化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1371-1383.DOI: 10.16085/j.issn.1000-6613.2020-0875
固-固相变储热材料的研究进展
- 1.华南理工大学化学与化工学院,教育部强化传热与节能重点实验室,广东 广州 510640
2.广东省高效蓄热与应用工程技术研究中心,广东 广州 510640
-
收稿日期:2020-05-20出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:方晓明 -
作者简介:周四丽(1993—),女,硕士研究生,研究方向为复合相变材料及其应用。E-mail:2741950361@qq.com 。 -
基金资助:国家自然科学基金(U1407132);国家重点研发计划(2020YFA0210704)
Research progress of solid-solid phase change materials for thermal energy storage
ZHOU Sili1( ), ZHANG Zhengguo1,2, FANG Xiaoming1,2(
), ZHANG Zhengguo1,2, FANG Xiaoming1,2( )
)
- 1.Key Laboratory of Enhanced Heat Transfer and Energy Conservation, the Ministry of Education, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, Guangdong, China
2.Guangdong Engineering Technology Research Center of Efficient Heat Storage and Application, South China University of Technology, Guangzhou 510640, Guangdong, China
-
Received:2020-05-20Online:2021-03-05Published:2021-03-17 -
Contact:FANG Xiaoming
摘要:
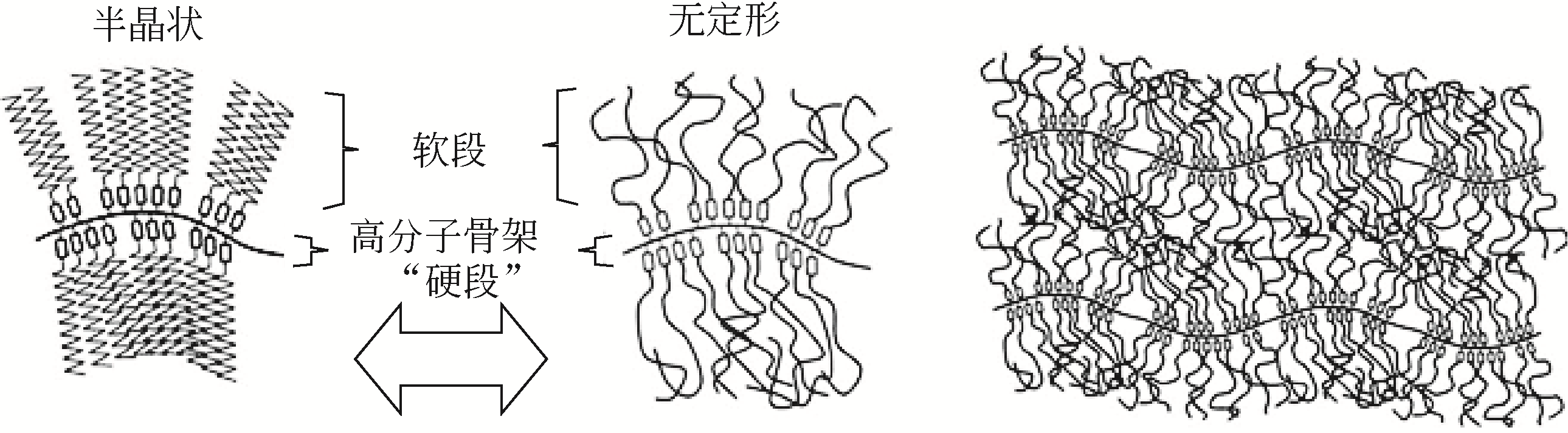
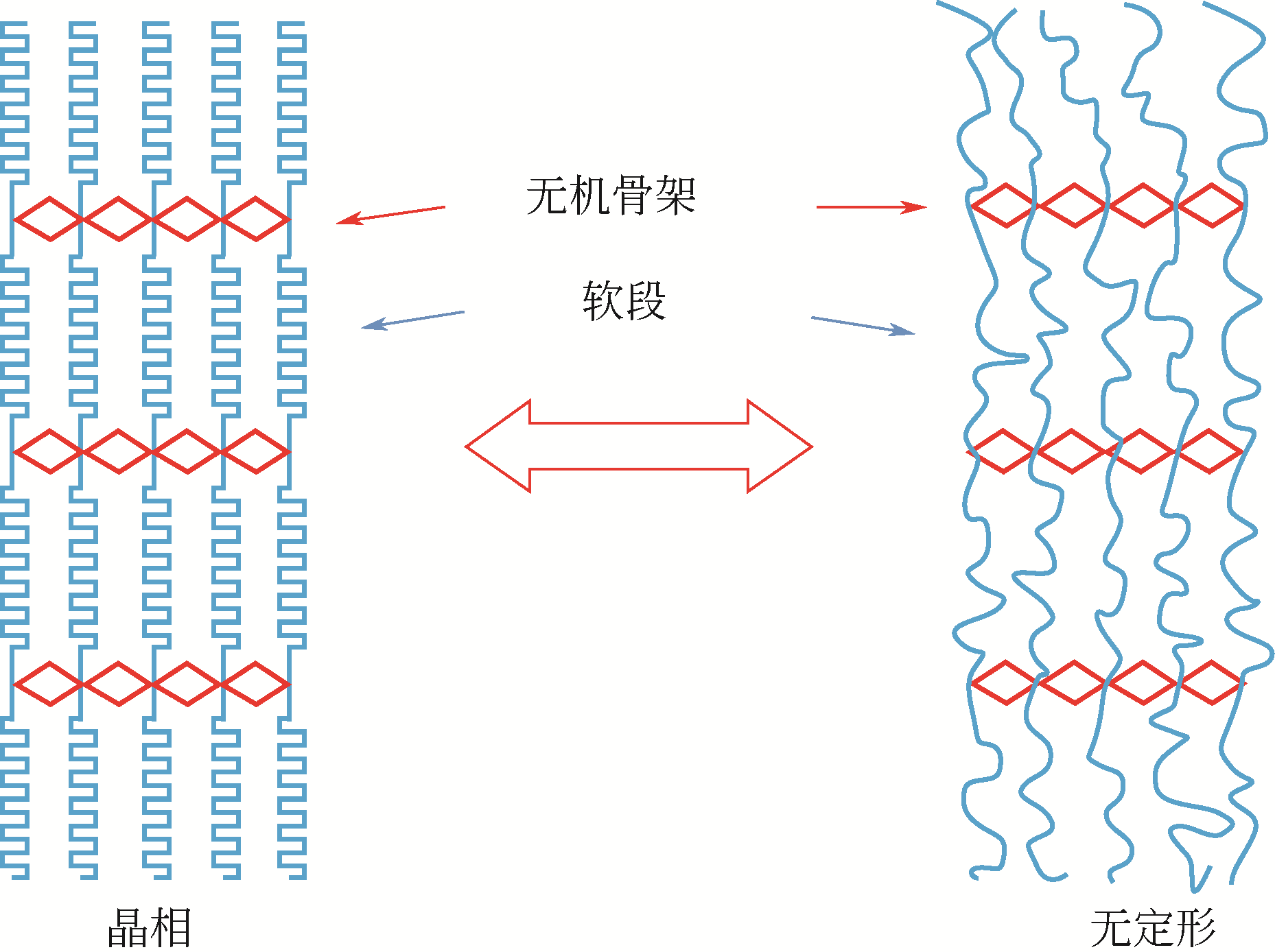
与固-液相变材料相比,固-固相变材料(SS-PCMs)受到的关注较少;鉴于SS-PCMs具有储能密度高、无毒且腐蚀性小、相变时无液体产生且体积变化较小、不易发生相分离以及过冷度小等优点,因而是一类具有发展潜力的相变材料。本文基于SS-PCMs的研究现状,对近年来几类重要SS-PCMs如多元醇SS-PCMs、高分子类SS-PCMs及无机盐类SS-PCMs的研究进展进行了综述。简要阐述了SS-PCMs的分类以及各类SS-PCMs的性能、相变储热机制和优缺点。同时介绍了选择固-固相变材料应用时的基本原则,并针对相变材料热导率低,过冷度大、稳定性差等问题的改性研究进行了综述,还简要综述了SS-PCMs的应用研究。最后指出,未来的研究应着眼于解决已合成SS-PCMs的缺陷,开发多功能的SS-PCMs,并在SS-PCMs的实际应用方面实现突破。
中图分类号:
引用本文
周四丽, 张正国, 方晓明. 固-固相变储热材料的研究进展[J]. 化工进展, 2021, 40(3): 1371-1383.
ZHOU Sili, ZHANG Zhengguo, FANG Xiaoming. Research progress of solid-solid phase change materials for thermal energy storage[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1371-1383.
| 多元醇 | 固-固转变 | 固-液转变 | ||
|---|---|---|---|---|
| 相变温度/℃ | 转变热/J·g-1 | 相变温度/℃ | 转变热/J·g-1 | |
| 2-氨基-2-甲基-1,3-丙二醇(AMP) | 56.96 过冷严重 | 114.06 — | 108.72 102.25 | 28.02(加热) 25.61(冷却) |
| 新戊二醇(NPG) | 44.09 17.51 | 116.54 81.90 | 123.05 119.04 | 28.80(加热) 25.61(冷却) |
| 三羟甲基乙烷(PG) | 81.76 | 172.58 | 199.63 | 42.65(加热) |
| 三羟甲基氨基甲烷(TAM) | 133.83 | 270.31 | 171.72 | 24.99(加热) |
| 季戊四醇(PE) | 185.45 165.19 | 209.45 180.93 | 257.07 244.99 | 33.61(加热) 13.70(冷却) |
表1 多元醇SS-PCMs的热性能[9]
| 多元醇 | 固-固转变 | 固-液转变 | ||
|---|---|---|---|---|
| 相变温度/℃ | 转变热/J·g-1 | 相变温度/℃ | 转变热/J·g-1 | |
| 2-氨基-2-甲基-1,3-丙二醇(AMP) | 56.96 过冷严重 | 114.06 — | 108.72 102.25 | 28.02(加热) 25.61(冷却) |
| 新戊二醇(NPG) | 44.09 17.51 | 116.54 81.90 | 123.05 119.04 | 28.80(加热) 25.61(冷却) |
| 三羟甲基乙烷(PG) | 81.76 | 172.58 | 199.63 | 42.65(加热) |
| 三羟甲基氨基甲烷(TAM) | 133.83 | 270.31 | 171.72 | 24.99(加热) |
| 季戊四醇(PE) | 185.45 165.19 | 209.45 180.93 | 257.07 244.99 | 33.61(加热) 13.70(冷却) |
| PCM | 熔化温度/℃ | 熔化焓/J·g-1 | 结晶温度/℃ | 结晶焓/J·g-1 | 升温速率/℃·min-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 纤维素-g-PEG | 40.7~60.1 | 77.6~203.2 | 25.1~40.6 | 44.7~203.2 | 10 | [ |
| CDA/PEG4000 | 40 | 73.6 | — | — | 5 | [ |
| NCC/PEG4000 | 47.2 | 103.8 | — | — | 5 | [ |
| 纤维素-g-mPEG | 40.2~50.4 | 133.0~175.4 | 12.2~28.9 | 97.8~157.7 | 10 | [ |
| HB-PUPCM6000 | 57.5~67.0 | 91.2~138.2 | 7.8~33.2 | 89.4~132.3 | 10 | [ |
| HB-PUPCM6000 | 66.6 | 124.8 | 33.1 | 121.4 | 10 | [ |
| HB-PUPCM | 56.15~56.99 | 117.1~146.6 | 36.61~36.98 | 88.35~125.6 | 10/5 | [ |
| PEG10000-MDI-BDO | 65.28 | 138.7 | 38.58 | 126.2 | 10 | [ |
| PEG6000-MDI-BDO/SA | 65.28 | 152.7 | — | — | 10 | [ |
| D-HBPU | 59.0 | 125.0 | 36.8 | 120.6 | 10 | [ |
| TPUPCM | 56.1 | 137.4 | 37.5 | 127.6 | — | [ |
| PU高聚物-6 | 57.1 | 142.5 | 33.2 | 137.7 | 10 | [ |
| PU高聚物-10 | 60.8 | 152.3 | 34.8 | 149.7 | 10 | [ |
| SPCM | 44.3 | 122.4 | 37.2 | 120.3 | 10 | [ |
| TPCM | 47.8 | 127.7 | 38.5 | 130.2 | 10 | [ |
| PEG/MDI/丙三醇 | 41.8 | 128.5 | 38.1 | 112.3 | 10 | [ |
| PEG/MDI/TMP | 42.5 | 119.3 | 38.3 | 106.7 | 10 | [ |
| PEG6000/PAPI | 50.48 | 111.7 | 41.38 | 110.4 | 10 | [ |
| BPU | 43.4/52.8 | 86.0/114.9 | 24.5/28.8 | 83.1/111.2 | 10 | [ |
| NPU | 55.8/55.7 | 136.5/137.5 | 27.7/33.6 | 128.0/129.8 | 10 | [ |
| Eu-PEG | 62.46~72.13 | 19.83~96.62 | 24.96~44.89 | 16.38~83.18 | — | [ |
| 聚苯乙烯-g-PEG6000 | 44.95~58.04 | 111.48~179.47 | 34.36~38.48 | 99.43~143.6 | 5 | [ |
| SMA-g-PEG6000 | 39.69~45.03 | 107.04~155.21 | 23.68~33.56 | 109.86~142.62 | 10 | [ |
| DC-PCM | 40.6~71.0 | 44.3~87.8 | 7.5~39.2 | 29.3~107.2 | 5 | [ |
| Fe3+-PEG | — | 97.6 | — | 92.9 | 5 | [ |
| Poly(S-PA-S) | 18.72~21.47 | 26.20~39.78 | 17.65~18.72 | 20.02~39.19 | 5 | [ |
| SAN-g-PA | 24.7~36.1 | 11.7~24.4 | 23.9~33.8 | 11.6~23.7 | 10 | [ |
| SMA-g-SA | 67.2~67.4 | 59.3~126.7 | 62.9~63.8 | 60.7~125.0 | 5 | [ |
| SMA-g-PA | 61.0~61.6 | 41.7~119.8 | 55.6~57.1 | 42.4~117.7 | 5 | [ |
| SMA-g-MA | 53.0~54 | 35.7~109.7 | 45.8~47.5 | 32.4~114.8 | 5 | [ |
| SMA-g-LA | 42.3~43.7 | 17.6~84.2 | 37.3~38.7 | 13.4~74.8 | 5 | [ |
| MBA/PEGA | 52.99~57.15 | 74.13~168.96 | 30.59~38.27 | 42.01~145.97 | 10 | [ |
| 纤维素-g-E2C16 | 28.2~28.6 | 21~33 | 12.9~14.9 | 18~30 | 30/10 | [ |
| CA-g-PAn | 17.4~53.2 | 15~95 | 5.1~36.7 | 15~92 | 10 | [ |
表2 高分子类SS-PCMs的性能
| PCM | 熔化温度/℃ | 熔化焓/J·g-1 | 结晶温度/℃ | 结晶焓/J·g-1 | 升温速率/℃·min-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 纤维素-g-PEG | 40.7~60.1 | 77.6~203.2 | 25.1~40.6 | 44.7~203.2 | 10 | [ |
| CDA/PEG4000 | 40 | 73.6 | — | — | 5 | [ |
| NCC/PEG4000 | 47.2 | 103.8 | — | — | 5 | [ |
| 纤维素-g-mPEG | 40.2~50.4 | 133.0~175.4 | 12.2~28.9 | 97.8~157.7 | 10 | [ |
| HB-PUPCM6000 | 57.5~67.0 | 91.2~138.2 | 7.8~33.2 | 89.4~132.3 | 10 | [ |
| HB-PUPCM6000 | 66.6 | 124.8 | 33.1 | 121.4 | 10 | [ |
| HB-PUPCM | 56.15~56.99 | 117.1~146.6 | 36.61~36.98 | 88.35~125.6 | 10/5 | [ |
| PEG10000-MDI-BDO | 65.28 | 138.7 | 38.58 | 126.2 | 10 | [ |
| PEG6000-MDI-BDO/SA | 65.28 | 152.7 | — | — | 10 | [ |
| D-HBPU | 59.0 | 125.0 | 36.8 | 120.6 | 10 | [ |
| TPUPCM | 56.1 | 137.4 | 37.5 | 127.6 | — | [ |
| PU高聚物-6 | 57.1 | 142.5 | 33.2 | 137.7 | 10 | [ |
| PU高聚物-10 | 60.8 | 152.3 | 34.8 | 149.7 | 10 | [ |
| SPCM | 44.3 | 122.4 | 37.2 | 120.3 | 10 | [ |
| TPCM | 47.8 | 127.7 | 38.5 | 130.2 | 10 | [ |
| PEG/MDI/丙三醇 | 41.8 | 128.5 | 38.1 | 112.3 | 10 | [ |
| PEG/MDI/TMP | 42.5 | 119.3 | 38.3 | 106.7 | 10 | [ |
| PEG6000/PAPI | 50.48 | 111.7 | 41.38 | 110.4 | 10 | [ |
| BPU | 43.4/52.8 | 86.0/114.9 | 24.5/28.8 | 83.1/111.2 | 10 | [ |
| NPU | 55.8/55.7 | 136.5/137.5 | 27.7/33.6 | 128.0/129.8 | 10 | [ |
| Eu-PEG | 62.46~72.13 | 19.83~96.62 | 24.96~44.89 | 16.38~83.18 | — | [ |
| 聚苯乙烯-g-PEG6000 | 44.95~58.04 | 111.48~179.47 | 34.36~38.48 | 99.43~143.6 | 5 | [ |
| SMA-g-PEG6000 | 39.69~45.03 | 107.04~155.21 | 23.68~33.56 | 109.86~142.62 | 10 | [ |
| DC-PCM | 40.6~71.0 | 44.3~87.8 | 7.5~39.2 | 29.3~107.2 | 5 | [ |
| Fe3+-PEG | — | 97.6 | — | 92.9 | 5 | [ |
| Poly(S-PA-S) | 18.72~21.47 | 26.20~39.78 | 17.65~18.72 | 20.02~39.19 | 5 | [ |
| SAN-g-PA | 24.7~36.1 | 11.7~24.4 | 23.9~33.8 | 11.6~23.7 | 10 | [ |
| SMA-g-SA | 67.2~67.4 | 59.3~126.7 | 62.9~63.8 | 60.7~125.0 | 5 | [ |
| SMA-g-PA | 61.0~61.6 | 41.7~119.8 | 55.6~57.1 | 42.4~117.7 | 5 | [ |
| SMA-g-MA | 53.0~54 | 35.7~109.7 | 45.8~47.5 | 32.4~114.8 | 5 | [ |
| SMA-g-LA | 42.3~43.7 | 17.6~84.2 | 37.3~38.7 | 13.4~74.8 | 5 | [ |
| MBA/PEGA | 52.99~57.15 | 74.13~168.96 | 30.59~38.27 | 42.01~145.97 | 10 | [ |
| 纤维素-g-E2C16 | 28.2~28.6 | 21~33 | 12.9~14.9 | 18~30 | 30/10 | [ |
| CA-g-PAn | 17.4~53.2 | 15~95 | 5.1~36.7 | 15~92 | 10 | [ |
| PCM | 相变温度/℃ | 相变焓/J·g-1 | 熔化温度/℃ | 熔化焓/J·g-1 | 参考文献 |
|---|---|---|---|---|---|
| (C12H25NH3)2ZnCl4 | 88.23 | 105.01 | — | — | [ |
| (C12H25NH3)2CoCl4 | 63.85,67.85 | 33.72,59.30 | — | — | [ |
| (C12H25NH3)2MnCl4 | 53.85,55.85 | 74.31,6.59 | — | — | [ |
| (C10H21NH3)2CuCl4 | 33.77 | 55.13 | 36.91 | 7.53 | [ |
| (C10H21NH3)2ZnCl4 | 80.14 | 82.90 | 162.79 | 18.14 | [ |
| (C10H21NH3)2CdCl4 | 27.0,61.0 | 34.5,8.25 | 121.0 | 13.13 | [ |
| (C10H21NH3)2HgCl4 | 36.73 | 28.02 | 203.0 | 50.42 | [ |
| (C10H21NH3)2MnCl4 | 32.80 | 77.44 | — | — | [ |
| (C10H21NH3)2CoCl4 | 77.71 | 74.38 | 165.0 | 13.70 | [ |
| (C10H21NH3)2NiCl4 | 32.0,58.0 | 15.2 | 135.0 | 65.76 | [ |
表3 部分(n-CnH2n+1NH3)2MCl4的相变性能
| PCM | 相变温度/℃ | 相变焓/J·g-1 | 熔化温度/℃ | 熔化焓/J·g-1 | 参考文献 |
|---|---|---|---|---|---|
| (C12H25NH3)2ZnCl4 | 88.23 | 105.01 | — | — | [ |
| (C12H25NH3)2CoCl4 | 63.85,67.85 | 33.72,59.30 | — | — | [ |
| (C12H25NH3)2MnCl4 | 53.85,55.85 | 74.31,6.59 | — | — | [ |
| (C10H21NH3)2CuCl4 | 33.77 | 55.13 | 36.91 | 7.53 | [ |
| (C10H21NH3)2ZnCl4 | 80.14 | 82.90 | 162.79 | 18.14 | [ |
| (C10H21NH3)2CdCl4 | 27.0,61.0 | 34.5,8.25 | 121.0 | 13.13 | [ |
| (C10H21NH3)2HgCl4 | 36.73 | 28.02 | 203.0 | 50.42 | [ |
| (C10H21NH3)2MnCl4 | 32.80 | 77.44 | — | — | [ |
| (C10H21NH3)2CoCl4 | 77.71 | 74.38 | 165.0 | 13.70 | [ |
| (C10H21NH3)2NiCl4 | 32.0,58.0 | 15.2 | 135.0 | 65.76 | [ |
| SS-PCM | 热导率/W·m-1·K-1 | 参考文献 |
|---|---|---|
| NPG | 0.12~0.24 | [ |
| PG | 0.232~0.36 | [ |
| PE | 0.106 | [ |
| 聚苯乙烯-g-PEG | 0.10~0.13 | [ |
| 聚(S-PA-S) | 0.13~0.2 | [ |
| 硬脂酸 | 0.18 | [ |
| S-AA-SE | 0.12~0.15 | [ |
| (n-C6H13)2NH2Br | 0.12~0.17 | [ |
表4 SS-PCMs的热导率
| SS-PCM | 热导率/W·m-1·K-1 | 参考文献 |
|---|---|---|
| NPG | 0.12~0.24 | [ |
| PG | 0.232~0.36 | [ |
| PE | 0.106 | [ |
| 聚苯乙烯-g-PEG | 0.10~0.13 | [ |
| 聚(S-PA-S) | 0.13~0.2 | [ |
| 硬脂酸 | 0.18 | [ |
| S-AA-SE | 0.12~0.15 | [ |
| (n-C6H13)2NH2Br | 0.12~0.17 | [ |
| SS-PCM | 过冷度/℃ | 参考文献 |
|---|---|---|
| PE | 24.3 | [ |
| NPG | 43.3 | [ |
| (C8H17NH3)2ZnCl4 | 9.9 | [ |
| (C10H21NH3)2ZnCl4 | 6.5 | [ |
| (C14H29NH3)2ZnCl4 | 6.6 | [ |
| (C8H17NH3)2CoCl4 | 9.3 | [ |
| (C8H17NH3)2CaCl4 | 8.4 | [ |
| (C10H21NH3)2CaCl4 | 8.7 | [ |
| (C14H29NH3)2CaCl4 | 9.1 | [ |
| (C18H37NH3)2CaCl4 | 9.5 | [ |
| (C10H21NH3)2ZnCl4-(C10H21NH3)2CoCl4 | 7 | [ |
| (C18H37NH3)2CuCl4-(C18H37NH3)2MnCl4 | 9 | [ |
| (C16H33NH3)2CuCl4-(C18H37NH3)2CoCl4 | 8 | [ |
| (C10H21NH3)2CoCl4-(C10H21NH3)2CaCl4 | 9 | [ |
| (C8H17NH3)2MnCl4 | 0.6 | [ |
| (C10H21NH3)2MnCl4 | 1.3 | [ |
| (C12H25NH3)2MnCl4 | 1.4 | [ |
表5 SS-PCMs的过冷度
| SS-PCM | 过冷度/℃ | 参考文献 |
|---|---|---|
| PE | 24.3 | [ |
| NPG | 43.3 | [ |
| (C8H17NH3)2ZnCl4 | 9.9 | [ |
| (C10H21NH3)2ZnCl4 | 6.5 | [ |
| (C14H29NH3)2ZnCl4 | 6.6 | [ |
| (C8H17NH3)2CoCl4 | 9.3 | [ |
| (C8H17NH3)2CaCl4 | 8.4 | [ |
| (C10H21NH3)2CaCl4 | 8.7 | [ |
| (C14H29NH3)2CaCl4 | 9.1 | [ |
| (C18H37NH3)2CaCl4 | 9.5 | [ |
| (C10H21NH3)2ZnCl4-(C10H21NH3)2CoCl4 | 7 | [ |
| (C18H37NH3)2CuCl4-(C18H37NH3)2MnCl4 | 9 | [ |
| (C16H33NH3)2CuCl4-(C18H37NH3)2CoCl4 | 8 | [ |
| (C10H21NH3)2CoCl4-(C10H21NH3)2CaCl4 | 9 | [ |
| (C8H17NH3)2MnCl4 | 0.6 | [ |
| (C10H21NH3)2MnCl4 | 1.3 | [ |
| (C12H25NH3)2MnCl4 | 1.4 | [ |
| 1 | 黄睿, 方晓明, 凌子夜, 等. 高性能三水醋酸钠-尿素-膨胀石墨混合相变材料的制备及其在电地暖中的应用性能[J]. 化工学报, 2020, 71(6): 2713-2723. |
| HUANG Rui, FANG Xiaoming, LING Ziye, et al. Preparation of high-performance sodium acetate trihydrate-urea-expanded graphite mixed phase change material and its application performance in electric floor heating[J]. CIESC Journal, 2020, 71(6): 2713-2723. | |
| 2 | RAJ C R, SURESH S, BHAVSAR R R, et al. Recent developments in thermo-physical property enhancement and applications of solid solid phase change materials[J]. Journal of Thermal Analysis and Calorimetry, 2019, 139(5): 3023-3049. |
| 3 | 李志生, 张姝婷. 相变材料的研究进展与应用综述[J]. 建筑节能, 2016, 44(4): 57-60, 107. |
| LI Zhisheng, ZHANG Shuting. Research progress and application of phase change materials[J]. Building Energy Efficiency, 2016, 44 (4): 57-60, 107. | |
| 4 | 于少明, 蒋长龙, 杭国培, 等. 固固相变贮能材料研究现状与进展[J]. 化工新型材料, 2002(7): 19-21. |
| YU Shaoming, JIANG Changlong, HANG Guopei, et al. Current situation and progress in studies of solid-solid phase change materials for energy storage[J]. New Chemical Materials, 2002(7): 19-21. | |
| 5 | FALLAHI A, GULDENTOPS G, TAO Mingjiang, et al. Review on solid-solid phase change materials for thermal energy storage: molecular structure and thermal properties[J]. Applied Thermal Engineering, 2017, 127: 1427-1441. |
| 6 | 樊耀峰, 张兴祥. 有机固-固相变材料的研究进展[J]. 材料导报, 2003(7): 50-53, 81. |
| FAN Yaofeng, ZHANG Xingxiang. Progress in studies of solid-solid phase change materials[J]. Materials Review, 2003(7): 50-53, 81. | |
| 7 | 王小伍, 徐海红. 多元醇固-固相变机理的研究[J]. 物理学报, 2011, 60(3): 136-139. |
| WANG Xiaowu, XU Haihong. Mechanism of polyalcohol solid-solid phase change[J]. Acta Physica Sinica, 2011, 60(3): 136-139. | |
| 8 | 邢澄清, 迟广山, 阮德水, 等. 多元醇二元体系固-固相变贮热的研究[J]. 太阳能学报, 1995(2): 131-137. |
| XING Dengqing, CHI Guangshan, Ruan Deshui, et al. Solid state phase transition in binary systems of polyhydric alcohols[J]. Acta Energiae Solaris Sinica, 1995(2): 131-137. | |
| 9 | 阮德水, 张太平, 张道圣, 等. 相变贮热材料的DSC研究[J]. 太阳能学报, 1994(1): 19-24. |
| RUAN Deshui, ZHANG Taiping, ZHANG Daosheng, et al. DSC investigation of phase change materials[J]. Acta Energiae Solaris Sinica, 1994(1): 19-24. | |
| 10 | FONT J, MUNTASELL J, NAVARRO J, et al. Time and temperature dependence of the exchanged energy on solid-solid transitions of pentaglycerine/neopentylglycol mixtures[J]. Solar Energy Materials, 1987, 15(5): 403-412. |
| 11 | FONT J, MUNTASELL J, NAVARRO J, et al. Calorimetric study of the mixtures PE/NPG and PG/NPG[J]. Solar Energy Materials, 1987, 15(4): 299-310. |
| 12 | 闫全英, 王威. 用于墙体中的固-固相变材料储热性能的研究[J]. 能源研究与利用, 2005(1): 19-20, 23. |
| YAN Quanying, WANG Wei. Research on the thermal storage performance of solid-solid phase-change material of wall[J]. Energy research and utilization, 2005(1): 19-20, 23. | |
| 13 | GAO Wenfeng, LIN Wenxian, LIU Tao, et al. An experimental study on the heat storage performances of polyalcohols NPG, TAM, PE, and AMPD and their mixtures as solid-solid phase-change materials for solar energy applications[J]. International Journal of Green Energy, 2007, 4(3): 301-311. |
| 14 | YAN Quanying, LIANG Chen. The thermal storage performance of monobasic, binary and triatomic polyalcohols systems[J]. Solar Energy, 2008, 82 (7): 656-662. |
| 15 | WANG Xiaowu, LU Enrong, LIN Wenxian, et al. Micromechanism of heat storage in a binary system of two kinds of polyalcohols as a solid-solid phase change material[J]. Energy Conversion and Management, 2000, 41(2): 135-144. |
| 16 | WANG Xiaowu, LU Enrong, LIN Wenxian, et al. Heat storage performance of the binary systems neopentyl glycol/pentaerythritol and neopentyl glycol/trihydroxy methyl-aminomethane as solid-solid phase change materials[J]. Energy Conversion and Management, 2000, 41(2): 129-134. |
| 17 | VENKITARAJ K P, SURESH S, PRAVEEN B, et al. Pentaerythritol with alumina nano additives for thermal energy storage applications[J]. Journal of Energy Storage, 2017, 13: 359-377. |
| 18 | CAO Fangyu, YE Jing, YANG Bao. Synthesis and characterization of solid-state phase change material microcapsules for thermal management applications[J]. Journal of Nanotechnology in Engineering and Medicine, 2014, 4(4): 040901-040905. |
| 19 | 杨磊, 姚远, 张冬冬, 等. 有机相变储能材料的研究进展[J]. 新能源进展, 2019, 7(5): 464-472. |
| YANG Lei, YAO Yuan, ZHANG Dongdong, et al. Progress of organic phase change energy storage materials[J]. Advances in New and Renewable Energy, 2019, 7 (5): 464-472. | |
| 20 | 钟秋, 张威, 赵春芳, 等. 固-固相变储热材料的研究进展[J]. 广州化工, 2016, 44(23): 4-6. |
| ZHONG Qiu, ZHANG Wei, ZHAO Chunfang, et al. Research progress on polymer solid-solid phase transition materials for thermal energy storage[J]. GuangZhou Chemical Industry and Technology, 2016, 44(23): 4-6. | |
| 21 | LI Yanxiang, WU Min, LIU Ruigang, et al. Cellulose-based solid-solid phase change materials synthesized in ionic liquid[J]. Solar Energy Materials and Solar Cells, 2009, 93(8): 1321-1328. |
| 22 | 姜勇, 丁恩勇, 杨玉芹, 等. 化学法和共混法制备的PEG/CDA相变材料的性能比较——微相结构与储热性能的关系[J]. 纤维素科学与技术, 2000(2): 36-41, 12. |
| JIANG Yong, DING Enyong, YANG Yuqin, et al. Performance comparison of PEG/CDA phase change materials prepared by chemical method and blending method — The relationship between microphase structure and heat storage performance[J]. Journal of Cellulose Science and Technology, 2000(2): 36-41, 12. | |
| 23 | 姜勇, 丁恩勇, 黎国康. 化学法和共混法制备的PEG/CDA相变材料的性能比较——储热性能与链结构的关系[J]. 纤维素科学与技术, 2000(1): 17-25. |
| JIANG Yong, DING Enyong, LI Guokang. Performance comparison of PEG/CDA phase change materials prepared by chemical method and blending method — the relationship between heat storage performance and chain structure[J]. Journal of Cellulose Science and Technology, 2000(1): 17-25. | |
| 24 | YUAN Xiaoping, DING Enyong. Synthesis and characterization of storage energy materials prepared from nano-crystalline cellulose/polyethylene glycol[J]. Chinese Chemical Letters, 2006, 17(8): 1129-1132. |
| 25 | 原小平, 丁恩勇. 纳米纤维素/聚乙二醇固-固相变材料的制备及其储能性能的研究[J]. 林产化学与工业, 2007(2): 67-70. |
| YUAN Xiaoping, DING Enyong. Preparation of nano-crystalline cellulose/polyethylene glycol solid-solid phase transition material and its energy storage characteristics[J]. Chemistry & Industry of Forest Products, 2007(2): 67-70. | |
| 26 | LI Yanxiang, LIU Ruigang, HUANG Yong. Synthesis and phase transition of cellulose-graft-poly(ethylene glycol) copolymers[J]. Journal of Applied Polymer Science, 2008, 110(3): 1797-1803. |
| 27 | CAO Qi, LIU Pengsheng. Hyperbranched polyurethane as novel solid-solid phase change material for thermal energy storage[J]. European Polymer Journal, 2006, 42(11): 2931-2939. |
| 28 | LIAO Li, CAO Qi, LIAO Hongqing. Investigation of a hyperbranched polyurethane as a solid-state phase change material[J]. Journal of Materials Science, 2010, 45(9): 2436-2441. |
| 29 | SUNDARARAJAN S, SAMUI A B, KULKARNI P S. Thermal energy storage using poly(ethylene glycol) incorporated hyperbranched polyurethane as solid-solid phase change material[J]. Industrial & Engineering Chemistry Research, 2017, 56(49): 14401-14409. |
| 30 | SU Jingcang, LIU Pengsheng. A novel solid-solid phase change heat storage material with polyurethane block copolymer structure[J]. Energy Conversion and Management, 2006, 47(18/19): 3185-3191. |
| 31 | 粟劲苍, 刘朋生. 微相分离促进剂对聚氨酯相变储能材料热性能的影响[J]. 中国塑料, 2006(4): 55-58. |
| SU Jingcang, LIU Pengsheng. Effect of micro-phase separation promoter on thermal behavior of phase change heat storage polyurethane materials[J]. China Plastics, 2006(4): 55-58. | |
| 32 | XI Peng, DUAN Yuqing, FEI Pengfei, et al. Synthesis and thermal energy storage properties of the polyurethane solid-solid phase change materials with a novel tetrahydroxy compound[J]. European Polymer Journal, 2012, 48(7): 1295-1303. |
| 33 | CHEN Changzhong, LIU Wenmin, WANG Hongwei, et al. Synthesis and performances of novel solid-solid phase change materials with hexahydroxy compounds for thermal energy storage[J]. Applied Energy, 2015, 152: 198-206. |
| 34 | CHEN Kai, LIU Ruowang, ZOU Chao, et al. Linear polyurethane ionomers as solid-solid phase change materials for thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2014, 130: 466-473. |
| 35 | CAO Hongwei, QI Feixuanyu, LIU Ruowang, et al. The influence of hydrogen bonding on N-methyldiethanolamine-extended polyurethane solid-solid phase change materials for energy storage[J]. RSC Advances, 2017, 7(19): 11244-11252. |
| 36 | YUAN Ye, WU Bo, JIANG Liang, et al. Nonconstant enthalpy of thermosetting solid-solid phase change materials controlled by light[J]. Energy and Buildings, 2020, 214: 109894. |
| 37 | ZANG Nan, DING Enyong. Energy storage properties of phase change materials prepared from PEG/CPP[J]. Chinese Chemical Letters, 2005, (10): 1375-1378. |
| 38 | GU Xiaohua, XI Peng, CHENG Bowen, et al. Synthesis and characterization of a novel solid-solid phase change luminescence material[J]. Polymer International, 2010, 59(6): 772-777. |
| 39 | SARI A, ALKAN C, BIÇER A. Synthesis and thermal properties of polystyrene-graft-PEG copolymers as new kinds of solid-solid phase change materials for thermal energy storage[J]. Materials Chemistry and Physics, 2012, 133(1): 87-94. |
| 40 | SARI A, BIÇER A, ALKAN C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid-solid phase change materials[J]. Solar Energy Materials and Solar Cells, 2017, 161: 219-225. |
| 41 | WU Bo, WANG Yi, LIU Zhimeng, et al. Thermally reliable, recyclable and malleable solid-solid phase-change materials through the classical Diels-Alder reaction for sustainable thermal energy storage[J]. Journal of Materials Chemistry A, 2019, 7(38): 21802-21811. |
| 42 | WANG Rui, XIAO Yao, LEI Jingxin. A solid-solid phase change material based on dynamic ion cross-linking with reprocessability at room temperature[J]. Chemical Engineering Journal, 2020, 390: 124586. |
| 43 | SARI A, ALKAN C, BIÇER A, et al. Synthesis and thermal energy storage characteristics of polystyrene-graft-palmitic acid copolymers as solid-solid phase change materials[J]. Solar Energy Materials and Solar Cells, 2011, 95(12): 3195-3201. |
| 44 | SARI A, ALKAN C, LAFÇI Ö. Synthesis and thermal properties of poly(styrene-co-ally alcohol)-graft-stearic acid copolymers as novel solid-solid PCMs for thermal energy storage[J]. Solar Energy, 2012, 86(9): 2282-2292. |
| 45 | SARI A, BIÇER A, ALKAN C. Poly(styrene-co-maleic anhydride)-graft-fatty acids as novel solid-solid PCMs for thermal energy storage[J]. Polymer Engineering and Science, 2019, 59(s2): E337-E347. |
| 46 | MU Siyang, GUO Jing, YU Chunfang, et al. A novel solid-solid phase change material based on poly(styrene-co-acrylonitrile) grafting with palmitic acid copolymers[J]. Journal of Macromolecular Science A, 2015, 52(8): 617-624. |
| 47 | ZHANG Hong, SUN Dike, WANG Qianqian, et al. Synthesis and characterization of polyethylene glycol acrylate crosslinking copolymer as solid-solid phase change materials[J]. Journal of Applied Polymer Science, 2014, 131(6). |
| 48 | HAN Na, LI Zhinan, ZHANG Xingxiang, et al. Synthesis and characterization of cellulose-g-polyoxyethylene (2) hexadecyl ether solid-solid phase change materials[J]. Cellulose, 2016, 23(3): 1663-1674. |
| 49 | ALKAN C, RATHGEBER C, HENNEMANN P, et al. Poly(ethylene-co-1-tetradecylacrylate) and poly(ethylene-co-1-octadecylacrylate) copolymers as novel solid-solid phase change materials for thermal energy storage[J]. Polymer Bulletin, 2018, 76(4): 2021-2039. |
| 50 | QIAN Yongqiang, HAN Na, BO Yiwen, et al. Homogeneous synthesis of cellulose acrylate-g-poly(n-alkyl acrylate) solid-solid phase change materials via free radical polymerization[J]. Carbohydr. Polym., 2018, 193: 129-136. |
| 51 | CAO Qi, LIU Pengsheng. Crystalline-amorphous phase transition of hyperbranched polyurethane phase change materials for energy storage[J]. Journal of Materials Science, 2007, 42(14): 5661-5665. |
| 52 | FU Xiaowei, KONG Weibo, ZHANG Yanyan, et al. Novel solid-solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage[J]. RSC Advances, 2015, 5(84): 68881-68889. |
| 53 | FU Xiaowei, ZHANG Yanyan, KONG Weibo, et al. Synthesis and properties of bulk-biodegradable phase change materials based on polyethylene glycol for thermal energy storage[J]. Journal of Thermal Analysis and Calorimetry, 2016, 128(2): 643-651. |
| 54 | KONG Weibo, FU Xiaowei, LIU Zhimeng, et al. A facile synthesis of solid-solid phase change material for thermal energy storage[J]. Applied Thermal Engineering, 2017, 117: 622-628. |
| 55 | 郭宁, 刘振海, 曾广赋, 等. [n-CnH2n+1NH3]2ZnCl4配合物相变研究[J]. 应用化学, 1991, 8(6): 53-56. |
| GUO Ning, LIU Zhenhai, ZENG Guangfu, et al. Phase transition of [n-CnH2n+1NH3]2ZnCl4[J]. Chinese Journal of Applied Chemistry, 1991, 8(6): 53-56. | |
| 56 | GUO Ning, ZENG Guangfu, XI Shiquan. An infrared spectroscopic study of the structural phase transition in the perovskite-type layer compound [n-C16H33NH3]2CoCl4[J]. Journal of Molecular Structure, 1992, 275: 85-94. |
| 57 | 阮德水, 邢澄清. 固-固相变贮热的研究——四氯合金属(Ⅱ)酸正十烷铵[J]. 华中师范大学学报(自然科学版), 1995, 29(2): 193-196. |
| RUAN Deshui, XING Chengqing, et al. Study of solid-solid phase change of decylammonium tetrachlorometallates(Ⅱ) for thermal energy storage[J]. Journal of Central China Normal University (Natural Sciences), 1995, 29(2): 193-196. | |
| 58 | LANDI E, VACATELLO M. Metal-dependent thermal behaviour in (n-CnH2n+1NH3)2MCl4[J]. Thermochimica Acta, 1975, 13(4): 441-447. |
| 59 | 李英品. 固-固相变储能物质——四氯合金属酸正烷基二铵及其二元体系的研究[D]. 石家庄: 河北师范大学, 2004. |
| LI Yingpin. Study of solid-solid phase change of tetrachlorometallic acid n-alkyl diammonium and its binary system[D]. Shijiazhuang: Hebei Normal University, 2004. | |
| 60 | 张超星. 固-固相变储能物质——(CnH2n+1NH3)2MX4及其二元体系的研究[D]. 石家庄: 河北师范大学, 2005. |
| ZAHNG Chaoxing. Study of solid-solid phase change of (CnH2n+1NH3)2MCl4 and their binary system for thermal energy storage[D]. Shijiazhuang: Hebei Normal University, 2005. | |
| 61 |
RUAN Deshui, LI Weiping, HE Luofu, et al. Phase diagrams of binary systems of alkylammonium tetrachlorometallates( )[J]. Journal of Thermal Analysis, 1995, 45(1): 235-242. )[J]. Journal of Thermal Analysis, 1995, 45(1): 235-242.
|
| 62 | 韩铁城. 固-固相变储能物质(CnH2n+1NH3)2MCl4及其二元体系的研究[D]. 石家庄: 河北师范大学, 2006. |
| HAN Tiecheng. Study of solid-solid phase change of (CnH2n+1NH3)2MCl4 and their binary system for thermal energy storage[D]. Shijiazhuang: Hebei Normal University, 2006. | |
| 63 | 武克忠, 李建玲, 王新东, 等. 贮热材料四氯合金属酸(Ⅱ)二烷基铵固-固相变动力学[J]. 北京科技大学学报, 2007(9): 928-931. |
| WU Kezhong, LI Jianling, WANG Xindong, et al. Nonisothermal kinetics of solid-solid phase transitions in C12Mn, C14Mn and their binary system[J]. Journal of University of Science and Technology Beijing, 2007(9): 928-931. | |
| 64 | SON C, MOREHOUSE J. An experimental investigation of solid-state phase-change materials for solar thermal storage[J]. Journal of Solar Energy Engineering, 1991, 113: 244. |
| 65 | PRAVEEN B, SURESH S. Experimental study on heat transfer performance of neopentyl glycol/CuO composite solid-solid PCM in TES based heat sink[J]. Engineering Science and Technology, an International Journal, 2018, 21(5): 1086-1094. |
| 66 | ZHANG Nan, SONG Yanlin, DU Yanxia, et al. A novel solid-solid phase change material: pentaglycerine/expanded graphite composite PCMs[J]. Advanced Engineering Materials, 2018, 20(10): 1800237. |
| 67 | WHITMAN C A, JOHNSON M B, WHITE M A. Characterization of thermal performance of a solid-solid phase change material, di-n-hexylammonium bromide, for potential integration in building materials[J]. Thermochimica Acta, 2012, 531: 54-59. |
| 68 | PIELICHOWSKA K, BIEDA J, SZATKOWSKI P. Polyurethane/graphite nano-platelet composites for thermal energy storage[J]. Renewable Energy, 2016, 91: 456-465. |
| 69 | WANG Feng, ZHANG Ping, MOU Yongren, et al. Synthesis of the polyethylene glycol solid-solid phase change materials with a functionalized graphene oxide for thermal energy storage[J]. Polymer Testing, 2017, 63: 494-504. |
| 70 | AFTAB W, MAHMOOD A, GUO Wenhan, et al. Polyurethane-based flexible and conductive phase change composites for energy conversion and storage[J]. Energy Storage Materials, 2019, 20: 401-409. |
| 71 | BARRIO M, FONT J, MUNTASELL J, et al. Applicability for heat storage of binary systems of neopentylglycol, pentaglycerine and pentaerythritol: a comparative analysis[J]. Solar Energy Materials, 1988, 18(1): 109-115. |
| 72 | 赵娜. 相变储能物质(CnH2n+1NH3)2MnCl4的研究及聚乙二醇复合储能材料的初步探索[D]. 石家庄: 河北师范大学, 2006. |
| ZHAO Na. Study of phase change of (CnH2n+1NH3)2MnCl4 and the preliminary exploration of polyethylene glycol composite energy storage material[D]. Shijiazhuang: Hebei Normal University, 2006. | |
| 73 | HU Peng, ZHAO Panpan, JIN Yi, et al. Experimental study on solid-solid phase change properties of pentaerythritol (PE)/nano-AlN composite for thermal storage[J]. Solar Energy, 2014, 102: 91-97. |
| 74 | GAO Chunfeng, WANG Liping, LI Qifeng, et al. Tuning thermal properties of latent heat storage material through confinement in porous media: the case of (1-CnH2n+1NH3)2ZnCl4 (n=10 and 12)[J]. Solar Energy Materials and Solar Cells, 2014, 128: 221-230. |
| 75 | LI Qifeng, WANG Chao, LAN Xiaozheng. Solid-solid phase transition of (1-C14H29NH3)2ZnCl4 in nanopores of silica gel for thermal energy storage[J]. Chinese Chemical Letters, 2017, 28(1): 49-54. |
| 76 | ZHOU Yan, LIU Xiangdong, SHENG Dekun, et al. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage[J]. Chemical Engineering Journal, 2018, 338: 117-125. |
| 77 | ZHOU Yan, SHENG Dekun, LIU Xiangdong, et al. Synthesis and properties of crosslinking halloysite nanotubes/polyurethane-based solid-solid phase change materials[J]. Solar Energy Materials and Solar Cells, 2018, 174: 84-93. |
| 78 | CHATTOPADHYAY D K, WEBSTER D C. Thermal stability and flame retardancy of polyurethanes[J]. Progress in Polymer Science, 2009, 34(10): 1068-1133. |
| 79 | VIGO T L. Intelligent fibrous substrates with thermal and dimensional memories[J]. Polymers for Advanced Technologies, 1997, 8(5): 281-288. |
| 80 | KUMAR A, KULKARNI P S, SAMUI A B. Polyethylene glycol grafted cotton as phase change polymer[J]. Cellulose, 2013, 21(1): 685-696. |
| 81 | 马骉, 段诗雨, 魏堃, 等. 道路用聚氨酯固-固相变材料的合成及性能研究[J]. 硅酸盐通报, 2018, 37(10): 3232-3238. |
| MA Biao, DUAN Shiyu, WEI Kun, et al. Synthesis and properties of polyurethane solid-solid phase change materials for highway[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(10): 3232-3238. |
| [1] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [2] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [3] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [4] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| [5] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [6] | 杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859. |
| [7] | 张晨宇, 王宁, 徐洪涛, 罗祝清. 纳米颗粒强化传热的多级潜热储热器性能评价[J]. 化工进展, 2023, 42(5): 2332-2342. |
| [8] | 李玲, 马超峰, 卢春山, 于万金, 石能富, 金佳敏, 张建君, 刘武灿, 李小年. 新型含氟替代品1,1,2-三氟乙烯的合成工艺与催化剂研究进展[J]. 化工进展, 2023, 42(4): 1822-1831. |
| [9] | 殷铭, 郭晋, 庞纪峰, 吴鹏飞, 郑明远. 铜催化剂在涉氢反应中的失活机制和稳定策略[J]. 化工进展, 2023, 42(4): 1860-1868. |
| [10] | 李云闯, 谢方明, 席亚男, 万新月, 孙玉虎, 赵永峰, 李根, 刘宏海, 高雄厚, 刘洪涛. 高水热稳定性介孔分子筛的低成本合成研究进展[J]. 化工进展, 2023, 42(4): 1877-1884. |
| [11] | 王钰琢, 李刚. 硫、氮共掺杂三维石墨烯的全固态超级电容器[J]. 化工进展, 2023, 42(4): 1974-1982. |
| [12] | 谭德新, 曾佳欣, 梁莉敏, 申思慧, 曾子倩, 王艳丽. 取代烷基变化对芳炔单体及其聚合物性能影响[J]. 化工进展, 2023, 42(4): 2031-2037. |
| [13] | 宗悦, 张瑞君, 高珊珊, 田家宇. “特殊稳定型”压力驱动薄膜复合(TFC)脱盐膜的研究进展[J]. 化工进展, 2023, 42(4): 2058-2067. |
| [14] | 李光文, 华渠成, 黄作鑫, 达志坚. 聚甲基丙烯酸酯类黏度指数改进剂的研究进展[J]. 化工进展, 2023, 42(3): 1562-1571. |
| [15] | 张晨光, 封硕, 邢玉烨, 沈伯雄, 苏立超. 柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展[J]. 化工进展, 2023, 42(3): 1321-1331. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||