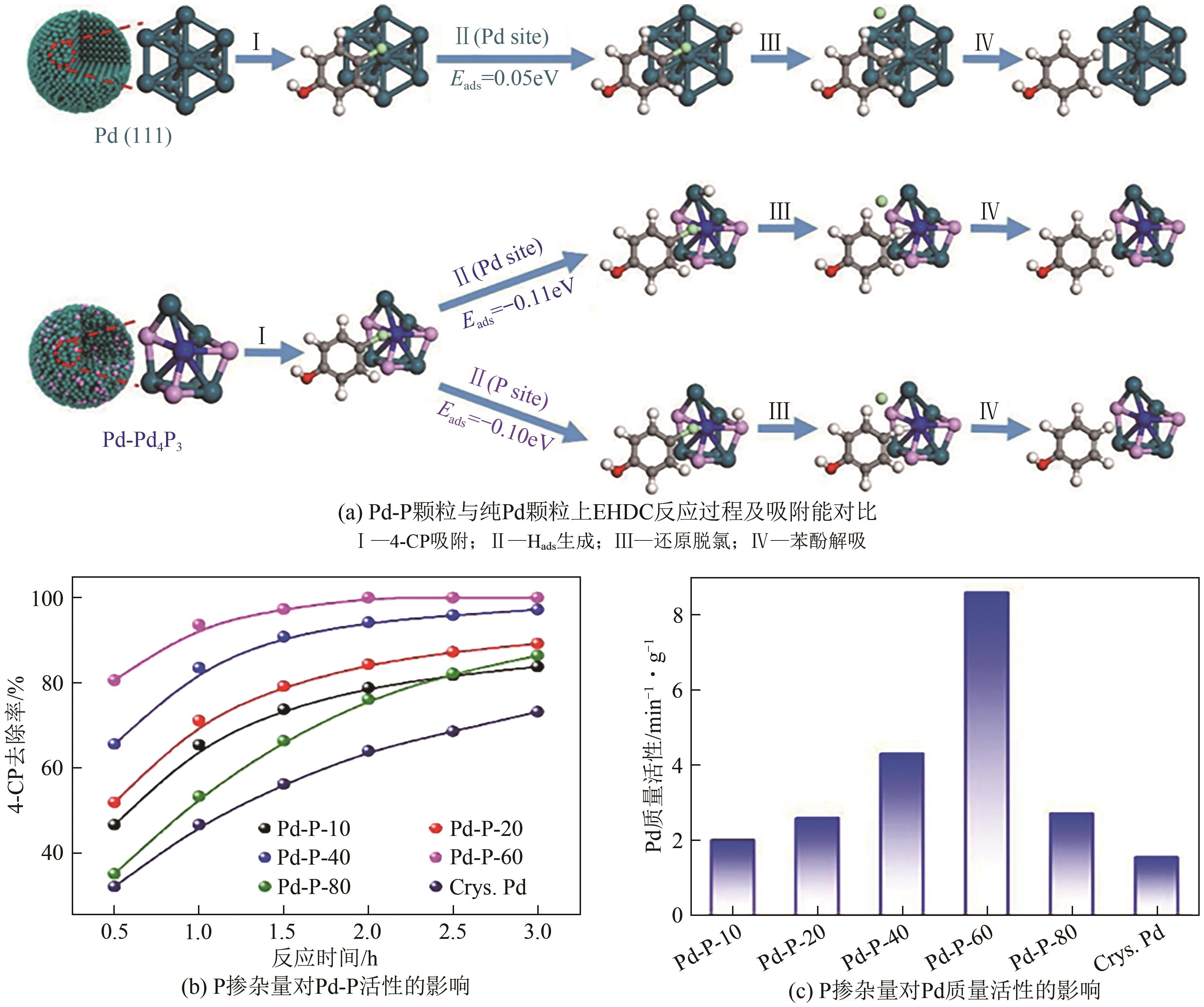
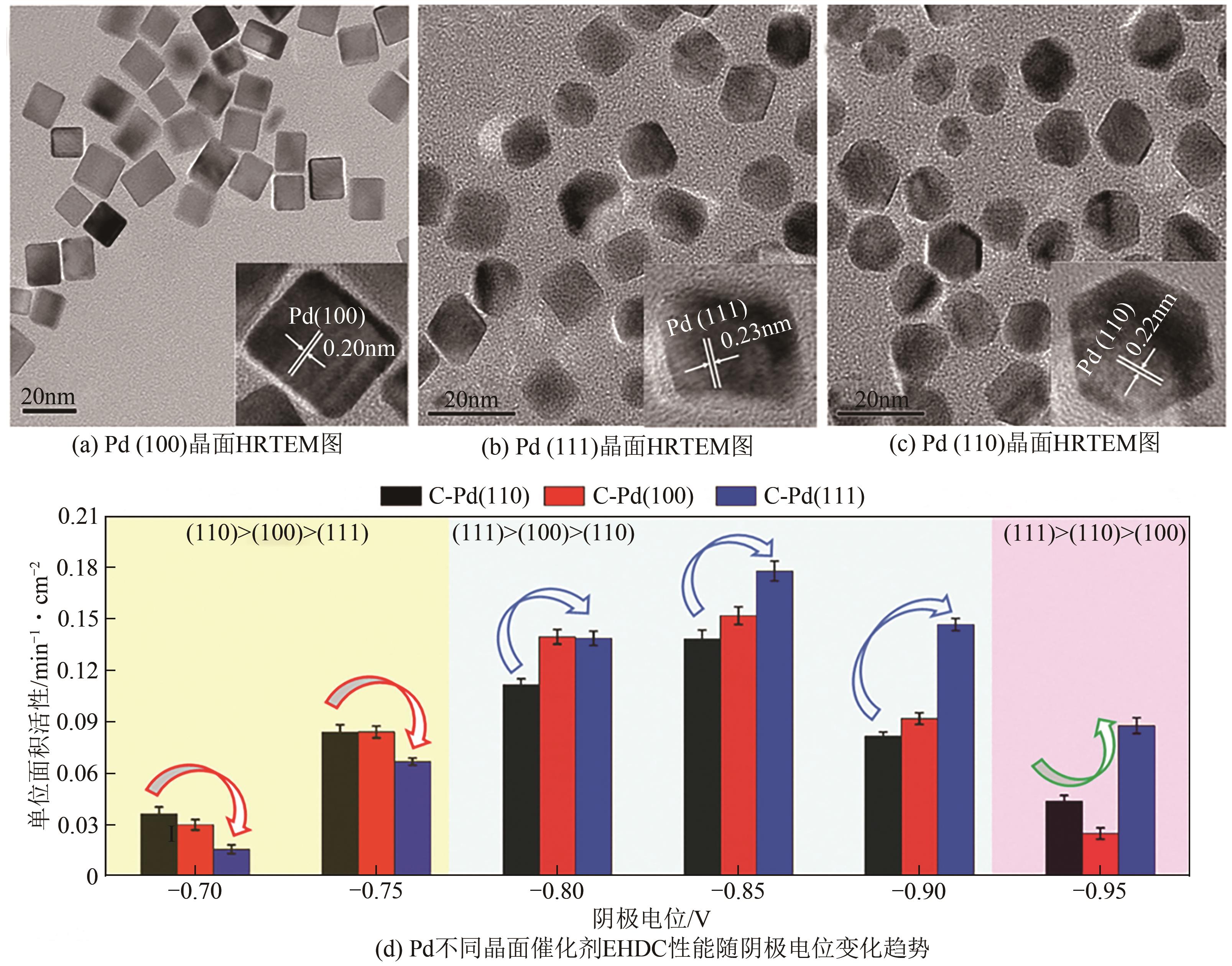
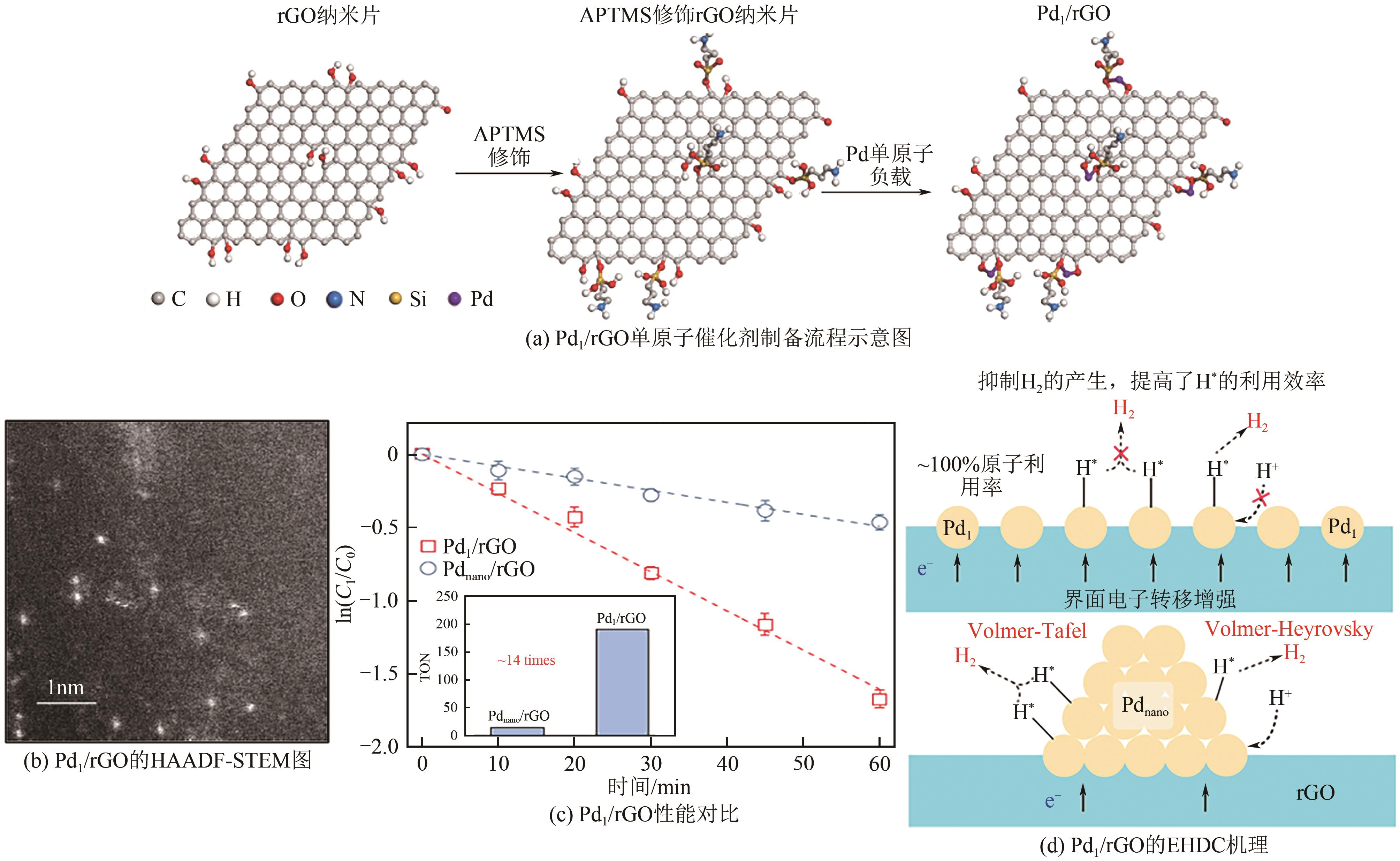
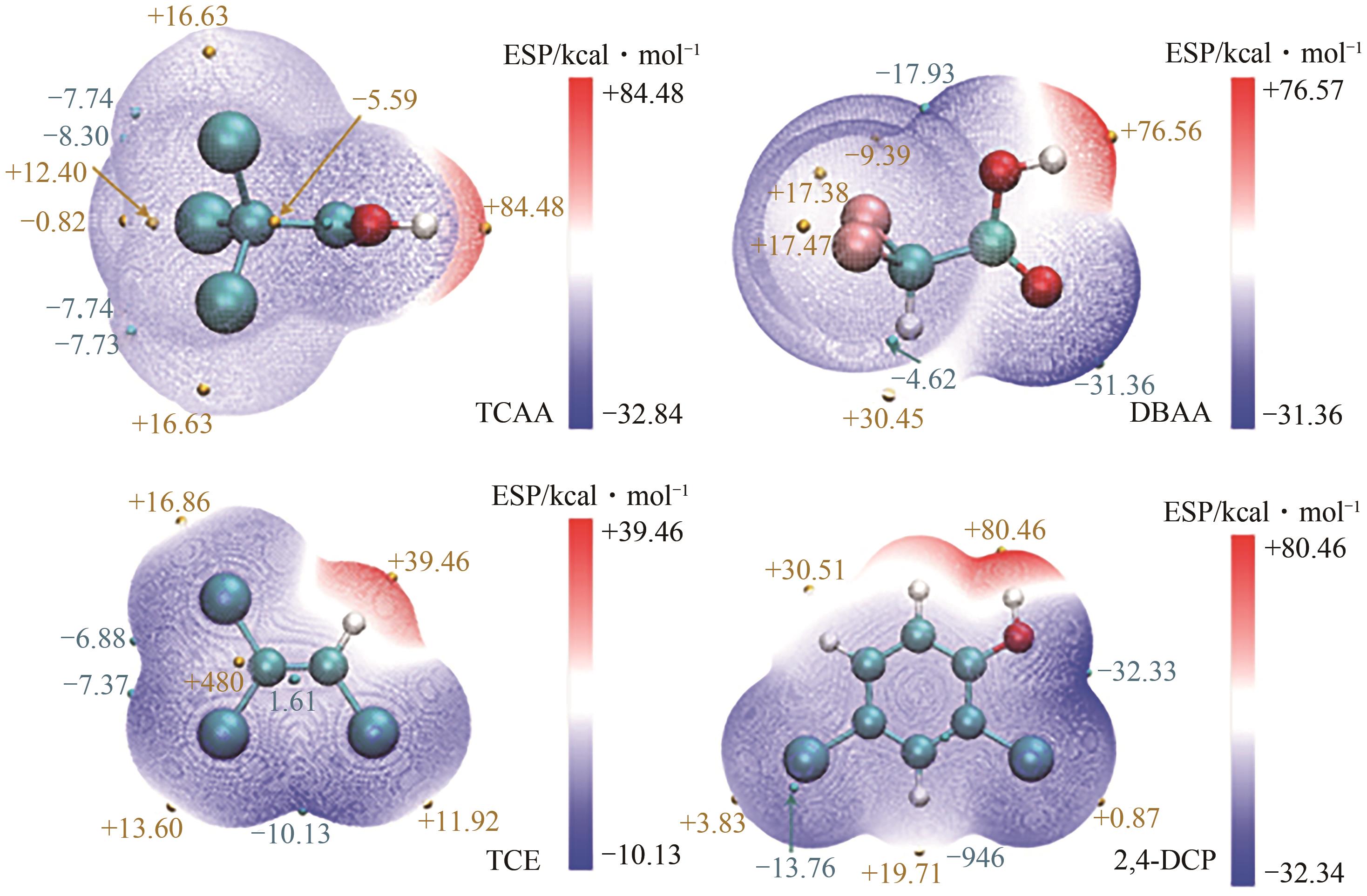
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (11): 6428-6442.DOI: 10.16085/j.issn.1000-6613.2023-1822
• Resources and environmental engineering • Previous Articles
Application and challenges of palladium-based catalysts in electrocatalytic hydrodechlorination
- State Key Laboratory of Heavy Oil Processing, College of Chemistry and Chemical Engineering, China University of Petroleum (East China), Qingdao 266580, Shandong, China
-
Received:2023-10-16Revised:2024-01-25Online:2024-12-07Published:2024-11-15 -
Contact:LIU Yunqi
钯基催化剂在电催化加氢脱氯技术的应用与挑战
- 中国石油大学(华东)化学化工学院重质油国家重点实验室,山东 青岛 266580
-
通讯作者:柳云骐 -
作者简介:李俊熙(1995—),男,博士研究生,研究方向为环境污染治理技术与材料。E-mail:B20030038@s.upc.edu.cn。 -
基金资助:国家自然科学基金(22178388)
CLC Number:
Cite this article
LI Junxi, LIU Yunqi. Application and challenges of palladium-based catalysts in electrocatalytic hydrodechlorination[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6428-6442.
李俊熙, 柳云骐. 钯基催化剂在电催化加氢脱氯技术的应用与挑战[J]. 化工进展, 2024, 43(11): 6428-6442.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1822
催化剂 (催化剂量) | 污染物 (添加量) | 测试条件 | 转化率/% | 电流效率/% | 参考文献 |
|---|---|---|---|---|---|
| Pd/MnO2/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | 10mA 10mmol/L Na2SO4;T=303K;pH=4.0 | 100(120min) | 70 | [ |
| TiC-Pd/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | -0.85V vs. Ag/AgCl 10mmol/L Na2SO4;T=(298±0.3K);pH=4 | 99.8(90min) | — | [ |
Pd/AC (8mg) | 2,4-二氯苯甲酸 (0.156mmol/L) | 10mA 10mmol/L Na2SO4;T=313K;pH=4.0 | 98(180min) | 6 | [ |
| Pd-Co3O4/Ni泡沫 (1.88mg/cm2) | 2,4-二氯苯氧基乙酸 (50mg/L) | 1.5mA/cm2 17mmol/L Na2SO4T=(298.15±1)K | 94.2(120min) | 12.1 | [ |
| Pd/TiN-Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯氧基乙酸 (0.23mmol/L) | 1.667mA/cm2 10mmol/L Na2SO4; T=298.15K | 100(120min) | — | [ |
| 碳载Ag32Pd68合金 (4.79mg) | 2,4-二氯苯酚 (0.31 mmol/L) | -0.70V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <90(240min) | <40 | [ |
| Pd-TiO2 | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <80(180min) | 25.8 | [ |
Pd/TiN [(3.20±0.4)mg] | 2,4-二氯苯酚 (0.31mmol/L) | -0.80V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | 96.4(240min) | <40 | [ |
Pd/MnO2/Ni泡沫 (0.205mg/cm2) | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(150min) | 26.3 | [ |
| Pd/Ni2P-Ni泡沫 (0.41mg/cm2) | 对氯苯酚 (100mg/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(120min) | 28.3 | [ |
| Pd/PPy-MWCNTs/Ti (3.72mg/cm2) | 对氯苯酚 (0.78mmol/L) | 1.0mA/cm2 0.1mol/L Na2SO4T=298.15K;pH=2.08 | 99.82(120min) | 13.8 | [ |
| Pd-P-60 NPs (0.8mg/cm2) | 对氯苯酚 (50mg/L) | -0.80V vs. SCE 25 mmol/L K2SO4; T=298.15K;pH=6.8 | 100(120min) | <20 | [ |
| 碳载Pd7Au3合金 | 对氯苯酚 (50mg/L) | -1.10V vs. SCE 50mmol/L K2SO4; T=298.15K;pH=6.8 | 98.35(240min) | <14 | [ |
催化剂 (催化剂量) | 污染物 (添加量) | 测试条件 | 转化率/% | 电流效率/% | 参考文献 |
|---|---|---|---|---|---|
| Pd/MnO2/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | 10mA 10mmol/L Na2SO4;T=303K;pH=4.0 | 100(120min) | 70 | [ |
| TiC-Pd/Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯甲酸 (0.2mmol/L) | -0.85V vs. Ag/AgCl 10mmol/L Na2SO4;T=(298±0.3K);pH=4 | 99.8(90min) | — | [ |
Pd/AC (8mg) | 2,4-二氯苯甲酸 (0.156mmol/L) | 10mA 10mmol/L Na2SO4;T=313K;pH=4.0 | 98(180min) | 6 | [ |
| Pd-Co3O4/Ni泡沫 (1.88mg/cm2) | 2,4-二氯苯氧基乙酸 (50mg/L) | 1.5mA/cm2 17mmol/L Na2SO4T=(298.15±1)K | 94.2(120min) | 12.1 | [ |
| Pd/TiN-Ni泡沫 (0.44mg/cm2) | 2,4-二氯苯氧基乙酸 (0.23mmol/L) | 1.667mA/cm2 10mmol/L Na2SO4; T=298.15K | 100(120min) | — | [ |
| 碳载Ag32Pd68合金 (4.79mg) | 2,4-二氯苯酚 (0.31 mmol/L) | -0.70V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <90(240min) | <40 | [ |
| Pd-TiO2 | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | <80(180min) | 25.8 | [ |
Pd/TiN [(3.20±0.4)mg] | 2,4-二氯苯酚 (0.31mmol/L) | -0.80V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=6.8 | 96.4(240min) | <40 | [ |
Pd/MnO2/Ni泡沫 (0.205mg/cm2) | 2,4-二氯苯酚 (0.31mmol/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(150min) | 26.3 | [ |
| Pd/Ni2P-Ni泡沫 (0.41mg/cm2) | 对氯苯酚 (100mg/L) | -0.85V vs. Ag/AgCl 50mmol/L Na2SO4; T=298.15K;pH=7.0 | 100(120min) | 28.3 | [ |
| Pd/PPy-MWCNTs/Ti (3.72mg/cm2) | 对氯苯酚 (0.78mmol/L) | 1.0mA/cm2 0.1mol/L Na2SO4T=298.15K;pH=2.08 | 99.82(120min) | 13.8 | [ |
| Pd-P-60 NPs (0.8mg/cm2) | 对氯苯酚 (50mg/L) | -0.80V vs. SCE 25 mmol/L K2SO4; T=298.15K;pH=6.8 | 100(120min) | <20 | [ |
| 碳载Pd7Au3合金 | 对氯苯酚 (50mg/L) | -1.10V vs. SCE 50mmol/L K2SO4; T=298.15K;pH=6.8 | 98.35(240min) | <14 | [ |
| 1 | RENAGULI Aikebaier, FERNANDO Sujan, HOPKE Philip K, et al. Nontargeted screening of halogenated organic compounds in fish fillet tissues from the great lakes[J]. Environmental Science & Technology, 2020, 54(23): 15035-15045. |
| 2 | ILUNGA Ali K, MAMBA Bhekie B, NKAMBULE Thabo T I. Catalytic hydrodehalogenation of halogenated disinfection byproducts for clean drinking water production: A review[J]. Journal of Water Process Engineering, 2021, 44: 102402. |
| 3 | HALSE Anne Karine, SCHLABACH Martin, SCHUSTER Jasmin K, et al. Endosulfan, pentachlorobenzene and short-chain chlorinated paraffins in background soils from Western Europe[J]. Environmental Pollution, 2015, 196: 21-28. |
| 4 | MCGOLDRICK Daryl J, MURPHY Elizabeth W. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008—2012)[J]. Environmental Pollution, 2016, 217: 85-96. |
| 5 | RANI Lata, THAPA Komal, KANOJIA Neha, et al. An extensive review on the consequences of chemical pesticides on human health and environment[J]. Journal of Cleaner Production, 2021, 283: 124657. |
| 6 | DENG Jia, HU Xinming, GAO Enlai, et al. Electrochemical reductive remediation of trichloroethylene contaminated groundwater using biomimetic iron-nitrogen-doped carbon[J]. Journal of Hazardous Materials, 2021, 419: 126458. |
| 7 | SHEN Yi, TONG Yiwen, XU Jinli, et al. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination[J]. Applied Catalysis B: Environmental, 2020, 264: 118505. |
| 8 | LOU Yaoyin, HAPIOT Philippe, FLONER Didier, et al. Efficient dechlorination of α-halocarbonyl and α-haloallyl pollutants by electroreduction on bismuth[J]. Environmental Science & Technology, 2020, 54(1): 559-567. |
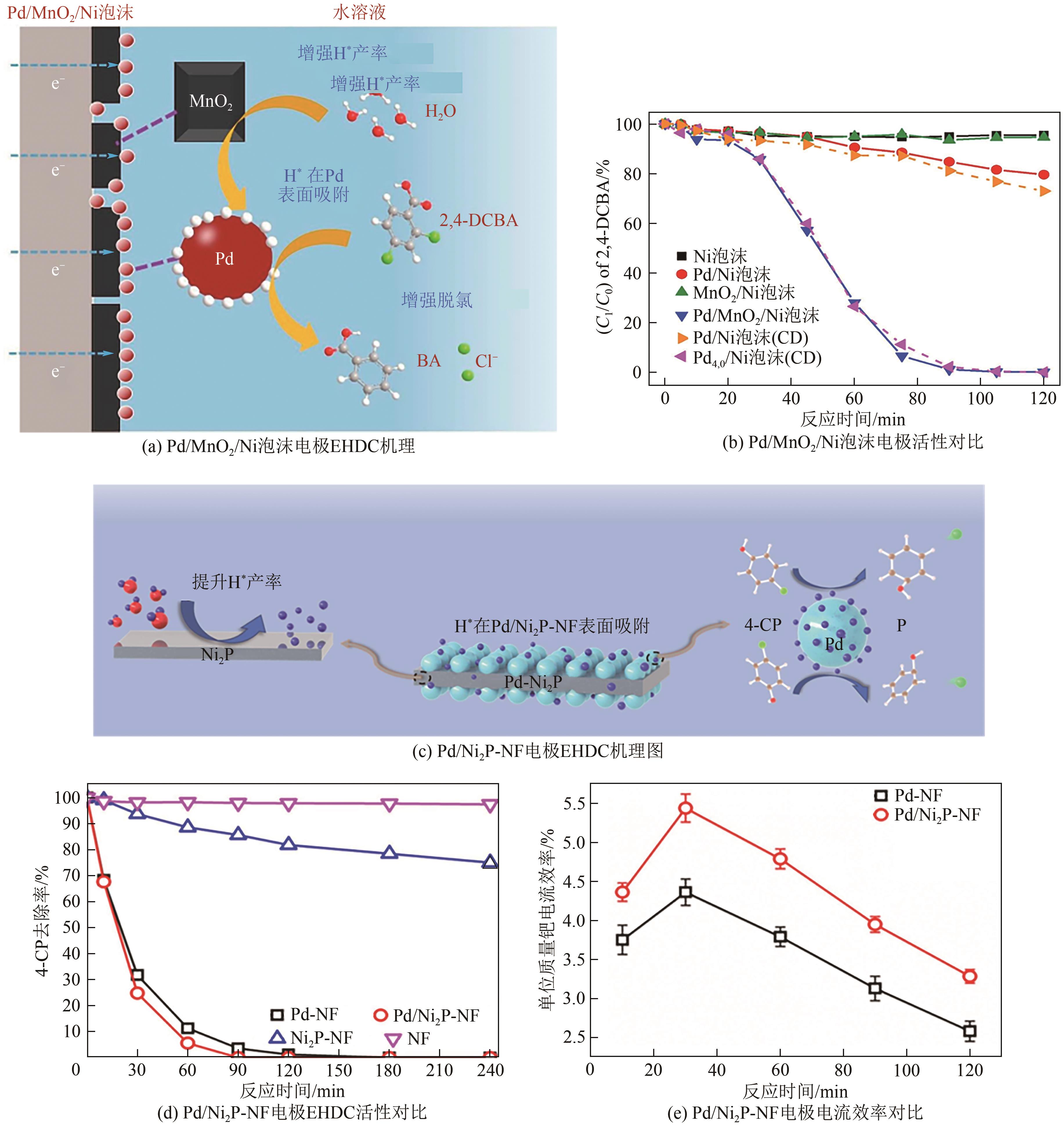
| 9 | LI Junxi, PENG Yiyin, ZHANG Wendong, et al. Hierarchical Pd/MnO2 nanosheet array supported on Ni foam: An advanced electrode for electrocatalytic hydrodechlorination reaction[J]. Applied Surface Science, 2020, 509: 145369. |
| 10 | JIANG Guangming, LI Xiangjun, SHEN Yu, et al. Mechanistic insight into the electrocatalytic hydrodechlorination reaction on palladium by a facet effect study[J]. Journal of Catalysis, 2020, 391: 414-423. |
| 11 | MAO Ran, HUANG Chao, ZHAO Xu, et al. Dechlorination of triclosan by enhanced atomic hydrogen-mediated electrochemical reduction: Kinetics, mechanism, and toxicity assessment[J]. Applied Catalysis B: Environmental, 2019, 241: 120-129. |
| 12 | JIANG Guangming, LAN Mengna, ZHANG Zhiyong, et al. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2, 4-dichlorophenol in water[J]. Environmental Science & Technology, 2017, 51(13): 7599-7605. |
| 13 | ZHOU Jiasheng, LOU Zimo, XU Jiang, et al. Enhanced electrocatalytic dechlorination by dispersed and moveable activated carbon supported palladium catalyst[J]. Chemical Engineering Journal, 2019, 358: 1176-1185. |
| 14 | LI Junjing, WANG Huan, QI Ziyan, et al. Kinetics and mechanisms of electrocatalytic hydrodechlorination of diclofenac on Pd-Ni/PPy-rGO/Ni electrodes[J]. Applied Catalysis B: Environmental, 2020, 268: 118696. |
| 15 | LIU Yong, LIU Lan, SHAN Jun, et al. Electrodeposition of palladium and reduced graphene oxide nanocomposites on foam-nickel electrode for electrocatalytic hydrodechlorination of 4-chlorophenol[J]. Journal of Hazardous Materials, 2015, 290: 1-8. |
| 16 | SHU Song, WANG Peng, ZHANG Wendong, et al. Pd nanoparticles on defective polymer carbon nitride: Enhanced activity and origin for electrocatalytic hydrodechlorination reaction[J]. Chinese Chemical Letters, 2020, 31(10): 2762-2768. |
| 17 | SUN Chen, LOU Zimo, LIU Yu, et al. Influence of environmental factors on the electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on nTiN doped Pd/Ni foam electrode[J]. Chemical Engineering Journal, 2015, 281: 183-191. |
| 18 | LIU Rui, ZHAO Huachao, ZHAO Xiaoyu, et al. Defect sites in ultrathin Pd nanowires facilitate the highly efficient electrochemical hydrodechlorination of pollutants by H*ads [J]. Environmental Science & Technology, 2018, 52(17): 9992-10002. |
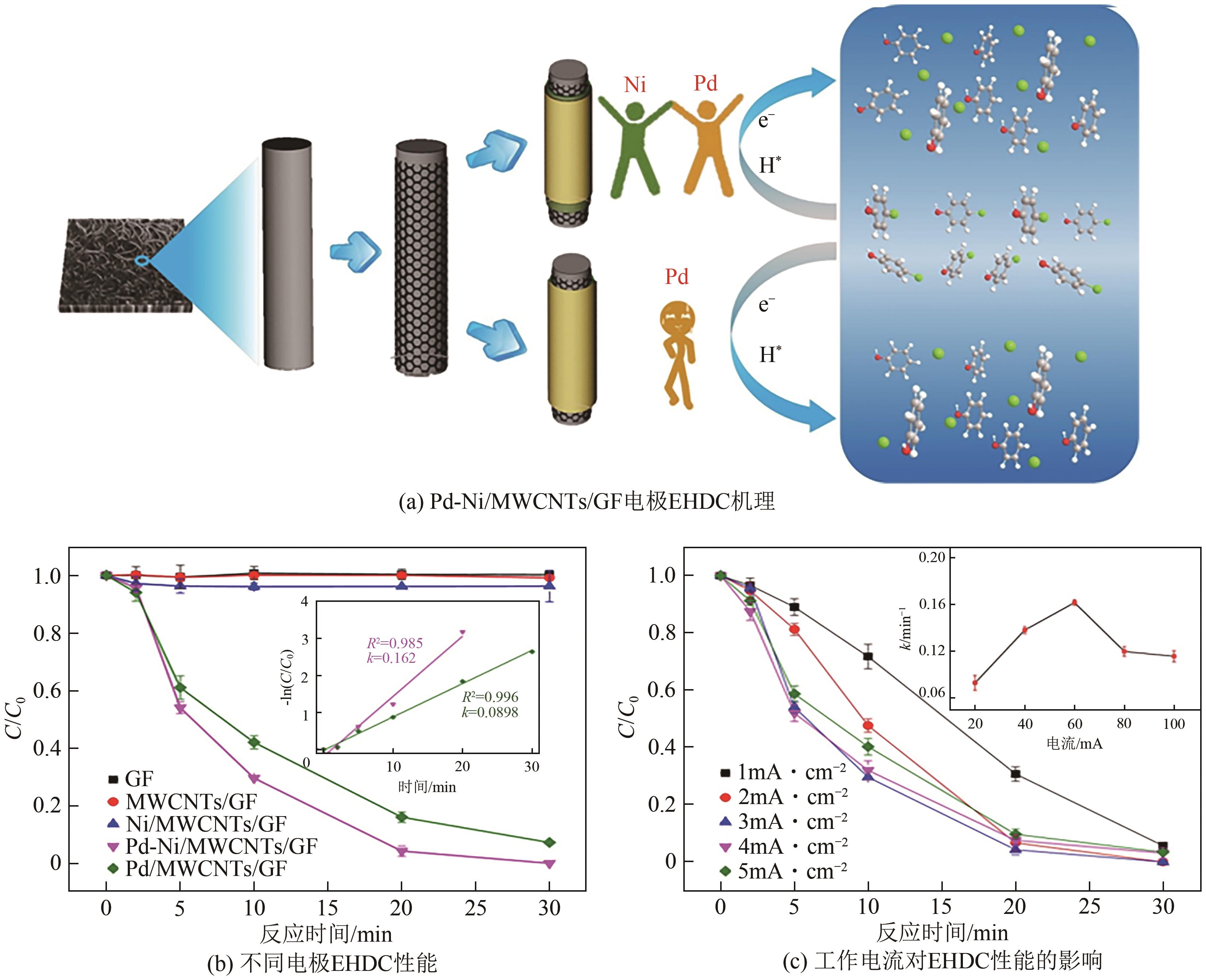
| 19 | WU Yifan, GAN Ling, ZHANG Shupeng, et al. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability[J]. Journal of Hazardous Materials, 2018, 356: 17-25. |
| 20 | WU Yifan, GAN Ling, ZHANG Shupeng, et al. Enhanced electrocatalytic dechlorination of para-chloronitrobenzene based on Ni/Pd foam electrode[J]. Chemical Engineering Journal, 2017, 316: 146-153. |
| 21 | LOU Zimo, XU Jiang, ZHOU Jiasheng, et al. Insight into atomic H* generation, H2 evolution, and cathode potential of MnO2 induced Pd/Ni foam cathode for electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2019, 374: 211-220. |
| 22 | YU Weiting, JIANG He, FANG Jinhui, et al. Designing an electron-deficient Pd/NiCo2O4 bifunctional electrocatalyst with an enhanced hydrodechlorination activity to reduce the consumption of Pd[J]. Environmental Science & Technology, 2021, 55(14): 10087-10096. |
| 23 | LI Junxi, CHEN Yanju, BAI Ruiyu, et al. Construction of Pd/Ni2P-Ni foam nanosheet array electrode by in situ phosphatization-electrodeposition strategy for synergistic electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2022, 435: 134932. |
| 24 | LOU Zimo, ZHOU Jiasheng, SUN Mei, et al. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs[J]. Chemical Engineering Journal, 2018, 352: 549-557. |
| 25 | CHEN Ge, WANG Zhenyao, XIA Dingguo. Electrochemically codeposited palladium/molybdenum oxide electrode for electrocatalytic reductive dechlorination of 4-chlorophenol[J]. Electrochemistry Communications, 2004, 6(3): 268-272. |
| 26 | LIAO Hanbin, WEI Chao, WANG Jingxian, et al. A multisite strategy for enhancing the hydrogen evolution reaction on a nano-Pd surface in alkaline media[J]. Advanced Energy Materials, 2017, 7(21): 1701129. |
| 27 | LUO Mingchuan, GUO Shaojun. Strain-controlled electrocatalysis on multimetallic nanomaterials[J]. Nature Reviews Materials, 2017, 2(11): 17059. |
| 28 | RATCLIFF Erin L, Clayton SHALLCROSS R, ARMSTRONG Neal R. Introduction: Electronic materials[J]. Chemical Reviews, 2016, 116(21): 12821-12822. |
| 29 | GAO Fei, ZHANG Yangping, SONG Pingping, et al. Self-template construction of Sub-24nmPd Ag hollow nanodendrites as highly efficient electrocatalysts for ethylene glycol oxidation[J]. Journal of Power Sources, 2019, 418: 186-192. |
| 30 | LI Angzhen, ZHAO Xu, HOU Yining, et al. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode[J]. Applied Catalysis B: Environmental, 2012, 111: 628-635. |
| 31 | CHEN Yali, XIONG Lu, SONG Xiangning, et al. Electrocatalytic hydrodehalogenation of atrazine in aqueous solution by Cu@Pd/Ti catalyst[J]. Chemosphere, 2015, 125: 57-63. |
| 32 | ESCOBEDO Ericson, KIM Jihun, Dasom OH, et al. Electrocatalytic dehalogenation of aqueous pollutants by dealloyed nanoporous Pd/Ti cathode[J]. Catalysis Today, 2021, 361: 63-68. |
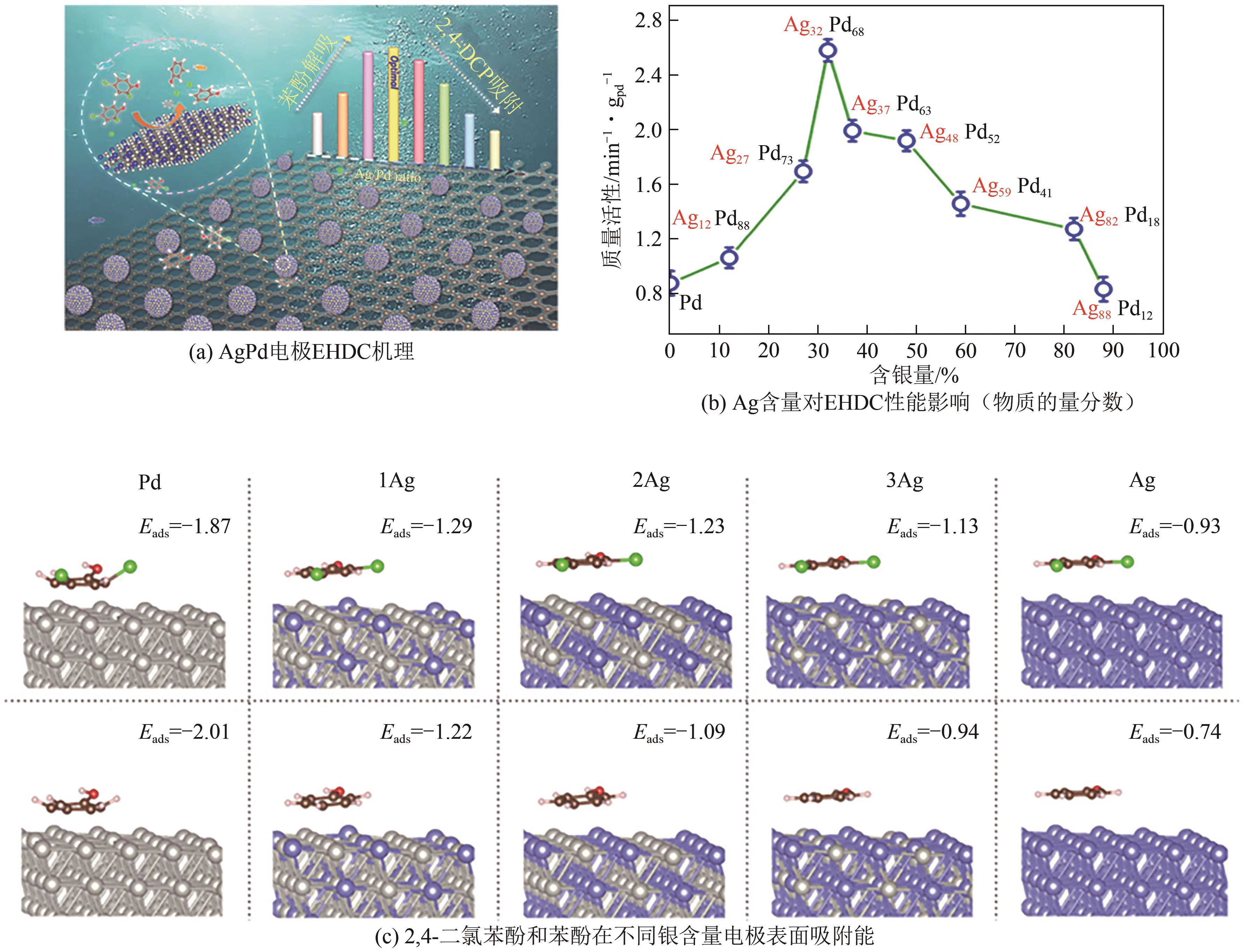
| 33 | PENG Yiyin, CUI Meiyang, ZHANG Zhiyong, et al. Bimetallic composition-promoted electrocatalytic hydrodechlorination reaction on silver-palladium alloy nanoparticles[J]. ACS Catalysis, 2019, 9(12): 10803-10811. |
| 34 | CHEN Yanju, FENG Chao, WANG Wenhong, et al. Electronic structure engineering of bimetallic Pd-Au alloy nanocatalysts for improving electrocatalytic hydrodechlorination performance[J]. Separation and Purification Technology, 2022, 289: 120731. |
| 35 | CHEN Yanju, LIU Zhi, LIU Shoujie, et al. In-situ doping-induced crystal form transition of amorphous Pd-P catalyst for robust electrocatalytic hydrodechlorination[J]. Applied Catalysis B: Environmental, 2021, 284: 119713. |
| 36 | WANG Kaifeng, SHU Song, CHEN Min, et al. Pd-TiO2 Schottky heterojunction catalyst boost the electrocatalytic hydrodechlorination reaction[J]. Chemical Engineering Journal, 2020, 381: 122673. |
| 37 | CHEN Min, SHU Song, LI Junxi, et al. Activating palladium nanoparticles via a Mott-Schottky heterojunction in electrocatalytic hydrodechlorination reaction[J]. Journal of Hazardous Materials, 2020, 389: 121876. |
| 38 | WANG Peng, SHI Xuelin, FU Chunhong, et al. Strong pyrrolic-N-Pd interactions boost the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2020, 12(2): 843-850. |
| 39 | LOU Zimo, YU Chaochao, WEN Xiaofei, et al. Construction of Pd nanoparticles/two-dimensional Co-MOF nanosheets heterojunction for enhanced electrocatalytic hydrodechlorination[J]. Applied Catalysis B: Environmental, 2022, 317: 121730. |
| 40 | FU Wenyang, SHU Song, LI Junxi, et al. Identifying the rate-determining step of the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2019, 11(34): 15892-15899. |
| 41 | FENG Jiyu, RAMACHANDRAN Ranjith K, SOLANO Eduardo, et al. Tuning size and coverage of Pd nanoparticles using atomic layer deposition[J]. Applied Surface Science, 2021, 539: 148238. |
| 42 | YUAN Qiuyi, DOAN Hieu A, GRABOW Lars C, et al. Finite size effects in submonolayer catalysts investigated by CO electrosorption on PtsML/Pd(100)[J]. Journal of the American Chemical Society, 2017, 139(39): 13676-13679. |
| 43 | ZHANG Zhiqiang, LU Jinsuo, ZHANG Bing, et al. Insight into the size effect of Pd nanoparticles on the catalytic reduction of nitrite in water over Pd/C catalysts[J]. Environmental Science: Nano, 2020, 7(7): 2117-2129. |
| 44 | HE Zhiqiao, JIAN Qiwei, TANG Juntao, et al. Improvement of electrochemical reductive dechlorination of 2,4-dichlorophenoxyacetic acid using palladium catalysts prepared by a pulsed electrodeposition method[J]. Electrochimica Acta, 2016, 222: 488-498. |
| 45 | SHU Xiaoyu, YANG Qi, YAO Fubing, et al. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes[J]. Chemical Engineering Journal, 2019, 358: 903-911. |
| 46 | MAO Mingyue, WU Jie, WANG Yi, et al. Active site and adsorption behavior engineering of subsize PdNi nanoparticles for boosting electrocatalytic hydrodechlorination of 4-chlorophenol[J]. Applied Surface Science, 2022, 600: 153988. |
| 47 | LEE Hyunjoo. Utilization of shape-controlled nanoparticles as catalysts with enhanced activity and selectivity[J]. RSC Advances, 2014, 4(77): 41017-41027. |
| 48 | WU Zhijie, PAN Tao, CHAI Yan, et al. Synthesis of palladium phosphides for aqueous phase hydrodechlorination: Kinetic study and deactivation resistance[J]. Journal of Catalysis, 2018, 366: 80-90. |
| 49 | QIN Shiyi, LEI Chao, WANG Xuxu, et al. Electrocatalytic activation of organic chlorides via direct and indirect electron transfer using atomic vacancy control of palladium-based catalyst[J]. Cell Reports Physical Science, 2022, 3(1): 100713. |
| 50 | LU Suwei, WENG Bo, CHEN Aizhu, et al. Facet engineering of Pd nanocrystals for enhancing photocatalytic hydrogenation: Modulation of the Schottky barrier height and enrichment of surface reactants[J]. ACS Applied Materials & Interfaces, 2021, 13(11): 13044-13054. |
| 51 | LOU Yaoyin, XIAO Chi, FANG Jiayi, et al. High activity of step sites on Pd nanocatalysts in electrocatalytic dechlorination[J]. Physical Chemistry Chemical Physics, 2022, 24(6): 3896-3904. |
| 52 | HE Zhen, DUAN Qiaohui, WANG Chengming, et al. Atom-stepped surface-regulated Pd nanowires for boosting alcohol oxidation activity[J]. Journal of Colloid and Interface Science, 2023, 646: 529-537. |
| 53 | WANG Minmin, ZHANG Hui, LIU Yunqi, et al. Research progress of precise structural regulation of single atom catalyst for accelerating electrocatalytic oxygen reduction reaction[J]. Journal of Energy Chemistry, 2022, 72: 56-72. |
| 54 | PAN Yuan, LI Min, MI Wanliang, et al. Single-atomic Mn sites coupled with Fe3C nanoparticles encapsulated in carbon matrixes derived from bimetallic Mn/Fe polyphthalocyanine conjugated polymer networks for accelerating electrocatalytic oxygen reduction[J]. Nano Research, 2022, 15(9): 7976-7985. |
| 55 | CHEN Zupeng, VOROBYEVA Evgeniya, MITCHELL Sharon, et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling[J]. Nature Nanotechnology, 2018, 13(8): 702-707. |
| 56 | PAN Yuan, ZHANG Chao, LIN Yan, et al. Electrocatalyst engineering and structure-activity relationship in hydrogen evolution reaction: From nanostructures to single atoms[J]. Science China Materials, 2020, 63(6): 921-948. |
| 57 | LI Min, ZHU Houyu, YUAN Qing, et al. Proximity electronic effect of Ni/Co diatomic sites for synergistic promotion of electrocatalytic oxygen reduction and hydrogen evolution[J]. Advanced Functional Materials, 2023, 33(4): 2210867. |
| 58 | HUANG Dahong, KIM David J, RIGBY Kali, et al. Elucidating the role of single-atom Pd for electrocatalytic hydrodechlorination[J]. Environmental Science & Technology, 2021, 55(19): 13306-13316. |
| 59 | MIN Yuan, ZHOU Xiao, CHEN Jiejie, et al. Integrating single-cobalt-site and electric field of boron nitride in dechlorination electrocatalysts by bioinspired design[J]. Nature Communications, 2021, 12(1): 303. |
| 60 | XU Yinghua, YAO Zeqing, MAO Zhechuan, et al. Single-Ni-atom catalyzes aqueous phase electrochemical reductive dechlorination reaction[J]. Applied Catalysis B: Environmental, 2020, 277: 119057. |
| 61 | CHU Chiheng, HUANG Dahong, GUPTA Srishti, et al. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis[J]. Nature Communications, 2021, 12(1): 5179. |
| 62 | LIU Zhijie, BETTERTON Eric A, ARNOLD Robert G. Electrolytic reduction of low molecular weight chlorinated aliphatic compounds: structural and thermodynamic effects on process kinetics[J]. Environmental Science & Technology, 2000, 34(5): 804-811. |
| 63 | DURANTE Christian, PERAZZOLO Valentina, PERINI Lorenzo, et al. Electrochemical activation of carbon-halogen bonds: Electrocatalysis at silver/copper nanoparticles[J]. Applied Catalysis B: Environmental, 2014, 158: 286-295. |
| 64 | GUO Yun, LI Yang, WANG Zhiwei. Electrocatalytic hydro-dehalogenation of halogenated organic pollutants from wastewater: A critical review[J]. Water Research, 2023, 234: 119810. |
| 65 | LI Zhouyan, LI Xuesong, LI Yang, et al. Efficient removal of micropollutants from low-conductance surface water using an electrochemical Janus ceramic membrane filtration system[J]. Water Research, 2022, 220: 118627. |
| 66 | SCIALDONE Onofrio, GUARISCO Chiara, GALIA Alessandro, et al. Electroreduction of aliphatic chlorides at silver cathodes in water[J]. Journal of Electroanalytical Chemistry, 2010, 641(1/2): 14-22. |
| 67 | GUO Kaiheng, WU Zihao, SHANG Chii, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
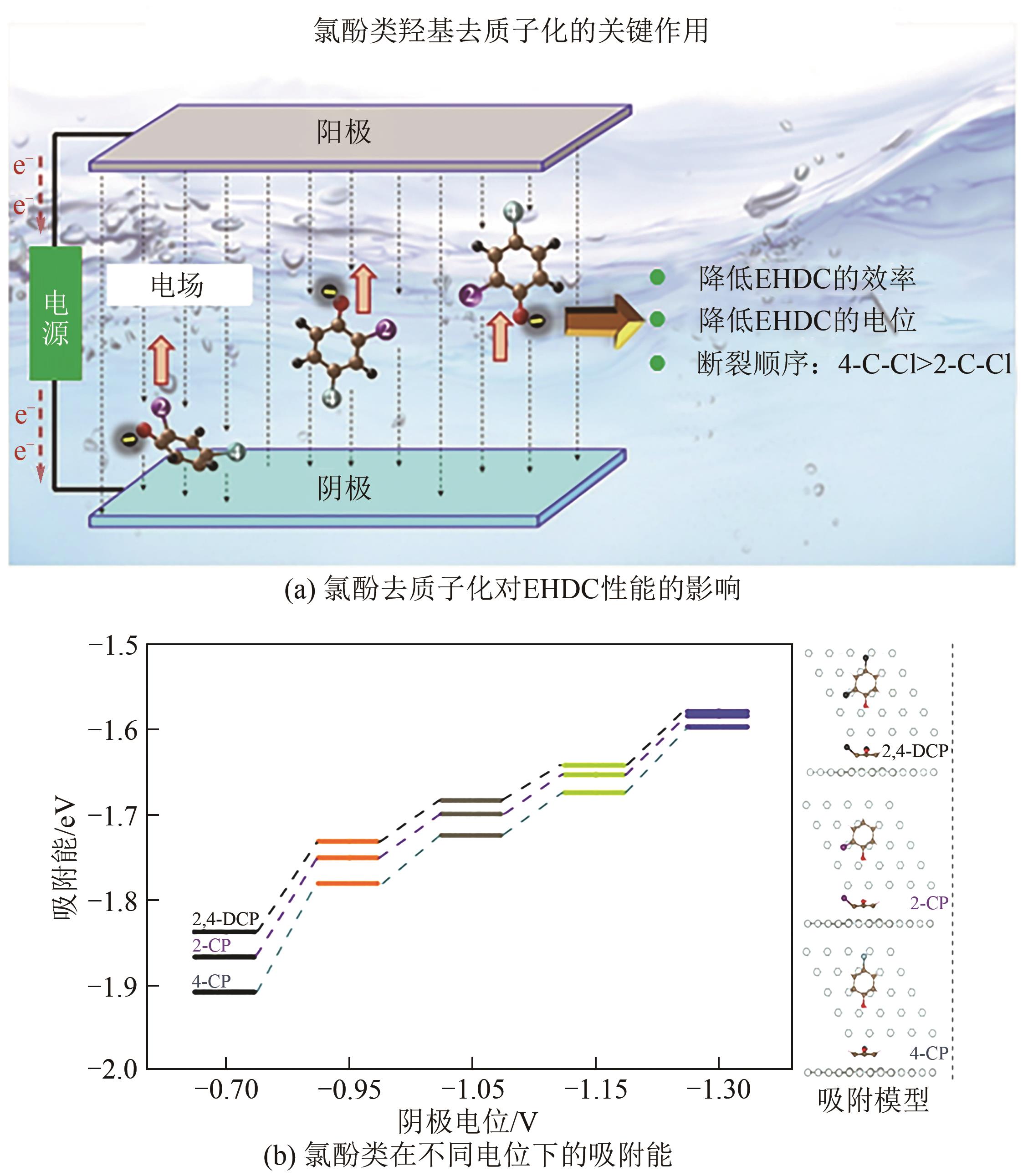
| 68 | SHU Song, FU Wenyang, WANG Peng, et al. Electrocatalytic hydrodechlorination of 2,4-dichlorophenol over palladium nanoparticles: The critical role of hydroxyl group deprotonation[J]. Applied Catalysis A: General, 2019, 583: 117146. |
| 69 | LI Junjing, WANG Huan, WANG Liang, et al. The preparation of Pd/foam-Ni electrode and its electrocatalytic hydrodechlorination for monochlorophenol isomers[J]. Catalysts, 2018, 8(9): 378. |
| 70 | HAN Jun, DEMING Richard L, TAO Fuming. Theoretical study of molecular structures and properties of the complete series of chlorophenols[J]. Journal of Physical Chemistry A, 2004, 108(38): 7736-7743. |
| 71 | LI Junjing, LIU Huiling, CHENG Xiuwen, et al. Stability of palladium-polypyrrole-foam nickel electrode and its electrocatalytic hydrodechlorination for dichlorophenol isomers[J]. Industrial & Engineering Chemistry Research, 2012, 51(48): 15557-15563. |
| 72 | CELIK Gokhan, AILAWAR Saurabh A, GUNDUZ Seval, et al. Aqueous-phase hydrodechlorination of trichloroethylene over Pd-based swellable organically modified silica: Catalyst deactivation due to sulfur species[J]. Industrial & Engineering Chemistry Research, 2019, 58(10): 4054-4064. |
| 73 | JIANG Cuishuang, YU Hongbin, WANG Xinhong, et al. Preparation of the palladium/polymeric pyrrole-multi-walled carbon nanotubes film/titanium electrode and its performance for the dechlorination of 4-chlorophenol[J]. International Journal of Electrochemical Science, 2017, 12(6): 5208-5219. |
| 74 | HUANG Binbin, LI Jing, CAO Xingkai, et al. Electrochemical reduction of p-chloronitrobenzene (p-CNB) at silver cathode in dimethylformamide[J]. Electrochimica Acta, 2019, 296: 980-988. |
| 75 | JIANG Guangming, WANG Kaifeng, LI Jieyuan, et al. Electrocatalytic hydrodechlorination of 2,4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution[J]. Chemical Engineering Journal, 2018, 348: 26-34. |
| 76 | SUN Zhirong, WEI Xuefeng, HAN Yanbo, et al. Complete dechlorination of 2,4-dichlorophenol in aqueous solution on palladium/polymeric pyrrole-cetyl trimethyl ammonium bromide/foam-nickel composite electrode[J]. Journal of Hazardous Materials, 2013, 244/245: 287-294. |
| 77 | SUN Zhirong, SHEN Haitao, WEI Xuefeng, et al. Electrocatalytic hydrogenolysis of chlorophenols in aqueous solution on Pd58Ni42 cathode modified with PPy and SDBS[J]. Chemical Engineering Journal, 2014, 241: 433-442. |
| 78 | LOU Zimo, LI Yizhou, ZHOU Jiasheng, et al. TiC doped palladium/nickel foam cathode for electrocatalytic hydrodechlorination of 2,4-DCBA: Enhanced electrical conductivity and reactive activity[J]. Journal of Hazardous Materials, 2019, 362: 148-159. |
| 79 | LIU Qiuxiang, SHEN Yanting, SONG Shuang, et al. Enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenoxyacetic acid by a Pd-Co3O4/Ni foam electrode[J]. RSC Advances, 2019, 9(21): 12124-12133. |
| 80 | SUN Chen, BAIG Shams ALI, LOU Zimo, et al. Electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions[J]. Applied Catalysis B: Environmental, 2014, 158/159: 38-47. |
| [1] | ZHU Hao, LIU Hanfei, GAO Yuan, HUANG Yiping, FEI Xiaocheng, HAN Weiqing. Effect of salt on electrocatalytic performance and mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 571-580. |
| [2] | LIN Meijie, MI Shuodong, BAO Cheng. Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 209-224. |
| [3] | MA Guixuan, XU Zitong, XIAO Zhihua, Ning Guoqing, WEI Qiang, XU Chunming. O,S co-doped carbon nanotube aqueous conductive additive assisted construction of high-performance graphite/SiO anode [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 443-456. |
| [4] | LIANG Hongcheng, ZHAO Dongni, QUAN Yin, LI Jingni, HU Xinyi. Influence of SEI film morphology and structure on the performance of lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5049-5062. |
| [5] | WU Jianyang, WANG Runa, CHEN Yao, SHEN Lanyao, YU Yongli, JIANG Ning, QIU Jingyi, ZHOU Henghui. Preparation process of high nickel cathode precursor for lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5079-5085. |
| [6] | LI Meixuan, CHENG Jianfeng, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Junlian, WANG Chunxia, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Synthesis and electrochemical mechanism of high voltage lithium nickel manganate cathode materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5086-5094. |
| [7] | SONG Zhanlong, TANG Tao, PAN Wei, ZHAO Xiqiang, SUN Jing, MAO Yanpeng, WANG Wenlong. Micro-nano bubbles enhance ozone oxidation and degradation of wastewater containing phenol [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4614-4623. |
| [8] | YANG Guang, JIANG Ruiting, ZHANG Yue, FU Zijian, LIU Wei. Application of vanadium pentoxide/carbon nanocomposites in supercapacitors [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3857-3871. |
| [9] | LUO Zhen, WANG Qingji, WANG Zhansheng, YANG Xueying, XIE Jiacai, WANG Hao. Strong oxidation coupled short range treatment of refining industry contaminated sites extraction water [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4155-4163. |
| [10] | LIU Mengfan, WANG Huawei, WANG Yanan, ZHANG Yanru, JIANG Xutong, SUN Yingjie. Efficiency and mechanism of Bio-FeMnCeO x activated PMS for degradation of tetracycline [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3492-3502. |
| [11] | WAN Chengfeng, LI Zhida, ZHANG Chunyue, LU Lu. Highly efficient electrocatalytic water splitting by MXene supported CoP nanorods [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3232-3239. |
| [12] | HUANG Peng, ZOU Ying, WANG Baohuan, WANG Xiaoyan, ZHAO Yong, LAING Xin, HU Di. Research progress of electrocatalysts towards electrocatalytic reduction reaction of carbon dioxide to syngas [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2760-2775. |
| [13] | ZHOU Anning, JIANG Yuhan, LIU Moxuan, ZHAO Wei, LI Zhen. Research progress in hydrogen production from electrolytic coal slurry: Effects of coal rank and minerals, and the evolution of coal structure [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2294-2310. |
| [14] | LI Si, TAO Yiyue, XIAO Zhenchong, ZHANG Liang, LI Jun, ZHU Xun, LIAO Qiang. Electrochemical characteristics of the coupled system of thermally regenerative battery stack and electrochemical CO2 reduction cell [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2568-2575. |
| [15] | FANG Yao, LIU Lei, GAO Zhihua, HUANG Wei, ZUO Zhijun. Advances in anode catalysts for photo-assisted direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2611-2628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||