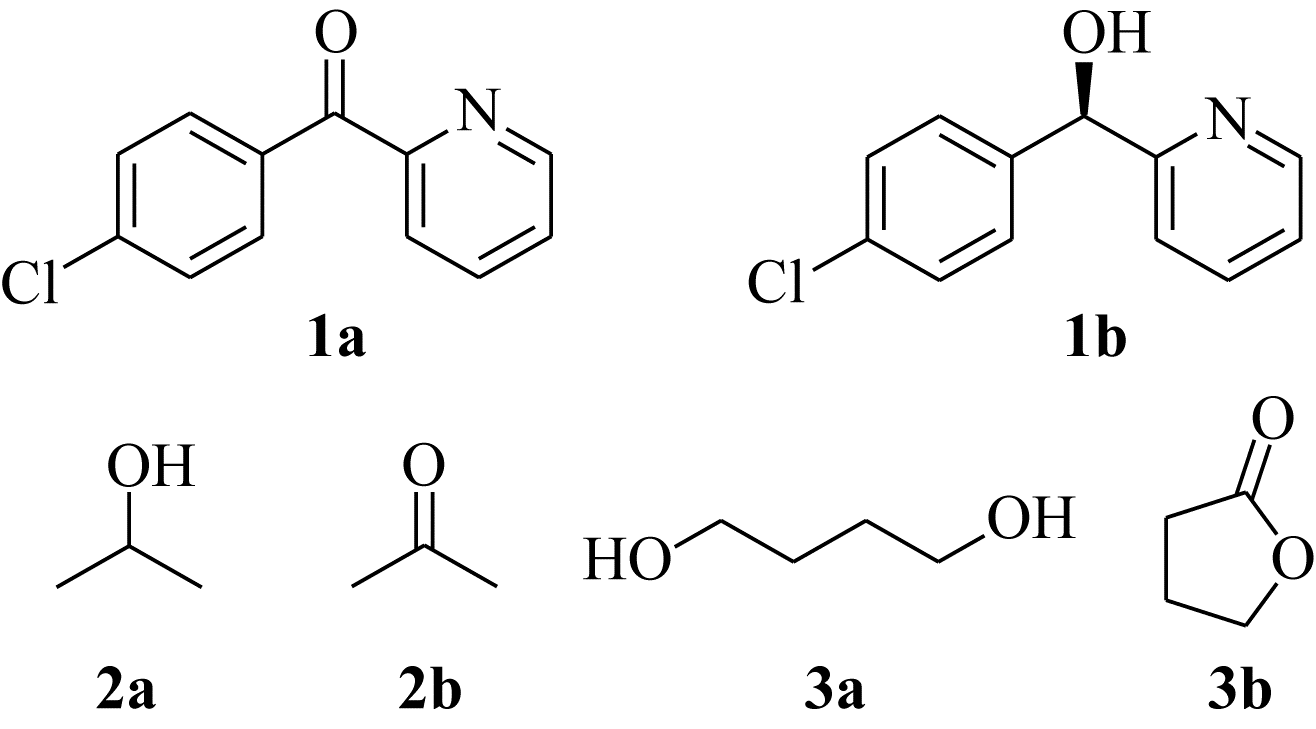
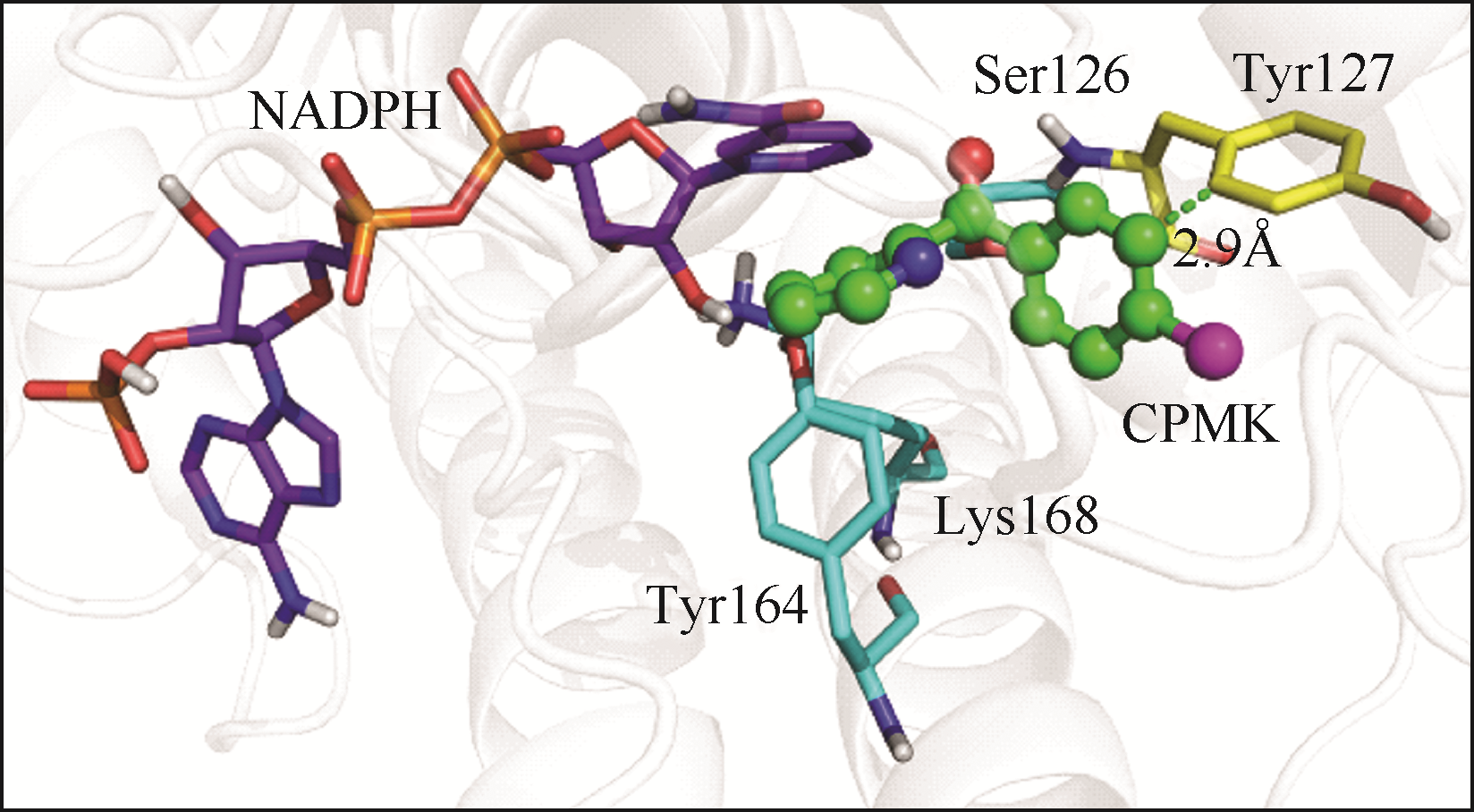
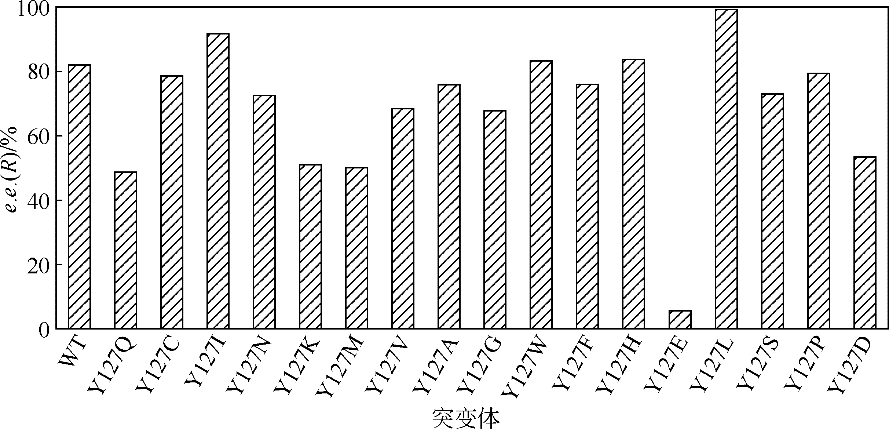
| 1 |
FARINA V, REEVES J T, SENANAYAKE C H, et al. Asymmetric synthesis of active pharmaceutical ingredients[J]. Chemical Reviews, 2006, 106(7): 2734-2793.
|
| 2 |
SCHMIDT F, STEMMLER R T, RUDOLPH J, et al. Catalytic asymmetric approaches towards enantiomerically enriched diarylmethanols and diarylmethylamines[J]. Chemical Society Reviews, 2006, 35(5): 454-470.
|
| 3 |
SUI Y Z, ZHANG X C, WU J W, et al. CuII-catalyzed asymmetric hydrosilylation of diaryl- and aryl heteroaryl ketones: application in the enantioselective synthesis of orphenadrine and neobenodine[J]. Chemistry: A European Journal, 2012, 18(24): 7486-7492.
|
| 4 |
HOLLMANN F, ARENDS I W, HOLTMANN D. Enzymatic reduction for the chemist[J]. Green Chemistry, 2011, 13(9): 2285-2314.
|
| 5 |
谌容, 王秋岩, 殷晓浦, 等. 醇脱氢酶不对称还原制备手性醇的研究进展[J].化工进展, 2011, 30(7): 1562-1569.
|
|
KAN R, WANG Q Y, YIN X P, et al. Progress of asymmetric reduction with alcohol dehydrogenase for preparation of chiral alcohols, [J].Chemical Industry and Engineering Progress, 2011, 30(7): 1562-1569.
|
| 6 |
TRUPPO M D, POLLARD D, DEVINE P. Enzyme-catalyzed enantioselective diaryl ketone reductions[J]. Organic Letters, 2007, 9(2): 335-338.
|
| 7 |
NI Y, ZHOU J Y, SUN Z H. Production of a key chiral intermediate of betahistine with a newly isolated Kluyveromyces sp. in an aqueous two-phase system[J]. Process Biochemistry, 2012, 47(7): 1042-1048.
|
| 8 |
ZHU D M, YANG Y, MAJKOWICZ S, et al. Inverting the enantioselectivity of a carbonyl reductase via substrate-enzyme docking guiding point mutation[J]. Organic Letters, 2008, 10(4): 525-528.
|
| 9 |
LI H M, ZHU D M, HUA L, et al. Enantioselective reduction of diaryl ketones catalyzed by a carbonyl reductase from Sporobolomyces salmonicolor and its mutant enzymes[J]. Advanced Synthesis & Catalysis, 2009, 351(4): 583-588.
|
| 10 |
唐铭烩, 许国超, 倪晔. 双芳基酮还原酶的基因挖掘及催化性质[J].食品与生物技术学报, 2018, 37(3): 240-249.
|
|
TANG M H, XU G C, NI Y. Genime mining and characterization of diaryl ketone reductase from kluyveromyces polysporus[J]. Journal of Food Science and Biotechnology, 2018, 37(3): 240-249.
|
| 11 |
XU G C, WANG Y, TANG M H, et al. Hydroclassified combinatorial saturation mutagenesis: reshaping substrate binding pockets of KpADH for enantioselective reduction of bulky-bulky ketones[J]. ACS Catalysis, 2018, 8(9): 8336-8345.
|
| 12 |
ZHOU J Y, WANG Y, XU G C, et al. Structural insight into enantioselective inversion of an alcohol dehydrogenase reveals a “polar gate” in stereorecognition of diaryl ketones[J]. Journal of the American Chemical Society, 2018, 140(39): 12645-12654.
|
| 13 |
ARNOLD F H. Combinatorial and computational challenges for biocatalyst design[J]. Nature, 2001, 409(6817): 253-257.
|
| 14 |
BRANNIGAN J A, WILKINSON A J. Protein engineering 20 years on[J]. Nature Reviews Molecular Cell Biology, 2002, 3(12): 964-970.
|
| 15 |
姜恬, 冯旭东, 李岩, 等. 底物特异性的生物催化与酶设计改造[J].化工进展, 2019, 38(1): 606-614.
|
|
JIANG T, FENG X D, LI Y, et al. The biocatalysis and enzyme modification of substrate specificity[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 606-614.
|
| 16 |
冯旭东, 吕波, 李春, 等. 酶分子稳定性改造研究进展[J].化工学报, 2016, 67(1): 277-284.
|
|
FENG X D, LÜ B, LI C,et al. Advances in enzyme stability modification[J]. CIESC Journal, 2016, 67(1): 277-284.
|
| 17 |
BETTS M J, RUSSELL R B. Bioinformatics for geneticists[M]. New York: Wiley, 2003: 291-315.
|
| 18 |
SUN Z T, LONSDALE R, KONG X D, et al. Reshaping an enzyme binding pocket for enhanced and inverted stereoselectivity: use of smallest amino acid alphabets in directed evolution[J]. Angewandte Chemie: International Edition, 2015, 54(42): 12410-12415.
|
| 19 |
KARA S, SPICKERMANN D, SCHEITTWIESER J H, et al. More efficient redox biocatalysis by utilising 1,4-butanediol as a ‘smart cosubstrate’[J]. Green Chemistry, 2013, 15(2): 330-335.
|
 ),Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI(
),Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI( )
)