化工进展 ›› 2021, Vol. 40 ›› Issue (9): 5156-5165.DOI: 10.16085/j.issn.1000-6613.2021-0632
热增强的光催化二氧化碳还原技术
罗志斌1,2( ), 龙冉2, 王小博1, 裴爱国1, 熊宇杰2(
), 龙冉2, 王小博1, 裴爱国1, 熊宇杰2( )
)
- 1.中国能源建设集团广东省电力设计研究院有限公司,广东 广州 510663
2.中国科学技术大学化学与材料科学学院,安徽 合肥 230026
-
收稿日期:2021-03-29修回日期:2021-07-04出版日期:2021-09-05发布日期:2021-09-13 -
通讯作者:熊宇杰 -
作者简介:罗志斌(1989年—),男,博士,博士后,研究方向为氢能以及二氧化碳利用技术产业化。E-mail:luozhibin@gedi.com.cn 。 -
基金资助:国家杰出青年科学基金(21725102);中国博士后科学基金(2020M682996)
Thermal-enhanced photocatalytic carbon dioxide reduction
LUO Zhibin1,2( ), LONG Ran2, WANG Xiaobo1, PEI Aiguo1, XIONG Yujie2(
), LONG Ran2, WANG Xiaobo1, PEI Aiguo1, XIONG Yujie2( )
)
- 1.China Energy Engineering Group Guangdong Electric Power Design Institute Co. , Ltd. , Guangzhou 510663, Guangdong, China
2.School of Chemistry and Materials Science, University of Science and Technology of China, Hefei 230026, Anhui, China
-
Received:2021-03-29Revised:2021-07-04Online:2021-09-05Published:2021-09-13 -
Contact:XIONG Yujie
摘要:
利用太阳光驱动二氧化碳(CO2)催化转化合成燃料是缓解能源危机和降低温室效应的理想途径。然而,当前面临的主要挑战在于CO2固有的化学稳定性使得光催化反应的转化效率低下。热量被认为是促进催化转化反应过程的重要推动力,可以有效提升光催化转化的效率。本文综述了不同形式的热增强光催化在CO2还原生产燃料方面的应用,包括外加热源的光催化CO2还原、光热效应促进的光催化CO2还原以及等离激元增强的光催化CO2还原体系。文章指出:热增强的光催化技术继承了光催化的高选择性和热催化的高反应活性的优势,实现了CO2还原反应的高效进行。具体分析如下:外加热源主要通过直接加热装置或者聚焦太阳光能实现,产物的生成效率明显增强,选择性影响不大;光热效应发挥着局部提高催化剂反应温度的作用,使能量利用效率更高,大幅度降低CO2还原反应所需的能量;等离激元效应除了发挥光热效应的作用,同时兼备增强光吸收、促进载流子分离和加速表面反应动力学的作用。文章最后指出,通过对反应机理进行深度研究,合理调控反应体系的反应条件,将极大促进热增强的光催化CO2还原技术发展,为CO2利用提供有效手段。
中图分类号:
引用本文
罗志斌, 龙冉, 王小博, 裴爱国, 熊宇杰. 热增强的光催化二氧化碳还原技术[J]. 化工进展, 2021, 40(9): 5156-5165.
LUO Zhibin, LONG Ran, WANG Xiaobo, PEI Aiguo, XIONG Yujie. Thermal-enhanced photocatalytic carbon dioxide reduction[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5156-5165.
| 催化材料 | 反应物 | 产物 | 能量来源 | 催化性能 | 参考文献 |
|---|---|---|---|---|---|
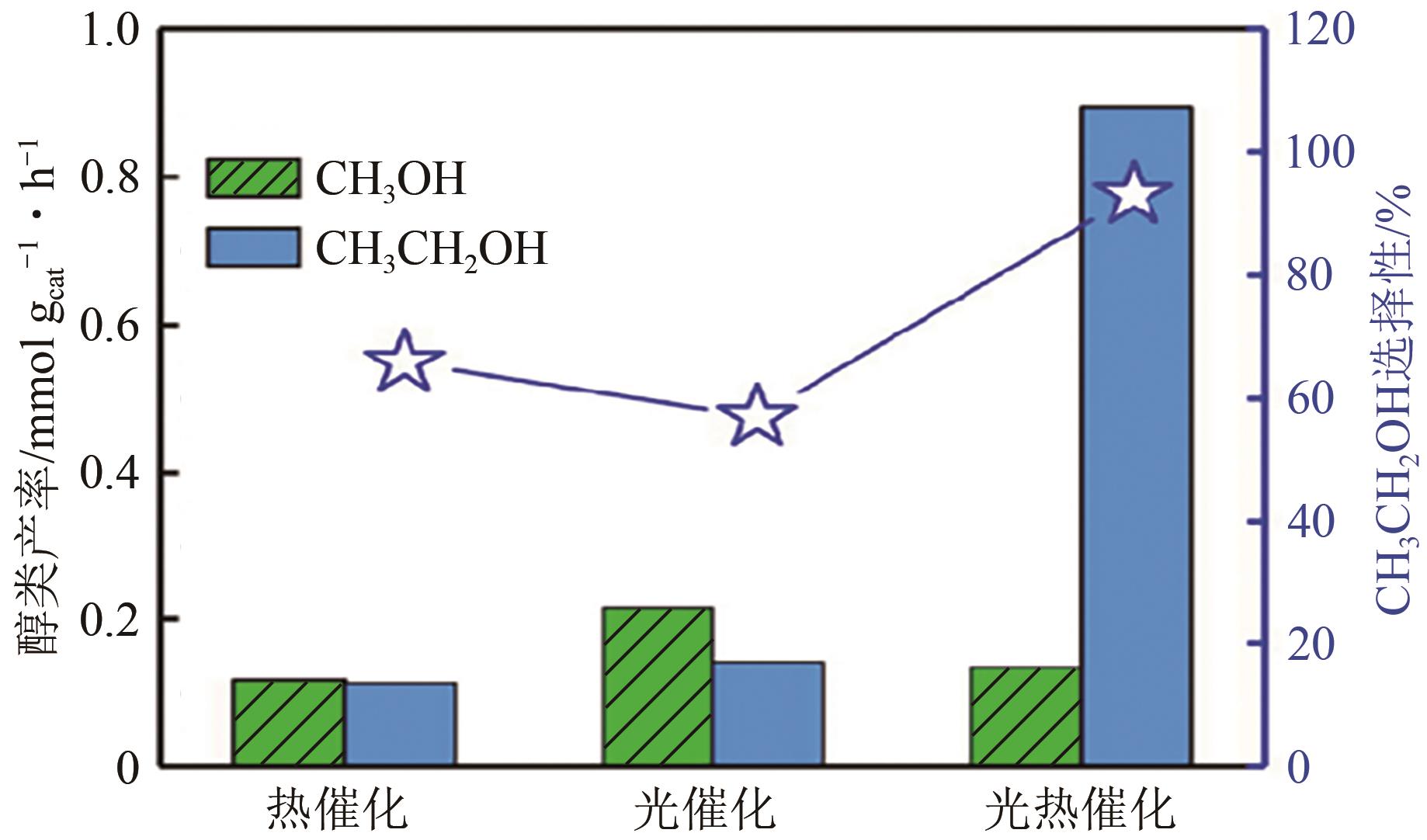
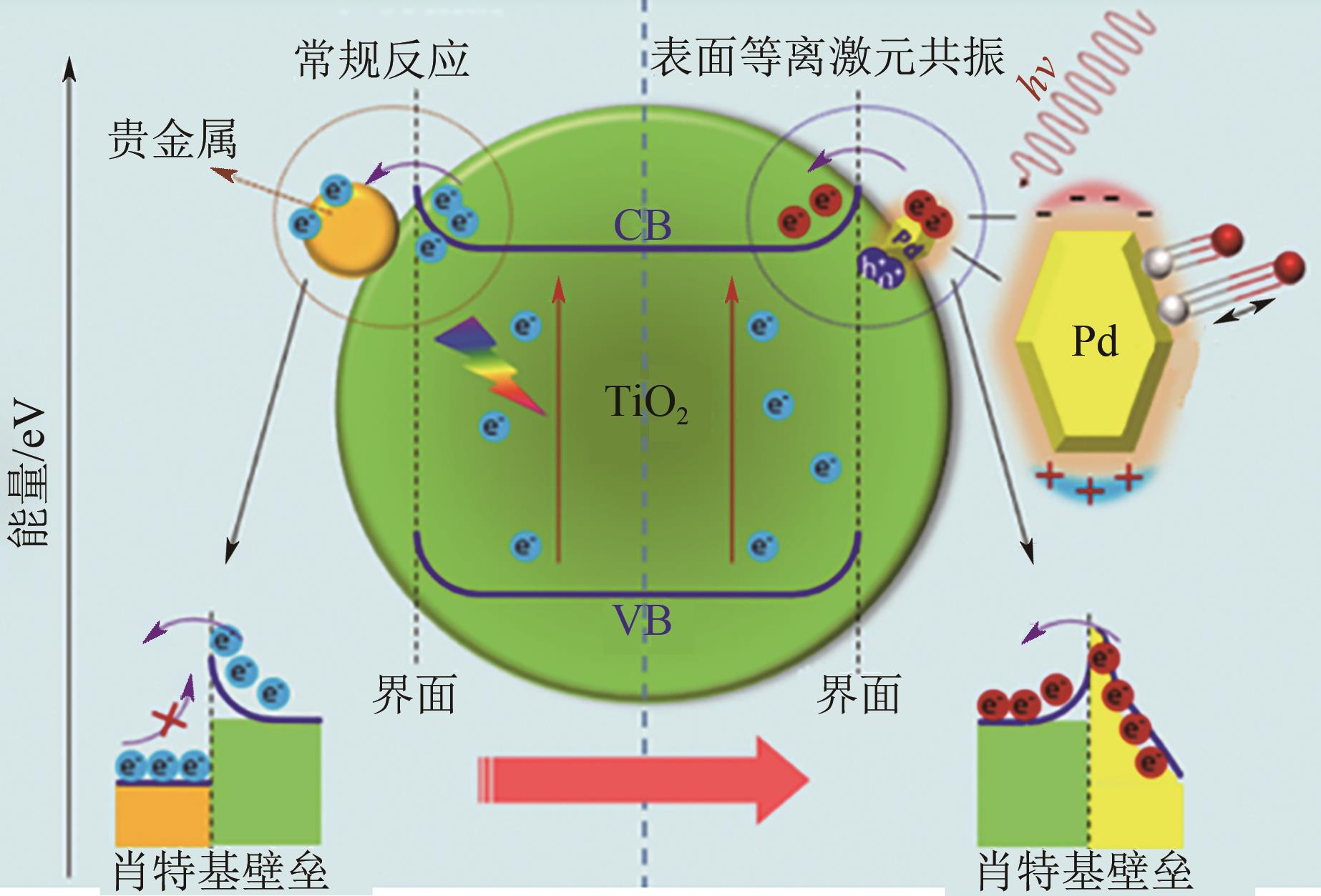
| AuCu/g-C3N4 | CO2 | CH3CH2OH | 300W氙灯(λ>420nm)+外热120℃ | 产率为0.89mmol · g | [ |
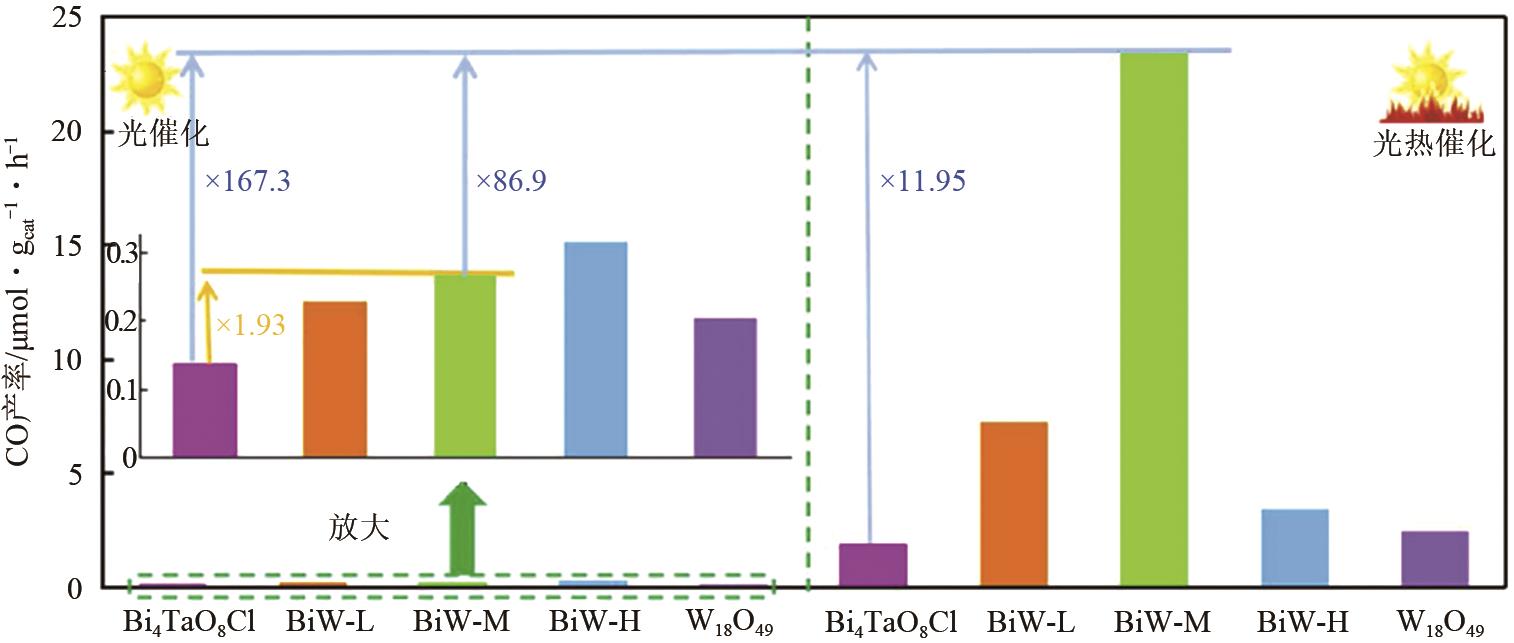
| Bi4TaO8Cl/W18O49异质结 | CO2 | CO | 模拟光源20mW · cm-2+外热120℃ | 产率为23.42μmol · g | [ |
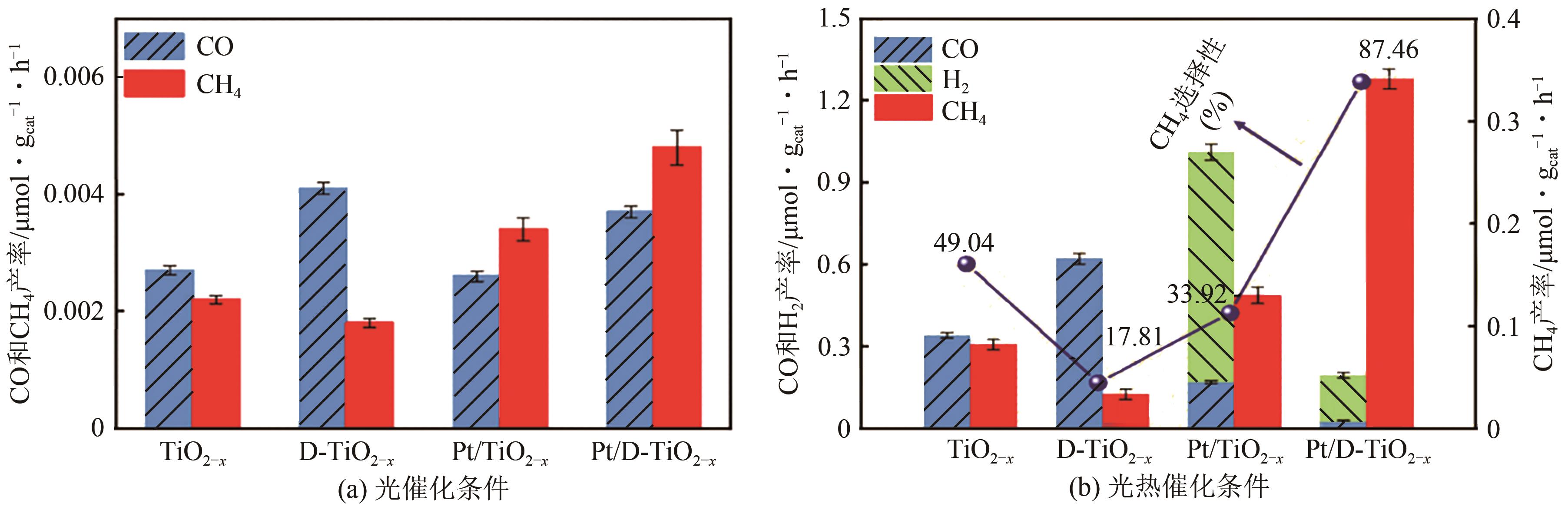
| Pt/TiO2-x | CO2 | CH4 | 模拟光源AM 1.5G+外热120℃ | 产率为0.3412μmol · g | [ |
| In2O3-x(OH)y | CO2+H2 | CO | 聚焦氙灯光源约20kW · m-2 | 产率为22.0μmol · g | [ |
| VIII族金属纳米颗粒 | CO2 | CH4 | 300W氙灯光源 | 相对于光催化方法,光热催化CO2还原产率级别提升了几个数量级,从μmol · g | [ |
| B纳米颗粒 | CO2 | CO、CH4 | 300W氙灯光源 | CO和CH4的产率分别为1.0μmol · g | [ |
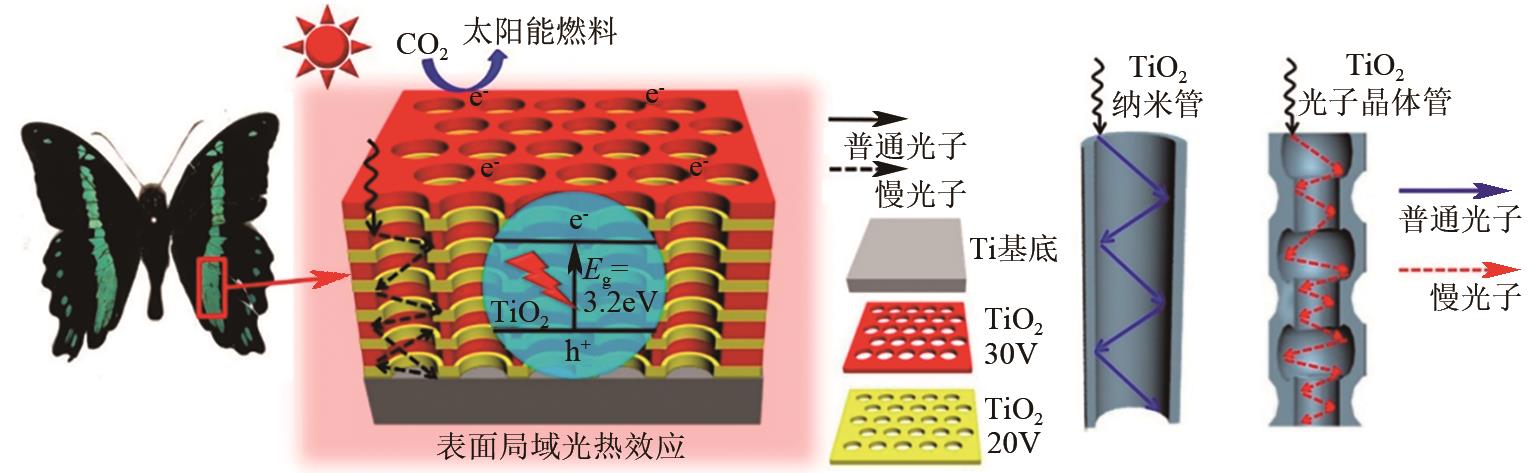
| TiO2光子晶体 | CO2 | CH4 | 300W氙灯光源 | CH4产率达到35.0μmol · h-1 · m-2,分别达到商用P25和TiO2纳米管的15.9倍和4.7倍 | [ |
| Ni/CexTiyO2 | CO2+H2 | CH4 | 300W氙灯光源 | CH4产率达到17.0mmol · g | [ |
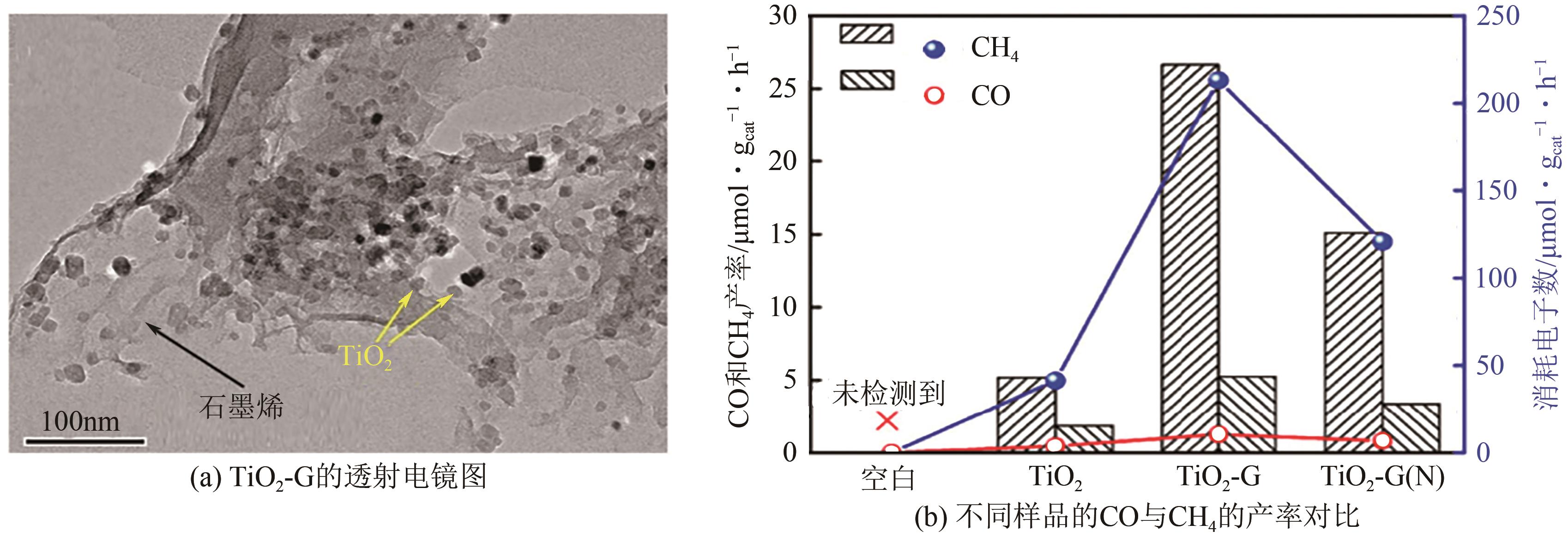
TiO2-石墨烯 复合材料 | CO2 | CO、CH4 | 300W氙灯光源 4.38kW · m-2 | CO的产率达到了5.2μmol · g-1cat · h-1,CH4的产率达到了26.7μmol · g | [ |
| Pd-TiO2 | CO2 | CO | 500W汞灯光源 | CO产率11.05μmol · g | [ |
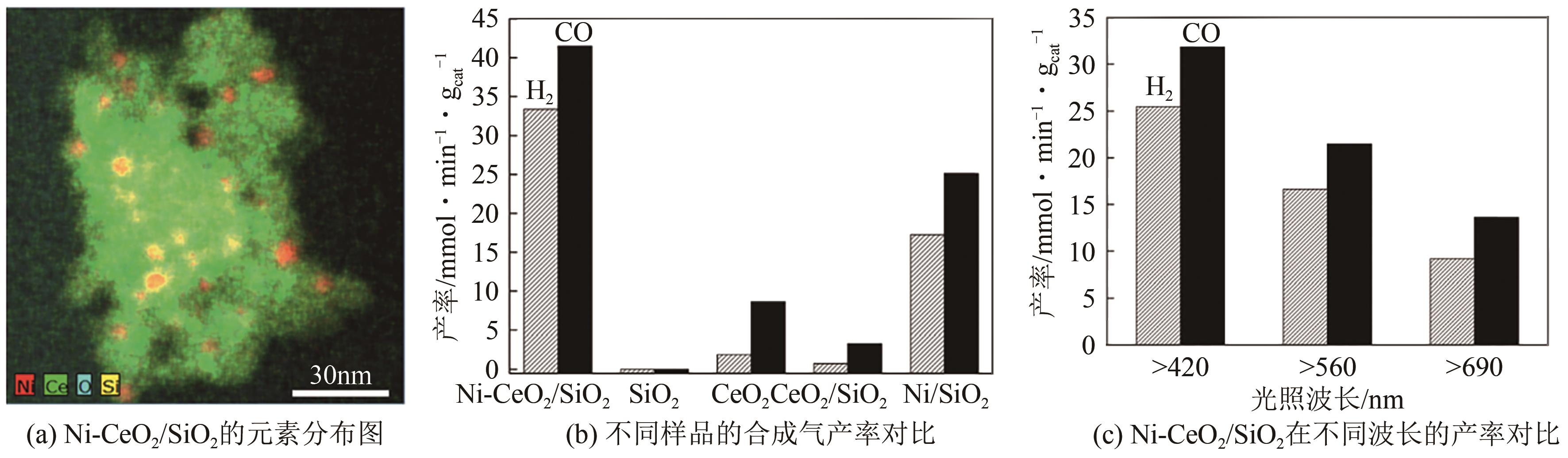
| Ni-CeO2/SiO2复合材料 | CO2+CH4 | H2、CO | 500W氙灯光源 | H2和CO的产率分别达到了33.42mmol · min-1 · g-1cat和41.53mmol · min-1 · g | [ |
| MoO3-x | CO2 | CO、CH4 | 模拟太阳光能 | CO产率达到10.3μmol · g | [ |
表1 热增强光催化CO2还原技术的代表性研究工作
| 催化材料 | 反应物 | 产物 | 能量来源 | 催化性能 | 参考文献 |
|---|---|---|---|---|---|
| AuCu/g-C3N4 | CO2 | CH3CH2OH | 300W氙灯(λ>420nm)+外热120℃ | 产率为0.89mmol · g | [ |
| Bi4TaO8Cl/W18O49异质结 | CO2 | CO | 模拟光源20mW · cm-2+外热120℃ | 产率为23.42μmol · g | [ |
| Pt/TiO2-x | CO2 | CH4 | 模拟光源AM 1.5G+外热120℃ | 产率为0.3412μmol · g | [ |
| In2O3-x(OH)y | CO2+H2 | CO | 聚焦氙灯光源约20kW · m-2 | 产率为22.0μmol · g | [ |
| VIII族金属纳米颗粒 | CO2 | CH4 | 300W氙灯光源 | 相对于光催化方法,光热催化CO2还原产率级别提升了几个数量级,从μmol · g | [ |
| B纳米颗粒 | CO2 | CO、CH4 | 300W氙灯光源 | CO和CH4的产率分别为1.0μmol · g | [ |
| TiO2光子晶体 | CO2 | CH4 | 300W氙灯光源 | CH4产率达到35.0μmol · h-1 · m-2,分别达到商用P25和TiO2纳米管的15.9倍和4.7倍 | [ |
| Ni/CexTiyO2 | CO2+H2 | CH4 | 300W氙灯光源 | CH4产率达到17.0mmol · g | [ |
TiO2-石墨烯 复合材料 | CO2 | CO、CH4 | 300W氙灯光源 4.38kW · m-2 | CO的产率达到了5.2μmol · g-1cat · h-1,CH4的产率达到了26.7μmol · g | [ |
| Pd-TiO2 | CO2 | CO | 500W汞灯光源 | CO产率11.05μmol · g | [ |
| Ni-CeO2/SiO2复合材料 | CO2+CH4 | H2、CO | 500W氙灯光源 | H2和CO的产率分别达到了33.42mmol · min-1 · g-1cat和41.53mmol · min-1 · g | [ |
| MoO3-x | CO2 | CO、CH4 | 模拟太阳光能 | CO产率达到10.3μmol · g | [ |
| 1 | XU C, KOHLER T A, LENTON T M, et al. Future of the human climate niche[J]. PNAS, 2020, 117(21): 11350-11355. |
| 2 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| GONG Jinlong. A brief overview on recent progress on chemical conversion of CO2[J]. CIESC Journal, 2017, 68(4): 1282-1285. | |
| 3 | 周威, 郭君康, 申升, 等. 光电催化二氧化碳还原研究进展[J]. 物理化学学报, 2020, 36(3): 71-81. |
| ZHOU Wei, GUO Junkang, SHEN Sheng, et al. Progress in photoelectrocatalytic reduction of carbon dioxide[J]. Acta Physico-Chimica Sinica, 2020, 36(3): 71-81. | |
| 4 | 陈庆云, 周苗, 王云海. BiVO4光催化剂的合成及其可见光下还原二氧化碳[J]. 化工进展, 2010, 29(S1): 443-445. |
| CHEN Qingyun, ZHOU Miao, WANG Yunhai. Synthesis of BiVO4 photocatalyst and reduction of carbon dioxide under visible light[J]. Chemical Industry and Engineering Progress, 2010, 29(S1): 443-445. | |
| 5 | SASTRE F, PUGA A V, LIU L C, et al. Complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation[J]. Journal of the American Chemical Society, 2014, 136(19): 6798-6801. |
| 6 | JIANG X, NIE X W, GUO X W, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 7 | 王丽敏, 王利清, 张一弛, 等. 光热协同催化技术在能源领域的应用[J]. 化工进展, 2017, 36(7): 2457-2463. |
| WANG Limin, WANG Liqing, ZHANG Yichi, et al. Photothermal synergistic catalytic technology in energy field[J]. Chemical Industry and Engineering Progress, 2017, 36(7): 2457-2463. | |
| 8 | WANG W, WANG S P, MA X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chemical Society Reviews, 2011, 40(7): 3703. |
| 9 | LI P Y, LIU L, AN W J, et al. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO2 to ethanol[J]. Applied Catalysis B: Environmental, 2020, 266: 118618. |
| 10 | YAN J Y, WANG C H, MA H, et al. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction[J]. Applied Catalysis B: Environmental, 2020, 268: 118401. |
| 11 | YU F, WANG C H, MA H, et al. Revisiting Pt/TiO2 photocatalysts for thermally assisted photocatalytic reduction of CO2[J]. Nanoscale, 2020, 12(13): 7000-7010. |
| 12 | MENG X G, WANG T, LIU L Q, et al. Photothermal conversion of CO2 into CH4 with H2 over Group Ⅷ nanocatalysts: an alternative approach for solar fuel production[J]. Angewandte Chemie International Edition, 2014, 53(43): 11478-11482. |
| 13 | XU C Y, HUANG W H, LI Z, et al. Photothermal coupling factor achieving CO2 reduction based on palladium-nanoparticle-loaded TiO2[J]. ACS Catalysis, 2018, 8(7): 6582-6593. |
| 14 | WEI G H, ZHENG D M, XU L J, et al. Photothermal catalytic activity and mechanism of LaNixCo1-xO3(0≤x≤1) perovskites for CO2 reduction to CH4 and CH3OH with H2O[J]. Materials Research Express, 2019, 6(8): 086221. |
| 15 | 赵晨辰, 何向明, 王莉, 等. 电化学还原CO2阴极材料研究进展[J]. 化工进展, 2013, 32(2): 373-380. |
| ZHAO Chenchen, HE Xiangming, WANG Li, et al. Progress of cathode materials for electrochemical reduction of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2013, 32(2): 373-380. | |
| 16 | 陈钱, 匡勤, 谢兆雄. 二维材料在光催化二氧化碳还原中的研究进展[J]. 化学学报, 2021, 79(1): 10-22. |
| CHEN Qian, KUANG Qin, XIE Zhaoxiong. Research progress of photocatalytic CO2 reduction based on two-dimensional materials[J]. Acta Chimica Sinica, 2021, 79(1): 10-22. | |
| 17 | 王路喜, 杨芳麒, 林欢欢, 等. Cu修饰的多孔碳材料高效电化学还原CO2为CO[J]. 化工进展, 2020, 39(9): 3685-3691. |
| WANG Luxi, YANG Fangqi, LIN Huanhuan, et al. Electrochemical reduction of CO2 to CO by Cu modified porous carbon materials[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3685-3691. | |
| 18 | LI Dashuai, HUANG Yu, LI Songmei, et al. Thermal coupled photoconductivity as a tool to understand the photothermal catalytic reduction of CO2[J]. Chinese Journal of Catalysis, 2020, 41(1): 154-160. |
| 19 | ZHAO Ziyan, DORONKIN D E, YE Yinghao, et al. Visible light-enhanced photothermal CO2 hydrogenation over Pt/Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2020, 41(2): 286-295. |
| 20 | QI Y H, SONG L Z, OUYANG S X, et al. Photoinduced defect engineering: enhanced photothermal catalytic performance of 2D black In2O3-x nanosheets with bifunctional oxygen vacancies[J]. Advanced Materials, 2020, 32(6): 1903915. |
| 21 | HOCH L B, O’BRIEN P G, JELLE A, et al. Nanostructured indium oxide coated silicon nanowire arrays: a hybrid photothermal/photochemical approach to solar fuels[J]. ACS Nano, 2016, 10(9): 9017-9025. |
| 22 | LIU G G, MENG X G, ZHANG H B, et al. Elemental boron for efficient carbon dioxide reduction under light irradiation[J]. Angewandte Chemie International Edtion, 2017, 129(20): 5662-5666. |
| 23 | LOW J, ZHANG L Y, ZHU B C, et al. TiO2Photonic crystals with localized surface photothermal effect and enhanced photocatalytic CO2 reduction activity[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15653-15661. |
| 24 | WANG L C, WANG Y, CHENG Y, et al. Hydrogen-treated mesoporous WO3 as a reducing agent of CO2 to fuels (CH4 and CH3OH) with enhanced photothermal catalytic performance[J]. Journal of Materials Chemistry A, 2016, 4(14): 5314-5322. |
| 25 | KHO E T, JANTARANG S, ZHENG Z K, et al. Harnessing the beneficial attributes of ceria and titania in a mixed-oxide support for nickel-catalyzed photothermal CO2 methanation[J]. Engineering, 2017, 3(3): 393-401. |
| 26 | JANTARANG S, LOVELL E C, TAN T H, et al. Role of support in photothermal carbon dioxide hydrogenation catalysed by Ni/CexTiyO2[J]. Progress in Natural Science: Materials International, 2018, 28(2): 168-177. |
| 27 | 李炳杰, 吴志坚, 陈澍, 等. Ni/Zn/Cr系复合金属氧化物的制备及其光催化还原二氧化碳性能研究[J]. 分子催化, 2014, 28(3): 268-274. |
| LI Bingjie, WU Zhijian, CHEN Shu, et al. Preparation and photocatalytic CO2 reduction activity of Ni/Zn/Cr composite metal oxides[J]. Journal of Molecular Catalysis, 2014, 28(3): 268-274. | |
| 28 | XU M, HU X T, WANG S L, et al. Photothermal effect promoting CO2 conversion over composite photocatalyst with high graphene content[J]. Journal of Catalysis, 2019, 377: 652-661. |
| 29 | LINIC S, CHRISTOPHER P, INGRAM D B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nature Materials, 2011, 10(12): 911-921. |
| 30 | MASCARETTI L, DUTTA A, KMENT Š, et al. Plasmon-enhanced photoelectrochemical water splitting for efficient renewable energy storage[J]. Advanced Materials, 2019, 31(31): 1805513. |
| 31 | CHIU Y H, CHANG K D, HSU Y J. Plasmon-mediated charge dynamics and photoactivity enhancement for Au-decorated ZnO nanocrystals[J]. Journal of Materials Chemistry A, 2018, 6(10): 4286-4296. |
| 32 | WANG J, PAN S L, CHEN M Y, et al. Gold nanorod-enhanced light absorption and photoelectrochemical performance of α-Fe2O3 thin-film electrode for solar water splitting[J]. The Journal of Physical Chemistry C, 2013, 117(42): 22060-22068. |
| 33 | WU D D, DENG K X, HU B, et al. Plasmon-assisted photothermal catalysis of low-pressure CO2 hydrogenation to methanol over Pd/ZnO catalyst[J]. ChemCatChem, 2019, 11(6): 1598-1601. |
| 34 | JIANG Z K, LI Y Z, ZHANG Q, et al. A novel nanocomposite of mesoporous silica supported Ni nanocrystals modified by ceria clusters with extremely high light-to-fuel efficiency for UV-vis-IR light-driven CO2 reduction[J]. Journal of Materials Chemistry A, 2019, 7(9): 4881-4892. |
| 35 | LI J, YE Y H, YE L Q, et al. Sunlight induced photo-thermal synergistic catalytic CO2 conversion via localized surface plasmon resonance of MoO3-x[J]. Journal of Materials Chemistry A, 2019, 7(6): 2821-2830. |
| 36 | 张继宏, 钟地长, 鲁统部. 钴(Ⅱ)基分子配合物用于光催化二氧化碳还原[J]. 物理化学学报, 2021, 37(5): 111-125. |
| ZHANG Jihong, ZHONG Dichang, LU Tongbu. Co(Ⅱ)-based molecular complexes for photochemical CO2 reduction[J]. Acta Physico-Chimica Sinica, 2021, 37(5): 111-125. | |
| 37 | 吴进, 刘京, 夏雾, 等. 基于CdS和CdSe纳米半导体材料的可见光催化二氧化碳还原研究进展[J]. 物理化学学报, 2021, 37(5): 103-110. |
| WU Jin, LIU Jing, XIA Wu, et al. Advances on photocatalytic CO2 reduction based on CdS and CdSe nano-semiconductors[J]. Acta Physico-Chimica Sinica, 2021, 37(5): 103-110. | |
| 38 | 常晓侠, 巩金龙. 表面反应在半导体光催化水分解过程中的重要性[J]. 物理化学学报, 2016, 32(1): 2-13. |
| CHANG Xiaoxia, GONG Jinlong. On the importance of surface reactions on semiconductor photocatalysts for solar water splitting[J]. Acta Physico-Chimica Sinica, 2016, 32(1): 2-13. | |
| 39 | ZHONG J W, YANG X F, WU Z L, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 40 | 林海周, 罗志斌, 裴爱国, 等. 二氧化碳与氢合成甲醇技术和产业化进展[J]. 南方能源建设, 2020, 7(2): 14-19. |
| LIN Haizhou, LUO Zhibin, PEI Aiguo, et al. Technology and industrialization progress on methanol synthesis from carbon dioxide and hydrogen[J]. Southern Energy Construction, 2020, 7(2): 14-19. |
| [1] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [2] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [3] | 郭立行, 庞蔚莹, 马克遥, 杨镓涵, 孙泽辉, 张盼, 付东, 赵昆. 层序空间多孔结构TiO2实现高效光催化CO2还原[J]. 化工进展, 2023, 42(7): 3643-3651. |
| [4] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [5] | 杨状, 李闰华, 强增寿, 王雅君, 姚文清. 废弃制冷剂R134a的光催化降解[J]. 化工进展, 2023, 42(4): 2109-2114. |
| [6] | 胥生元, 郝玮, 王杰, 高文生, 谢克锋. 半导体光催化剂BiOCl异质结的构建及应用[J]. 化工进展, 2023, 42(3): 1493-1507. |
| [7] | 陈邦富, 欧阳平, 李宇涵, 段有雨, 董帆. ZnSn(OH)6 基纳米材料在环境光催化中的应用[J]. 化工进展, 2023, 42(2): 756-764. |
| [8] | 姚稳, 张雨晨, 滕文馨, 黎江玲. 表面活性剂对制备Ca掺杂β-In2S3微观结构的影响及其光催化降解甲基橙性能[J]. 化工进展, 2023, 42(2): 774-782. |
| [9] | 程荣, 邓子祺, 夏锦程, 李江, 石磊, 郑祥. 光催化系统灭活微生物气溶胶的研究进展[J]. 化工进展, 2023, 42(2): 957-968. |
| [10] | 多佳, 姚国栋, 王英霁, 曾旭, 金滨滨. 改性Au-TiO2光降解废水中诺氟沙星的影响[J]. 化工进展, 2023, 42(2): 624-630. |
| [11] | 宋亚丽, 李紫燕, 杨彩荣, 黄龙, 张宏忠. 非金属元素掺杂石墨相氮化碳光催化材料的研究进展[J]. 化工进展, 2023, 42(10): 5299-5309. |
| [12] | 章萍萍, 丁书海, 高晶晶, 赵敏, 俞海祥, 刘玥宏, 谷麟. 碳量子点修饰半导体复合光催化剂降解水中有机污染物[J]. 化工进展, 2023, 42(10): 5487-5500. |
| [13] | 张金辉, 张焕, 朱新锋, 宋忠贤, 康海彦, 刘红盼, 邓炜, 侯广超, 李桂亭, 黄真真. UiO-66复合材料用于典型有机污染物吸附和光催化氧化的研究进展[J]. 化工进展, 2023, 42(1): 445-456. |
| [14] | 寇佳伟, 程淑艳, 程芳琴. 类水滑石基催化剂光催化二氧化碳还原研究进展[J]. 化工进展, 2022, 41(S1): 190-198. |
| [15] | 刘怡璇, 林跃朝, 马伟芳. 可见光催化降解水中卤代有机污染物的研究进展[J]. 化工进展, 2022, 41(S1): 571-579. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||