化工进展 ›› 2021, Vol. 40 ›› Issue (S2): 89-100.DOI: 10.16085/j.issn.1000-6613.2021-0649
质子交换膜燃料电池高稳定性低铂载量膜电极的研究进展
李丹1( ), 张博雅1, 刘柏鸿1, 陶阳2, 熊子昂3, 侯三英1(
), 张博雅1, 刘柏鸿1, 陶阳2, 熊子昂3, 侯三英1( )
)
- 1.南华大学化学化工学院,湖南 衡阳 421001
2.南华大学资源环境与安全工程学院,湖南 衡阳 421001
3.中南大学粉末冶金研究院,湖南 长沙 410083
-
收稿日期:2021-03-30修回日期:2021-04-19出版日期:2021-11-12发布日期:2021-11-12 -
通讯作者:侯三英 -
作者简介:李丹(1997—),女,本科,研究方向为能源化工。E-mail:1009116307@qq.com 。 -
基金资助:湖南省自然科学基金青年项目(2020JJ5470);湖南省教育厅青年项目(18B275);国家级大学生创新创业训练项目(S202010555034)
Research progress on low platinum load and high stable membrane electrode assembly of proton exchange membrane fuel cell
LI Dan1( ), ZHANG Boya1, LIU Bohong1, TAO Yang2, XIONG Zi’ang3, HOU Sanying1(
), ZHANG Boya1, LIU Bohong1, TAO Yang2, XIONG Zi’ang3, HOU Sanying1( )
)
- 1.School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, Hunan, China
2.School of Resource Environment and Safety Engineering, University of South China, Hengyang 421001, Hunan, China
3.Powder Metallurgy Research Institute, Central South University, Changsha 410083, Hunan, China
-
Received:2021-03-30Revised:2021-04-19Online:2021-11-12Published:2021-11-12 -
Contact:HOU Sanying
摘要:
质子交换膜燃料电池由于高能量转化率、零污染、低温启动等优点在新能源领域备受关注,但其成本和耐久性仍是本领域的挑战性课题。本文首先回顾了近年来国内外研究者在降低燃料电池成本和提高其耐久性方面取得的成就,从催化剂制备技术、膜电极结构优化、耐久性提升三个方面介绍了近年来国内外在降低膜电极铂载量、提高膜电极功率密度和耐久性方面的发展趋势,通过构筑铂基合金、核壳结构和纳米结构等催化剂能有效地降低铂载量,从而降低燃料电池成本;通过构筑多孔结构催化层或气体扩散层可以改善膜电极的微结构,从而提高电池的功率密度;通过开发新型质子交换膜、更换催化剂载体等方法可以提高膜电极的耐久性。最后,本文针对目前研究进展阐述提高膜电极稳定性仍然是目前的研究难题,并对未来的研究方向进行了展望。
中图分类号:
引用本文
李丹, 张博雅, 刘柏鸿, 陶阳, 熊子昂, 侯三英. 质子交换膜燃料电池高稳定性低铂载量膜电极的研究进展[J]. 化工进展, 2021, 40(S2): 89-100.
LI Dan, ZHANG Boya, LIU Bohong, TAO Yang, XIONG Zi’ang, HOU Sanying. Research progress on low platinum load and high stable membrane electrode assembly of proton exchange membrane fuel cell[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 89-100.
| 1 | WHISTON M M, AZEVEDO I L, LITSTER S, et al.Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles[J]. PNAS, 2019, 116: 4899-4904. |
| 2 | ADE N, WILHITE B, GOYETTE H, et al. Intensifying vehicular proton exchange membrane fuel cells for safer and durable, design and operation[J]. International Journal of Hydrogen Energy, 2020, 45: 5039-5054. |
| 3 | ABDELKAREEM M A, ELSAID K, WILBERFORCE T, et al. Environmental aspects of fuel cells: a review[J]. Science of the Total Environment, 2021, 141803: 752-767. |
| 4 | KONGKANAND A, MATHIAS M F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells[J]. Journal of Physical Chemistry Letters, 2016, 7: 1127-1137. |
| 5 | OEZASLAN M, HASCHE F, STRASSER P. Pt-based core-shell catalyst architectures for oxygen fuel cell electrodes[J]. Journal of Physical Chemistry Letters, 2013, 4: 3273-3291. |
| 6 | 沈丹丹, 林瑞.质子交换膜燃料电池铂基合金催化剂研究进展[J]. 世界有色金属, 2018(21): 215. |
| SHEN D D, LIN R. Research progress of platinum based alloy catalysts for proton exchange membrane fuel cells[J]. World Nonferrous Metals, 2018(21): 215. | |
| 7 | LI W, CHEN Z, XU L, et al. A solution-phase synthesis method to highly active Pt-Co/C electrocatalysts for proton exchange membrane fuel cell[J]. Journal of Power Source, 2010, 195: 2534-2540. |
| 8 | GUO S, LI D, ZHU H, et al. FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2013, 125: 3549-3552. |
| 9 | FIDIANI E, THIRUNAVUKKARASU G, LI Y, et al. Ultrathin AgPt alloy nanorods as low-cost oxygen reduction reaction electrocatalysts in proton exchange membrane fuel cells[J]. Journal of Materials Chemistry A, 2020, 8: 11874-11883. |
| 10 | KUTTIYIEL K A, SASAKI K, DONG S, et al. Pt monolayer on Au-stabilized PdNi core-shell nanoparticles for oxygen reduction reaction[J]. Electrochimica Acta, 2013, 110: 267-272. |
| 11 | HUANG X, ZHAO Z, CAO L, et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction[J]. Science, 2015, 348: 1230-1234. |
| 12 | KIM I G, NAH I W, OH I, et al. Crumpled rGO-supported Pt-Ir bifunctional catalyst prepared by spray pyrolysis for unitized regenerative fuel cells[J]. Power Sources, 2017, 364: 215-225. |
| 13 | TIAN X, ZHAO X, SU Y Q, et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells[J]. Science, 2019, 366: 850-856. |
| 14 | ZHOU M, WANG H, ELNABAWY A O, et al. Facile one-pot synthesis of Pd@Pt 1L octahedra with enhanced activity and durability toward oxygen reduction[J]. Chemistry of Materials, 2019, 31: 1370-1380. |
| 15 | KHATEEB S, GUERREO S, SU D, et al. Fuel cell performance of palladium-platinum core-shell electrocatalysts synthesized in gram-scale batches[J]. The Electrochemical Society, 2016, 163: F708-F713. |
| 16 | LI S, LIU J W, ZHU G L, et al. Pd@Pt core-shell nanodots arrays for efficient electrocatalytic oxygen reduction[J]. ACS Applied Nano Materials, 2019, 2: 3695-3700. |
| 17 | JACKSON A, STRICKLER A, HIGGINS D, et al. Engineering Ru@Pt core-shell catalysts for enhanced electrochemical oxygen reduction mass activity and stability[J]. Nanomaterials, 2018, 8: 38-52. |
| 18 | ALAINA L S, ARIEL J, THOMAS F J. Active and stable Ir@Pt core-shell catalysts for electrochemical oxygen reduction[J]. ACS Energy Letters, 2017, 2: 244-249. |
| 19 | DAI Y, CHEN S. Oxygen reduction electrocatalyst of Pt on Au nanoparticles through spontaneous deposition[J]. ACS Applied Materials & Interfaces, 2015, 7: 823-829. |
| 20 | PARK H Y, PARK J H, KIM P, et al. Hollow PdCu2@Pt core@shell nanoparticles with ordered intermetallic cores as efficient and durable oxygen reduction reaction electrocatalysts[J]. Applied Catalysis B: Environmental, 2018, 225: 84-90. |
| 21 | NAN H, TIAN X, LUO J, et al. A core-shell Pd1Ru1Ni2@Pt/C catalyst with a ternary alloy core and Pt monolayer: enhanced activity and stability towards the oxygen reduction reaction by the addition of Ni[J]. Journal of Materials Chemistry A, 2016, 4: 847-855. |
| 22 | NAN H, SU Y Q, TANG C, et al. Engineering the electronic and strained interface for high activity of PdMcore@Ptmonolayer electrocatalysts for oxygen reduction reaction[J]. Science Bulletin, 2020, 65: 1396-1404. |
| 23 | TIAN X, TANG H, LUO J, et al. High-performance core-shell catalyst with nitride nanoparticles as a core: well-defined titanium copper nitride coated with an atomic Pt layer for the oxygen reduction reaction[J]. ACS Catalysis, 2017, 7: 3810-3817. |
| 24 | HUNT S T, MILINA M, ALBA-RUBIO A C, et al. Self-assembly of noble metal monolayerson transition metal carbide nanoparticle catalysts[J]. Science, 2016, 352: 974-978. |
| 25 | BU L, GUO S, ZHANG X, et al. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis[J]. Nature Communications, 2016, 7: 11850. |
| 26 | LI M F, ZHAO Z P, CHENG T, et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction[J]. Science, 2016, 354: 1414-1419. |
| 27 | LIU L F, PIPPEL E. Low-platinum-content quaternary PtCuCoNi nanotubes with markedly enhanced oxygen reduction activity[J]. Angewandte Chemie International Edition, 2011, 50: 2729-2733. |
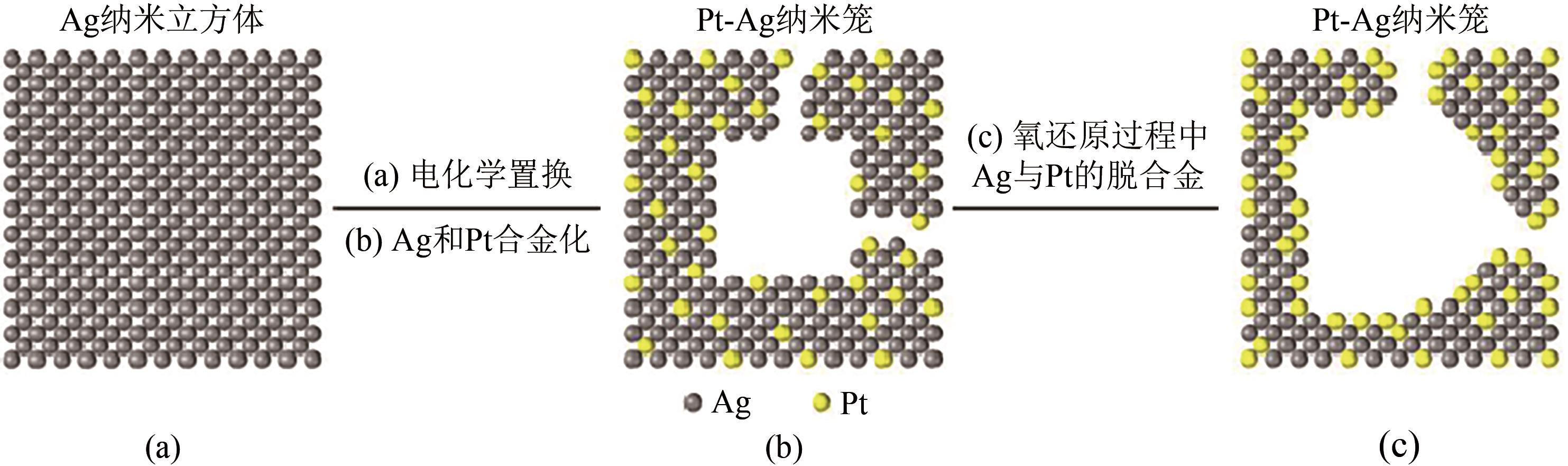
| 28 | YANG X, ROLING L T, VARA M, et al. Synthesis and characterization of Pt-Ag alloy nanocages with enhanced activity and durability toward oxygen reduction[J]. Nano Letters, 2016, 16: 6644-6649. |
| 29 | XU Y, ZHANG B. Recent advances in porous Pt-based nanostructures: synthesis and electrochemical applications recent advances in porous Pt-based nanostructures: synthesis and electrochemical applications[J]. Chemical Society Reviews, 2014, 43: 2439-2450. |
| 30 | SHUI J L, CHEN C, LI J C M. Evolution of nanoporous Pt-Fe alloy nanowires by dealloying and their catalytic property for oxygen reduction reaction[J]. Advanced Functional Materials, 2011, 21: 3357-3362. |
| 31 | LIU W, RODRIGUEZ P, BORCHARDT L, et al. Bimetallic aerogels: high-performance electrocatalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2013, 52: 9849-9852. |
| 32 | HITOTSUYANAGI A, NAKAMURA M, HOSHI N. Structural effects on the activity for the oxygen reduction reaction on n(111)-(100) series of Pt: correlation with the oxide film formation[J]. Electrochimica Acta, 2012, 82: 512-516. |
| 33 | HE D S, HE D, WANG J, et al. Ultrathin icosahedral Pt-enriched nanocage with excellent oxygen reduction reaction activity[J]. Journal of the American Chemical Society, 2016, 138: 1494-1497. |
| 34 | ZAMEL N. The catalyst layer and its dimensionality a look into its ingredients and how to characterize their effects[J]. Power Sources, 2016, 309: 141-159. |
| 35 | WEI Z, SU K H, SUI S, et al. High performance polymer electrolyte membrane fuel cells (PEMFCs) with gradient Pt nanowire cathodes prepared by decal transfer method[J]. Hydrogen Energy, 2015, 40: 3068-3074. |
| 36 | QIU Y, ZHANG H, ZHONG H, et al. A novel cathode structure with double catalyst layers and low Pt loading for proton exchange membrane fuel cells[J]. Hydrogen Energy, 2013, 38: 5836-5844. |
| 37 | XIONG Z, LIAO S, DANG D, et al.Enhanced water management in the cathode of an air-breathing PEMFC using a dual catalyst layer and optimizing the gas diffusion and microporous layers[J]. Hydrogen Energy, 2015, 40: 3961-3967. |
| 38 | CHI B, YE Y, LU X, et al. Enhancing membrane electrode assembly performance by improving the porous structure and hydrophobicity of the cathode catalyst layer[J]. Power Sources, 2019, 443: 227-284. |
| 39 | GOKCE S A, BERKER F, INCI E. Influence of FEP nanoparticles in catalyst layer on water management and performance of PEM fuel cell with high Pt loading[J]. Hydrogen Energy, 2016, 42: 496-506. |
| 40 | LI A, HAN M, CHAN S H, et al. Effects of hydrophobicity of the cathode catalyst layer on the performance of a PEM fuel cell[J]. Electrochim. Acta, 2010, 55: 2706-2711. |
| 41 | CHOUN M, NAURYZBAYEV D, SHIN D, et al. Polydimethylsiloxane treated cathode catalyst layer to prolong hydrogen fuel cell lifetime[J]. Catalysis Today, 2016, 262: 155-160. |
| 42 | CHI B, HOU S, LIU G, et al. Tuning hydrophobic-hydrophilic balance of cathode catalyst layer to improve cell performance of proton exchange membrane fuel cell (PEMFC) by mixing polytetrafluoroethylene (PTFE)[J]. Electrochimica Acta, 2018, 277: 110-115. |
| 43 | DOGAN D C, CHO S, HWANG S M, et al. Highly durable supportless Pt hollow spheres designed for enhanced oxygen transport in cathode catalyst layers of proton exchange membrane fuel cells[J]. ACS Applied Materials & Interfaces, 2016, 8: 27730-27739. |
| 44 | LEE M, UCHIDA M, TRYK D A. The effectiveness of platinum/carbon electrocatalysts: dependence on catalyst layer thickness and Pt alloy catalytic effects[J]. Electrochimica Acta, 2011, 56: 4783-4790. |
| 45 | PARK Y C, TOKIWA H, KAKINUMA K, et al. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells[J]. Power Sources, 2015, 315: 179-191. |
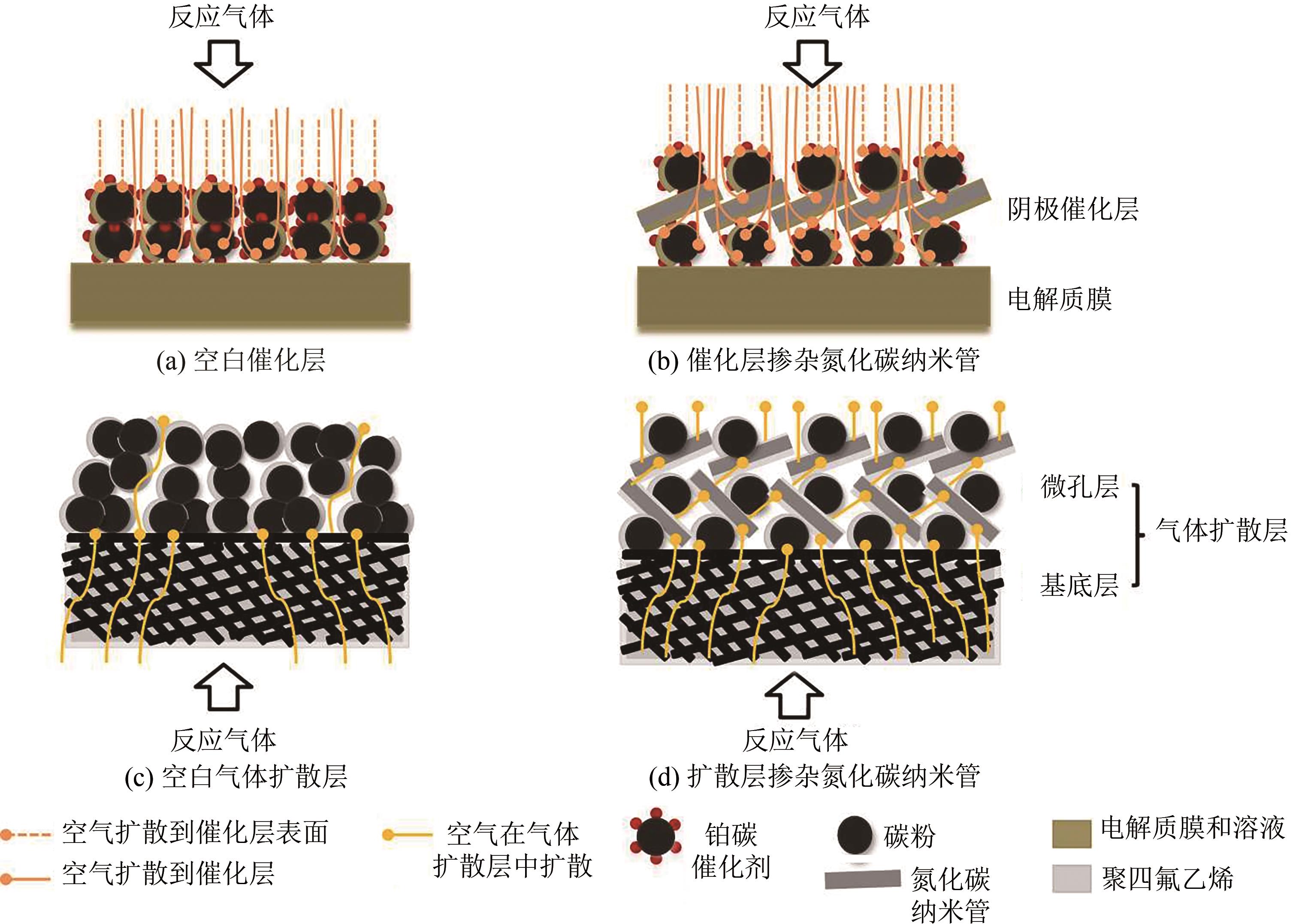
| 46 | HOU S, CHI B, LIU G, et al. Enhanced performance of proton exchange membrane fuel cell by introducing nitrogen-doped CNTs in both catalyst layer and gas diffusion layer[J]. Electrochimica Acta, 2017, 253: 142-150. |
| 47 | YAN W M, WU D K, WANG X D, et al. Optimal microporous layer for proton exchange membrane fuel cell[J]. Power Sources, 2010, 195: 5731-5734. |
| 48 | SCHWEISS R, STEEB M, WILDE P M, et al. Enhancement of proton exchange membrane fuel cell performance by doping microporous layers of gas diffusion layers with multiwall carbon nanotubes[J]. Power Sources, 2012, 220: 79-83. |
| 49 | JUNG G B, TZENG W J, JAO T C. Investigation of porous carbon and carbon nanotube layer for proton exchange membrane fuel cells[J]. Applied Energy, 2013, 101: 457-464. |
| 50 | THOMAS Y R J, BENAYAD A, SCHRODER M, et al. New method for super hydrophobic treatment of gas diffusion layers for proton exchange membrane fuel cells using electrochemical reduction of diazonium salts[J]. ACS Applied Materials & Interfaces, 2015, 7: 15068-15707. |
| 51 | KITAHARA T, NAKAJIMA H, INAMOTO M. Novel hydrophilic and hydrophobic double microporous layer coated gas diffusion layer to enhance performance of polymer electrolyte fuel cells under both low and high humidity[J]. Power Sources, 2013, 234: 129-138. |
| 52 | OZTÜRK A, BERKER F, EROGLU I, et al. Facilitation of water management in low Pt loaded PEM fuel cell by creating hydrophobic microporous layer with PTFE, FEP and PDMS polymers: effect of polymer and carbon amounts[J]. Hydrogen Energy, 2017, 42: 21226-21249. |
| 53 | WANG Y, WANG L, ADVANI S G, et al. Double-layer gas diffusion media for improved water management in polymer electrolyte membrane fuel cells[J]. Power Sources, 2015, 292: 39-48. |
| 54 | ADNAN O, SAMANEH S, JIAN Z, et al. Assessment of graphene as an alternative microporous layer material for proton exchange membrane fuel cells[J]. Fuel, 2018, 215: 726-734. |
| 55 | MARGLE P, JI X, WU J, et al. Thin film electrodes from Pt nanorods supported on aligned N-CNTs for proton exchange membrane fuel cells[J]. Applied Catalysis B: Environmental, 2020, 260: 118031. |
| 56 | CHOI H, KIM O H, KIM M, et al. Next-generation polymer-electrolyte-membrane fuel cells using titanium foam as gas diffusion Layer[J]. ACS Applied Materials & Interfaces, 2014, 6(10): 7665-7671. |
| 57 | KINUMOTO T, MASTSUMURA T, YAMAGUCHI K, et al. Material processing of bamboo for use as a gas diffusion layer in proton exchange membrane fuel cells[J]. ACS Sustainable Chemistry & Engineering, 2015, 3: 1374-1380. |
| 58 | JOSEPH D, BUSSELMANN J, HARMS C, et al. Porous Nafion membranes[J]. Journal of Membrane Science, 2016, 520: 723-730. |
| 59 | XU G, LI J, MA L, et al. Performance dependence of swelling-filling treated Nafion membrane on nano-structure of macromolecular filler[J]. Journal of Membrane Science, 2017, 534: 68-72. |
| 60 | LI J, TANG H, WANG Z, et al. Microstructure evolution of Nafion/silica membrane under humidity conditions[J]. Power Sources, 2013, 234: 333-339. |
| 61 | XU G, WEI Z, LI S, et al. In-situ sulfonation of targeted silica-filled Nafion for high-temperature PEM fuel cell application[J]. Hydrogen Energy, 2019, 44: 29711-29716. |
| 62 | IBRAHIM A, HOSSAIN O, CHAGGAR J, et al. GO-nafion composite membrane development for enabling intermediate temperature operation of polymer electrolyte fuel cell[J]. Hydrogen Energy, 2020, 45: 5526-5534. |
| 63 | YIN C, LI J, ZHOU Y, et al. Enhancement in proton conductivity and thermal stability in Nafion membranes induced by incorporation of sulfonated carbon nanotubes [J]. ACS Applied Materials & Interfaces, 2018, 10: 14026. |
| 64 | JANG S, KANG Y S, ChOI J W, et al. Prism patterned TiO2 layers/Nafion® composite membrane for elevated temperature/low relative humidity fuel cell operation[J]. Journal of Industrial and Engineering Chemistry, 2020, 90: 327-332. |
| 65 | 马双春, 鲁伊恒, 陈颖, 等.杂多酸对燃料电池质子交换膜稳定性的影响[J].电源技术, 2015, 39(3): 484. |
| MA S C, LU Y H, CHEN Y, et al. Effect of heteropoly acid on the stability of proton exchange membrane for fuel cells[J]. Chinese Journal of Power Sources, 2015, 39(3): 484-487. | |
| 66 | 王景涛, 张浩勤, 和亚昆, 等.一种纳米复合质子交换膜及其制备方法和应用: CN103715438A[P]. 2014-09-30. |
| WANG J T, ZHANG H Q,HE Y K,et al. The producing method and application of a nano-composite proton exchange membrane: CN103715438A[P]. 2014-09-30. | |
| 67 | 王禛, 侯明, 姜永燚, 等. 磺化聚醚醚酮/Nafion复合质子交换膜的制备及性能[J].电源技术, 2018, 42(1): 50-54. |
| WANG Z, HOU M, JIANG Y Y, et al. The preparation and performance of sulfonated polyetherketone composite proton exchange membrane[J]. Chinese Journal of Power Sources, 2018, 42(1): 50-54. | |
| 68 | LIU J M, YU L W, CAI X K, et al. Sandwiching h-BN monolayer films between sulfonated poly(ether ether ketone) and Nafion for proton exchange membranes with improved ion selectivity[J]. ACS Nano, 2019, 13: 2094-2102. |
| 69 | MIYAKE J, TAKI R, MOCHIZUKI T, et al. Design of flexible polyphenylene proton-conducting membrane for next-generation fuel cells[J]. Science Advances, 2017, 3: eaao0476. |
| 70 | LEE K H, CHU J Y, KIM A R, et al. Enhanced performance of a sulfonated poly(arylene ether ketone) block copolymer bearing pendant sulfonic acid groups for polymer electrolyte membrane fuel cells operating at 80% relative humidity[J]. ACS Applied Materials & Interfaces, 2018, 10: 20835-20844. |
| 71 | KUN S, DONG D X, RYSZARD W, et al. Synthesis and characterization of poly(para-phenylene disulfonic acid), its copolymers and their n-alkylbenzene grafts as proton exchange membranes: high conductivity at low relative humidity[J]. Journal of Materials Chemistry, 2012, 22: 20907-20917. |
| 72 | 陶丹.高支化侧链型磺化聚芳醚砜质子交换膜的制备与性能研究[D]. 深圳: 深圳大学, 2015. |
| TAO D. The preparation and properties of sulfonated poly(aryl ether sulfone) proton exchange membrane with high branched side chain[D]. Shenzhen: Shenzhen University, 2015. | |
| 73 | YAOWARAT W, LI O L H, SAITO N. Highly durable silica-coated Pt/carbon nanotubes for proton-exchange membrane fuel cells application[J]. Japanese Journal of Applied Physics, 2016, 55: 1-5. |
| 74 | OH E J, HEMPELMANN R, NICA V, et al. New catalyst supports prepared by surface modification of graphene- and carbon nanotube structures with nitrogen containing carbon coatings [J]. Power Sources, 2017, 341: 240-249. |
| 75 | LI L, CHEN S G, WEI Z D, et al. Experimental and DFT study of thiol-stabilized Pt/CNTs catalysts[J]. Physical Chemistry Chemical Physics, 2012, 14: 16581-16587. |
| 76 | GAO W, ZHANG Z, DOU M, et al. Highly dispersed and crystalline Ta2O5 anchored Pt electrocatalyst with improved activity and durability towards oxygen reduction: promotion by atomic-scale Pt-Ta2O5 interactions[J]. ACS Catalysis, 2019, 9: 3278. |
| 77 | MEENAKSHI S G, RAMAPRABHU S. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for proton exchange membrane fuel cell[J]. Hydrogen Energy, 2019, 44: 10951. |
| 78 | 刘硕, 高远, 马婕, 等.质子交换膜燃料电池用碳气凝胶载铂催化电极[J].南京工业大学学报, 2016, 38(2): 60-63. |
| LIU S, GAO Y, MA J, et al. Carbon aerogel platinum-loaded catalytic electrode for proton exchange membrane fuel cells[J]. Journal of Nanjing Tech University, 2016, 38(2): 60-63. | |
| 79 | ALEGRE C, SEBASTIAN D, GALVEZ M E, et al. Sulfurized carbon xerogels as Pt support with enhanced activity for fuel cell applications[J]. Applied Catalysis B: Environmental, 2016, 192: 260. |
| 80 | 朱米. 燃料电池低铂催化剂结构优化及新型催化剂载体设计[D]. 西安: 西安理工大学, 2020. |
| ZHU M. Stucture optimization of low platinum catalyst for fuel cell and design of new catalyst support[D]. Xi’an: Xi’an University of Technology, 2020. | |
| 81 | NAEEM R, AHMED R, ANSARI M S. TiO2 and Al2O3 promoted Pt/C nanocomposites as low temperature fuel cell catalysts for electro oxidation of methanol in acidic media[J]. IOP Conference Series: Materials Science and Engineering, 2014, 60: 1-8. |
| 82 | ESFAHANI R A M, GAVIDIA L M R, GARCIA G, et al. Highly active platinum supported on Mo-doped titanium nanotubes suboxide (Pt/TNTS-Mo) electrocatalyst for oxygen reduction reaction in PEMFC[J]. Renewable Energy, 2018, 120: 209-219. |
| 83 | CHENG H, SANKARASUBRAMANIAN S, MATANOVIC I, et al. Understanding the oxygen reduction reaction activity and oxidative stability of Pt supported on Nb‐doped TiO2[J]. ChemSusChem, 2019, 12: 3468-3480. |
| 84 | CHIKUNOVA L O, SEMEYKINA V S, KUZNETSOV A N, et al. Template-assisted synthesis and electrochemical properties of SnO2 as a cathode catalyst support for PEMFC[J]. Ionics, 2020, 26: 1861-1873. |
| 85 | LIU Y, MUSTAIN W E. High stability, high activity Pt/ITO oxygen reduction electrocatalysts [J]. Journal of the American Chemical Society, 2013, 135: 530-533. |
| 86 | OZOUF G, COGNARD G, MAILLARD F, et al. Sb-doped SnO2 aerogels based catalysts for proton exchange membrane fuel cells: Pt deposition routes [J]. Journal of the Electrochemical Society, 2018, 165: F3036-F3044. |
| 87 | MUHAMMAD T A, YAN X H, MUHAMMAD R A, et al. MoS2-rGO hybrid architecture as durable support for cathode catalyst in proton exchange membrane fuel cells[J]. Chinese Journal of Catalysis,2019, 40(8): 1160-1167. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [11] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [12] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||