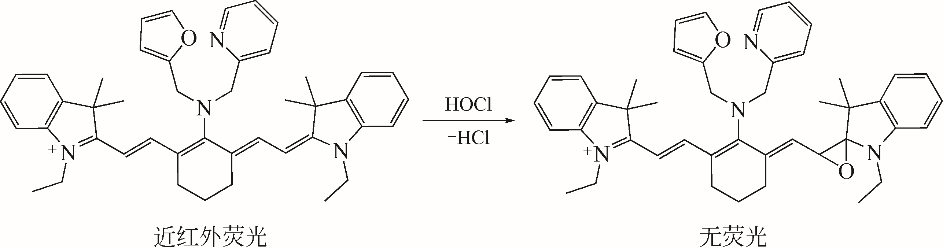
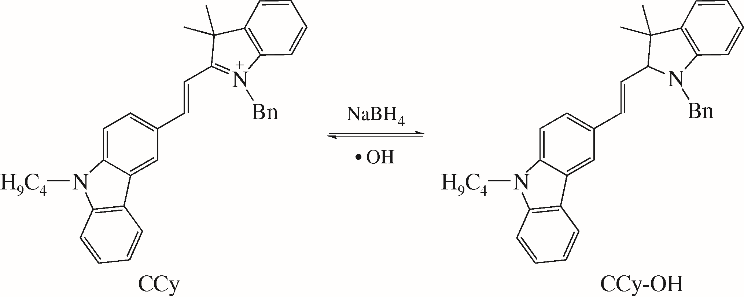
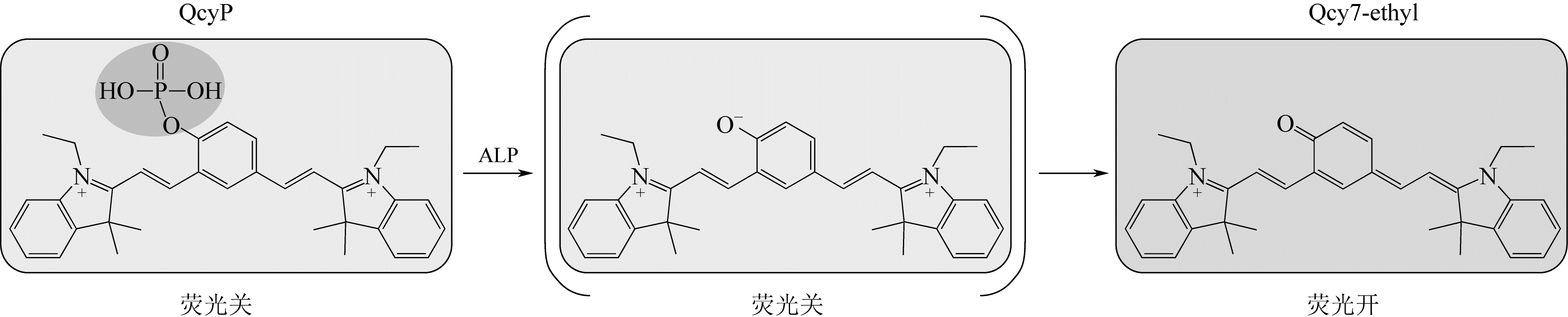
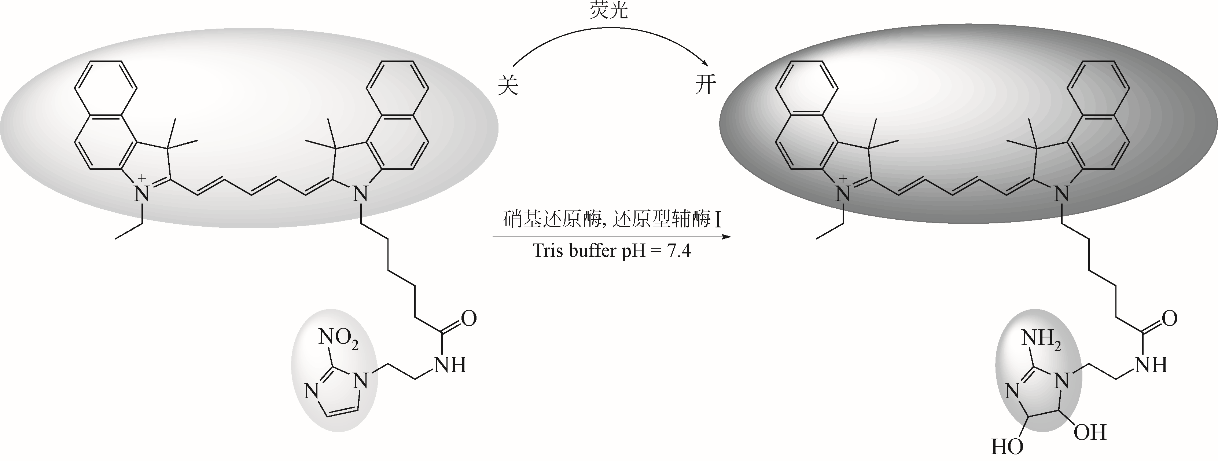
化工进展 ›› 2019, Vol. 38 ›› Issue (12): 5492-5503.DOI: 10.16085/j.issn.1000-6613.2019-0442
花菁类近红外荧光探针在生物检测中的应用研究进展
于雪1( ),包青青2,姜超2,张思霞2,张丰旗2,陈驰2,陶娅妮2,张跃伟2(
),包青青2,姜超2,张思霞2,张丰旗2,陈驰2,陶娅妮2,张跃伟2( )
)
- 1. 吉林化工学院科学技术处,吉林 吉林 132022
2. 吉林化工学院化学与制药工程学院,吉林 吉林 132022
-
收稿日期:2019-03-24出版日期:2019-12-05发布日期:2019-12-05 -
通讯作者:张跃伟 -
作者简介:于雪(1987—),女,博士,副教授,主要从事有机合成、精细化学品合成及化工助剂研究。E-mail:dongjibinghuayuxue@163.com 。 -
基金资助:吉林省科技发展计划(20160520130JH);吉林省教育厅“十三五”科学技术研究规划项目(JJKH20170214KJ);吉林化工学院博士启动基金(2017007);吉林化工学院重大科技项目(2016004);吉林化工学院科学技术研究项目(2017011);吉林市科技创新发展计划(201750231)
Applied research progress on cyanine-based near-infrared fluorescent probe in biological detection
Xue YU1( ),Qingqing BAO2,Chao JIANG2,Sixia ZHANG2,Fengqi ZHANG2,Chi CHEN2,Yani TAO2,Yuewei ZHANG2(
),Qingqing BAO2,Chao JIANG2,Sixia ZHANG2,Fengqi ZHANG2,Chi CHEN2,Yani TAO2,Yuewei ZHANG2( )
)
- 1. Division of Science and Technology, Jilin Institute Chemical Technology, Jilin 132022, Jilin, China
2. School of Chemistry and Pharmaceutical Engineering, Jilin Institute Chemical Technology, Jilin 132022, Jilin, China
-
Received:2019-03-24Online:2019-12-05Published:2019-12-05 -
Contact:Yuewei ZHANG
摘要:
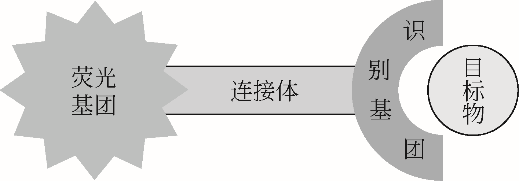
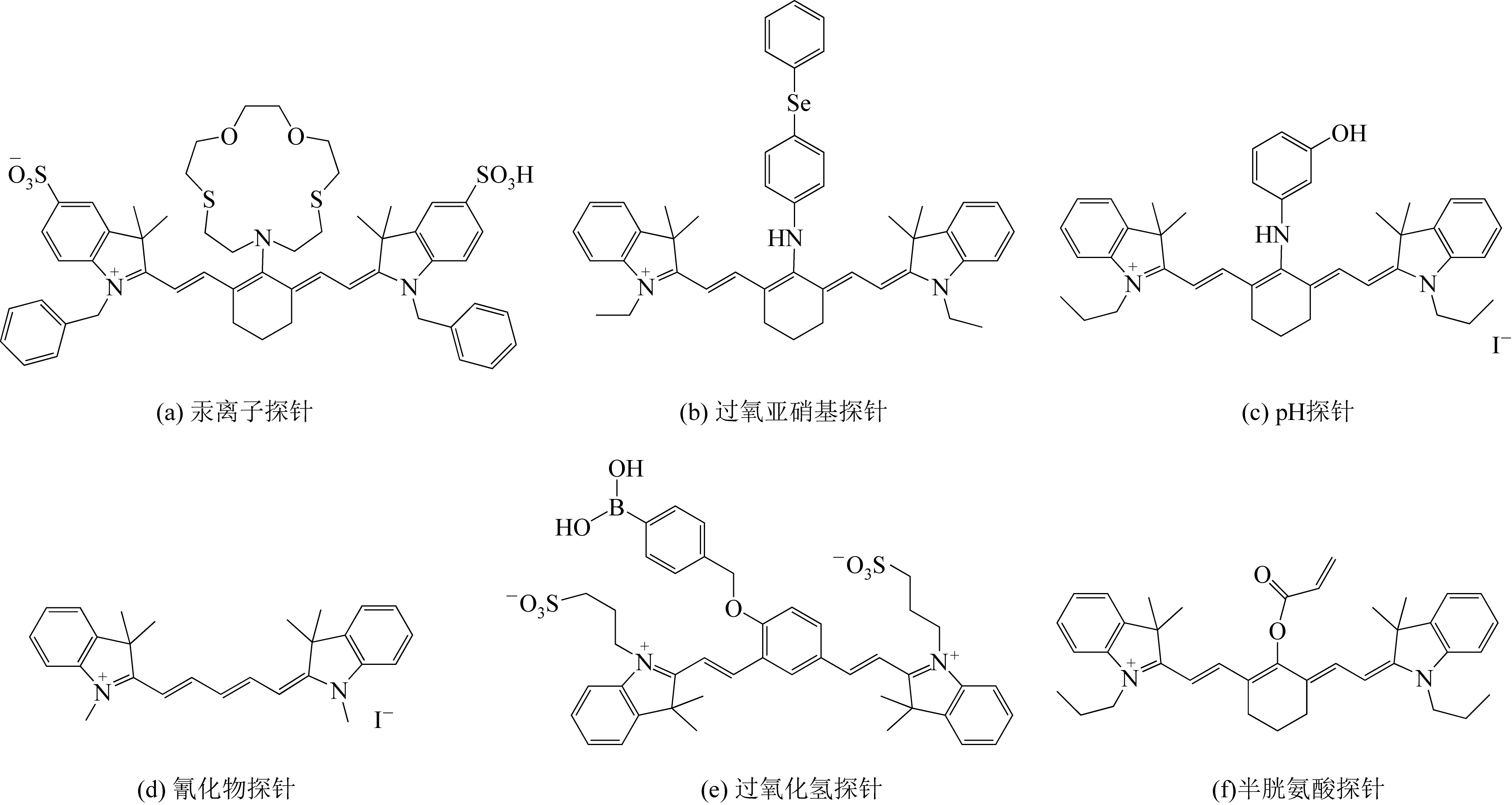
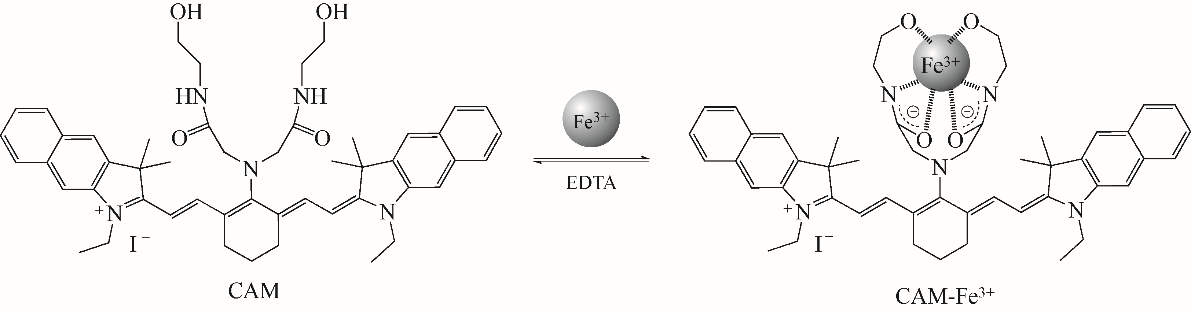
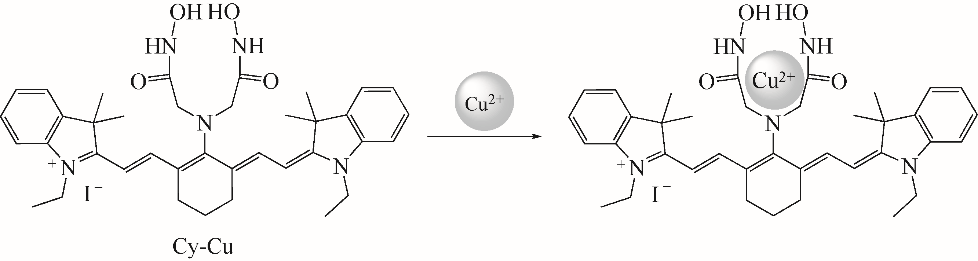
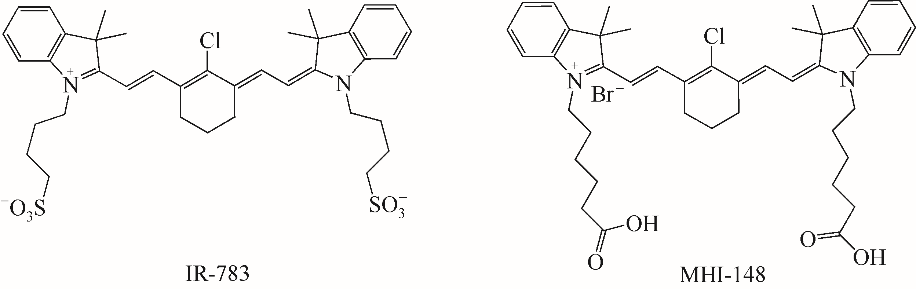
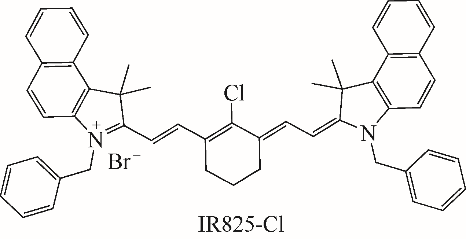
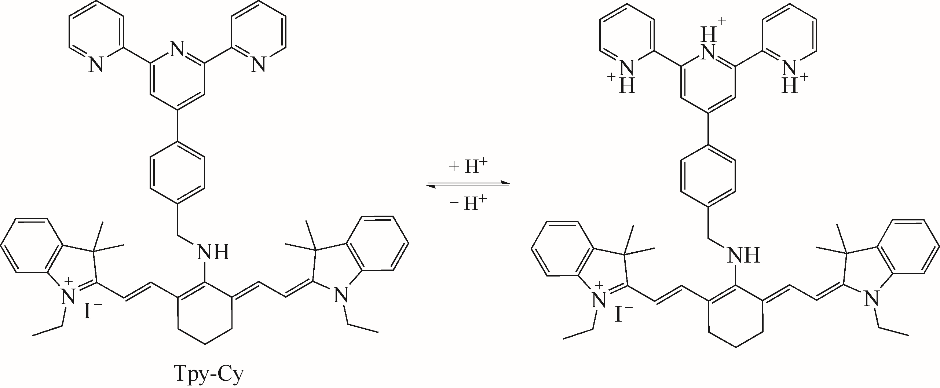
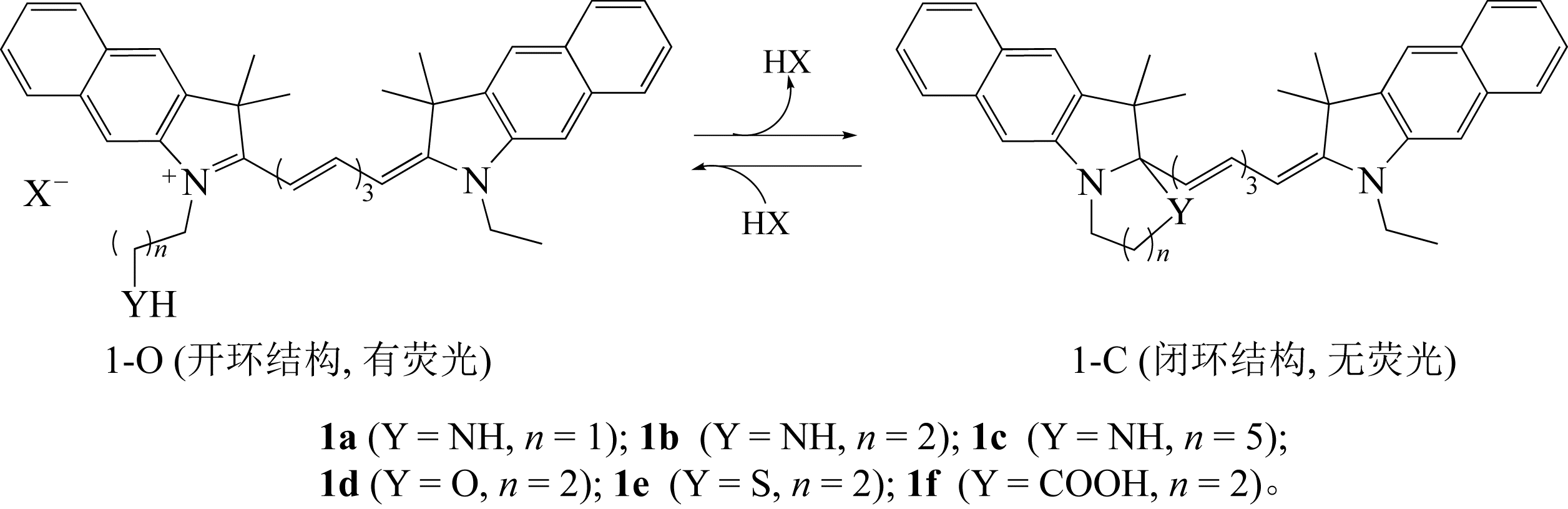
随着现代生物学和生物技术的发展,近红外荧光探针作为一种重要的技术手段在许多领域都展现出了重要的应用价值。因具有背景干扰低、细胞损伤小、样品穿透性强、检测灵敏度高等优点,经常用于分子识别、医学诊断、生物分子检测以及生物成像等方面。本综述从金属离子检测、含硫小分子检测、活性氧(ROS)/活性氮(RNS)检测、酶识别、肿瘤细胞识别及治疗以及细胞内pH响应等方面,介绍了近年来花菁类荧光探针在生物检测中的应用研究进展。同时指出了花菁类荧光探针亟待解决的问题,通过花菁母体的结构修饰和改造提高探针的光稳定性、灵敏度、靶向性和水溶性,有望使其在生物检测以及疾病的诊断方面得到进一步应用发展。
中图分类号:
引用本文
于雪,包青青,姜超,张思霞,张丰旗,陈驰,陶娅妮,张跃伟. 花菁类近红外荧光探针在生物检测中的应用研究进展[J]. 化工进展, 2019, 38(12): 5492-5503.
Xue YU,Qingqing BAO,Chao JIANG,Sixia ZHANG,Fengqi ZHANG,Chi CHEN,Yani TAO,Yuewei ZHANG. Applied research progress on cyanine-based near-infrared fluorescent probe in biological detection[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5492-5503.
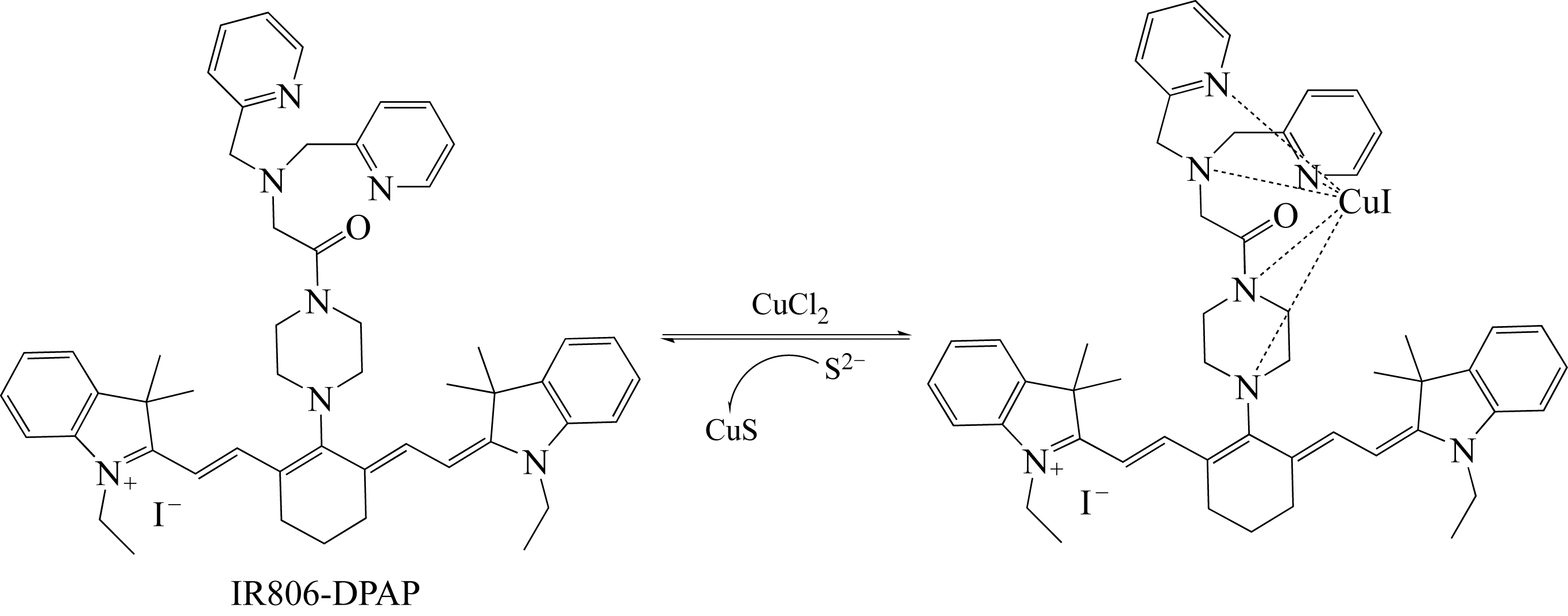
| 1 | BAKER M. Whole-animal imaging: the whole picture[J]. Nature, 2010, 463(7283): 977-980. |
| 2 | 张聪, 高云玲. 反应型荧光探针在检测金属离子中的研究进展[J]. 化工进展, 2016, 35(10): 3288-3294. |
| ZHANG C, GAO Y L. Reaction-based fluorescence probe for the detection of metal ions[J]. Chemical Industry and Engineering Progress, 2016, 35(10): 3288-3294. | |
| 3 | 池雨, 高云玲, 潘勇, 等. 氮杂氟硼荧(Aza-BODIPY)类荧光探针的研究进展[J]. 化工进展, 2018, 37(3): 1137-1144. |
| CHI Y, GAO Y L, PAN Y, et al. New progress in Aza-BODIPY-based fluorescence probes[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1137-1144. | |
| 4 | CHINEN A B, GUAN C M, FERRER J R, et al. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence[J]. Chemical Reviews, 2015, 115(19): 10530-10574. |
| 5 | HE D D, LIU W, SUN R, et al. N-pyridineium-2-yl darrow red analogue: unique near-infrared lysosome-biomarker for the detection of cancer cells[J]. Analytical Chemistry, 2015, 87(3): 1499-1502. |
| 6 | TANG F, WANG C, WANG X Y, et al. Facile synthesis of biocompatible fluorescent nanoparticles for cellular imaging and targeted detection of cancer cells[J]. ACS Applied Materials & Interfaces, 2015, 7(45): 25077-25083. |
| 7 | LI X H, GAO X H, SHI W, et al. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes[J]. Chemical Reviews, 2014, 114(1): 590-659. |
| 8 | 刘毋凡, 陈楚芳, 潘文慧, 等. 受激辐射损耗超分辨荧光成像探针研究进展[J]. 化工进展, 2019, 38(2): 726-739. |
| LIU W F, CHEN C F, PAN W H, et al. Research progress on stimulated emission depletion using fluorescent probes[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 726-739. | |
| 9 | YAO J, YANG M, DUAN Y X. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: new insights into biosensing, bioimaging, genomics, diagnostics, and therapy[J]. Chemical Reviews, 2014, 114(12): 6130-6178. |
| 10 | LIU B, WANG H, YANG D, et al. A cyanine-based colorimetric and fluorescent probe for highly selective sensing and bioimaging of phosphate ions[J]. Dyes and Pigments, 2016, 133: 127-131. |
| 11 | HE Q W, MILLER E W, WONG A P, et al. A selective fluorescent sensor for detecting lead in living cells[J]. Journal of the American Chemical Society, 2006, 128(29): 9316-9317. |
| 12 | QI X, JUN E J, XU L, et al. New bodipy derivatives as off-on fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: cooperative selectivity enhancement toward Cu2+[J]. The Journal of Organic Chemistry, 2006, 71(7): 2881-2884. |
| 13 | MARUYAMA S, KIKUCHI K, HIRANO T, et al. A novel, cell-permeable, fluorescent probe for ratiometric imaging of zinc ion[J]. Journal of the American Chemical Society, 2002, 124(36): 10650-10651. |
| 14 | MIZUKAMI S, NAGANO T, URANO Y, et al. A fluorescent anion sensor that works in neutral aqueous solution for bioanalytical application[J]. Journal of the American Chemical Society, 2002, 124(15): 3920-3925. |
| 15 | CLAPP A R, MEDINTZ I L, MAURO J M, et al. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors[J]. Journal of the American Chemical Society, 2004, 126(1): 301-310. |
| 16 | GUO Z Q, ZHU W H, ZHU M M, et al. Near-infrared cell-permeable Hg2+-selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines[J]. Chemistry:European Journal, 2010, 16(48): 14424-14432. |
| 17 | ZHU M M, SHI C X, XU X T, et al. Near-infrared cyanine-based sensor for Fe3+ with high sensitivity: its intracellular imaging application in colorectal cancer cells[J]. RSC Advances, 2016, 6(103): 100759-100764. |
| 18 | LI C M, LIU Z X, MIAO Y, et al. A reversible fluorescent chemosensor for selective and sequential detection of copper ion and sulfide[J]. Dyes and Pigments, 2016, 125: 292-298. |
| 19 | ZHANG Y J, GUAN L M, YU H, et al. Reversible fluorescent probe for selective detection and cell imaging of oxidative stress indicator bisulfite[J]. Analytical Chemistry, 2016, 88 (8): 4426-4431. |
| 20 | BLAMEY N J F, RYDER A G. Hydrocarbon fluid inclusion fluorescence: a review[J]. Rev. Fluoresc., 2009, 4: 299-334. |
| 21 | GUO Z Q, NAM S W, PARK S S, et al. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging[J]. Chemical Science, 2012, 3(9): 2760-2765. |
| 22 | XUE C, LEI Y J, ZHANG S C, et al. A cyanine-derived “turn-on” fluorescent probe for imaging nitroreductase in hypoxic tumor cells[J]. Analytical Methods, 2015, 7(24): 10125-10128. |
| 23 | BHATTARAI P, DAI Z. Cyanine based nanoprobes for cancer theranostics[J]. Advanced Healthcare Materials, 2017, 6(14): 1700262. |
| 24 | CAO X J, CHEN L N, ZHANG X, et al. A NBD-based simple but effective fluorescent pH probe for imaging of lysosomes in living cells[J]. Analytica Chimica Acta, 2016, 920: 86-93. |
| 25 | CAO X W, LIN W Y, WAN W. Development of a near-infrared fluorescent probe for imaging of endogenous Cu+ in live cells[J]. Chemical Communications, 2012, 48(50): 6247-6249. |
| 26 | LI P, DUAN X, CHEN Z Z, et al. A near-infrared fluorescent probe for detecting copper(Ⅱ) with high selectivity and sensitivity and its biological imaging applications[J]. Chemical Communications, 2011, 47(27): 7755-7757. |
| 27 | YANG X J, SHEN L Q, BAO H B, et al. A tricarbocyanine near-infrared fluorescent probe for sulfide through a copper displacement mechanism[J]. Sensors and Actuators B: Chemical, 2015, 220: 1361-1367. |
| 28 | MARET W. Analyzing free zinc(Ⅱ) ion concentrations in cell biology with fluorescent chelating molecules[J]. Metallomics, 2015, 7(2): 202-211. |
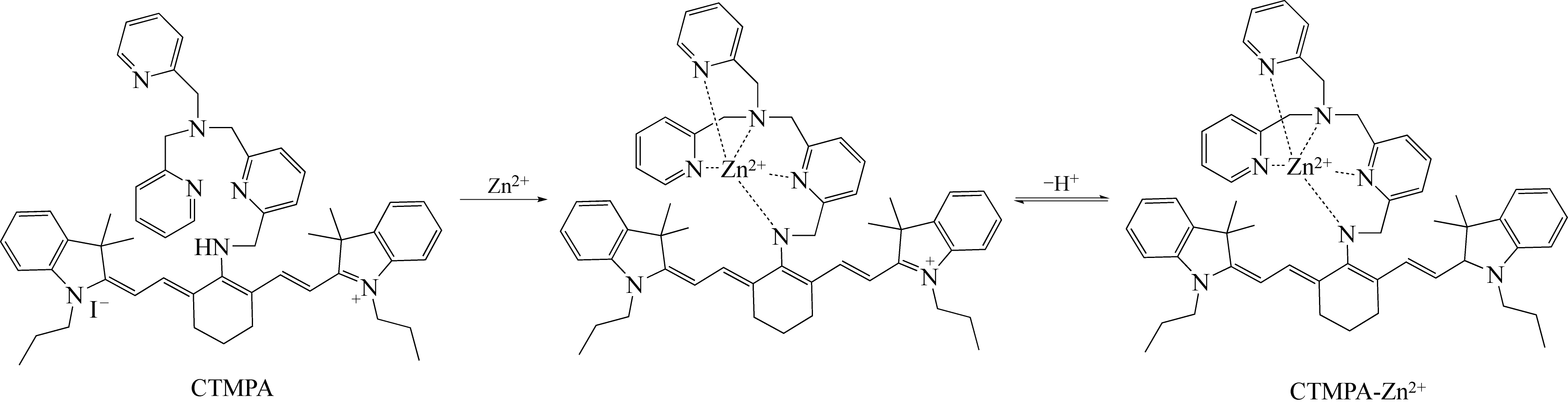
| 29 | GUO Z Q, KIM G H, SHIN I, et al. A cyanine-based fluorescent sensor for detecting endogenous zinc ions in live cells and organisms[J]. Biomaterials, 2012, 33(31): 7818-7827. |
| 30 | SHELLAIAH M, RAJAN Y C, BALU P, et al. A pyrene based Schiff base probe for selective fluorescence turn-on detection of Hg2+ ions with live cell application[J]. New Journal of Chemistry, 2015, 39(4): 2523-2531. |
| 31 | ZHU M, YUAN M J, LIU X F, et al. Visible near-infrared chemosensor for mercury ion[J]. Organic Letters, 2008, 10(7): 1481-1484. |
| 32 | JIN X, WU S, SHE M, et al. Novel fluorescein-based fluorescent probe for detecting H2S and its real applications in blood plasma and biological imaging[J]. Analytical Chemistry, 2016, 88(22): 11253-11260. |
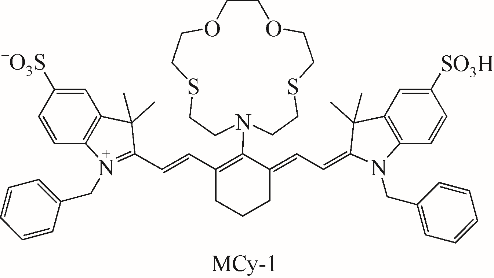
| 33 | WANG R, YU F B, CHEN L X, et al. A highly selective turn-on near-infrared fluorescent probe for hydrogen sulfide detection and imaging in living cells[J]. Chemical Communications, 2012, 48(96): 11757-11759. |
| 34 | WEERAPANA E, WANG C, SIMON G M, et al. Quantitative reactivity profiling predicts functional cysteines in proteomes[J]. Nature, 2010, 468: 790. |
| 35 | ZHANG J J, WANG J X, LIU J T, et al. Near-infrared and naked-eye fluorescence probe for direct and highly selective detection of cysteine and its application in living cells[J]. Analytical Chemistry, 2015, 87(9): 4856-4863. |
| 36 | YU F B, LI P, WANG B S, et al. Reversible near-infrared fluorescent probe introducing tellurium to mimetic glutathione peroxidase for monitoring the redox cycles between peroxynitrite and glutathione invivo[J]. Journal of the American Chemical Society, 2013, 135(20): 7674-7680. |
| 37 | LEE S Y, JEONG K S, LUO X, et al. Near-infrared fluorescent probes for the detection of glutathione and their application in the fluorescence imaging of living cells and tumor-bearing mice[J]. Journal of Materials Chemistry B, 2018, 6(17): 2541-2546. |
| 38 | GAO X Y, FENG G, MANGHNANI P N, et al. A two-channel responsive fluorescent probe with AIE characteristics and its application for selective imaging of superoxide anions in living cells[J]. Chemical Communications, 2017, 53(10): 1653-1656. |
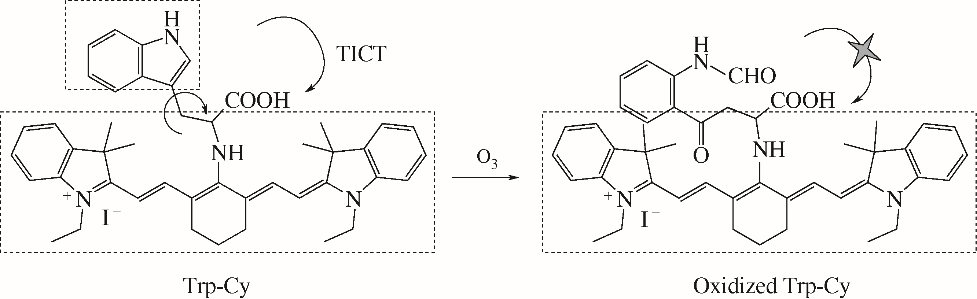
| 39 | XU K H, SUN S X, LI J, et al. A near-infrared fluorescent probe for monitoring ozone and imaging in living cells[J]. Chemical Communications, 2012, 48(5): 684-686. |
| 40 | SUN M T, YU H, ZHU H J, et al. Oxidative cleavage-based near-infrared fluorescent probe for hypochlorous acid detection and myeloperoxidase activity evaluation[J]. Analytical Chemistry, 2014, 86(1): 671-677. |
| 41 | HOU T X, ZHANG K, KANG X X, et al. Sensitive detection and imaging of endogenous peroxynitrite using a benzo[d]thiazole derived cyanine probe[J]. Talanta, 2019, 196: 345-351. |
| 42 | WANG J Y, LIU Z R, REN M G, et al. A fast-responsive turn on fluorescent probe for detecting endogenous hydroxyl radicals based on a hybrid carbazole-cyanine platform[J]. Sensors and Actuators B: Chemical, 2016, 236: 60-66. |
| 43 | CHENG G H, FAN J L, SUN W, et al. A near-infrared fluorescent probe for selective detection of HClO based on Se-sensitized aggregation of heptamethine cyanine dye[J]. Chemical Communications, 2014, 50(8): 1018-1020. |
| 44 | GAO Z W, SUN J Y, GAO M, et al. A unique off-on near-infrared cyanine-based probe for imaging of endogenous alkaline phosphatase activity in cells and in vivo[J]. Sensors and Actuators B: Chemical, 2018, 265: 565-574. |
| 45 | XU S N, WANG Q H, ZHANG Q Y, et al. Real time detection of ESKAPE pathogens by a nitroreductase-triggered fluorescence turn-on probe[J]. Chemical Communications, 2017, 53(81): 11177-11180. |
| 46 | SHI C H, WU J B, PAN D F. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy[J]. Journal of Biomedical Optics, 2016, 21(5): 050901 |
| 47 | ZHANG C, LIU T, SU Y P, et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging[J]. Biomaterials, 2010, 31(25): 6612-6617. |
| 48 | YANG X J, SHI C M, TONG R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging[J]. Clinical Cancer Research, 2010, 16(10): 2833. |
| 49 | YANG X J, SHAO C, WANG R X, et al. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes[J]. Journal of Urology, 2013, 189(2): 702-710. |
| 50 | PAN G Y, JIA H R, ZHU Y X, et al. Dual channel activatable cyanine dye for mitochondrial imaging and mitochondria-targeted cancer theranostics[J]. ACS Biomaterials Science & Engineering, 2017, 3(12): 3596-3606. |
| 51 | JIN D, WANG B W, HOU Y Q, et al. Novel near-infrared pH-sensitive cyanine-based fluorescent probes for intracellular pH monitoring[J]. Dyes and Pigments, 2019, 170: 107612. |
| 52 | TANG B, YU F B, LI P, et al. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: imaging in living HepG2 and HL-7702 cells[J]. Journal of the American Chemical Society, 2009, 131(8): 3016-3023. |
| 53 | MIKI K, KOJIMA K, ORIDE K, et al. pH-responsive near-infrared fluorescent cyanine dyes for molecular imaging based on pH sensing[J]. Chemical Communications, 2017, 53(55): 7792-7795. |
| 54 | HOU J R, JIN D, CHEN B, et al. Two near-infrared highly sensitive cyanine fluorescent probes for pH monitoring[J]. Chinese Chemical Letters, 2017, 28(8): 1681-1687. |
| [1] | 张志伟, 杨伟鑫, 张隽佶. 长波长驱动光开关染料分子研究进展[J]. 化工进展, 2023, 42(8): 4058-4075. |
| [2] | 张芳, 郭鹍鹏, 梁春平, 尤雪瑞, 张志超. 一种具有聚集诱导发光效应有机荧光小分子的设计合成及功能应用[J]. 化工进展, 2023, 42(6): 3097-3104. |
| [3] | 安宁, 高云玲. 反应型HClO/ClO-荧光探针的研究进展[J]. 化工进展, 2021, 40(6): 3346-3362. |
| [4] | 胡丽萍, 黄生权, 田淑华, 黄延盛, 胡流云, 李璇, 舒逸聃, 王学重. 近红外光谱技术在中草药口服液在线质量监控中的模型建立和模型转移[J]. 化工进展, 2020, 39(8): 3263-3272. |
| [5] | 刘清浩, 何艳飞, 梁丽娜, 念继鹏, 胡志勇, 郭金春, 梁栋, 刘红彦. 基于氮掺杂碳量子点的荧光微球制备和Fe3+检测[J]. 化工进展, 2018, 37(10): 3936-3942. |
| [6] | 贺凯迅, 曹鹏飞. 基于智能优化算法的软测量模型建模样本优选及应用[J]. 化工进展, 2018, 37(07): 2516-2523. |
| [7] | 李晶晶, 周昭露, 黄生权, 鲁亮, 黄延盛, 田淑华, FALOLA Akinola A, 李璇, 李杰, 舒逸聃, 王学重. 近红外光谱技术应用于中草药口服液在线质量控制的化学计量学建模[J]. 化工进展, 2018, 37(05): 1923-1932. |
| [8] | 池雨, 高云玲, 潘勇, 刘孟, 赵辉. 氮杂氟硼荧(Aza-BODIPY)类荧光探针的研究进展[J]. 化工进展, 2018, 37(03): 1137-1144. |
| [9] | 李珍珍, 黄华莹, 任长靖, 张其翼, 赵强. 多功能复合载药脂质体的制备方法及性能[J]. 化工进展, 2016, 35(S2): 279-282. |
| [10] | 许利娜, 黄坤, 李守海, 李梅, 夏建陵. 木质素磺酸钙-石墨烯复合量子点的制备及性能[J]. 化工进展, 2016, 35(11): 3595-3595. |
| [11] | 周昭露, 李杰, 黄生权, 田淑华, 刘玉娇, 鲁亮, 张扬, 黄延盛, 王学重. 近红外光谱技术在中药质量控制应用中的化学计量学建模:综述和展望[J]. 化工进展, 2016, 35(06): 1627-1645. |
| [12] | 吴清平1,马延霞1,2,3,张菊梅1,韦献虎1,2,3. 香豆素类荧光底物的合成及其在微生物检测中的应用进展[J]. 化工进展, 2014, 33(09): 2444-2449. |
| [13] | 程 明,吉 静. 近红外区具有高反射率的建筑节能涂料的研究进展 [J]. 化工进展, 2008, 27(1): 12-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||