化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1384-1394.DOI: 10.16085/j.issn.1000-6613.2020-0889
金属基材料电催化CO2还原的研究进展
- 中国石油大学(北京)重质油国家重点实验室,北京 102249
-
收稿日期:2020-05-22出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:范煜 -
作者简介:苏文礼(1995—),男,博士研究生,研究方向为能源转化与优化利用。E-mail:wenli.su@foxmail.com 。
Progress of electrocatalytic reduction of CO2 on metal-based materials
- State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
-
Received:2020-05-22Online:2021-03-05Published:2021-03-17 -
Contact:FAN Yu
摘要:
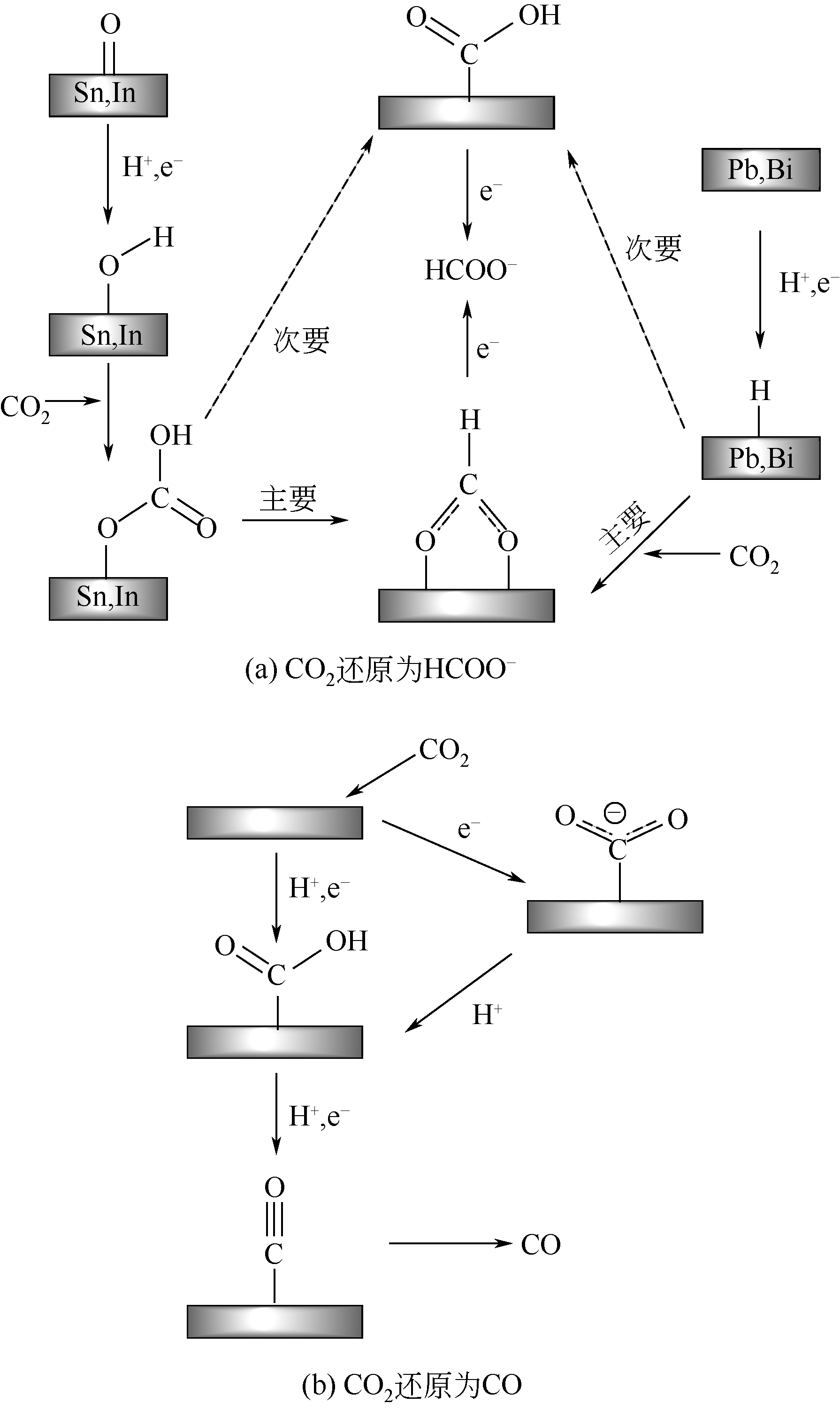
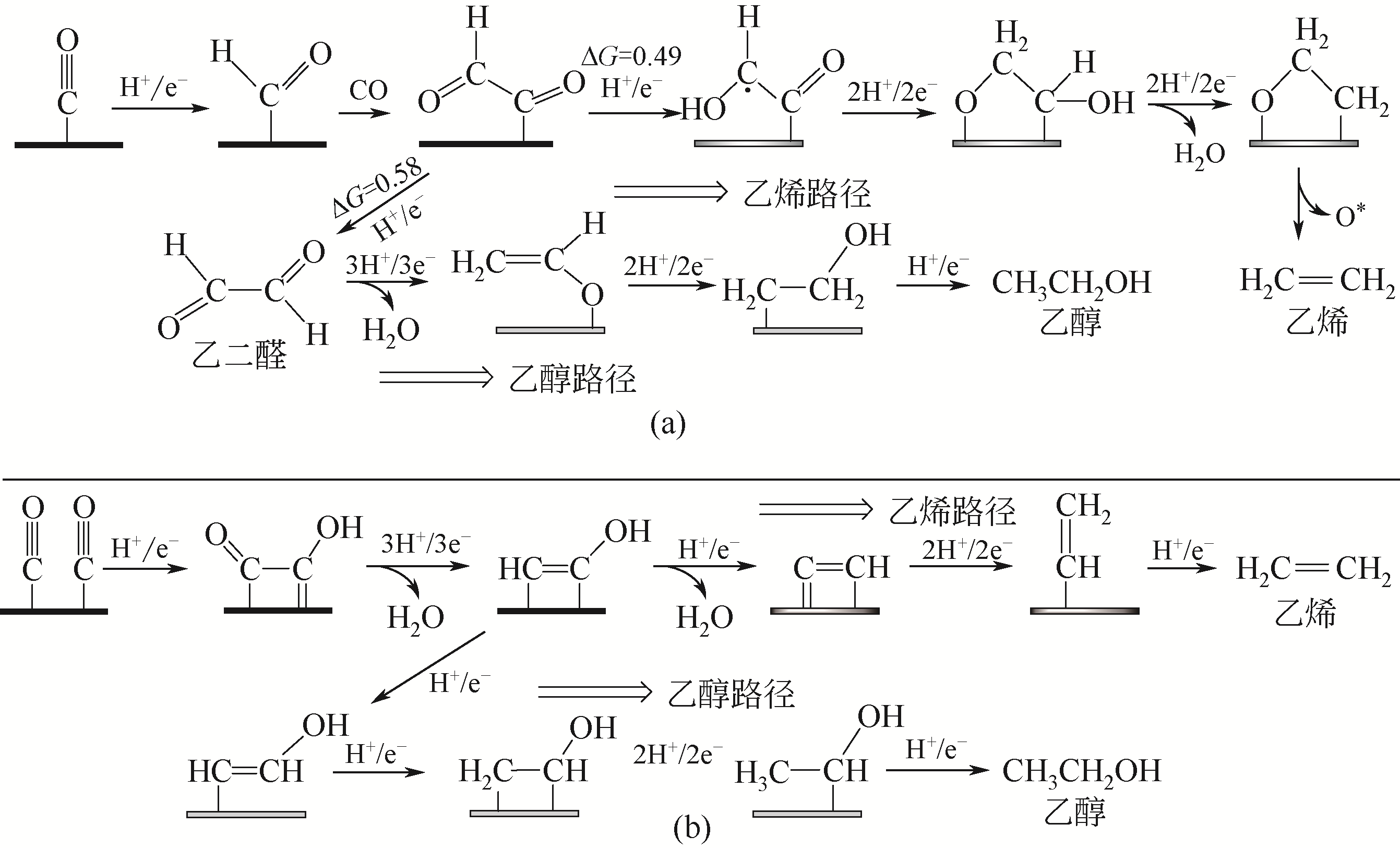
电催化CO2还原为具有附加价值的燃料和化学品,在缓解全球气候变暖和有效储存可再生能源方面极具潜力,近年来受到了广泛的关注。本文首先简述了水溶液中CO2电化学还原为不同产物反应途径的研究成果,当前简单C1产物的生成路径较为清晰,但生成多碳烃类和醇类的反应途径尚缺乏明确的证据,需要进一步探索。然后综述了用于CO2电化学还原的金属基电催化材料的研究进展,聚焦于产物选择性、催化活性和稳定性,分别对金属纳米类、金属合金类、金属氧化物类、金属基复合物以及最近出现的单原子金属催化材料的研究现状进行了介绍。最后,展望了电催化CO2还原的研究前景,指出不断优化电催化材料的性能是将来研究的主要方向之一,特别是有望取代Au、Ag等贵金属的单原子催化剂以及能高效生成多碳产物的铜基材料;同时,更精确的理论计算结合原位光谱表征,深入探究CO2电化学还原反应的机理,将极大地促进高效电催化材料的开发。
中图分类号:
引用本文
苏文礼, 范煜. 金属基材料电催化CO2还原的研究进展[J]. 化工进展, 2021, 40(3): 1384-1394.
SU Wenli, FAN Yu. Progress of electrocatalytic reduction of CO2 on metal-based materials[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1384-1394.
| 电化学半反应 | 电极电位(vs. SHE)/V |
|---|---|
| 2H++2e- | -0.42 |
| CO2+2H++2e- | -0.52 |
| CO2+2H++2e- | -0.61 |
| CO2+4H++4e- | -0.51 |
| CO2+6H++6e- | -0.38 |
| CO2+8H++8e- | -0.24 |
| 2CO2+12H++12e- | 0.064 |
| 2CO2+12H++12e- | 0.084 |
表1 电化学还原CO2半反应的电极电位(标准试验条件)[4]
| 电化学半反应 | 电极电位(vs. SHE)/V |
|---|---|
| 2H++2e- | -0.42 |
| CO2+2H++2e- | -0.52 |
| CO2+2H++2e- | -0.61 |
| CO2+4H++4e- | -0.51 |
| CO2+6H++6e- | -0.38 |
| CO2+8H++8e- | -0.24 |
| 2CO2+12H++12e- | 0.064 |
| 2CO2+12H++12e- | 0.084 |
| 催化剂 | 电解质 | 电位(vs. RHE)/V | 电流密度/mA·cm-2 | 主要产物(FE) | 稳定性/h | 参考文献 |
|---|---|---|---|---|---|---|
| Cu NCs | 0.1mol·L-1 KHCO3 | -1.1 | 5.6 | C2H4(41%) | — | [ |
| Cu基 NP/C | 0.1mol·L-1 KHCO3 | -1.1 | 约60mA·mgCu-1 | C2H4(57.3%) | 10 | [ |
| CuOx颗粒 | 0.1mol·L-1 CsHCO3 | -0.9 | 11 | C2+(80%) | 3 | [ |
| Au NWs | 0.5mol·L-1 KHCO3 | -0.35 | 1.84A·gAu-1 | CO(94%) | 6 | [ |
| Cu NWs | 0.1mol·L-1 KHCO3 | -1.25 | 约15 | CH4(55%) | — | [ |
| 2D Cu纳米片 | 2mol·L-1 KOH | -0.74 | 约272 | C2+(70%),其中CH3COO-(48%) | 3 | [ |
| 3D Cu NFs | 0.1mol·L-1 KHCO3 | -1.0~-1.6 | 18.7~21 | HCOOH(45%) | 9 | [ |
| 3D hp-In | 0.1mol·L-1 KHCO3 | -1.2 | 65.7 | HCOOH(90%) | 24 | [ |
| Cu-In合金 | 0.1mol·L-1 KHCO3 | -0.7 | 约1.6 | CO(95%) | 7 | [ |
| CuPd合金 | 0.5mol·L-1 KHCO3 | -0.87 | 约4.5 | CO(88%) | 20 | [ |
| Sn-Cu合金 | 0.5mol·L-1 KCl | -1.14 | 约100 | HCOOH(82.3%) | 11 | [ |
| In-Zn NCs | 0.5mol·L-1 KHCO3 | -1.2 | 22 | HCOOH(95%) | 24 | [ |
| Cu-Ag合金 | 1mol·L-1 KOH | -0.7 | 约300 | C2H4(60%),C2H5OH(25%) | — | [ |
| AuCu/Cu-SCA | 0.5mol·L-1 KHCO3 | -1.0 | 约19 | C2H5OH(29%),C2H4(16%) | 24 | [ |
| OD-Au | 0.5mol·L-1 NaHCO3 | -0.35 | 2~4 | CO(96%) | 8 | [ |
| Cu2O薄膜 | 0.1mol·L-1 KHCO3 | -0.99 | 30~35 | C2H4(34%~39%),C2H5OH(9%~16%) | — | [ |
| OD-Cu | 0.1mol·L-1 KHCO3 | -0.9 | 11 | C2H4(60%) | — | [ |
| 等离子体处理的Cu | 0.1mol·L-1 KHCO3 | -1.0 | 约34 | C2H4(45%),C2H5OH(22%) | — | [ |
| Cu/CNS | 0.1mol·L-1 KHCO3 | -1.2 | 2 | C2H5OH(63%) | 6 | [ |
| In2O3-rGO | 0.1mol·L-1 KHCO3 | -1.2 | 26 | HCOOH(84.6%) | 10 | [ |
| Ag-G-NCF | 0.1mol·L-1 KHCO3 | -0.6 | 0.31 | C2H5OH (85.2%) | 10 | [ |
| Cu Ps/BCF | 0.1mol·L-1 KHCO3 | -1.0 | 50 | C2H4(63.7%) | 24 | [ |
| Ni SAs/N-C | 0.5mol·L-1 KHCO3 | -1.0 | 10.48 | CO(71%) | 60 | [ |
| Ni-N-C | 0.1mol·L-1 KHCO3 | -0.75 | 7.51 | CO(96%) | 10 | [ |
| Co-N2 | 0.5mol·L-1 KHCO3 | -0.68 | 18.1 | CO(95%) | 60 | [ |
| Ni-NG | 0.5mol·L-1 KHCO3 | -0.65 | 约11 | CO(95%) | 20 | [ |
| SANi-GO | 0.5mol·L-1 KHCO3 | -0.63 | 约21 | CO(96.5%) | 50 | [ |
表2 代表性金属基材料电催化剂CO2还原的性能比较
| 催化剂 | 电解质 | 电位(vs. RHE)/V | 电流密度/mA·cm-2 | 主要产物(FE) | 稳定性/h | 参考文献 |
|---|---|---|---|---|---|---|
| Cu NCs | 0.1mol·L-1 KHCO3 | -1.1 | 5.6 | C2H4(41%) | — | [ |
| Cu基 NP/C | 0.1mol·L-1 KHCO3 | -1.1 | 约60mA·mgCu-1 | C2H4(57.3%) | 10 | [ |
| CuOx颗粒 | 0.1mol·L-1 CsHCO3 | -0.9 | 11 | C2+(80%) | 3 | [ |
| Au NWs | 0.5mol·L-1 KHCO3 | -0.35 | 1.84A·gAu-1 | CO(94%) | 6 | [ |
| Cu NWs | 0.1mol·L-1 KHCO3 | -1.25 | 约15 | CH4(55%) | — | [ |
| 2D Cu纳米片 | 2mol·L-1 KOH | -0.74 | 约272 | C2+(70%),其中CH3COO-(48%) | 3 | [ |
| 3D Cu NFs | 0.1mol·L-1 KHCO3 | -1.0~-1.6 | 18.7~21 | HCOOH(45%) | 9 | [ |
| 3D hp-In | 0.1mol·L-1 KHCO3 | -1.2 | 65.7 | HCOOH(90%) | 24 | [ |
| Cu-In合金 | 0.1mol·L-1 KHCO3 | -0.7 | 约1.6 | CO(95%) | 7 | [ |
| CuPd合金 | 0.5mol·L-1 KHCO3 | -0.87 | 约4.5 | CO(88%) | 20 | [ |
| Sn-Cu合金 | 0.5mol·L-1 KCl | -1.14 | 约100 | HCOOH(82.3%) | 11 | [ |
| In-Zn NCs | 0.5mol·L-1 KHCO3 | -1.2 | 22 | HCOOH(95%) | 24 | [ |
| Cu-Ag合金 | 1mol·L-1 KOH | -0.7 | 约300 | C2H4(60%),C2H5OH(25%) | — | [ |
| AuCu/Cu-SCA | 0.5mol·L-1 KHCO3 | -1.0 | 约19 | C2H5OH(29%),C2H4(16%) | 24 | [ |
| OD-Au | 0.5mol·L-1 NaHCO3 | -0.35 | 2~4 | CO(96%) | 8 | [ |
| Cu2O薄膜 | 0.1mol·L-1 KHCO3 | -0.99 | 30~35 | C2H4(34%~39%),C2H5OH(9%~16%) | — | [ |
| OD-Cu | 0.1mol·L-1 KHCO3 | -0.9 | 11 | C2H4(60%) | — | [ |
| 等离子体处理的Cu | 0.1mol·L-1 KHCO3 | -1.0 | 约34 | C2H4(45%),C2H5OH(22%) | — | [ |
| Cu/CNS | 0.1mol·L-1 KHCO3 | -1.2 | 2 | C2H5OH(63%) | 6 | [ |
| In2O3-rGO | 0.1mol·L-1 KHCO3 | -1.2 | 26 | HCOOH(84.6%) | 10 | [ |
| Ag-G-NCF | 0.1mol·L-1 KHCO3 | -0.6 | 0.31 | C2H5OH (85.2%) | 10 | [ |
| Cu Ps/BCF | 0.1mol·L-1 KHCO3 | -1.0 | 50 | C2H4(63.7%) | 24 | [ |
| Ni SAs/N-C | 0.5mol·L-1 KHCO3 | -1.0 | 10.48 | CO(71%) | 60 | [ |
| Ni-N-C | 0.1mol·L-1 KHCO3 | -0.75 | 7.51 | CO(96%) | 10 | [ |
| Co-N2 | 0.5mol·L-1 KHCO3 | -0.68 | 18.1 | CO(95%) | 60 | [ |
| Ni-NG | 0.5mol·L-1 KHCO3 | -0.65 | 约11 | CO(95%) | 20 | [ |
| SANi-GO | 0.5mol·L-1 KHCO3 | -0.63 | 约21 | CO(96.5%) | 50 | [ |
| 1 | LIU Anmin, GAO Mengfan, REN Xuefeng, et al. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts[J]. Journal of Materials Chemistry A, 2020, 8(7): 3541-3562. |
| 2 | KUHL K P, CAVE E R, ABRAM D N, et al. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces[J]. Energy & Environmental Science, 2012, 5(5): 7050-7059. |
| 3 | Seunghwa LEE, CHOI Minjun, Jaeyoung LEE. Looking back and looking ahead in electrochemical reduction of CO2[J]. Chemical Record, 2020, 20(2): 89-101. |
| 4 | ZHU Dongdong, LIU Jinlong, QIAO Shizhang. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials, 2016, 28(18): 3423-3452. |
| 5 | HORI Y, MURATA A, TAKAHASHI R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1989, 85(8): 2309-2326. |
| 6 | 殷中枢, 郭建伟, 王博. 二氧化碳电化学还原催化剂[J]. 科学技术与工程, 2013, 13(35): 10560-10570. |
| YIN Zhongshu, GUO Jianwei, WANG Bo. Catalyst for electrochemical reduction of carbon dioxide[J]. Science Technology and Engineering, 2013, 13(35): 10560-10570. | |
| 7 | QIAO Jinli, LIU Yuyu, HONG Feng, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675. |
| 8 | DE SALLES PUPO M M, KORTLEVER R. Electrolyte effects on the electrochemical reduction of CO2[J]. ChemPhysChem, 2019, 20(22): 2926-2935. |
| 9 | MIZUNO T, OHTA K, SASAKI A, et al. Effect of temperature on electrochemical reduction of high-pressure CO2 with In, Sn, and Pb electrodes[J]. Energy Sources, 1995, 17(5): 503-508. |
| 10 | HARA K, KUDO A, SAKATA T. Electrochemical CO2 reduction on a glassy carbon electrode under high pressure[J]. Journal of Electroanalytical Chemistry, 1997, 421(1): 1-4. |
| 11 | FRESE JR K W, LEACH S. Electrochemical reduction of carbon dioxide to methane, methanol, and CO on Ru electrodes[J]. Journal of the Electrochemical Society, 1985, 132(1): 259-260. |
| 12 | HORI Y, KIKUCHI K, MURATA A, et al. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution[J]. Chemistry Letters, 1986, 15(6): 897-898. |
| 13 | HORI Y. Electrochemical CO2 reduction on metal electrodes[M]. Berlin: Springer, 2008: 89-189. |
| 14 | DEWULF D W, JIN Tuo, BARD A J. Electrochemical and surface studies of carbon dioxide reduction to methane and ethylene at copper electrodes in aqueous solutions[J]. Journal of the Electrochemical Society, 1989, 136(6): 1686. |
| 15 | PETERSON A A, ABILDPEDERSEN F, STUDT F, et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels[J]. Energy and Environmental Science, 2010, 3(9): 1311-1315. |
| 16 | PETERSON A A, NORSKOV J K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts[J]. Journal of Physical Chemistry Letters, 2012, 3(2): 251-258. |
| 17 | DENG Yilin, Boon Siang YEO. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando raman spectroscopy[J]. ACS Catalysis, 2017, 7(11): 7873-7889. |
| 18 | ADARSH K S, CHANDRASEKARAN N, CHAKRAPANI V. In-situ spectroscopic techniques as critical evaluation tools for electrochemical carbon dioxide reduction: a mini review[J]. Frontiers in Chemistry, 2020, 8: 137. |
| 19 | TING Louisa Rui Lin, Boon Siang YEO. Recent advances in understanding mechanisms for the electrochemical reduction of carbon dioxide[J]. Current Opinion in Electrochemistry, 2018, 8: 126-134. |
| 20 | CUI Chaonan, HAN Jinyu, ZHU Xinli, et al. Promotional effect of surface hydroxyls on electrochemical reduction of CO2 over SnOx/Sn electrode[J]. Journal of Catalysis, 2016, 343: 257-265. |
| 21 | PANDER J E, BARUCH M F, BOCARSLY A B. Probing the mechanism of aqueous CO2 reduction on post-transition-metal electrodes using ATR-IR spectroelectrochemistry[J]. ACS Catalysis, 2016, 6(11): 7824-7833. |
| 22 | Jong Suk YOO, CHRISTENSEN R H B, VEGGE T, et al. Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid[J]. ChemSusChem, 2016, 9(4): 358-363. |
| 23 | FEASTER J T, SHI Chuan, CAVE E R, et al. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes[J]. ACS Catalysis, 2017, 7(7): 4822-4827. |
| 24 | BACK Seoin, KIM Junhyuk, KIM Yongtae, et al. On the mechanism of high product selectivity for HCOOH using Pb in CO2 electroreduction[J]. Physical Chemistry Chemical Physics, 2016, 18(14): 9652-9657. |
| 25 | KORTLEVER R, SHEN Jing, SCHOUTEN K J P, et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide[J]. Journal of Physical Chemistry Letters, 2015, 6(20): 4073-4082. |
| 26 | FIRET N J, SMITH W A. Probing the reaction mechanism of CO2 electroreduction over Ag films via operando infrared spectroscopy[J]. ACS Catalysis, 2017, 7(1): 606-612. |
| 27 | Le TRI NGUYEN Dang, KIM Younghye, HWANG Yun Jeong, et al. Progress in development of electrocatalyst for CO2 conversion to selective CO production[J]. Carbon Energy, 2020, 2(1): 72-98. |
| 28 | HANSEN H A, VARLEY J B, PETERSON A A, et al. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO[J]. Journal of Physical Chemistry Letters, 2013, 4(3): 388-392. |
| 29 | KUMAR B, BRIAN J P, ATLA V, et al. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction[J]. Catalysis Today, 2016, 270: 19-30. |
| 30 | ZHENG Yao, VASILEFF A, ZHOU Xianlong, et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts[J]. Journal of the American Chemical Society, 2019, 141(19): 7646-7659. |
| 31 | GARZA A, BELL A T, HEAD-GORDON M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products[J]. ACS Catalysis, 2018, 8(2): 1490-1499. |
| 32 | XIAO Hai, CHENG Tao, GODDARD W A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111)[J]. Journal of the American Chemical Society, 2017, 139(1): 130-136. |
| 33 | REN Dan, SU-HUI ANG B, Boon Siang YEO. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts[J]. ACS Catalysis, 2016, 6(12): 8239-8247. |
| 34 | Seunghwa LEE, PARK Gibeom, Jaeyoung LEE. Importance of Ag-Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol[J]. ACS Catalysis, 2017, 7(12): 8594-8604. |
| 35 | DUTTA A, RAHAMAN Mr, LUEDI N C, et al. Morphology matters: tuning the product distribution of CO2 electroreduction on oxide-derived Cu foam catalysts[J]. ACS Catalysis, 2016, 6(6): 3804-3814. |
| 36 | HANDOKO A D, CHAN Kuang Wen, Boon Siang YEO. -CH3 mediated pathway for the electroreduction of CO2 to ethane and ethanol on thick oxide-derived copper catalysts at low overpotentials[J]. ACS Energy Letters, 2017, 2(9): 2103-2109. |
| 37 | KIBRIA M G, EDWARDS J P, GABARDO C M, et al. Electrochemical CO2 reduction into chemical feedstocks: from mechanistic electrocatalysis models to system design[J]. Advanced Materials, 2019, 31(31): e1807166. |
| 38 | HAN Na, DING Pan, HE Le, et al. Promises of main group metal-based nanostructured materials for electrochemical CO2 reduction to formate[J]. Advanced Energy Materials, 2020, 10(11): 19. |
| 39 | HOU Liang, YAN Jingze, TAKELE L, et al. Current progress of metallic and carbon-based nanostructure catalysts towards the electrochemical reduction of CO2[J]. Inorganic Chemistry Frontiers, 2019, 6(12): 3363-3380. |
| 40 | KIM Cheonghee, DIONIGI F, BEERMANN V, et al. Alloy nanocatalysts for the electrochemical oxygen reduction (ORR) and the direct electrochemical carbon dioxide reduction reaction (CO2RR)[J]. Advanced Materials, 2019, 31: 19. |
| 41 | PANDER J E, REN Dan, HUANG Yun, et al. Understanding the heterogeneous electrocatalytic reduction of carbon dioxide on oxide-derived catalysts[J]. Chemelectrochem, 2018, 5(2): 219-237. |
| 42 | FAN Qun, ZHANG Mingli, JIA Mingwen, et al. Electrochemical CO2 reduction to C2+ species: heterogeneous electrocatalysts, reaction pathways, and optimization strategies[J]. Materials Today Energy, 2018, 10: 280-301. |
| 43 | CHENG Yi, YANG Shize, JIANG Sanping, et al. Supported single atoms as new class of catalysts for electrochemical reduction of carbon dioxide[J]. Small Methods, 2019, 3(9): 16. |
| 44 | Fang LYU, BAO Haihong, MI Yuying, et al. Electrochemical CO2 reduction: from nanoclusters to single atom catalysts[J]. Sustainable Energy & Fuels, 2020, 4(3): 1012-1028. |
| 45 | LOIUDICE A, LOBACCARO P, KAMALI E A, et al. Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction[J]. Angewandte Chemie: International Edition, 2016, 55(19): 5789-5792. |
| 46 | JUNG Hyejin, Si Young LEE, Chan Woo LEE, et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C-C coupling from CO2 reduction reaction[J]. Journal of The American Chemical Society, 2019, 141(11): 4624-4633. |
| 47 | JEONG Hyung Mo, KWON Youngkook, Jong Ho WON, et al. Atomic-scale spacing between copper facets for the electrochemical reduction of carbon dioxide[J]. Advanced Energy Materials, 2020, 10: 10. |
| 48 | ZHU Wenlei, ZHANG Yinjia, ZHANG Hongyi, et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires[J]. Journal of the American Chemical Society, 2014, 136(46): 16132-16135. |
| 49 | LI Yifan, CUI Fan, ROSS M B, et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires[J]. Nano Lett, 2017, 17(2): 1312-1317. |
| 50 | LUC W, FU Xianbiao, SHI Jianjian, et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate[J]. Nature Catalysis, 2019, 2(5): 423-430. |
| 51 | XIE Jiafang, HUANG Yuxi, LI Wenwei, et al. Efficient electrochemical CO2 reduction on a unique chrysanthemum-like Cu nanoflower electrode and direct observation of carbon deposite[J]. Electrochimica Acta, 2014, 139: 137-144. |
| 52 | LUO Wen, XIE Wei, LI Mo, et al. 3D hierarchical porous indium catalyst for highly efficient electroreduction of CO2[J]. Journal of Materials Chemistry A, 2019, 7(9): 4505-4515. |
| 53 | RASUL S, ANJUM D H, JEDIDI A, et al. A highly selective copper-indium bimetallic electrocatalyst for the electrochemical reduction of aqueous CO2 to CO[J]. Angewandte Chemie: International Edition, 2015, 54(7): 2146-2150. |
| 54 | CHEN Dong, WANG Yanlei, LIU Danye, et al. Surface composition dominates the electrocatalytic reduction of CO2 on ultrafine CuPd nanoalloys[J]. Carbon Energy, 2020, DOI: 1002/cey2.38. |
| 55 | YE Ke, CAO Ang, SHAO Jiaqi, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity[J]. Science Bulletin, 2020, 65(9): 711-719. |
| 56 | KWON Ik Seon, DEBELA T T, KWAK In Hye, et al. Selective electrochemical reduction of carbon dioxide to formic acid using indium-zinc bimetallic nanocrystals[J]. Journal of Materials Chemistry A, 2019, 7(40): 22879-22883. |
| 57 | HOANG T T H, VERMA S, MA Sichao, et al. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol[J]. Journal of the American Chemical Society, 2018, 140(17): 5791-5797. |
| 58 | SHEN Sibo, PENG Xianyun, SONG Lida, et al. AuCu alloy nanoparticle embedded Cu submicrocone arrays for selective conversion of CO2 to ethanol[J]. Small, 2019, 15(37): 7. |
| 59 | CHEN Y H, LI C W, KANAN M W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles[J]. Journal of the American Chemical Society, 2012, 134(49): 19969-19972. |
| 60 | REN Dan, DENG Yilin, HANDOKO A D, et al. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(Ⅰ) oxide catalysts[J]. ACS Catalysis, 2015, 5(5): 2814-2821. |
| 61 | MISTRY H, VARELA A S, BONIFACIO C S, et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene[J]. Nature Communications, 2016, 7(1): 12123 |
| 62 | GAO Dunfeng, ZEGKINOGLOU I, DIVINS N J, et al. Plasma-activated copper nanocube catalysts for efficient carbon dioxide electroreduction to hydrocarbons and alcohols[J]. ACS Nano, 2017, 11(5): 4825-4831. |
| 63 | SONG Yang, PENG Rui, HENSLEY D K, et al. High-selectivity electrochemical conversion of CO2 to ethanol using a copper nanoparticle/N-doped graphene electrode[J]. ChemistrySelect, 2016, 1(19): 6055-6061. |
| 64 | ZHANG Z R, AHMAD F, ZHAO W H, et al. Enhanced electrocatalytic reduction of CO2via chemical coupling between indium oxide and reduced graphene oxide[J]. Nano Letters, 2019, 19(6): 4029-4034. |
| 65 | Kuilin LYU, FAN Yanchen, ZHU Ying, et al. Elastic Ag-anchored N-doped graphene/carbon foam for the selective electrochemical reduction of carbon dioxide to ethanol[J]. Journal of Materials Chemistry A, 2018, 6(12): 5025-5031. |
| 66 | HUO Yajiao, PENG Xianyun, LIU Xijun, et al. High selectivity toward C2H4 production over Cu particles supported by butterfly-wing-derived carbon frameworks[J]. ACS Applied Materials & Interfaces, 2018, 10(15): 12618-12625. |
| 67 | ZHAO Changming, DAI Xinyao, YAO Tao, et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2[J]. Journal of the American Chemical Society, 2017, 139(24): 8078-8081. |
| 68 | PAN Fuping, ZHANG Hanguang, LIU Zhenyu, et al. Atomic-level active sites of efficient imidazolate framework-derived nickel catalysts for CO2 reduction[J]. Journal of Materials Chemistry A, 2019, 7(46): 26231-26237. |
| 69 | WANG Xiaoqian, CHEN Zhao, ZHAO Xuyan, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2[J]. Angewandte Chemie: International Edition, 2018, 57(7): 1944-1948. |
| 70 | JIANG Kun, SIAHROSTAMI S, ZHENG Tingting, et al. Isolated Ni single atoms in graphene nanosheets for highperformance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 71 | ZHAO Shiyong, CHEN Guangxu, ZHOU Guangmin, et al. A universal seeding strategy to synthesize single atom catalysts on 2D materials for electrocatalytic applications[J]. Advanced Functional Materials, 2020, 30(6): 1906157. |
| 72 | RESKE R, MISTRY H, BEHAFARID F, et al. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles[J]. Journal of the American Chemical Society, 2014, 136(19): 6978-6986. |
| 73 | MISTRY H, BEHAFARID F, RESKE R, et al. Tuning catalytic selectivity at the mesoscale via interparticle interactions[J]. ACS Catalysis, 2016, 6(2): 1075-1080. |
| 74 | HOSSAIN M N, CHEN Shuai, CHEN Aicheng. Thermal-assisted synthesis of unique Cu nanodendrites for the efficient electrochemical reduction of CO2[J]. Applied Catalysis B: Environmental, 2019, 259: 118096. |
| 75 | KORTLEVER R, PETERS I, BALEMANS C, et al. Palladium-gold catalyst for the electrochemical reduction of CO2 to C1-C5 hydrocarbons[J]. Chemical Communications, 2016, 52(67): 10229-10232. |
| 76 | FRESE K W. Electrochemical reduction of CO2 at intentionally oxidized copper electrodes[J]. Journal of the Electrochemical Society, 1991, 138(11): 3338-3344. |
| 77 | LI C W, KANAN M W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films[J]. Journal of the American Chemical Society, 2012, 134(17): 7231-7234. |
| 78 | ZHAO Jian, XUE Song, BARBER J, et al. An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction[J]. Journal of Materials Chemistry A, 2020, 8(9): 4700-4734. |
| 79 | SCHOLTEN F, SINEV I, BERNAL M, et al. Plasma-modified dendritic Cu catalyst for CO2 electroreduction[J]. ACS Catalysis, 2019, 9(6): 5496-5502. |
| 80 | ENGELBRECHT A, HÄMMERLE M, MOOS R, et al. Improvement of the selectivity of the electrochemical conversion of CO2 to hydrocarbons using cupreous electrodes with in-situ oxidation by oxygen[J]. Electrochimica Acta, 2017, 224: 642-648. |
| 81 | MANDAL L, YANG Ke R, MOTAPOTHULA M R, et al. Investigating the role of copper oxide in electrochemical CO2 reduction in real time[J]. ACS Applied Materials & Interfaces, 2018, 10(10): 8574-8584. |
| 82 | Yanwei LUM, AGER Joel W. Stability of residual oxides in oxide-derived copper catalysts for electrochemical CO2 reduction investigated with 18O labeling[J]. Angewandte Chemie: International Edition, 2018, 57(2): 551-554. |
| 83 | FAN Lei, XIA Chuan, YANG Fangqi, et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products[J]. Science Advances, 2020, 6(8): 17. |
| 84 | XIAO Hai, GODDARD W A, CHENG Tao, et al. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(26): 6685-6688. |
| 85 | FAVARO M, XIAO Hai, CHENG Tao, et al. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(26): 6706-6711. |
| 86 | ZHOU Yansong, CHE Fanglin, LIU Min, et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons[J]. Nature Chemistry, 2018, 10(9): 974-980. |
| 87 | JIANG Kun, SANDBERG R B, AKEY A J, et al. Metal ion cycling of Cu foil for selective C-C coupling in electrochemical CO2 reduction[J]. Nature Catalysis, 2018, 1(2): 111-119. |
| 88 | QIAO Botao, WANG Aiqin, YANG Xiaofeng, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 89 | YE Yifan, CAI Fan, LI Haobo, et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction[J]. Nano Energy, 2017, 38: 281-289. |
| 90 | WANG Yifei, CHEN Zheng, HAN Peng, et al. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4[J]. ACS Catalysis, 2018, 8(8): 7113-7119. |
| 91 | JIAO Yan, ZHENG Yao, CHEN Ping, et al. Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol[J]. Journal of the American Chemical Society, 2017, 139(49): 18093-18100. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [7] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [8] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [9] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [12] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||