化工进展 ›› 2021, Vol. 40 ›› Issue (S2): 380-388.DOI: 10.16085/j.issn.1000-6613.2021-0988
高级氧化技术处理1,4-二 烷污染研究进展
烷污染研究进展
张轩1,2( ), 宋小三1, 赵珀1, 董元华2,3, 刘云2,3(
), 宋小三1, 赵珀1, 董元华2,3, 刘云2,3( )
)
- 1.兰州交通大学环境与市政工程学院, 甘肃 兰州 730070
2.中国科学院南京土壤研究所土壤环境与污染修复重点实验室, 江苏 南京 210008
3.中国科学院大学,北京 100049
-
收稿日期:2021-05-10修回日期:2021-05-13出版日期:2021-11-12发布日期:2021-11-12 -
通讯作者:刘云 -
作者简介:张轩(1997—),男,硕士研究生,研究方向为水处理技术。E-mail:2584653665@qq.com 。 -
基金资助:国家自然科学基金(41877504)
A critical review of advanced oxidation technology to treat 1,4-dioxane pollution
ZHANG Xuan1,2( ), SONG Xiaosan1, ZHAO Po1, DONG Yuanhua2,3, LIU Yun2,3(
), SONG Xiaosan1, ZHAO Po1, DONG Yuanhua2,3, LIU Yun2,3( )
)
- 1.School of Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou 730070, Gansu, China
2.CAS Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, Jiangsu, China
3.University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2021-05-10Revised:2021-05-13Online:2021-11-12Published:2021-11-12 -
Contact:LIU Yun
摘要:
随着工业的发展,各种新兴有机污染物不断出现,1,4-二 烷作为其中之一,广泛存在于地表水和氯代烃污染的地下水中。由于其稳定的杂环结构及高水溶性,常规的水处理技术很难完全去除1,4-二
烷作为其中之一,广泛存在于地表水和氯代烃污染的地下水中。由于其稳定的杂环结构及高水溶性,常规的水处理技术很难完全去除1,4-二 烷,高级氧化技术利用?OH、
烷,高级氧化技术利用?OH、 烷。本文首先阐述了1,4-二
烷。本文首先阐述了1,4-二 烷的污染现状,重点概述了近年来国内外1,4-二
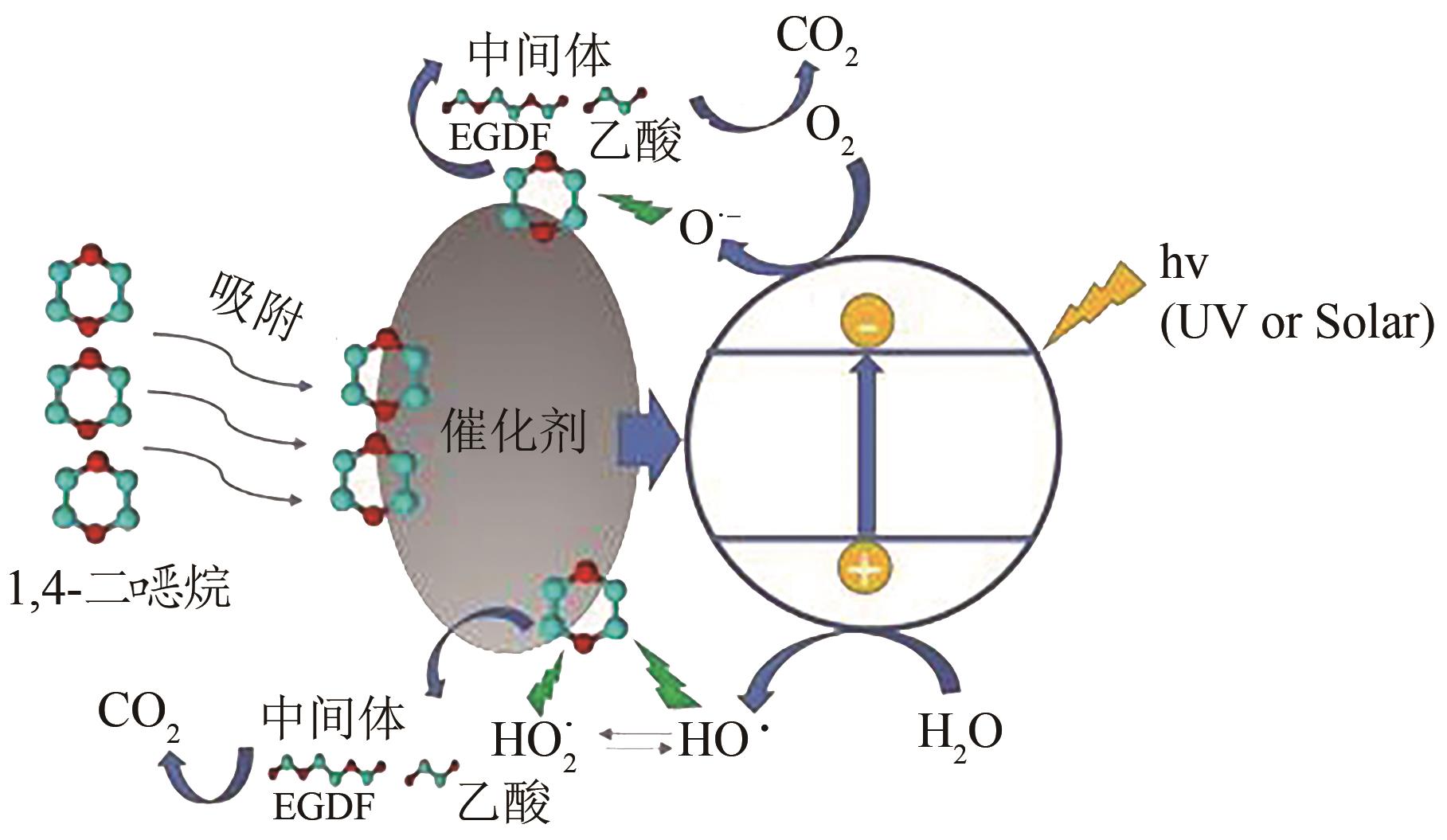
烷的污染现状,重点概述了近年来国内外1,4-二 烷降解的高级氧化技术,主要包括光催化氧化法、电化学氧化法和活化过硫酸盐氧化法等,深入分析了这些高级氧化技术对1,4-二
烷降解的高级氧化技术,主要包括光催化氧化法、电化学氧化法和活化过硫酸盐氧化法等,深入分析了这些高级氧化技术对1,4-二 烷的降解机理,并总结了这些技术用于处理1,4-二
烷的降解机理,并总结了这些技术用于处理1,4-二 烷的降解效果及影响因素,最后提出了高级氧化技术处理1,4-二
烷的降解效果及影响因素,最后提出了高级氧化技术处理1,4-二 烷目前存在的问题及未来的研究和发展方向,以推动1,4-二
烷目前存在的问题及未来的研究和发展方向,以推动1,4-二 烷污染处理技术的发展,为实际污染水体的治理奠定理论基础。
烷污染处理技术的发展,为实际污染水体的治理奠定理论基础。
中图分类号:
引用本文
张轩, 宋小三, 赵珀, 董元华, 刘云. 高级氧化技术处理1,4-二 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388.
烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388.
ZHANG Xuan, SONG Xiaosan, ZHAO Po, DONG Yuanhua, LIU Yun. A critical review of advanced oxidation technology to treat 1,4-dioxane pollution[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 380-388.
| 项目 | 数值 |
|---|---|
| 分子式 | C4H8O2 |
| 水溶性/mg·L-1 | 4.31×105 |
| 分子量 | 88.11 |
| 亨利系数/atm·m3·mol-1 | 4.88×10-6 |
| 辛醇-水分配系数 | 0.27 |
| 沸点/℃ | 101.3 |
表1 1,4-DX理化性质
| 项目 | 数值 |
|---|---|
| 分子式 | C4H8O2 |
| 水溶性/mg·L-1 | 4.31×105 |
| 分子量 | 88.11 |
| 亨利系数/atm·m3·mol-1 | 4.88×10-6 |
| 辛醇-水分配系数 | 0.27 |
| 沸点/℃ | 101.3 |
| 氧化剂种类 | 氧化电位/eV | 相对强度(氯=1) |
|---|---|---|
| ·OH | 2.7 | 2 |
| 2.6 | 1.8 | |
| O3 | 2.2 | 1.5 |
| 2.1 | 1.5 | |
| H2O2 | 1.8 | 1.3 |
| 1.7 | 1.2 | |
| Cl- | 1.4 | 1 |
| O2 | 1.2 | 0.9 |
表2 常见氧化剂氧化电位[1]
| 氧化剂种类 | 氧化电位/eV | 相对强度(氯=1) |
|---|---|---|
| ·OH | 2.7 | 2 |
| 2.6 | 1.8 | |
| O3 | 2.2 | 1.5 |
| 2.1 | 1.5 | |
| H2O2 | 1.8 | 1.3 |
| 1.7 | 1.2 | |
| Cl- | 1.4 | 1 |
| O2 | 1.2 | 0.9 |
| 氧化技术 | 处理条件 | 影响因素 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| UV/H2O2 | [DX]0=100μg·L-1 H2O2=10mg·L-1 UV=550MJ·cm-2 | 天然有机物(NOM)pH、硝酸盐、铁 | pH为5~7之间,1,4-DX在3.5min内降解率达到90%。高浓度硝酸盐使去除率降低约25% | [ |
| 真空紫外(VUV) | [DX]0=100μg·L-1 H2O2=1~5mg·L-1 光催化剂由非晶态Si02纤维制成的无纺布片,表面层为TiO2 | 紫外线、催化剂、pH | 1,4-DX的分解速率: | [ |
| 氯氨/UV/H2O2 | [DX]0=250μmol·L-1 pH=5.8NH2Cl=50mmol·L-1 NHCl2=25mmol·L-1 UV=3500MJ·cm-2 | NH2Cl、NHCl2、 | 当氯氨处于低剂量时,NH2Cl比NHCl2对DX去除率高约60%~80%,NH2Cl为2mmol·L-1时降解速率最大 | [ |
| UV/H2O2、UV/氯氨UV/游离氯 | [DX]0=15μg·L-1 低/高剂量:Cl2=2.7mg·L-1/6.8mg·L-1 NH2Cl=1.3mg·L-1/4.6mg·L-1 H2O2=3.1mg·L-1/6.2mg·L-1 | 催化剂种类 | DX去除效率:UV> UV/H2O2> UV/氯氨;达到90%以上,是传统UV/H2O2理想的代替技术 | [ |
| UV/O3 | [DX]0=150mg·L-1 [O3]0=36.7mg·L-1 pH=6~8 | pH、O3、催化剂 | 1,4-DX被完全降解 | [ |
| UV/TiO | UV=0.58W·L-1 [DX]0=850μg·L-1 催化剂:5g·L-1 | 膜制备过程、污染程度、催化剂浓度、紫外线强度 | 1,4-DX被完全降解,通过定期反冲洗保持膜的渗透性良好 | [ |
表3 紫外光氧化技术降解1,4-DX
| 氧化技术 | 处理条件 | 影响因素 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| UV/H2O2 | [DX]0=100μg·L-1 H2O2=10mg·L-1 UV=550MJ·cm-2 | 天然有机物(NOM)pH、硝酸盐、铁 | pH为5~7之间,1,4-DX在3.5min内降解率达到90%。高浓度硝酸盐使去除率降低约25% | [ |
| 真空紫外(VUV) | [DX]0=100μg·L-1 H2O2=1~5mg·L-1 光催化剂由非晶态Si02纤维制成的无纺布片,表面层为TiO2 | 紫外线、催化剂、pH | 1,4-DX的分解速率: | [ |
| 氯氨/UV/H2O2 | [DX]0=250μmol·L-1 pH=5.8NH2Cl=50mmol·L-1 NHCl2=25mmol·L-1 UV=3500MJ·cm-2 | NH2Cl、NHCl2、 | 当氯氨处于低剂量时,NH2Cl比NHCl2对DX去除率高约60%~80%,NH2Cl为2mmol·L-1时降解速率最大 | [ |
| UV/H2O2、UV/氯氨UV/游离氯 | [DX]0=15μg·L-1 低/高剂量:Cl2=2.7mg·L-1/6.8mg·L-1 NH2Cl=1.3mg·L-1/4.6mg·L-1 H2O2=3.1mg·L-1/6.2mg·L-1 | 催化剂种类 | DX去除效率:UV> UV/H2O2> UV/氯氨;达到90%以上,是传统UV/H2O2理想的代替技术 | [ |
| UV/O3 | [DX]0=150mg·L-1 [O3]0=36.7mg·L-1 pH=6~8 | pH、O3、催化剂 | 1,4-DX被完全降解 | [ |
| UV/TiO | UV=0.58W·L-1 [DX]0=850μg·L-1 催化剂:5g·L-1 | 膜制备过程、污染程度、催化剂浓度、紫外线强度 | 1,4-DX被完全降解,通过定期反冲洗保持膜的渗透性良好 | [ |
| 1 | ADAMSON D T, ANDERSON R H, MAHENDRA S, et al.Evidence of 1,4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1,4-dioxane[J]. Environmental Science & Technology, 2015, 49(11): 6510-6518. |
| 2 | International Agency for Research on Cancer (IARC).Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide[M]. Lyon, France: 1999: 589-602. |
| 3 | TANABE A, TSUCHIDA Y, IBARAKI T, et al. Impact of 1,4-dioxane from domestic effluent on the agano and Shinano Rivers, Japan[J]. Bulletin of Environmental Contamination & Toxicology, 2006, 76(1):44. |
| 4 | FENG Y, LI H L, LIN L, et al. Degradation of 1, 4-dioxane via controlled generation of radicals by pyrite-activated oxidants: synergistic effects, role of disulfides, and activation sites[J]. Chemical Engineering Journal, 2018, 336: 416-426. |
| 5 | ADAMSON D T, MAHENDRA S, WALKER K L, et al. A multisite survey to identify the scale of the 1, 4-dioxane problem at contaminated groundwater sites[J]. Environmental Science & Technology Letters, 2014, 1(5): 254-258. |
| 6 | ANDERSON R H, ANDERSON J K, BOWER P A. Co-occurrence of 1, 4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: fact or fiction[J]. Integrated Environmental Assessment and Management, 2012, 8(4): 731-737. |
| 7 | KARGES U, BECKER J, PÜTTMANN W. 1, 4-Dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume[J]. Science of the Total Environment, 2018, 619/620: 712-720. |
| 8 | GODRI POLLITT K J, KIM J H, PECCIA J, et al. 1, 4-Dioxane as an emerging water contaminant: state of the science and evaluation of research needs[J]. Science of the Total Environment, 2019, 690: 853-866. |
| 9 | CARRERA G, VEGUÉ L, VENTURA F, et al. Dioxanes and dioxolanes in source waters: occurrence, odor thresholds and behavior through upgraded conventional and advanced processes in a drinking water treatment plant[J]. Water Research, 2019, 156: 404-413. |
| 10 | TAHARA M, OBAMA T, IKARASHI Y. Development of analytical method for determination of 1, 4-dioxane in cleansing products[J]. International Journal of Cosmetic Science, 2013, 35(6): 575-580. |
| 11 | TANABE A, KAWATA K. Determination of 1,4-dioxane in household detergents and cleaners[J]. Journal of AOAC International, 2008, 91(2): 439-444. |
| 12 | BLACK R E, HURLEY F J, HAVERY D C. Occurrence of 1, 4-dioxane in cosmetic raw materials and finished cosmetic products[J]. Journal of AOAC International, 2001, 84(3): 666-670. |
| 13 | GUO W Q, BRODOWSKY H. Determination of the trace 1,4-dioxane[J]. Microchemical Journal, 2000, 64(2): 173-179. |
| 14 | GROSTERN A, SALES C M, ZHUANG W Q, et al. Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by Pseudonocardia dioxanivorans strain CB1190[J]. Applied and Environmental Microbiology, 2012, 78(9): 3298-3308. |
| 15 | DOURSON M L, HIGGINBOTHAM J, CRUM J, et al. Update: mode of action (MOA) for liver tumors induced by oral exposure to 1,4-dioxane[J]. Regulatory Toxicology and Pharmacology, 2017, 88: 45-55. |
| 16 | GI M, FUJIOKA M, KAKEHASHI A, et al.In vivo positive mutagenicity of 1,4-dioxane and quantitative analysis of its mutagenicity and carcinogenicity in rats[J]. Archives of Toxicology, 2018, 92(10): 3207-3221. |
| 17 | GÖEN T, HELDEN F, ECKERT E, et al. Metabolism and toxicokinetics of 1,4-dioxane in humans after inhalational exposure at rest and under physical stress[J]. Archives of Toxicology, 2016, 90(6): 1315-1324. |
| 18 | NOMURA Y, FUKAHORI S, FUJIWARA T. Removal of 1, 4-dioxane from landfill leachate by a rotating advanced oxidation contactor equipped with activated carbon/TiO2 composite sheets[J]. Journal of Hazardous Materials, 2020, 383: 121005. |
| 19 | LAFRANCONI M, BUDINSKY R, COREY L, et al. A 90-day drinking water study in mice to characterize early events in the cancer mode of action of 1, 4-dioxane[J]. Regulatory Toxicology and Pharmacology, 2021, 119: 104819. |
| 20 | DIGUISEPPI W, WALECKA-HUTCHISON C, HATTON J. 1,4-dioxane treatment technologies[J]. Remediation Journal, 2016, 27(1): 71-92. |
| 21 | PATTON S, LI W, COUCH K D, et al. Impact of the ultraviolet photolysis of monochloramine on 1,4-dioxane removal: new insights into potable water reuse[J]. Environmental Science & Technology Letters, 2017, 4(1): 26-30. |
| 22 | LI W, PATTON S, GLEASON J M, et al. UV photolysis of chloramine and persulfate for 1,4-dioxane removal in reverse-osmosis permeate for potable water reuse[J]. Environmental Science & Technology, 2018, 52(11): 6417-6425. |
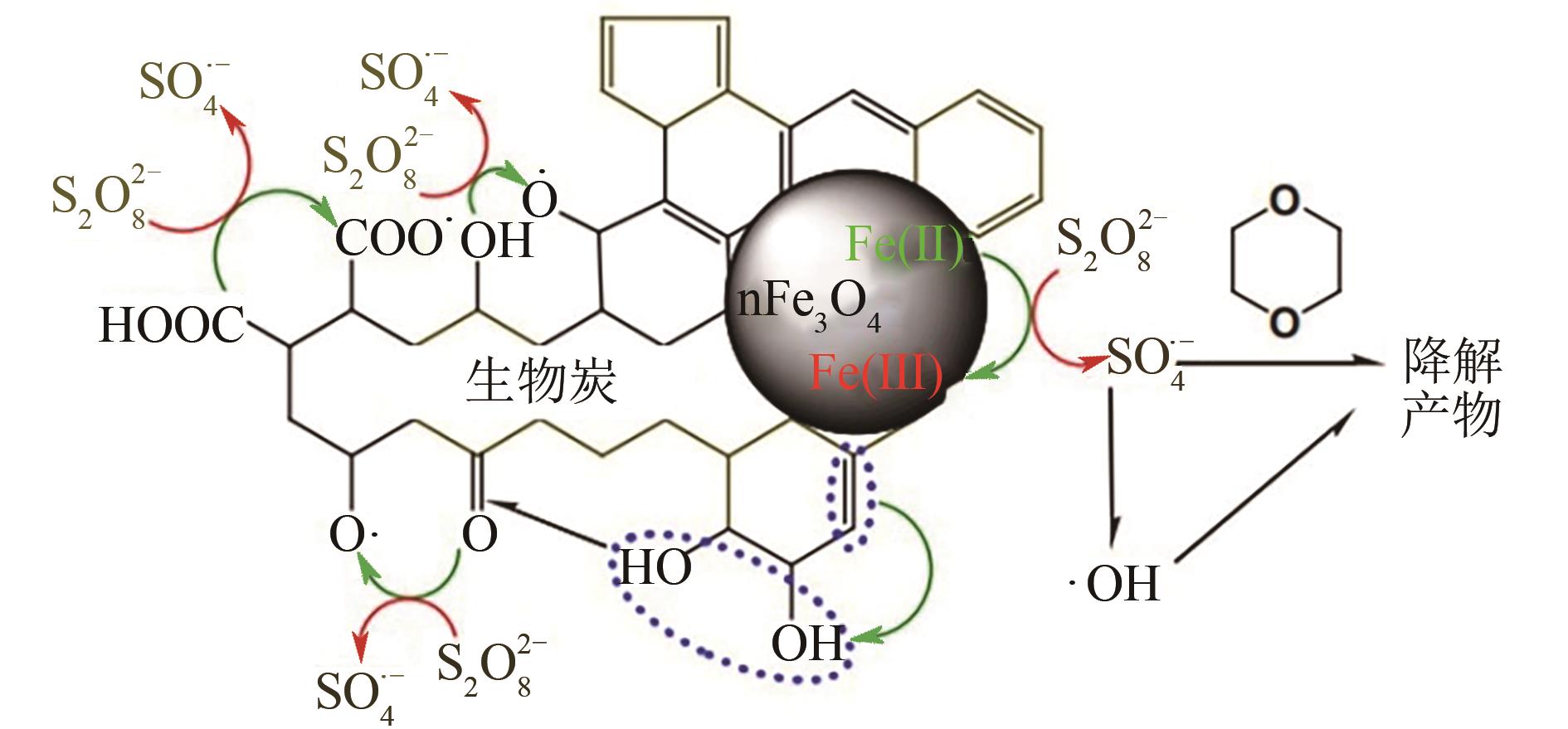
| 23 | OUYANG D, YAN J C, QIAN L B, et al. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate[J]. Chemosphere, 2017, 184: 609-617. |
| 24 | BLOTEVOGEL J, PIJLS C, SCHEFFER B, et al. Pilot-scale electrochemical treatment of a 1,4-dioxane source zone[J]. Groundwater Monitoring & Remediation, 2019, 39(1): 36-42. |
| 25 | DIGUISEPPI W, WALECKA-HUTCHISON C, HATTON J. 1,4‐Dioxane treatment technologies[J]. Remediation Journal, 2016, 27(1):71-92. |
| 26 | PATTON S, ROMANO M, NADDEO V, et al. Photolysis of mono- and dichloramines in UV/hydrogen peroxide: effects on 1,4-dioxane removal and relevance in water reuse[J]. Environmental Science & Technology, 2018, 52(20): 11720-11727. |
| 27 | BARNDÕK H, BLANCO L, HERMOSILLA D, et al. Heterogeneous photo-Fenton processes using zero valent iron microspheres for the treatment of wastewaters contaminated with 1,4-dioxane[J]. Chemical Engineering Journal, 2016, 284: 112-121. |
| 28 | MERAYO N, HERMOSILLA D, CORTIJO L, et al. Optimization of the Fenton treatment of 1,4-dioxane and on-line FTIR monitoring of the reaction[J]. Journal of Hazardous Materials, 2014, 268: 102-109. |
| 29 | XU X Y, LIU S M, SMITH K, et al. Light-driven breakdown of 1,4-dioxane for potable reuse: a review[J]. Chemical Engineering Journal, 2019, 373: 508-518. |
| 30 | MAZIERSKI P, MIKOLAJCZYK A, BAJOROWICZ B, et al. The role of lanthanides in TiO2-based photocatalysis: a review[J]. Applied Catalysis B: Environmental, 2018, 233: 301-317. |
| 31 | MEHRVAR M, ANDERSON W A, MOO-YOUNG M. Photocatalytic degradation of aqueous tetrahydrofuran, 1,4-dioxane, and their mixture with TiO2[J]. International Journal of Photoenergy, 2000, 2: 67-80. |
| 32 | 赵振悦. 可见光催化-臭氧催化氧化联用降解酚类污染物[D]. 武汉: 武汉科技大学, 2020. |
| ZHAO Zhenyue. Visible-light-driven photocatalytic ozonation for degradation of phenolic pollutants[D]. Wuhan: Wuhan University of Science and Technology, 2020. | |
| 33 | PINO E, ENCINAS M V. Photocatalytic degradation of chlorophenols on TiO2-325mesh and TiO2-P25. an extended kinetic study of photodegradation under competitive conditions[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2012, 242: 20-27. |
| 34 | FRIEDMANN D, MENDIVE C, BAHNEMANN D. TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis[J]. Applied Catalysis B: Environmental, 2010, 99(3/4): 398-406. |
| 35 | LEE C S, VENKATESAN A K, WALKER H W, et al. Impact of groundwater quality and associated byproduct formation during UV/hydrogen peroxide treatment of 1,4-dioxane[J]. Water Research, 2020, 173: 115534. |
| 36 | MATSUSHITA T, HIRAI S, ISHIKAWA T, et al. Decomposition of 1,4-dioxane by vacuum ultraviolet irradiation: study of economic feasibility and by-product formation[J]. Process Safety and Environmental Protection, 2015, 94: 528-541. |
| 37 | ZHANG Z, CHUANG Y H, SZCZUKA A, et al. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation process treatment for potable reuse[J]. Water Research, 2019, 164: 114939. |
| 38 | TAKAHASHI N, HIBINO T, TORII H, et al. Evaluation of O3/UV and O3/H2O2 as practical advanced oxidation processes for degradation of 1,4-dioxane[J]. Ozone: Science & Engineering, 2013, 35(5): 331-337. |
| 39 | LEE K C, BEAK H J, CHOO K H. Membrane photoreactor treatment of 1,4-dioxane-containing textile wastewater effluent: performance, modeling, and fouling control[J]. Water Research, 2015, 86: 58-65. |
| 40 | ZHAO L, HOU H, FUJII A, et al. Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation[J]. Environmental Science and Pollution Research, 2014, 21(12): 7457-7465. |
| 41 | ANTONIOU M G, ANDERSEN H R. Comparison of UVC/S2O82- with UVC/H2O2 in terms of efficiency and cost for the removal of micropollutants from groundwater[J]. Chemosphere, 2015, 119: S81-S88. |
| 42 | MISTURA C M, SCHNEIDER I, VIEIRA Y. Heterogeneous photocatalytic degradation of dyes in water/alcohol solution used by the brazilian agate industry[J]. Geomaterials, 2019, 9(1):29-39. |
| 43 | PARK I S, CHUNG K H, KIM S C, et al. Photocatalytic degradation of 1,4-dioxane and hydrogen production using liquid phase plasma on N- and Ni- codoped TiO2 photocatalyst[J]. Materials Letters, 2021, 283: 128751. |
| 44 | PARK Y K, CHUNG K H, PARK I S, et al. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts[J]. Journal of Hazardous Materials, 2020, 399: 123087. |
| 45 | MUESES M A, MACHUCA-MARTINEZ F, LI PUMA G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors[J]. Chemical Engineering Journal, 2013, 215/216: 937-947. |
| 46 | MIN B K, HEO J E, YOUN N K, et al. Tuning of the photocatalytic 1,4-dioxane degradation with surface plasmon resonance of gold nanoparticles on titania[J]. Catalysis Communications, 2009, 10(5): 712-715. |
| 47 | YOUN N K, HEO J E, JOO O S, et al. The effect of dissolved oxygen on the 1,4-dioxane degradation with TiO2 and Au-TiO2 photocatalysts[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 216-221. |
| 48 | BANIĆ N, ABRAMOVIĆ B, KRSTIĆ J, et al. Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo-Fenton catalyst[J]. Applied Catalysis B: Environmental, 2011, 107(3/4): 363-371. |
| 49 | BARNDÕK H, HERMOSILLA D, HAN C, et al. Degradation of 1,4-dioxane from industrial wastewater by solar photocatalysis using immobilized NF-TiO2 composite with monodisperse TiO2 nanoparticles[J]. Applied Catalysis B: Environmental, 2016, 180: 44-52. |
| 50 | MAMEDA N, PARK H J, CHOO K H. Membrane electro-oxidizer: a new hybrid membrane system with electrochemical oxidation for enhanced organics and fouling control[J]. Water Research, 2017, 126: 40-49. |
| 51 | TIWARI D, JAMSHEERA A, ZIRLIANNGURA, et al. Use of hybrid materials in the trace determination of As(V) from aqueous solutions: an electrochemical study[J]. Environmental Engineering Research, 2017, 22(2): 186-192. |
| 52 | SCIALDONE O, PROIETTO F, GALIA A. Electrochemical production and use of chlorinated oxidants for the treatment of wastewater contaminated by organic pollutants and disinfection[J]. Current Opinion in Electrochemistry, 2021, 27: 100682. |
| 53 | BRILLAS E. Recent development of electrochemical advanced oxidation of herbicides.A review on its application to wastewater treatment and soil remediation[J]. Journal of Cleaner Production, 2021, 290: 125841. |
| 54 | LI X Y, CUI Y H, FENG Y J, et al. Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes[J]. Water Research, 2005, 39(10): 1972-1981. |
| 55 | ZHAO H Z, SUN Y, XU L N, et al. Removal of acid orange 7 in simulated wastewater using a three-dimensional electrode reactor: removal mechanisms and dye degradation pathway[J]. Chemosphere, 2010, 78(1): 46-51. |
| 56 | ZHANG C, JIANG Y H, LI Y L, et al. Three-dimensional electrochemical process for wastewater treatment: a general review[J]. Chemical Engineering Journal, 2013, 228: 455-467. |
| 57 | 曲久辉, 刘会娟. 水处理电化学原理与技术[M].北京: 科学出版社, 2007: 205-212. |
| QU Jiuhui, LIU Huijuan. Principles and technology of water treatment electrochemistry [M]. Beijing: Science Press, 2007: 205-212. | |
| 58 | CHOI J Y, LEE Y J, SHIN J, et al. Anodic oxidation of 1, 4-dioxane on boron-doped diamond electrodes for wastewater treatment[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 762-768. |
| 59 | DE CLERCQ J, DE STEENE E VAN, VERBEKEN K, et al. Electrochemical oxidation of 1,4-dioxane at boron-doped diamond electrode[J]. Journal of Chemical Technology & Biotechnology, 2010, 85(8): 1162-1167. |
| 60 | JASMANN J R, BORCH T, SALE T C, et al. Advanced electrochemical oxidation of 1, 4-dioxane via dark catalysis by novel titanium dioxide (TiO2) pellets[J]. Environmental Science & Technology, 2016, 50(16): 8817-8826. |
| 61 | PARK H, MAMEDA N, CHOO K H. Catalytic metal oxide nanopowder composite Ti mesh for electrochemical oxidation of 1, 4-dioxane and dyes[J]. Chemical Engineering Journal, 2018, 345: 233-241. |
| 62 | MAMEDA N, PARK H, SHAH S S A, et al. Highly robust and efficient Ti-based Sb-SnO2 anode with a mixed carbon and nitrogen interlayer for electrochemical 1,4-dioxane removal from water[J]. Chemical Engineering Journal, 2020, 393: 124794. |
| 63 | NAKAGAWA H, TAKAGI S, MAEKAWA J. Fered-Fenton process for the degradation of 1, 4-dioxane with an activated carbon electrode: a kinetic model including active radicals[J]. Chemical Engineering Journal, 2016, 296: 398-405. |
| 64 | ADELAJA O, KESHAVARZ T, KYAZZE G. Treatment of phenanthrene and benzene using microbial fuel cells operated continuously for possible in situ and ex situ applications[J]. International Biodeterioration & Biodegradation, 2017, 116: 91-103. |
| 65 | ZHOU L, DENG D D, ZHANG D, et al. Microbial electricity generation and isolation of exoelectrogenic bacteria based on petroleum hydrocarbon-contaminated soil[J]. Electroanalysis, 2016, 28(7): 1510-1516. |
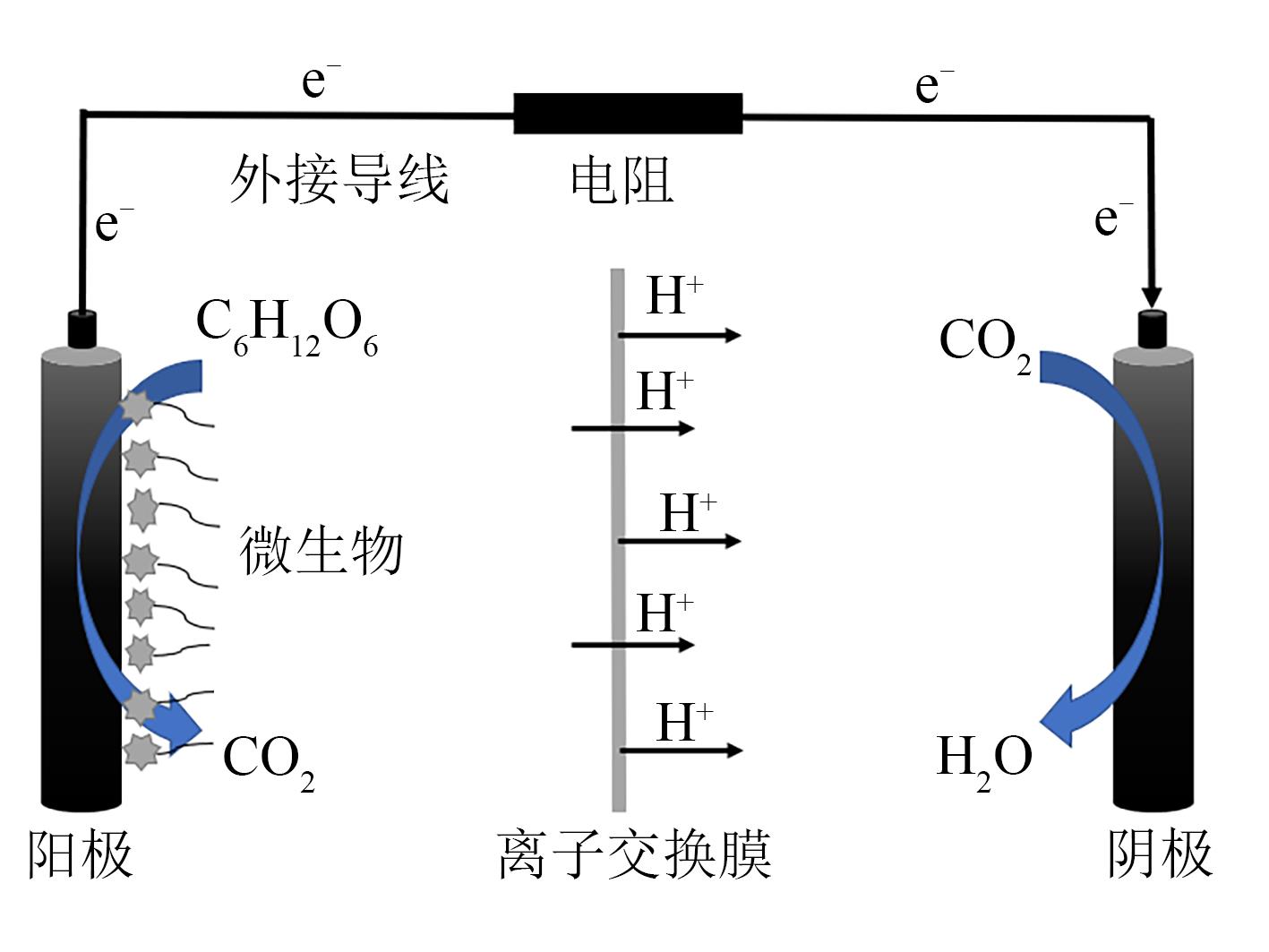
| 66 | ARYAL R, XIA C J, LIU J. 1, 4-Dioxane-contaminated groundwater remediation in the anode chamber of a microbial fuel cell[J]. Water Environment Research, 2019, 91(11): 1537-1545. |
| 67 | JASMANN J R, GEDALANGA P B, BORCH T, et al. Synergistic treatment of mixed 1, 4-dioxane and chlorinated solvent contaminations by coupling electrochemical oxidation with aerobic biodegradation[J]. Environmental Science & Technology, 2017, 51(21): 12619-12629. |
| 68 | KISHIMOTO N, NAKAGAWA T, ASANO M, et al. Ozonation combined with electrolysis of 1, 4-dioxane using a two-compartment electrolytic flow cell with solid electrolyte[J]. Water Research, 2008, 42(1/2): 379-385. |
| 69 | YANG C. Enhanced PMS activation property of Cu decorated MnO catalyst for antibiotic degradation[J]. Functional Materials Letters, 2021, 14(1): 2150003. |
| 70 | KISHIMOTO N, YASUDA Y, MIZUTANI H, et al. Applicability of ozonation combined with electrolysis to 1, 4-dioxane removal from wastewater containing radical scavengers[J]. Ozone: Science & Engineering, 2007, 29(1): 13-22. |
| 71 | LUTZE H V, BIRCHER S, RAPP I, et al. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter[J]. Environmental Science & Technology, 2015, 49(3): 1673-1680. |
| 72 | 田婷婷, 李朝阳, 王召东, 等. 过渡金属活化过硫酸盐降解有机废水技术研究进展[J]. 化工进展, 2021, 40(6): 3480-3488. |
| TIAN Tingting, LI Chaoyang, WANG Zhaodong, et al. Research progress in the degradation of organic wastewater by persulfate activated by transition metals[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3480-3488. | |
| 73 | WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 74 | GHAUCH A, TUQAN A M, KIBBI N. Ibuprofen removal by heated persulfate in aqueous solution: a kinetics study[J]. Chemical Engineering Journal, 2012, 197: 483-492. |
| 75 | FURMAN O S, TEEL A L, WATTS R J. Mechanism of base activation of persulfate[J]. Environmental Science & Technology, 2010, 44(16): 6423-6428. |
| 76 | YAN N, LIU F, LIU B Y, et al. Treatment of 1, 4-dioxane and trichloroethene co-contamination by an activated binary persulfate-peroxide oxidation process[J]. Environmental Science and Pollution Research, 2018, 25(32): 32088-32095. |
| 77 | ZHONG H, BRUSSEAU M L, WANG Y K, et al. In-situ activation of persulfate by iron filings and degradation of 1, 4-dioxane[J]. Water Research, 2015, 83: 104-111. |
| 78 | BRIDGES L, MOHAMED R A M, KHAN N A, et al. Comparison of manganese dioxide and permanganate as amendments with persulfate for aqueous 1, 4-dioxane oxidation[J]. Water, 2020, 12(11): 3061. |
| 79 | LI B Z, ZHU J. Simultaneous degradation of 1, 1, 1-trichloroethane and solvent stabilizer 1,4-dioxane by a sono-activated persulfate process[J]. Chemical Engineering Journal, 2016, 284: 750-763. |
| 80 | ZHU J, LI B Z. Degradation kinetic and remediation effectiveness of 1,4-dioxane-contaminated groundwater by a sono-activated persulfate process[J]. Journal of Environmental Engineering, 2018, 144(10): 04018098. |
| 81 | EBERLE D, BALL R, BOVING T B. Peroxone activated persulfate treatment of 1, 4-dioxane in the presence of chlorinated solvent co-contaminants[J]. Chemosphere, 2016, 144: 728-735. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [5] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [6] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [7] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [8] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [9] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [10] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [11] | 张鹏, 潘原. 单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展[J]. 化工进展, 2023, 42(6): 2944-2953. |
| [12] | 陈少华, 王义华, 胡强飞, 胡坤, 陈立爱, 李洁. 电化学修饰电极在检测Cr(Ⅵ)中的研究进展[J]. 化工进展, 2023, 42(5): 2429-2438. |
| [13] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [14] | 郭朋举, 何小波, 银凤翔. 电催化氮还原合成氨MOF基催化剂研究进展[J]. 化工进展, 2023, 42(4): 1797-1810. |
| [15] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||