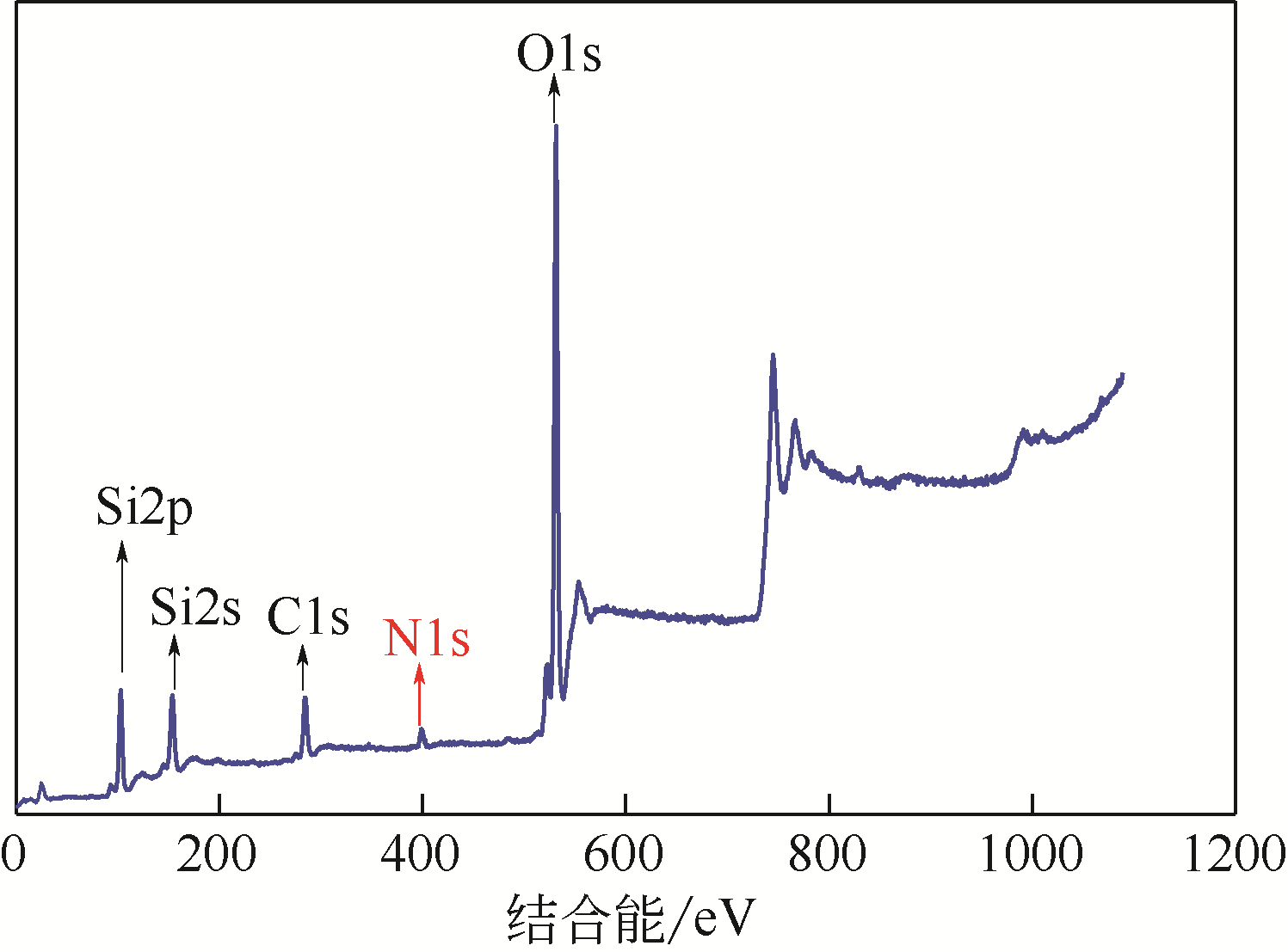
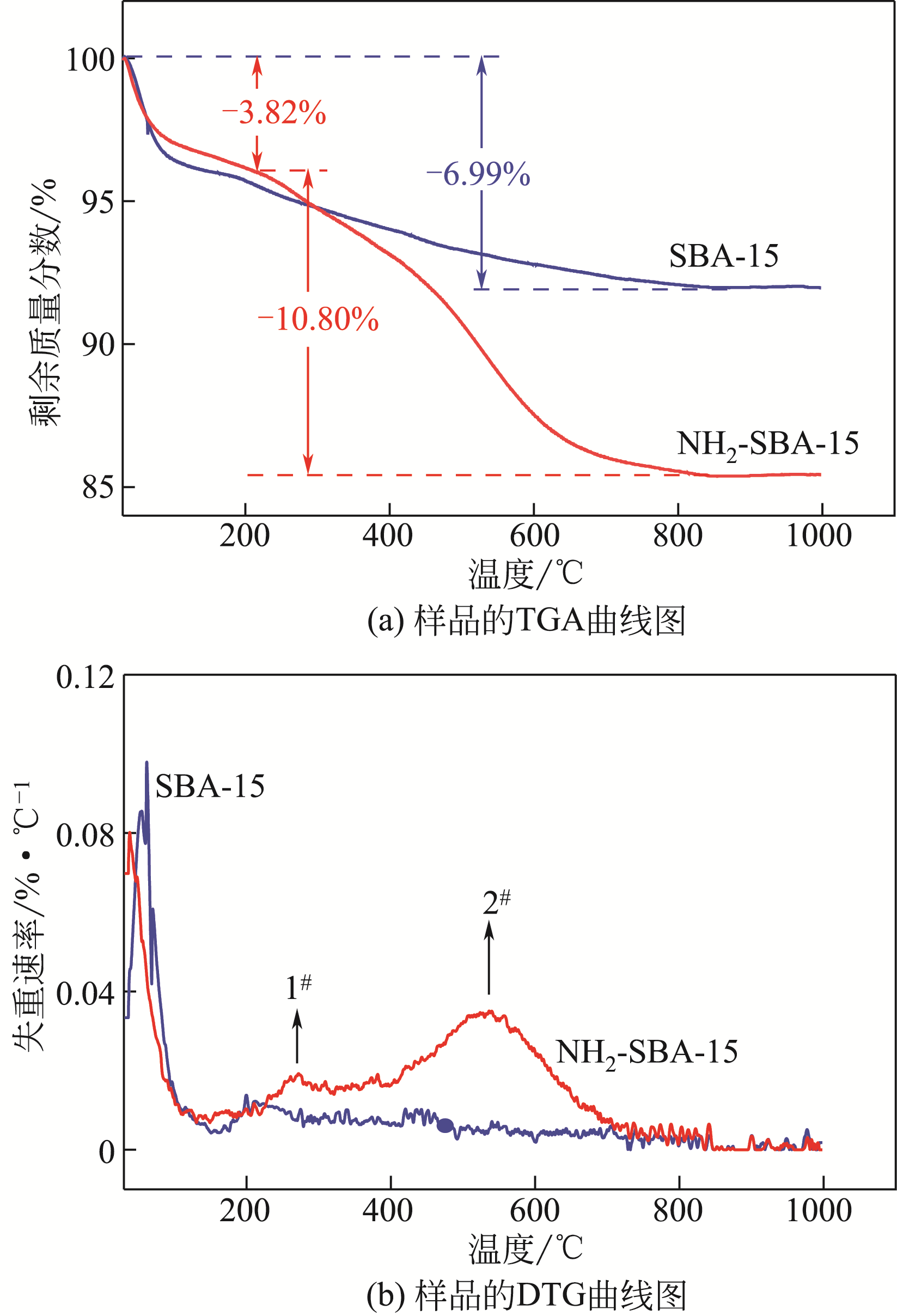
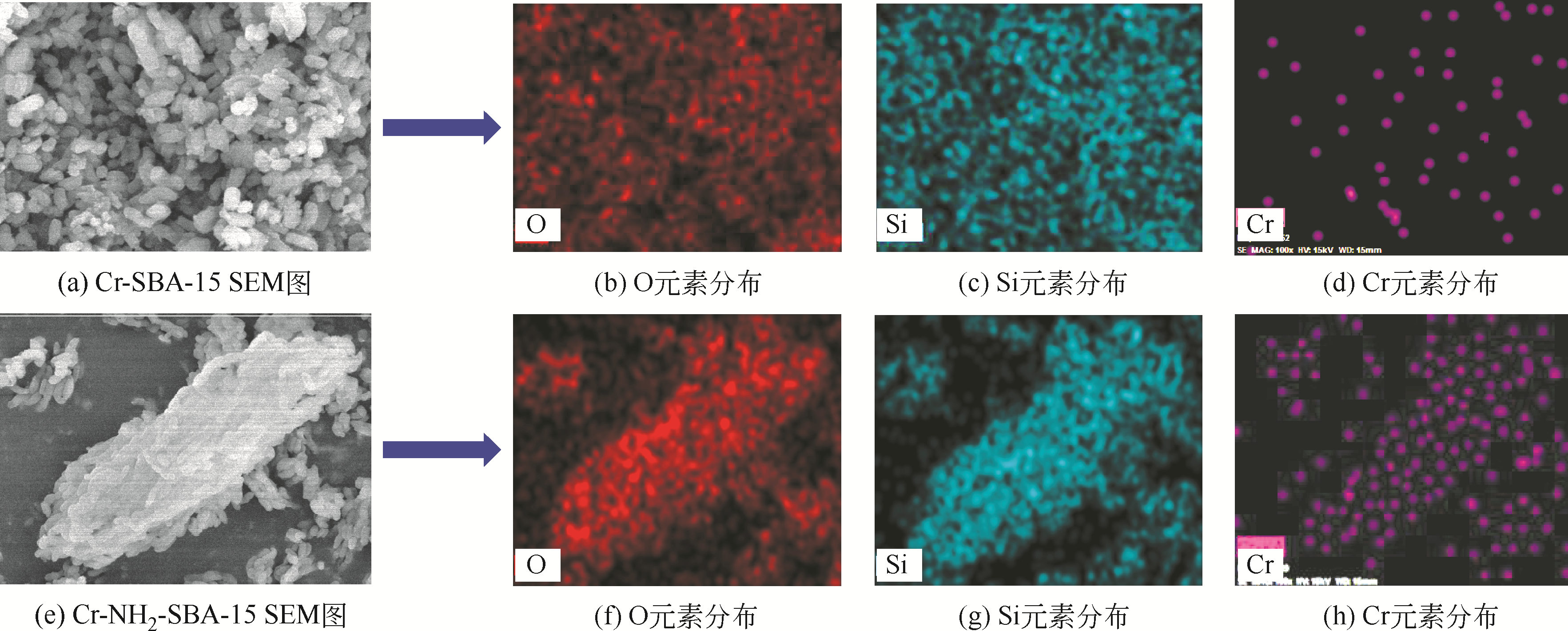
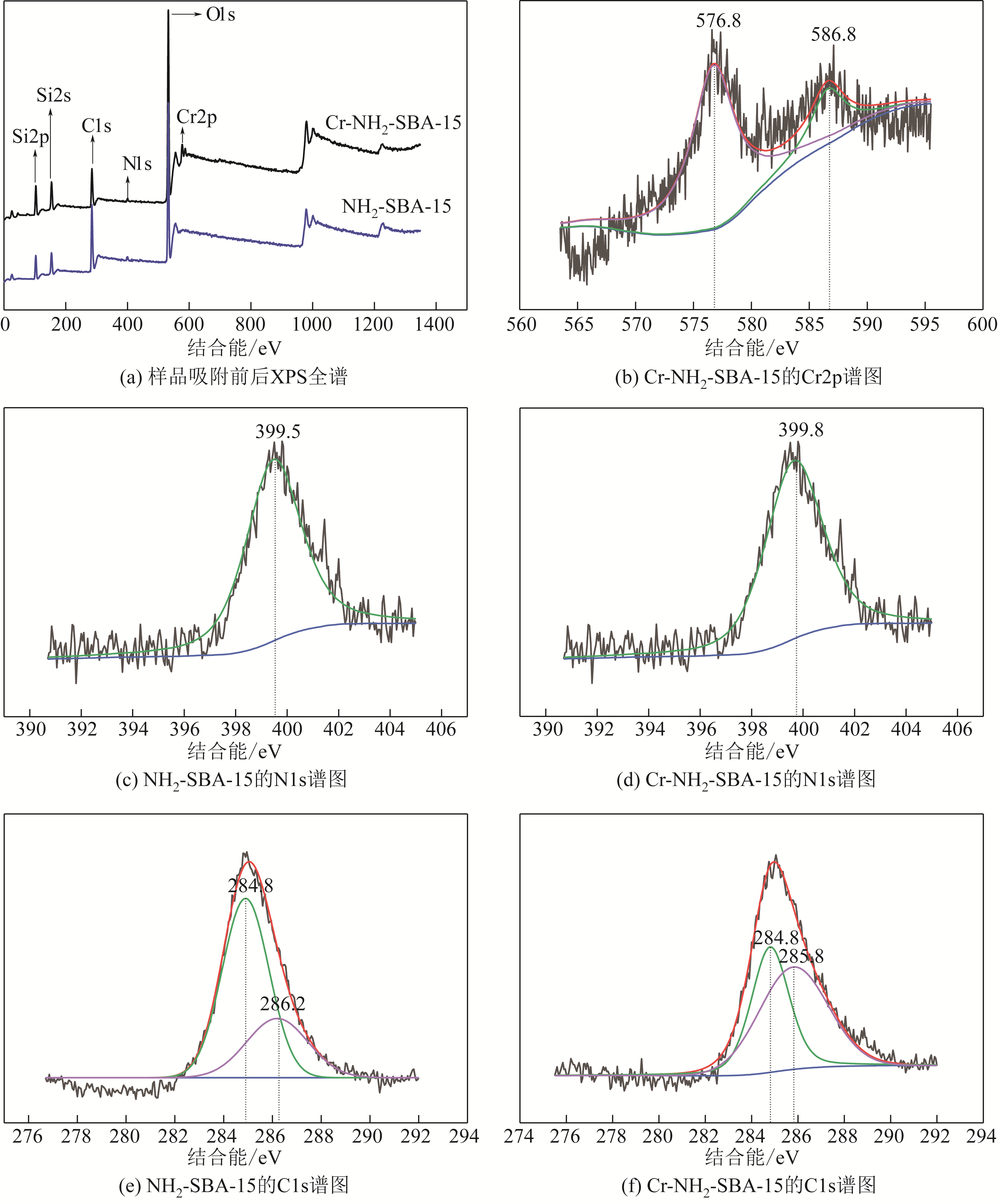
化工进展 ›› 2020, Vol. 39 ›› Issue (1): 257-266.DOI: 10.16085/j.issn.1000-6613.2019-0747
氨基功能化介孔硅吸附剂的制备及其对铬(Ⅲ)的吸附行为
- 辽宁工业大学化学与环境工程学院,辽宁 锦州 121001
-
收稿日期:2019-05-08出版日期:2020-01-05发布日期:2020-01-14 -
通讯作者:郭宇 -
作者简介:肖昱(1994—),男,硕士研究生,研究方向为化工新材料。E-mail:xiaoyuwo2359@foxmail.com 。 -
基金资助:国家自然科学基金(21601075);辽宁省高校基本科研项目重点项目(JZL201715403);辽宁省自然科学基金(20170540435);辽宁省教育厅科学研究重点攻关项目(JZL201915402)
Adsorption of chromium (Ⅲ) ions with amino functionalized mesoporous silica adsorbent
Yu XIAO( ),Yu GUO(
),Yu GUO( ),Hongmei WU,Xiaoqing JIANG
),Hongmei WU,Xiaoqing JIANG
- School of Chemical and Environmental Engineering, Liaoning University of Technology, Jinzhou 121001, Liaoning, China
-
Received:2019-05-08Online:2020-01-05Published:2020-01-14 -
Contact:Yu GUO
摘要:
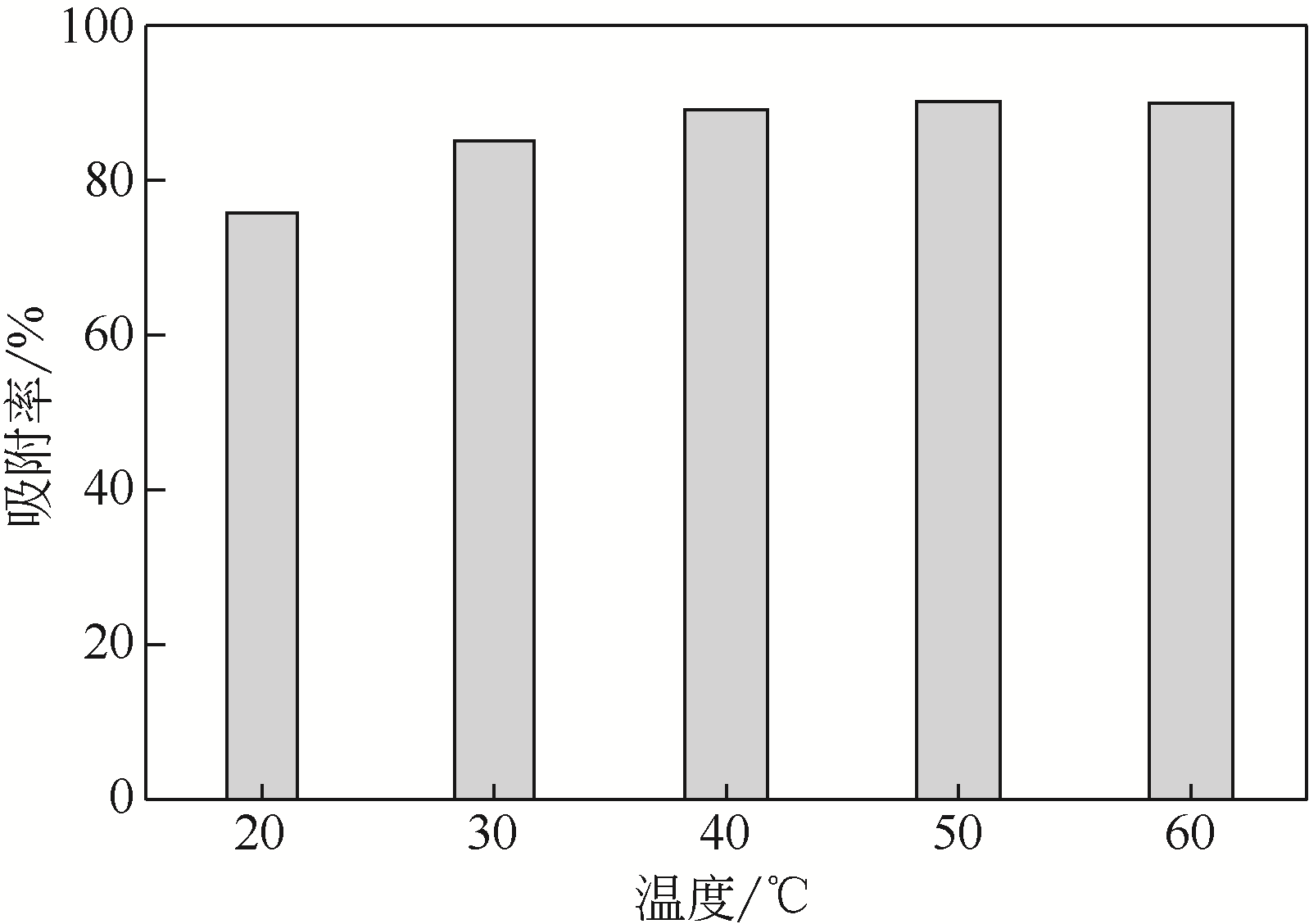
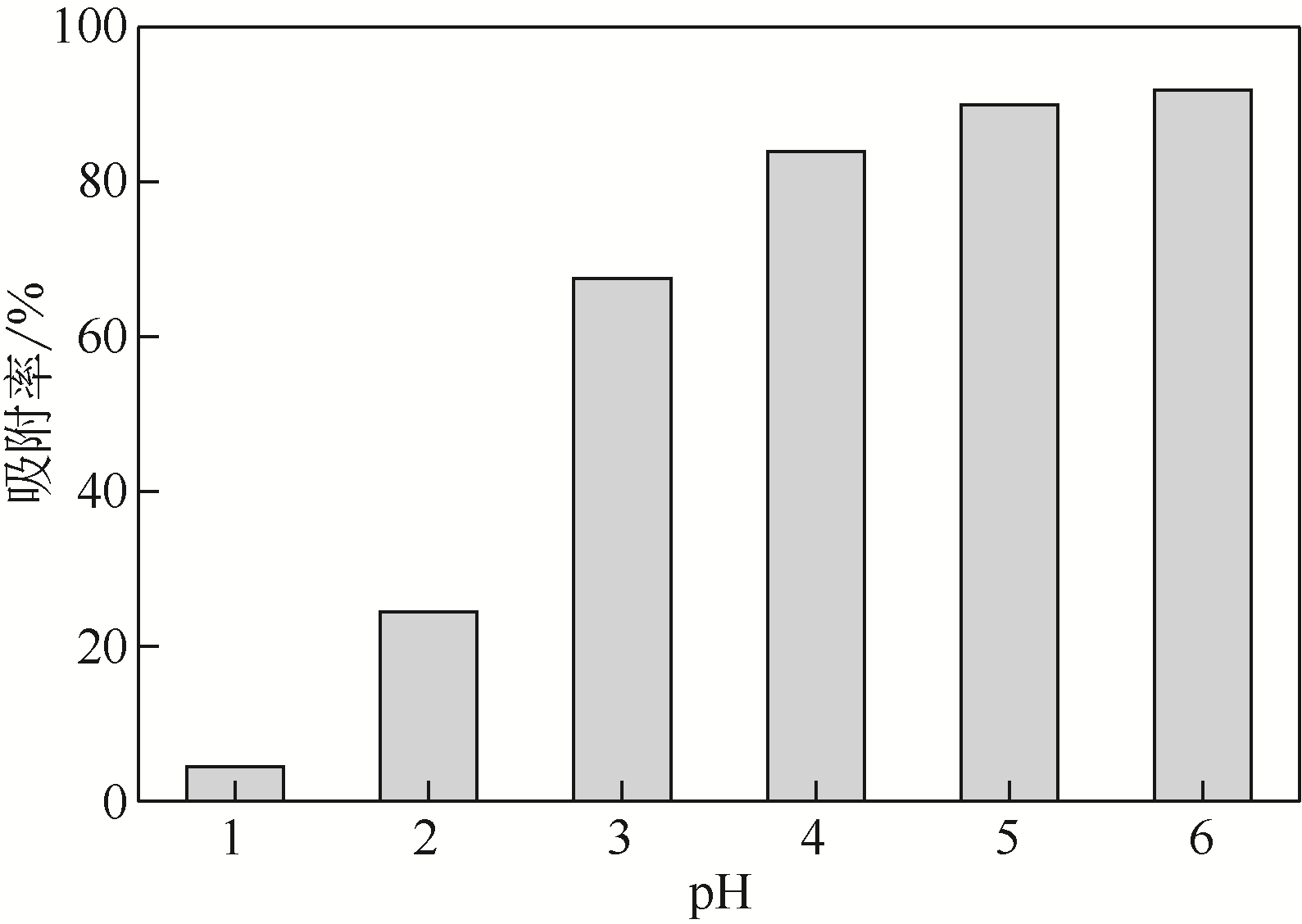
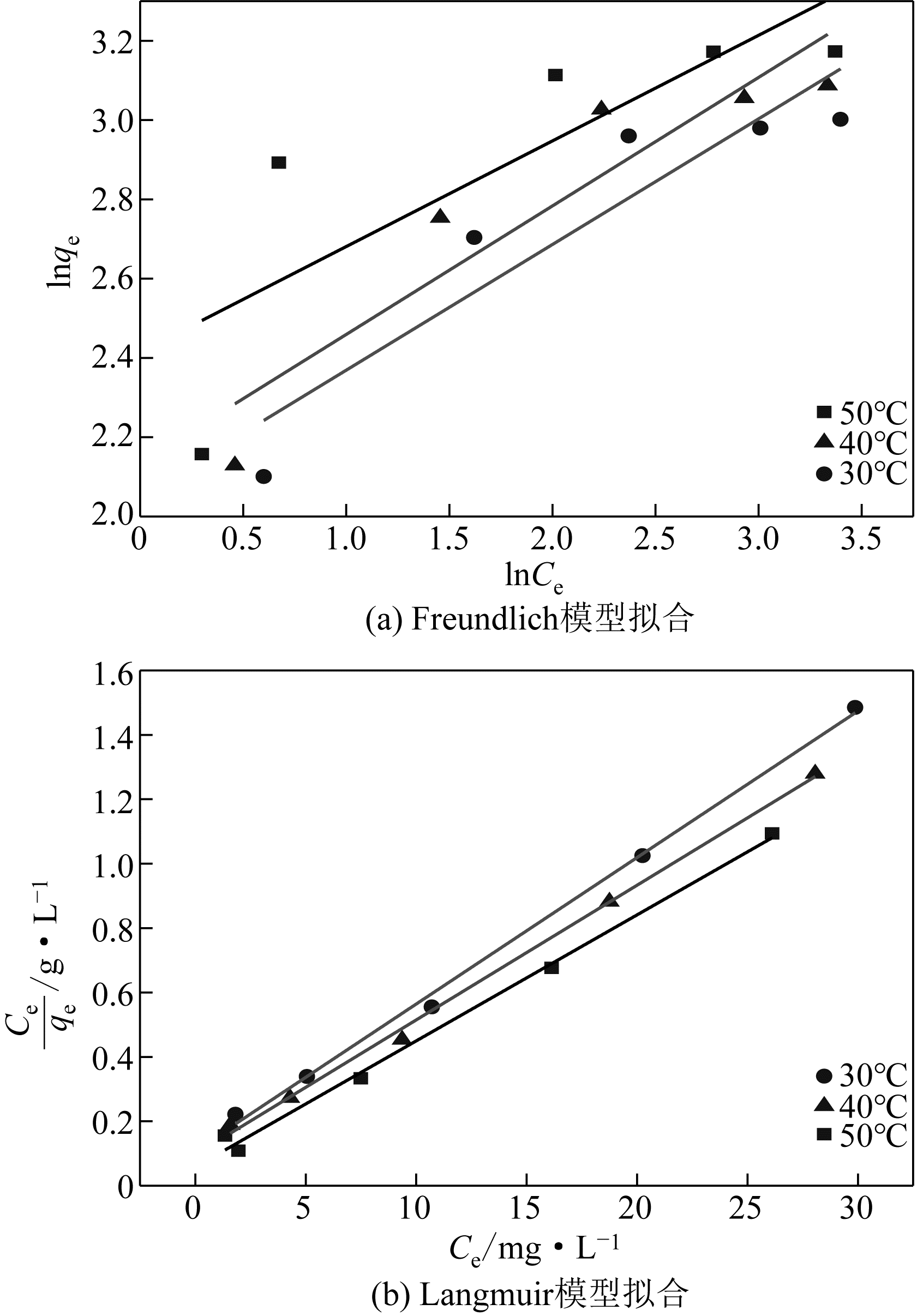
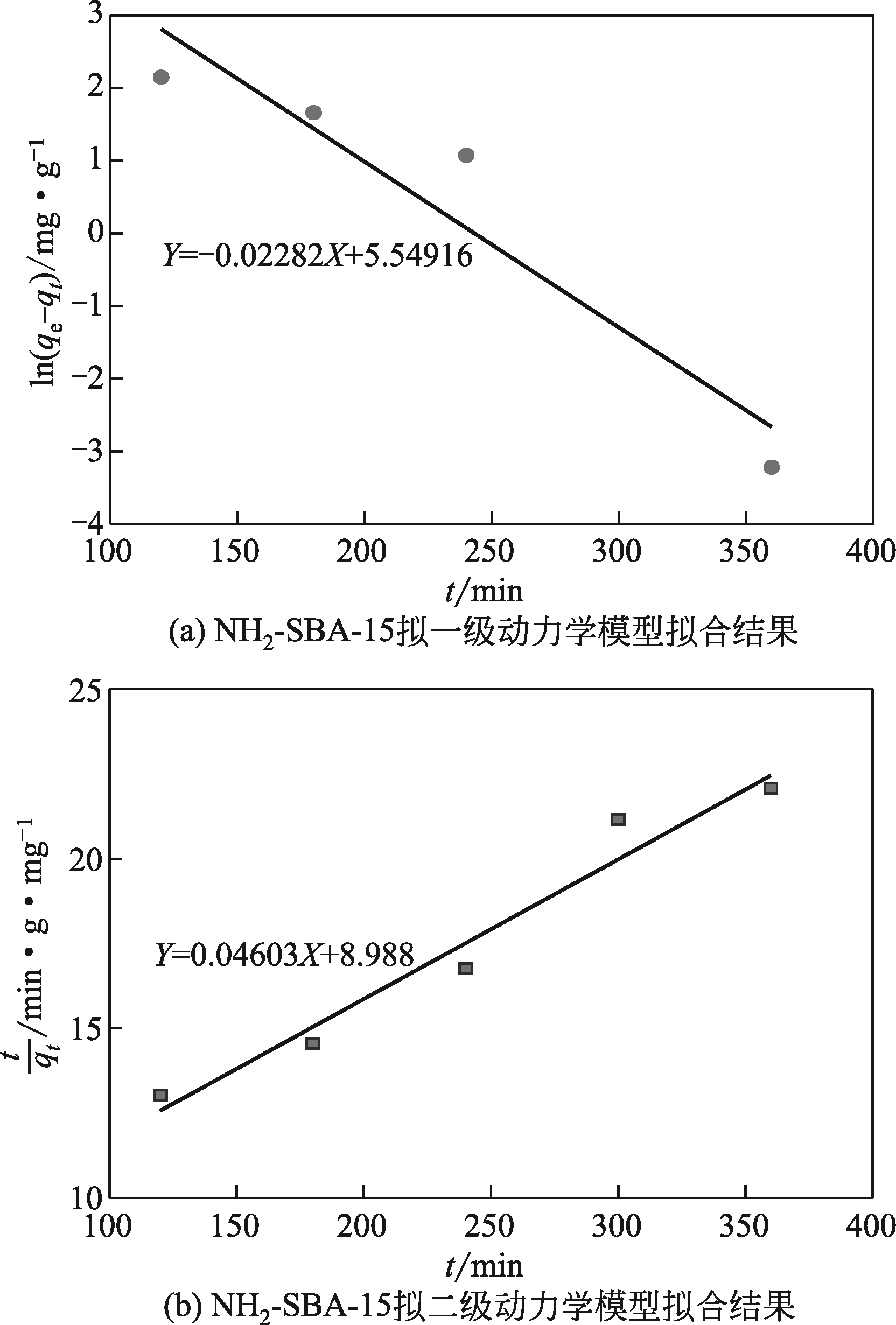
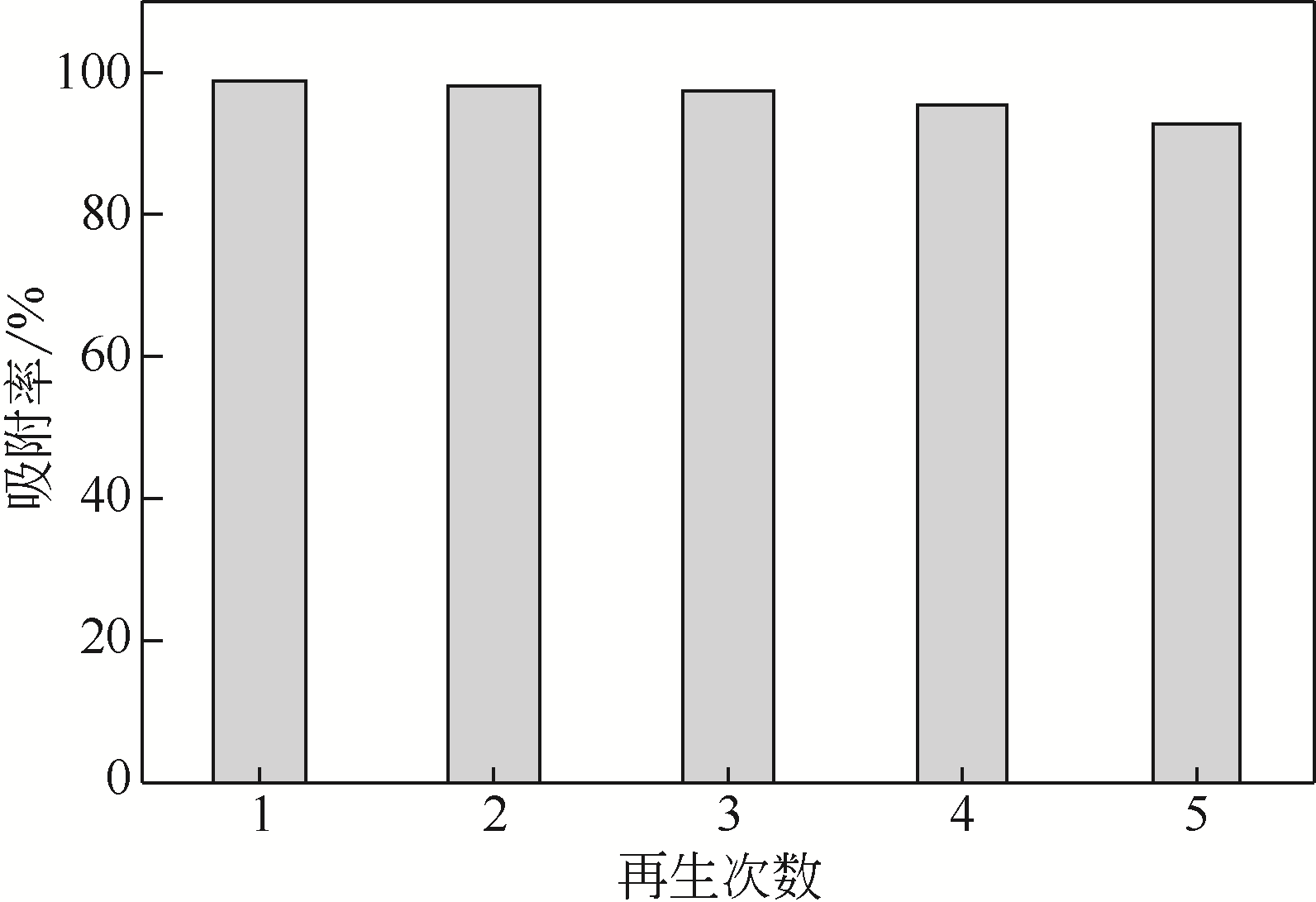
采用3-氨丙基三甲氧基硅烷(APTMS)对SBA-15介孔硅进行改性,获得氨基功能化介孔硅吸附剂(NH2-SBA-15),从而赋予其螯合重金属离子的能力。利用XRD、SEM、TEM、EDX、TGA、BET和XPS等手段对吸附剂的表面形貌、孔道结构、元素分布和表面化学性质进行了表征。研究了NH2-SBA-15吸附剂对水溶液中铬(Ⅲ)的吸附性能,分析了吸附动力学、吸附热力学和再生性能。结果表明,SBA-15吸附剂经过氨基功能化后,其原有的结晶结构没有明显变化,且对铬(Ⅲ)的吸附性能显著提高。NH2-SBA-15对铬(Ⅲ)的吸附行为符合Langmuir等温吸附模型和拟二级吸附动力学方程。NH2-SBA-15对铬(Ⅲ)的吸附过程主要依靠其表面—NH2与铬(Ⅲ)的配位螯合作用,且为吸热过程。经过5次循环利用后,NH2-SBA-15对铬(Ⅲ)的吸附率仍然保持在92%以上。该氨基功能化介孔硅吸附剂在吸附铬(Ⅲ)方面具有潜在的应用前景。
中图分类号:
引用本文
肖昱,郭宇,吴红梅,姜晓庆. 氨基功能化介孔硅吸附剂的制备及其对铬(Ⅲ)的吸附行为[J]. 化工进展, 2020, 39(1): 257-266.
Yu XIAO,Yu GUO,Hongmei WU,Xiaoqing JIANG. Adsorption of chromium (Ⅲ) ions with amino functionalized mesoporous silica adsorbent[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 257-266.
| 样品 | 比表面积/m2·g-1 | 孔径/nm |
|---|---|---|
| SBA-15 | 757 | 9.69 |
| NH2-SBA-15 | 565 | 6.54 |
表1 样品的孔结构分析
| 样品 | 比表面积/m2·g-1 | 孔径/nm |
|---|---|---|
| SBA-15 | 757 | 9.69 |
| NH2-SBA-15 | 565 | 6.54 |
| 温度/℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| q m/mg·g-1 | K L | R 2 | K F | 1/n | R 2 | ||
| 30 | 21.982 | 0.417 | 0.996 | 7.785 | 0.317 | 0.816 | |
| 40 | 23.911 | 0.434 | 0.996 | 8.459 | 0.324 | 0.814 | |
| 50 | 25.550 | 0.672 | 0.993 | 11.207 | 0.266 | 0.555 | |
表2 吸附等温线常数
| 温度/℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| q m/mg·g-1 | K L | R 2 | K F | 1/n | R 2 | ||
| 30 | 21.982 | 0.417 | 0.996 | 7.785 | 0.317 | 0.816 | |
| 40 | 23.911 | 0.434 | 0.996 | 8.459 | 0.324 | 0.814 | |
| 50 | 25.550 | 0.672 | 0.993 | 11.207 | 0.266 | 0.555 | |
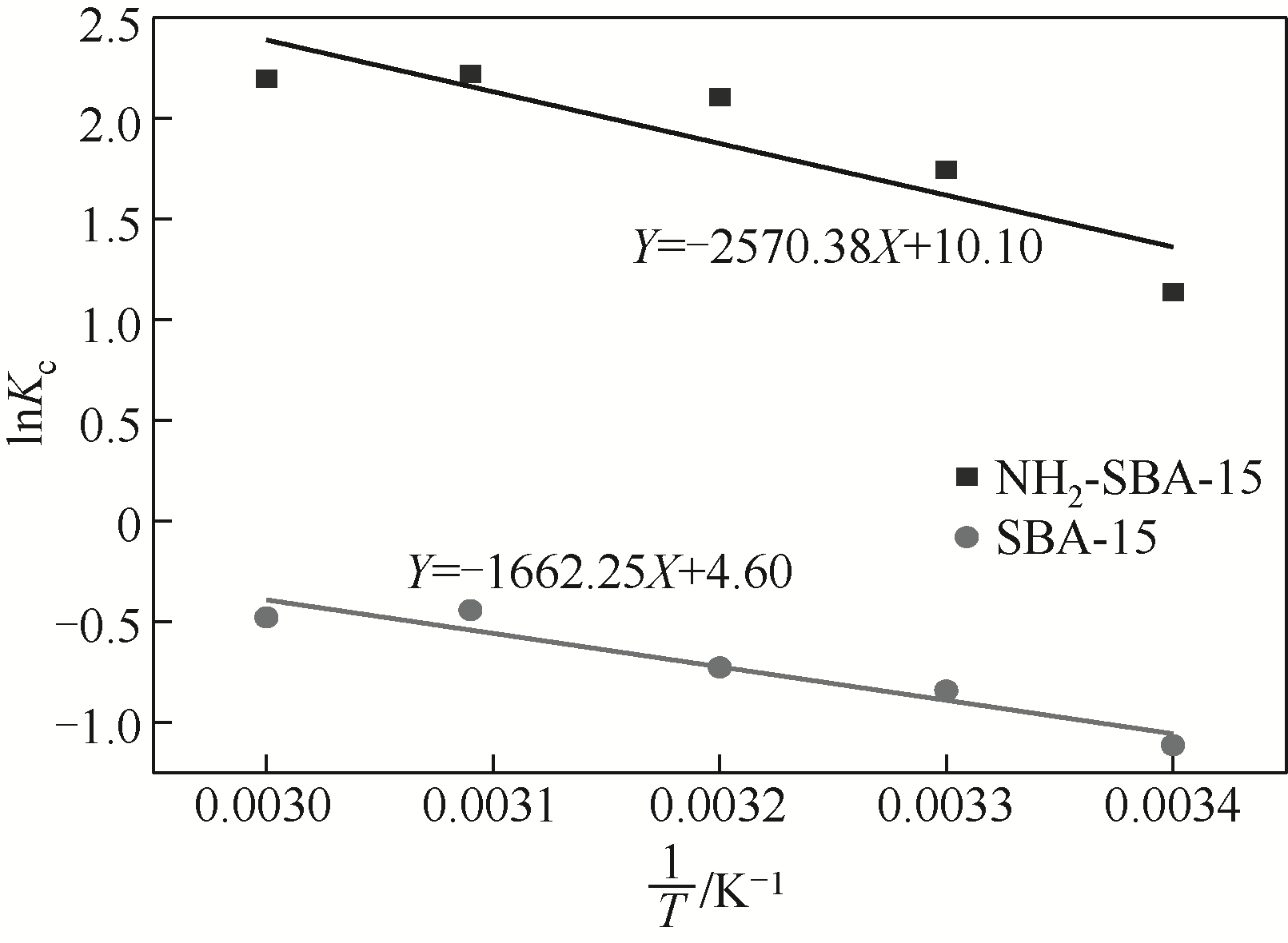
| 样品 | 温度 /K | K c | ΔG /kJ?mol-1 | ΔS/kJ?mol?K-1 | ΔH/kJ?mol-1 |
|---|---|---|---|---|---|
| SBA-15 | 293 | 0.329 | 2.686 | 0.038 | 13.82 |
| 303 | 0.432 | 2.306 | |||
| 313 | 0.484 | 1.926 | |||
| 323 | 0.643 | 1.546 | |||
| 333 | 0.621 | 1.166 | |||
| NH2-SBA-15 | 293 | 3.124 | -3.242 | 0.084 | 21.37 |
| 303 | 5.734 | -4.082 | |||
| 313 | 8.217 | -4.922 | |||
| 323 | 9.204 | -5.762 | |||
| 333 | 9.000 | -6.602 |
表3 吸附铬(Ⅲ)的平衡常数和热力学参数
| 样品 | 温度 /K | K c | ΔG /kJ?mol-1 | ΔS/kJ?mol?K-1 | ΔH/kJ?mol-1 |
|---|---|---|---|---|---|
| SBA-15 | 293 | 0.329 | 2.686 | 0.038 | 13.82 |
| 303 | 0.432 | 2.306 | |||
| 313 | 0.484 | 1.926 | |||
| 323 | 0.643 | 1.546 | |||
| 333 | 0.621 | 1.166 | |||
| NH2-SBA-15 | 293 | 3.124 | -3.242 | 0.084 | 21.37 |
| 303 | 5.734 | -4.082 | |||
| 313 | 8.217 | -4.922 | |||
| 323 | 9.204 | -5.762 | |||
| 333 | 9.000 | -6.602 |
| 吸附剂 | q e(实验)/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|---|
| K 1/min-1 | q e(计算)/mg·g-1 | R 2 | K 2/g·mg-1·min-1 | q e(计算)/mg·g-1 | R 2 | |||
| NH2-SBA-15 | 18.10 | 0.0228 | 252.924 | 0.852 | 2.36×10-4 | 21.724 | 0.942 | |
表4 NH2-SBA-15吸附铬(Ⅲ)动力学方程回归数据
| 吸附剂 | q e(实验)/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|---|
| K 1/min-1 | q e(计算)/mg·g-1 | R 2 | K 2/g·mg-1·min-1 | q e(计算)/mg·g-1 | R 2 | |||
| NH2-SBA-15 | 18.10 | 0.0228 | 252.924 | 0.852 | 2.36×10-4 | 21.724 | 0.942 | |
| 吸附剂 | pH | q m/mg·g-1 | 参考文献 |
|---|---|---|---|
| 多壁碳纳米管 | 3 | 13.2 | [ |
| 活性炭 | 6 | 12.23 | [ |
| 改性氧化石墨烯 | 5 | 13.3 | [ |
| 硫醇功能化MCM-41 | 5 | 15.34 | [ |
| 臭氧化活性炭 | 6 | 13.31 | [ |
| NH2-SBA-15 | 6 | 24.88 | 本工作 |
表5 各种吸附剂对铬(Ⅲ)的吸附性能数据
| 吸附剂 | pH | q m/mg·g-1 | 参考文献 |
|---|---|---|---|
| 多壁碳纳米管 | 3 | 13.2 | [ |
| 活性炭 | 6 | 12.23 | [ |
| 改性氧化石墨烯 | 5 | 13.3 | [ |
| 硫醇功能化MCM-41 | 5 | 15.34 | [ |
| 臭氧化活性炭 | 6 | 13.31 | [ |
| NH2-SBA-15 | 6 | 24.88 | 本工作 |
| 1 | 周蒙蒙, 王会玲, 姚民秀 . 铬镁离子对糖代谢异常患者的影响及研究进展[J/CD].中华临床医师杂志: 电子版, 2015, 9(9): 1701-1705. |
| ZHOU Mengmeng , WANG Huiling , YAO Minxiu . Research progress and advances in chromium magnesium ion effect on patients with abnormal glucose metabolism[J/CD]. Chinese Journal of Clinicians:Electronic Editien, 2015, 9(9): 1701-1705. | |
| 2 | JAMSHIDI M , GHAEDI M , DASHTIAN K , et al . New ion-imprinted polymer-functionalized mesoporous SBA-15 for selective separation and preconcentration of Cr(Ⅲ) ions: modeling and optimization[J]. RSC Advances, 2015, 5(128): 105789-105799. |
| 3 | 王谦,李延,孙平,等 .含铬废水处理技术及研究进展[J].环境科学与技术, 2013, 36(12): 150-156. |
| WANG Qian , LI Yan , SUN Ping , et al . The treatment technology and research progress of hexavalent chromium-containing wastewater[J]. Environmental Science and Technology, 2013, 36(2): 150-156. | |
| 4 | THONG Z , HAN G , CUI Y , et al . Novel nanofiltration membranes consisting of a sulfonated pentablock copolymer rejection layer for heavy metal removal[J]. Environmental Science & Technology, 2014, 48(23): 13880-13887. |
| 5 | DUAN Q , LEE J, LIU Y , et al . Distribution of heavy metal pollution in surface soil samples in china: a graphical review[J]. Bulletin of Environmental Contamination and Toxicology, 2016, 97(3): 303-309. |
| 6 | ZHENG W , LI H , CHEN W , et al . Recyclable colorimetric detection of trivalent cations in aqueous media using zwitterionic gold nanoparticles[J]. Analytical Chemistry, 2016, 88(7): 4140-4146. |
| 7 | ENGATES K E , SHIPLEY H J . Adsorption of Pb, Cd, Cu, Zn and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration and exhaustion[J]. Environmental Science & Pollution Research, 2011, 18(3): 386-395. |
| 8 | MURTAZA G , HAYNES R J , NAIDU R . et al . Natural attenuation of Zn, Cu, Pb and Cd in three biosolids-amended soils of contrasting pH measured using rhizon pore water samplers[J]. Water, Air, and Soil Pollution, 2011, 221: 351-363. |
| 9 | OTTOSEN L M , HANSE H K , JENSEN P E . Relation between pH and desorption of Cu, Cr, Zn and Pb from industrially polluted soils[J]. Water, Air, and Soil Pollution, 2009, 201: 295-304. |
| 10 | 张振国, 张铭栋, 顾平, 等 . 沸石材料吸附水中放射性锶和铯的研究进展[J]. 化工进展, 2019, 38(4): 1984-1995. |
| ZHANG Zhenguo , ZHANG Mingdong , GU Ping , et al . Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1984-1995. | |
| 11 | 史明明, 刘美艳, 曾佑林, 等 . 硅藻土和膨润土对重金属离子Zn2+、Pb2+及 Cd2+的吸附特性[J]. 环境化学, 2012, 31(2): 162-167. |
| SHI Mingming , LIU Meiyan , ZENG Youlin , et al . Study on adsorption of Zn2+, Pb2+ and Cd2+ on diatomite and bentonite[J]. Environmental Chemistry, 2012, 31(2): 162-167. | |
| 12 | MADAENI S S , MANSOURPANAH Y . COD removal from concentrated wastewater using membranes[J]. Filtration & Separation, 2003, 40(6): 40-46. |
| 13 | QIN J J , WAI M N, OO M H . A feasibility study on the treatment and recycling of a wastewater from metal plating[J]. Journal of Membrane Science, 2002, 208(1/2): 213-221. |
| 14 | LI J , JIN P , TANG C C . Cr(Ⅲ) adsorption by fluorinated activated boron nitride: a combined experimental and theoretical investigation[J]. RSC Advances, 2014, 4(29): 14815-14821. |
| 15 | SANTHANA K K A , JIANG S J , WARCHOL J K . Synthesis and characterization of two-dimensional transition metal dichalcogenide magnetic MoS2@Fe3O4 nanoparticles for adsorption of Cr(Ⅵ)/Cr(Ⅲ)[J]. ACS Omega, 2017, 2(9): 6187-6200. |
| 16 | DAI C , HU Y . Correction to Fe(Ⅲ) hydroxide nucleation and growth on quartz in the presence of Cu(Ⅱ), Pb(Ⅱ), and Cr(Ⅲ): metal hydrolysis and adsorption[J]. Environmental Science & Technology, 2015, 49(3): 292-300. |
| 17 | ZHU H , JIN W , MING F , et al . Synthesis of a core-shell magnetic Fe3O4-NH2@PmPD nanocomposite for efficient removal of Cr(Ⅵ) from aqueous media[J]. RSC Advances, 2017, 7(58): 36231-36241. |
| 18 | VARTULI J C , SCHMITT K D , KRESGE C T , et al . Development of a formation mechanism for M41S materials[J]. Studies in Surface Science & Catalysis, 1994, 84(8): 53-60. |
| 19 | MURESEANU M , REISS A , STEFANESEU I , et a1 . Modified SBA-15 mesoporous silica for heavy metal ions remediation[J]. Chemosphere, 2008, 73(9): 1499-1504. |
| 20 | ALGARRA M , JIMENEZ M , RODRIGUEZ C , et a1 . Heavy metals removal from electroplating wastewater by aminopropyl-Si MCM-41[J]. Chemosphere, 2005, 59(6): 779-786. |
| 21 | JIANG Y J , GAO Q M , YU H G , et a1 . Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic-organic hybrid materials[J]. Microporous and Mesoporous Materials, 2007, 103(1/2/3): 316-324. |
| 22 | BURKE A M , HANRAHAN J P , HEALYD D A , et a1 . Large pore bifunctionalised mesoporous silica for metal ion pollution treatment[J]. Journal of Hazardous Materials, 2009, 164(1): 229-234. |
| 23 | ZHAO D Y , HUO Q S , FENG J L , et al . Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered,hydrothermally stable,mesoporous silica structures[J]. Journal of the American Chemical Society, 1998, 120 (24): 6024-6036. |
| 24 | LASHGARI N , BADIEI A , MOHAMMADI Z G . Selective detection of Hg2+ ion in aqueous medium with the use of 3-(pyrimidin-2-ylimino) indolin-2-functionalized SBA-15[J]. Applied Organometallic Chemistry, 2018, 32(1): e3991. |
| 25 | FELLENZ N , PEREZ-ALONSO F J , MARTIN P P , et al . Chromium(Ⅵ) removal from water by means of adsorption-reduction at the surface of amino -functionalized MCM-41 sorbents[J]. Microporous & Mesoporous Materials, 2017, 239: 138-146. |
| 26 | CHEN F , HONG M , YOU W , et al . Simultaneous efficient adsorption of Pb2+ and MnO4 - ions by MCM-41 functionalized with amine and nitrilotriacetic acid anhydride[J]. Applied Surface Science, 2015, 357: 856-865. |
| 27 | LEYVA-RAMOS R , FUENTES-RUBIO L , GUERRERO-CORONADO R M , et al . Adsorption of trivalent chromium from aqueous solutions onto activated carbon[J]. Journal of Chemical Technology & Biotechnology, 2010, 62(1): 64-67. |
| 28 | WANG T , LIU W , XIONG L , et al . Influence of pH ionic strength and humic acid on competitive adsorption of Pb(Ⅱ), Cd(Ⅱ) and Cr(Ⅲ) onto titanate nanotubes[J]. Chemical Engineering Journal, 2013, 215(3): 366-374. |
| 29 | RIVERA-UTRILLA J , SANCHEZ-POLO M . Adsorption of Cr(Ⅲ) on ozonised activated carbon. Importance of Cπ-cation interactions[J]. Journal of Ornithology, 2003, 37(14): 3335-3340. |
| 30 | 施华珍, 刘坤, 汤睿, 等 . 有机改性磁性碱性钙基膨润土的制备及对Cu(Ⅱ)和Mn(Ⅱ)的吸附[J]. 化工进展, 2018, 37(11): 4509-4521. |
| SHI Huazhen , LIU Kun , TANG Rui , et al . Organically modification of magnetic alkaline Ca-bentonite and its adsorption for Cu(Ⅱ) and Mn(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4509-4521. | |
| 31 | 张艺钟, 刘珊, 刘志文, 等 . 壳聚糖凝胶球对Cu(Ⅱ)和Cr(Ⅵ)吸附行为的对比[J]. 化工进展, 2017, 36(2): 712-719. |
| ZHANG Yizhong , LIU Shan , LIU Zhiwen , et al . Comparison for Cu(Ⅱ) and Cr(Ⅵ) adsorption behavior onto chitosan hydrogel beads[J]. Chemical Industry and Engineering Progress, 2017, 36(2): 712-719. | |
| 32 | PONCE-LIRA B , OTAZO-SÁNCHEZ E M , REGUERA E , et al . Lead removal from aqueous solution by basaltic scoria: adsorption equilibrium and kinetics[J]. International Journal of Environmental Science and Technology, 2017, 14: 1181-1196. |
| 33 | SANTHANA K K A , JIANG S J , WEI L T . Effective adsorption of Cr (Ⅵ)/Cr(Ⅲ) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent[J]. Journal of Materials Chemistry A, 2015, 3, 7044-7057. |
| 34 | OHAN D , SINGH K P , SINGH V K . Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth[J]. Journal of Hazardous Materials, 2006, 135(1/3): 280-295. |
| 35 | JANIK P , ZWISZA B , TALIK E , et al . Selective adsorption and determination of hexavalent chromium ions using graphene oxide modified with amino silanes[J]. Microchimica Acta, 2018, 185(2): 117. |
| 36 | WU S J , LI F T , XU R , et al . Synthesis of thiol-functionalized MCM-41 mesoporous silicas and its application in Cu(Ⅱ), Pb(Ⅱ), Ag(Ⅰ), and Cr(Ⅲ) removal[J]. Journal of Nanoparticle Research, 2010, 12(6): 2111–2124. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 陈俊俊, 费昌恩, 段金汤, 顾雪萍, 冯连芳, 张才亮. 高生物活性聚醚醚酮化学改性研究进展[J]. 化工进展, 2023, 42(8): 4015-4028. |
| [3] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [4] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [5] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [6] | 陈明星, 王新亚, 张威, 肖长发. 纤维基耐高温空气过滤材料研究进展[J]. 化工进展, 2023, 42(5): 2439-2453. |
| [7] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [8] | 郭帅帅, 陈锦路, 金梁程龙, 陶醉, 陈小丽, 彭国文. 基于海水提铀的多孔芳香框架材料研究进展[J]. 化工进展, 2023, 42(3): 1426-1436. |
| [9] | 杨凯璐, 陈明星, 王新亚, 张威, 肖长发. 染料废水处理用纳滤膜制备及改性研究进展[J]. 化工进展, 2023, 42(10): 5470-5486. |
| [10] | 王胜楠, 郑旭. 空气取水用活性炭纤维复合吸附剂的研究[J]. 化工进展, 2023, 42(10): 5567-5573. |
| [11] | 曹正凯, 米晓斌, 吴子明, 孙士可, 曹均丰, 彭德强, 梁相程. 煤合成气净化除尘装置压降问题分析及应用优化[J]. 化工进展, 2022, 41(S1): 15-21. |
| [12] | 王子航, 梁瑞升, 邓超和, 王佳韵. 离子凝胶复合吸附剂的制备及空气取水性能[J]. 化工进展, 2022, 41(S1): 389-396. |
| [13] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [14] | 黄岳峰, 马丽莎, 张莉莉, 王志国. 木质纤维素复合生物质薄膜材料的功能化应用研究进展[J]. 化工进展, 2022, 41(9): 4840-4854. |
| [15] | 边宇, 张百超, 郑红. 多级孔COFs材料的设计、合成及应用[J]. 化工进展, 2022, 41(9): 4866-4883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||