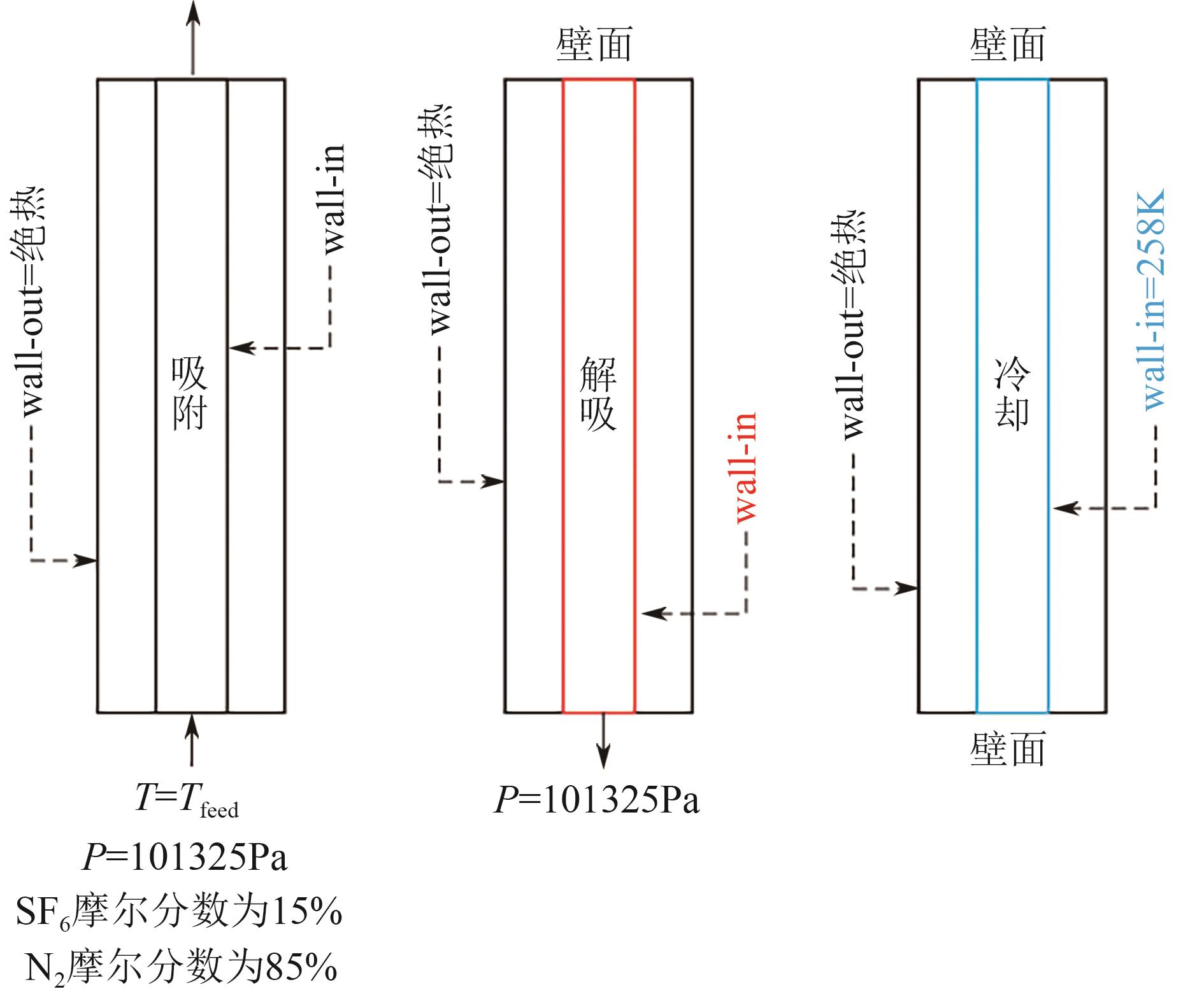
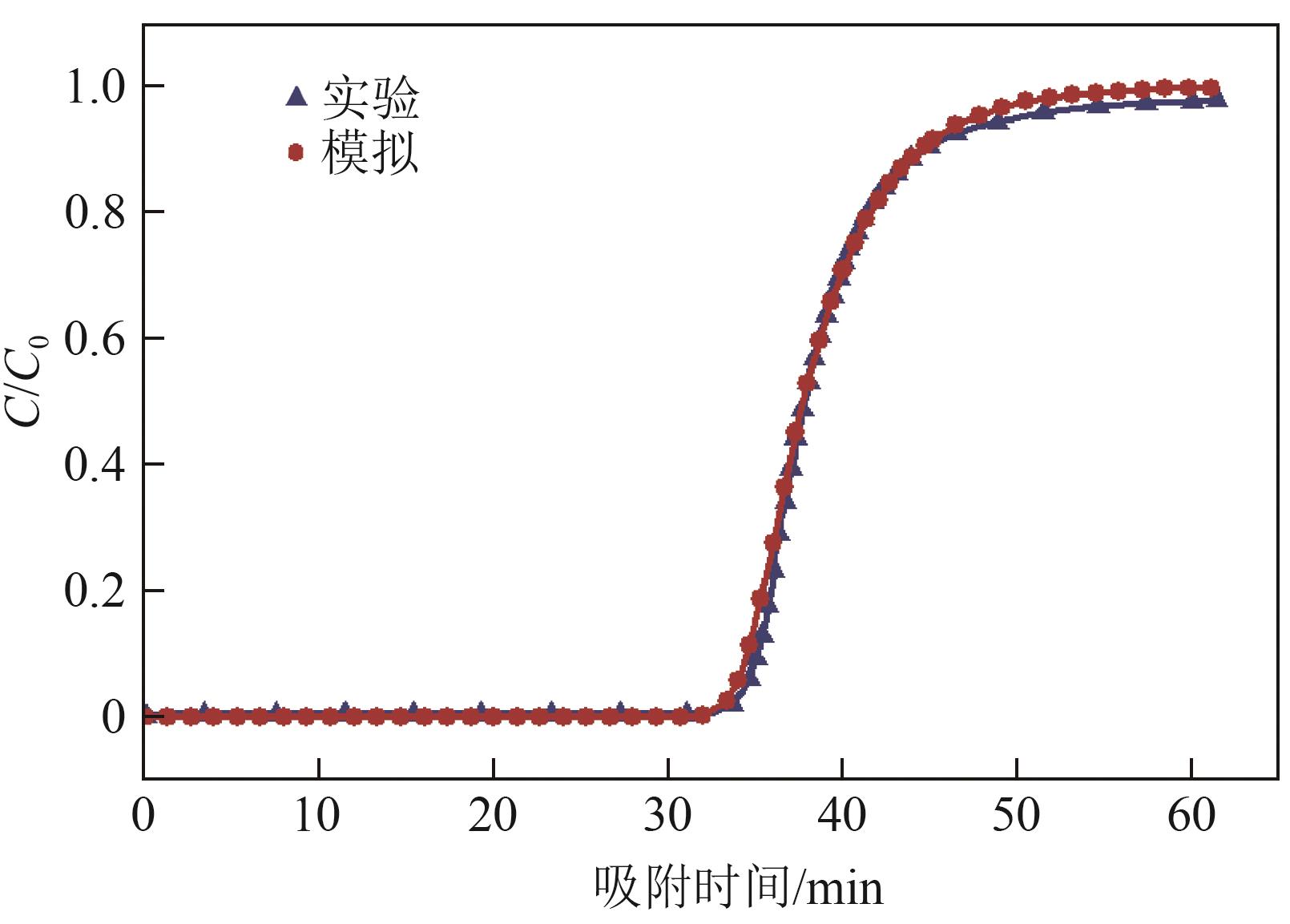
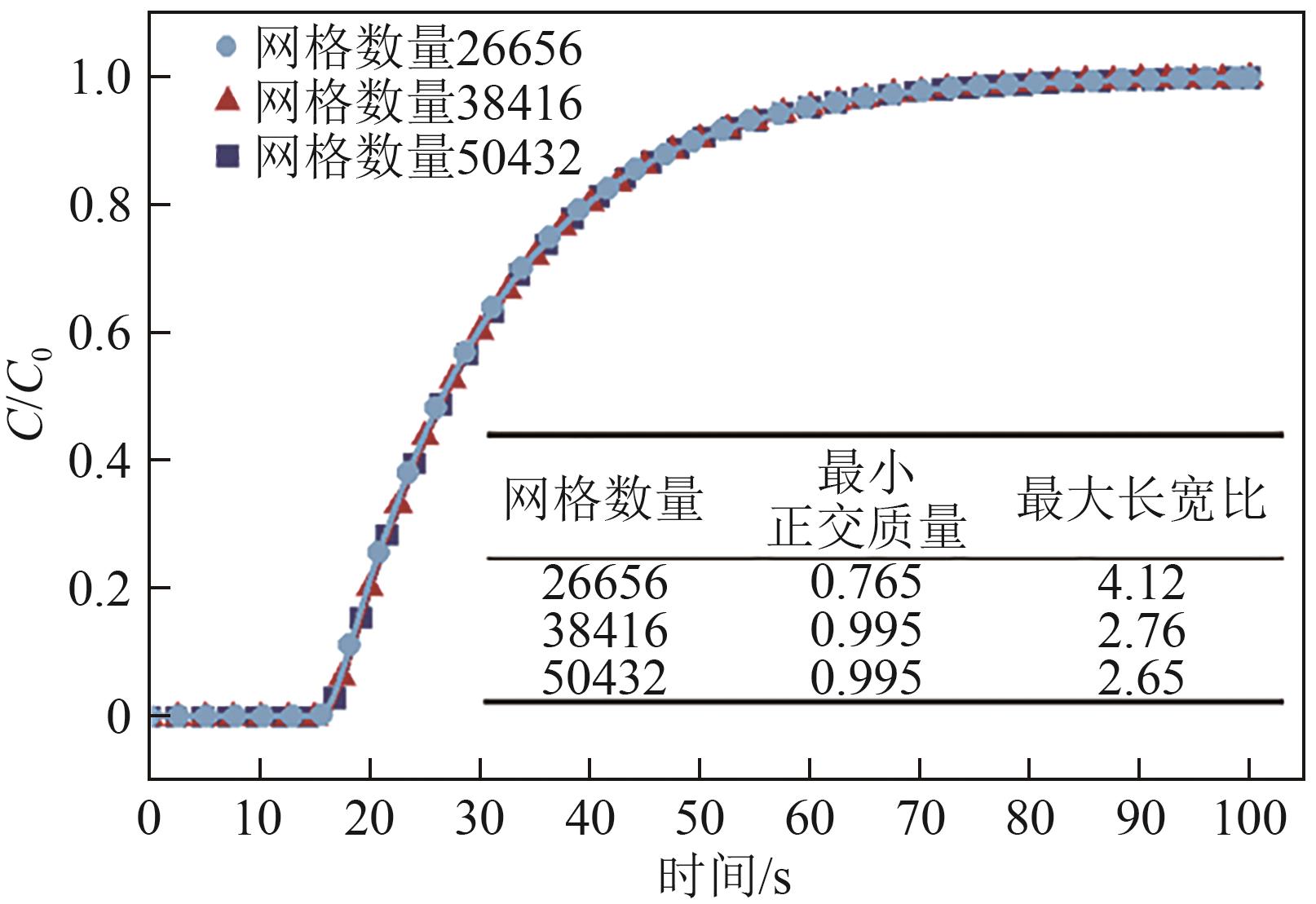
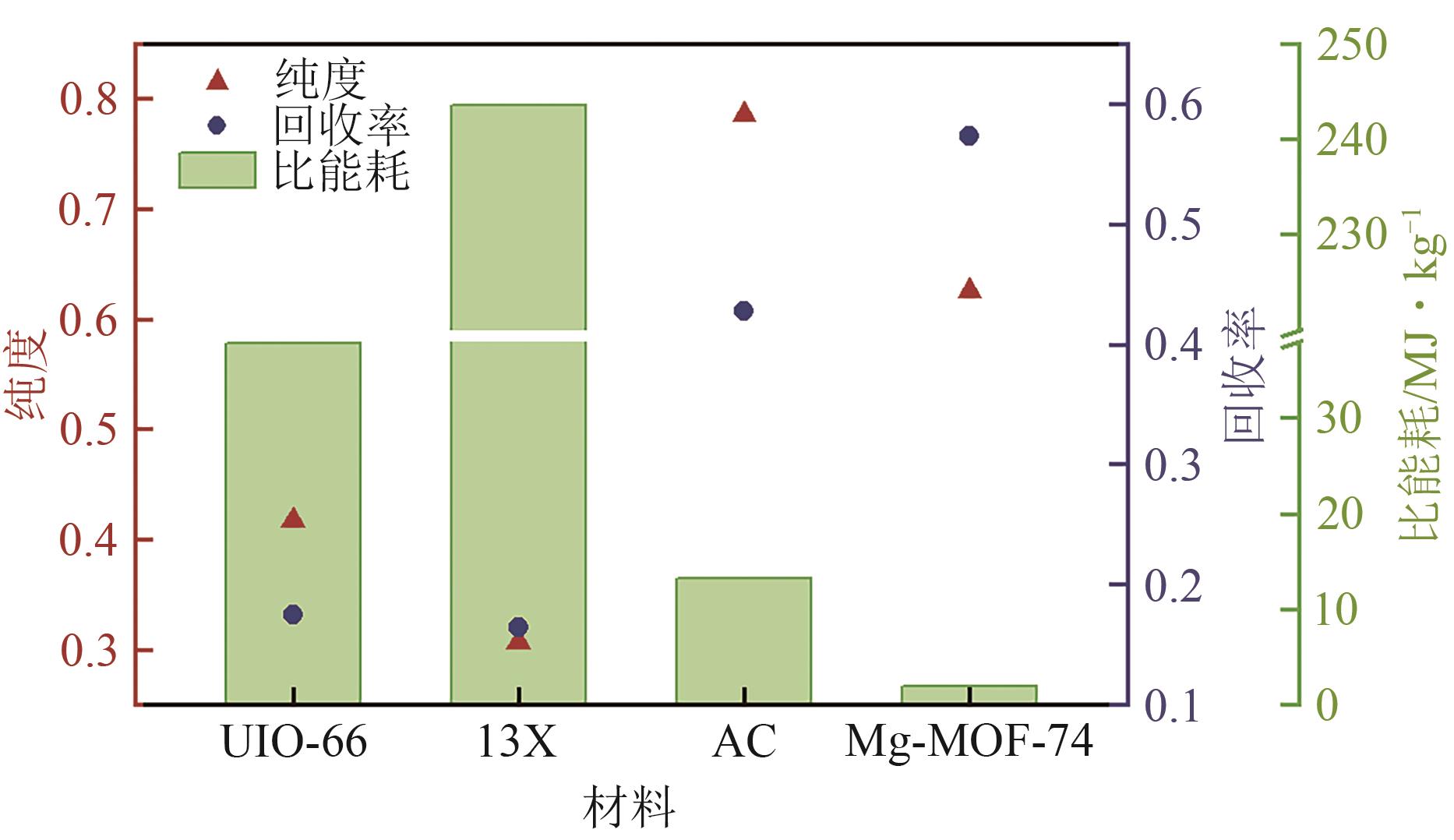
化工进展 ›› 2025, Vol. 44 ›› Issue (S1): 19-28.DOI: 10.16085/j.issn.1000-6613.2024-2129
混合绝缘气体变温吸附分离回收SF6的数值模拟
武锦怡1( ), 赵睿恺1,2(
), 赵睿恺1,2( ), 邓帅1,2, 张家麒1, 高春霄1, 刘葳桦1, 赵力1
), 邓帅1,2, 张家麒1, 高春霄1, 刘葳桦1, 赵力1
- 1.天津大学先进内燃动力全国重点实验室,天津 300350
2.天津市超低能耗碳捕集国际联合研究中心,天津 300350
-
收稿日期:2024-12-31修回日期:2025-03-17出版日期:2025-10-25发布日期:2025-11-24 -
通讯作者:赵睿恺 -
作者简介:武锦怡(2003—),女,硕士研究生,研究方向为SF6吸附分离回收。E-mail:wjinyi124@163.com。 -
基金资助:国家自然科学基金青年科学基金(52306265);天津市自然科学基金重点项目(22JCZDJC00540)
Numerical simulation of temperature swing adsorption for SF6 recovery from mixed insulating gas
WU Jinyi1( ), ZHAO Ruikai1,2(
), ZHAO Ruikai1,2( ), DENG Shuai1,2, ZHANG Jiaqi1, GAO Chunxiao1, LIU Weihua1, ZHAO Li1
), DENG Shuai1,2, ZHANG Jiaqi1, GAO Chunxiao1, LIU Weihua1, ZHAO Li1
- 1.State Key Laboratory of Engines, Tianjin University, Tianjin 300350, China
2.International Joint Research Center for Ultra-Low Energy Carbon Capture, Tianjin University, Tianjin 300350, China
-
Received:2024-12-31Revised:2025-03-17Online:2025-10-25Published:2025-11-24 -
Contact:ZHAO Ruikai
摘要:
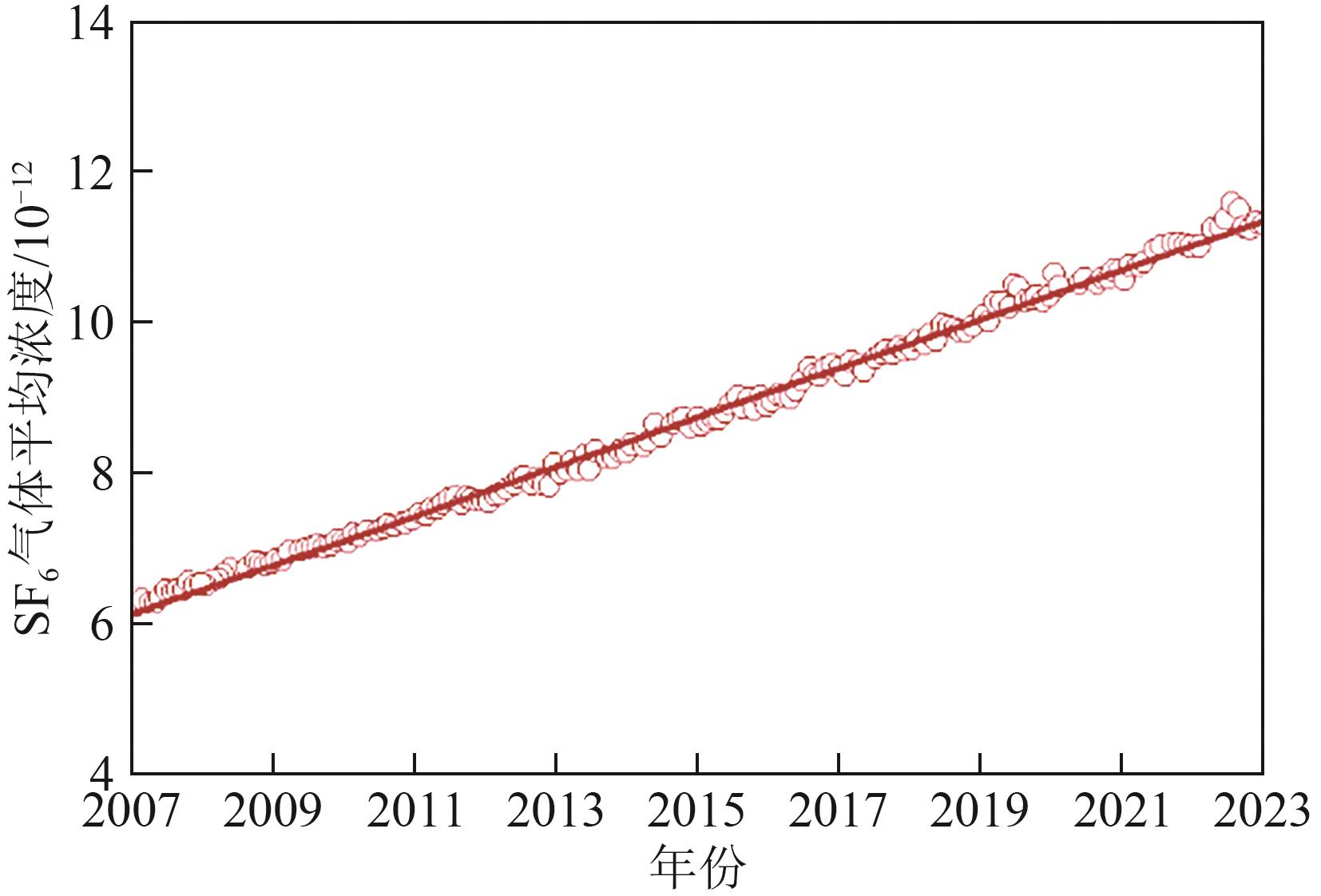
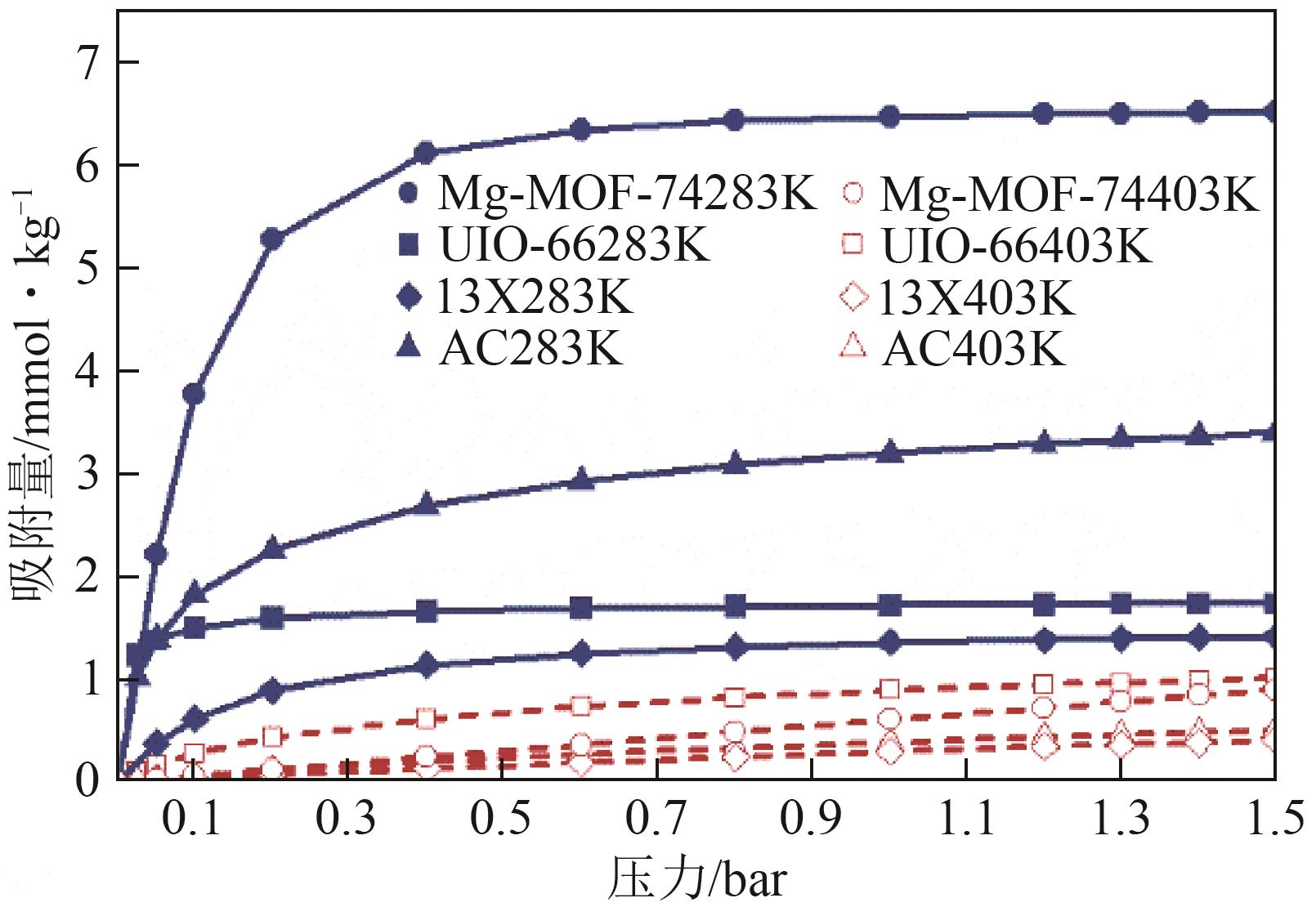
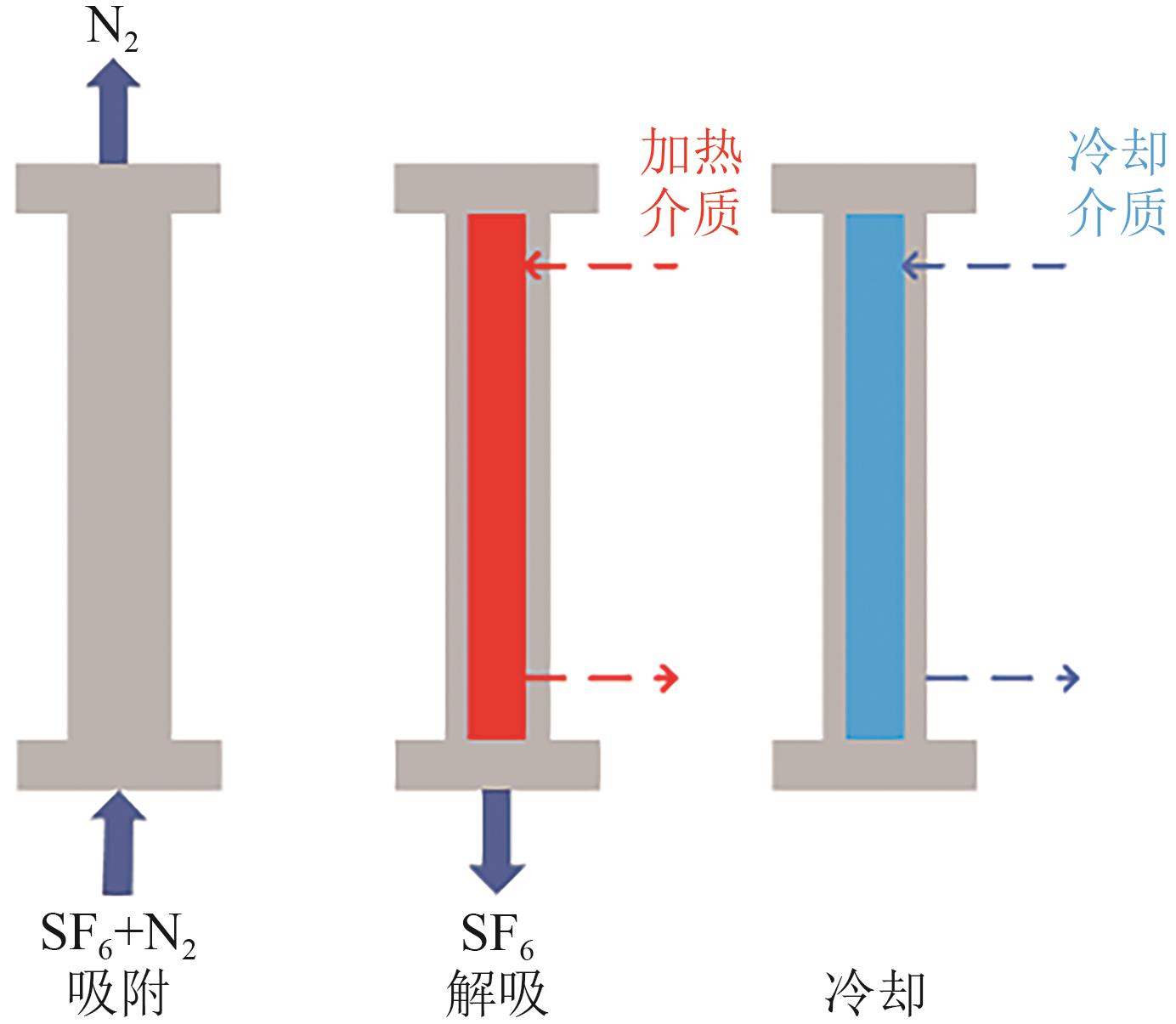
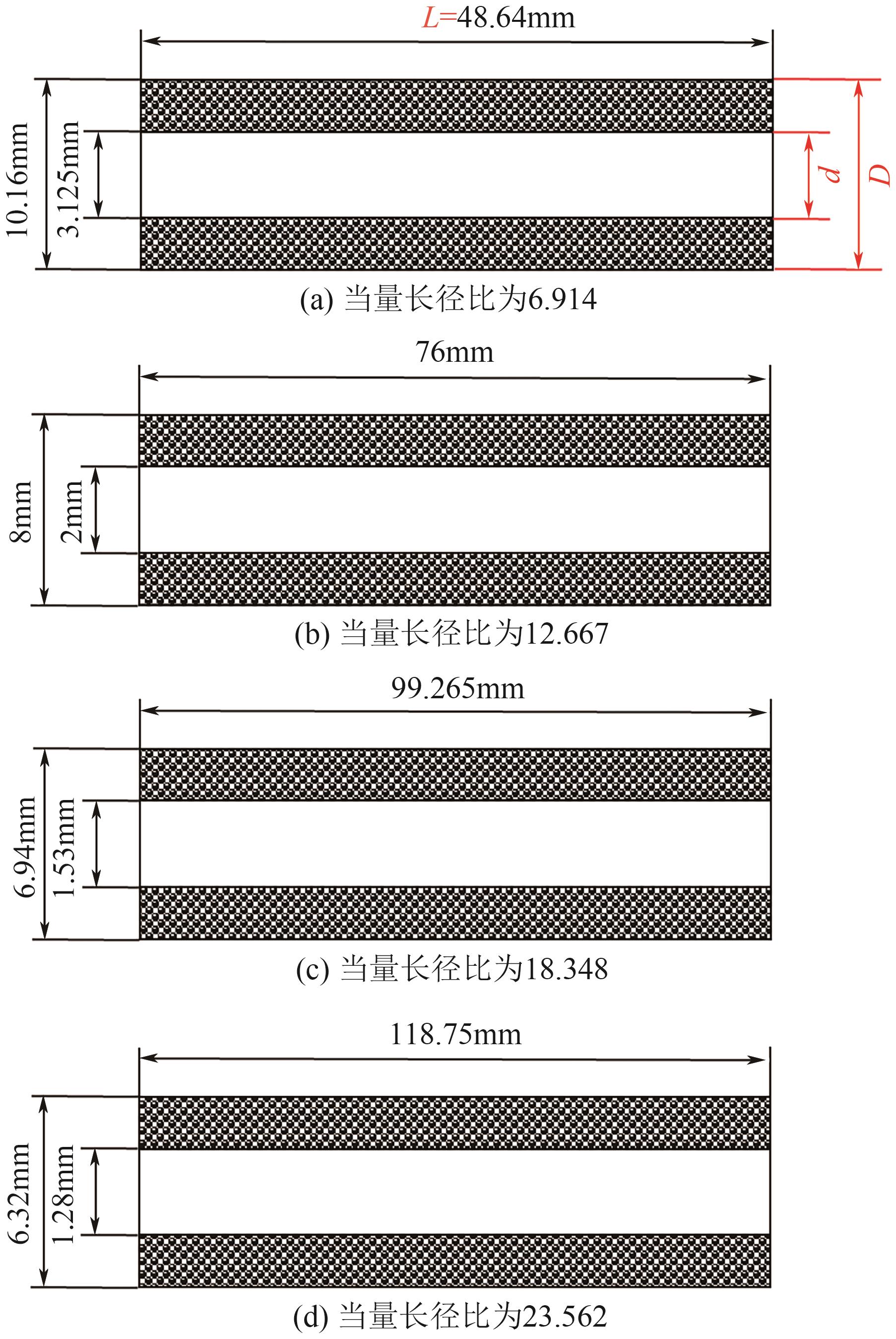
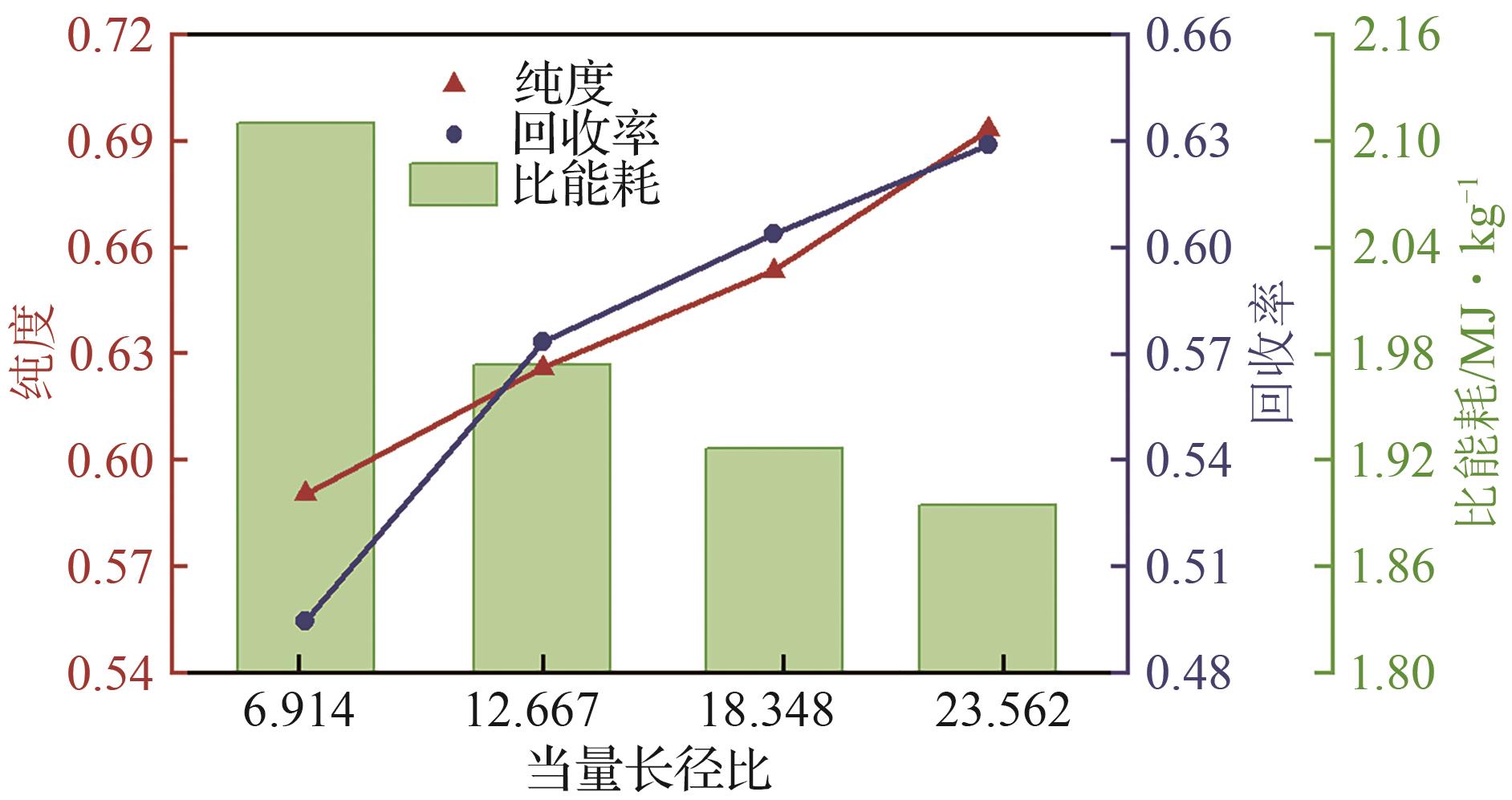
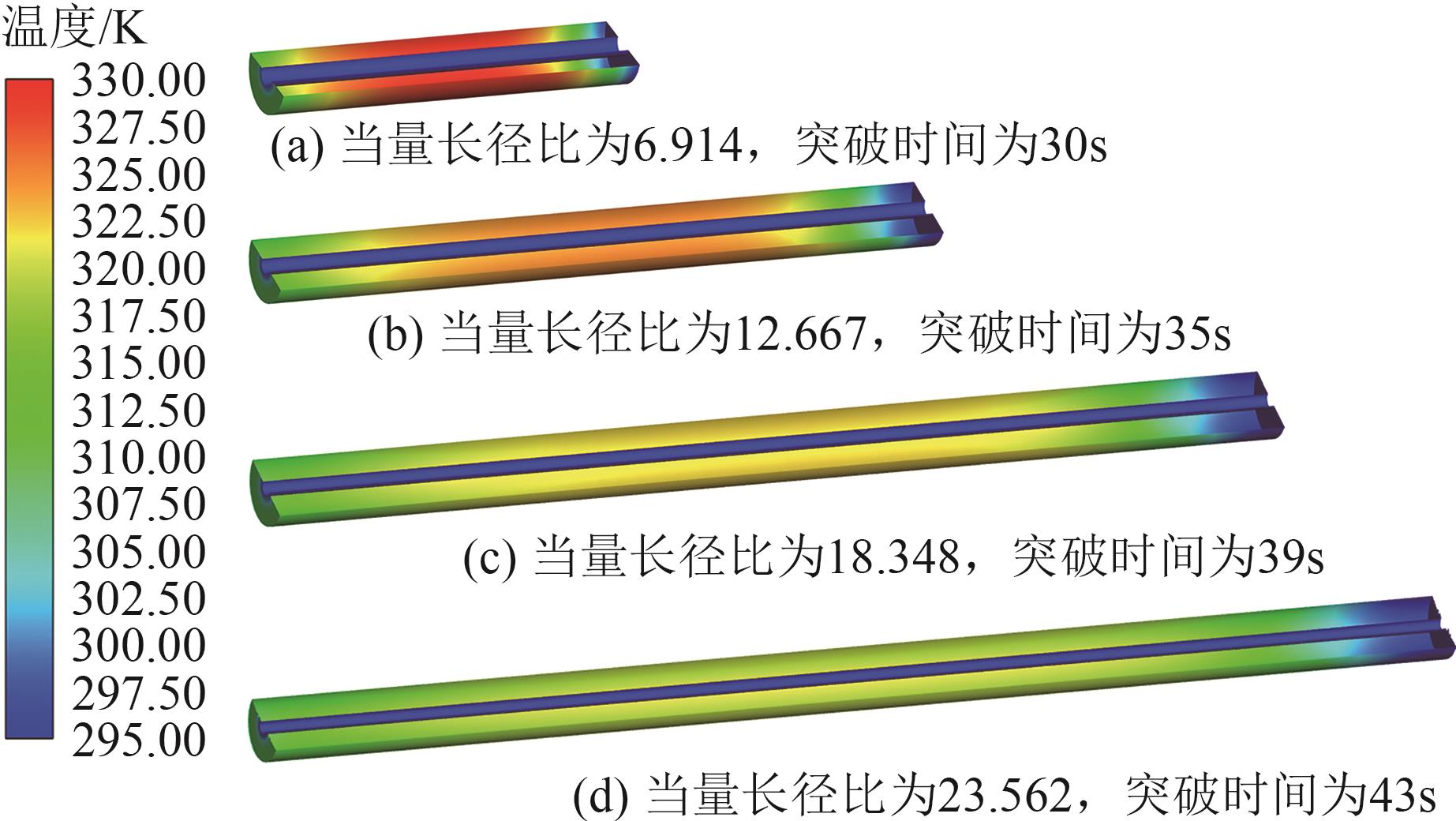
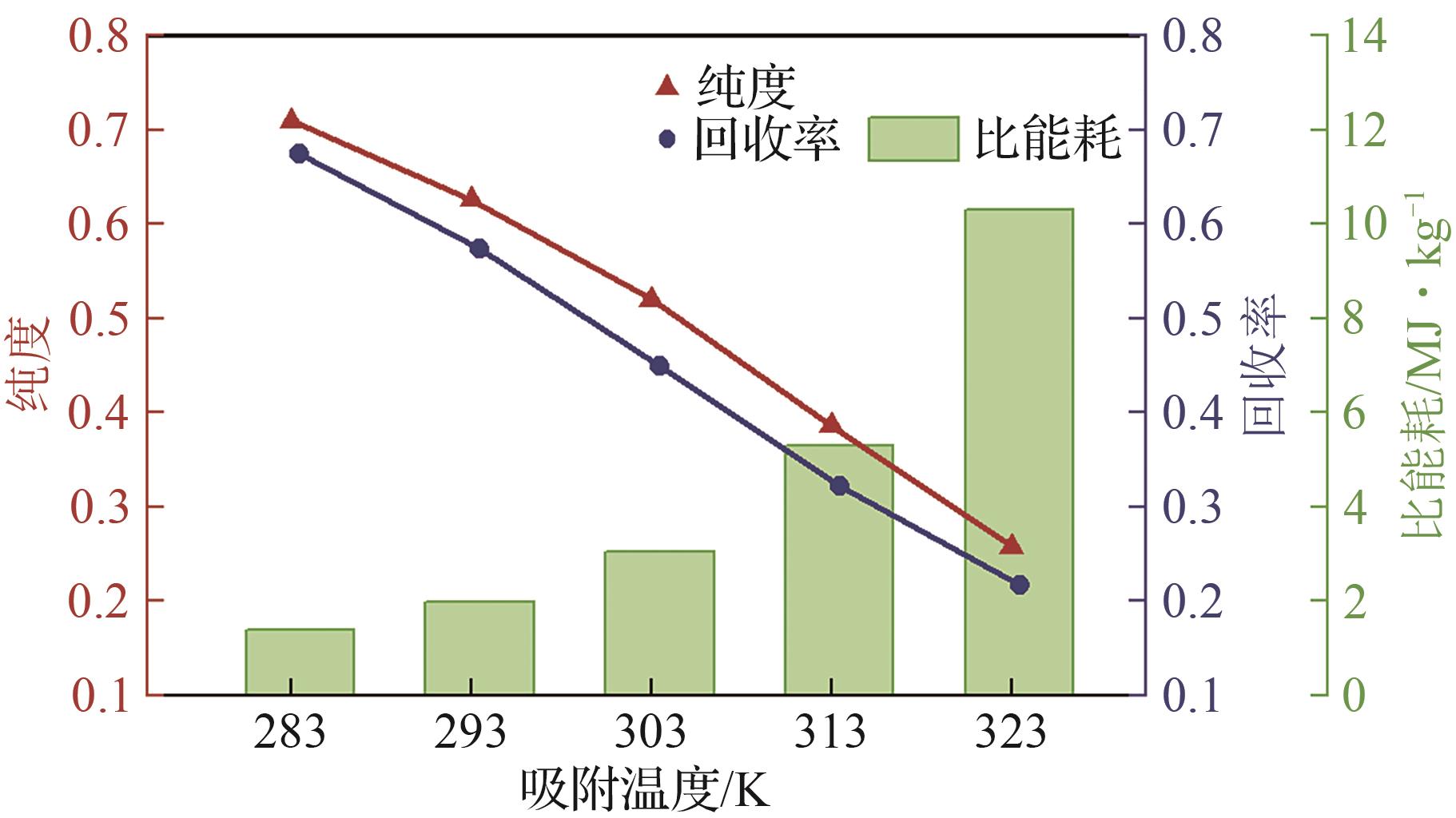
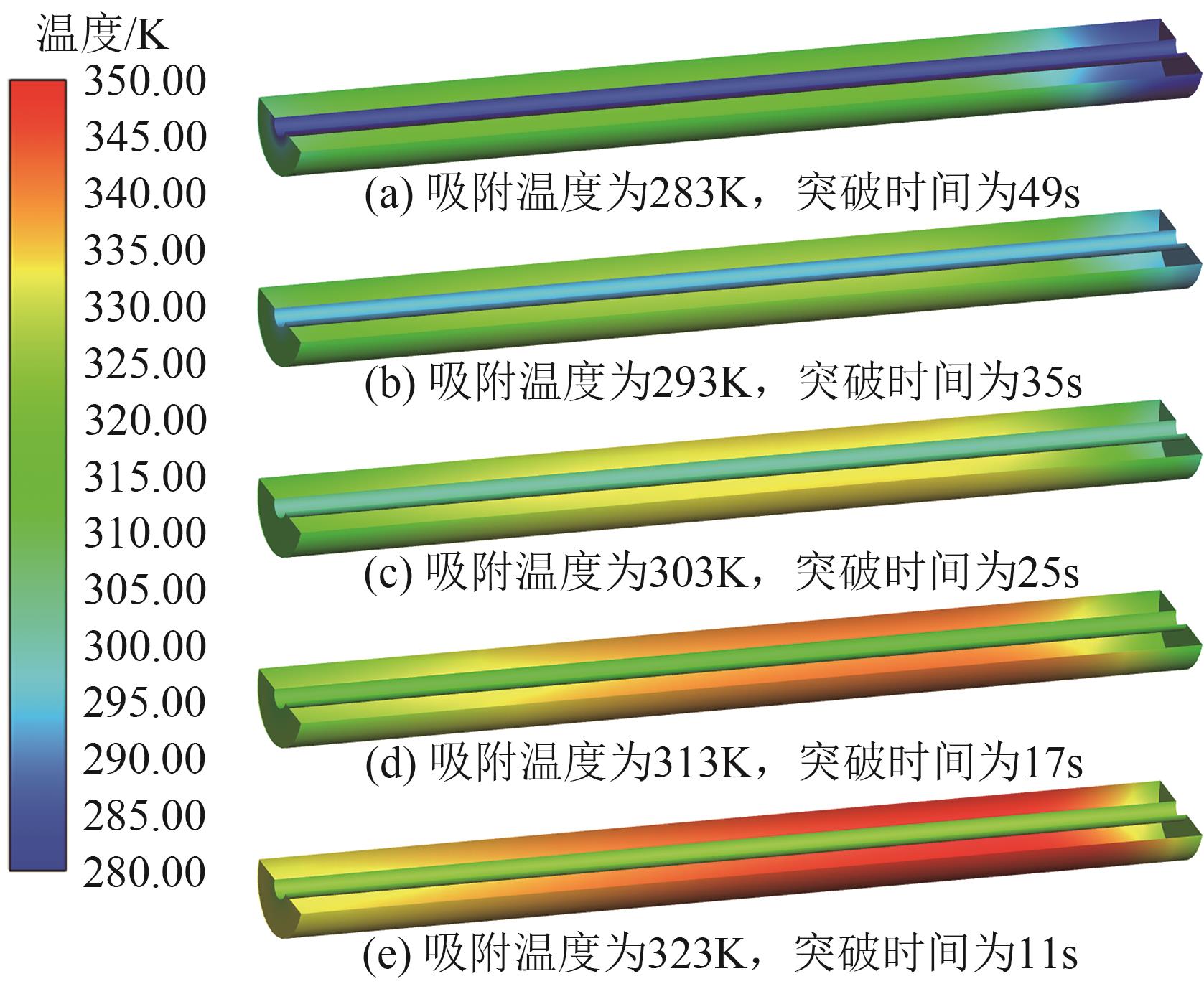
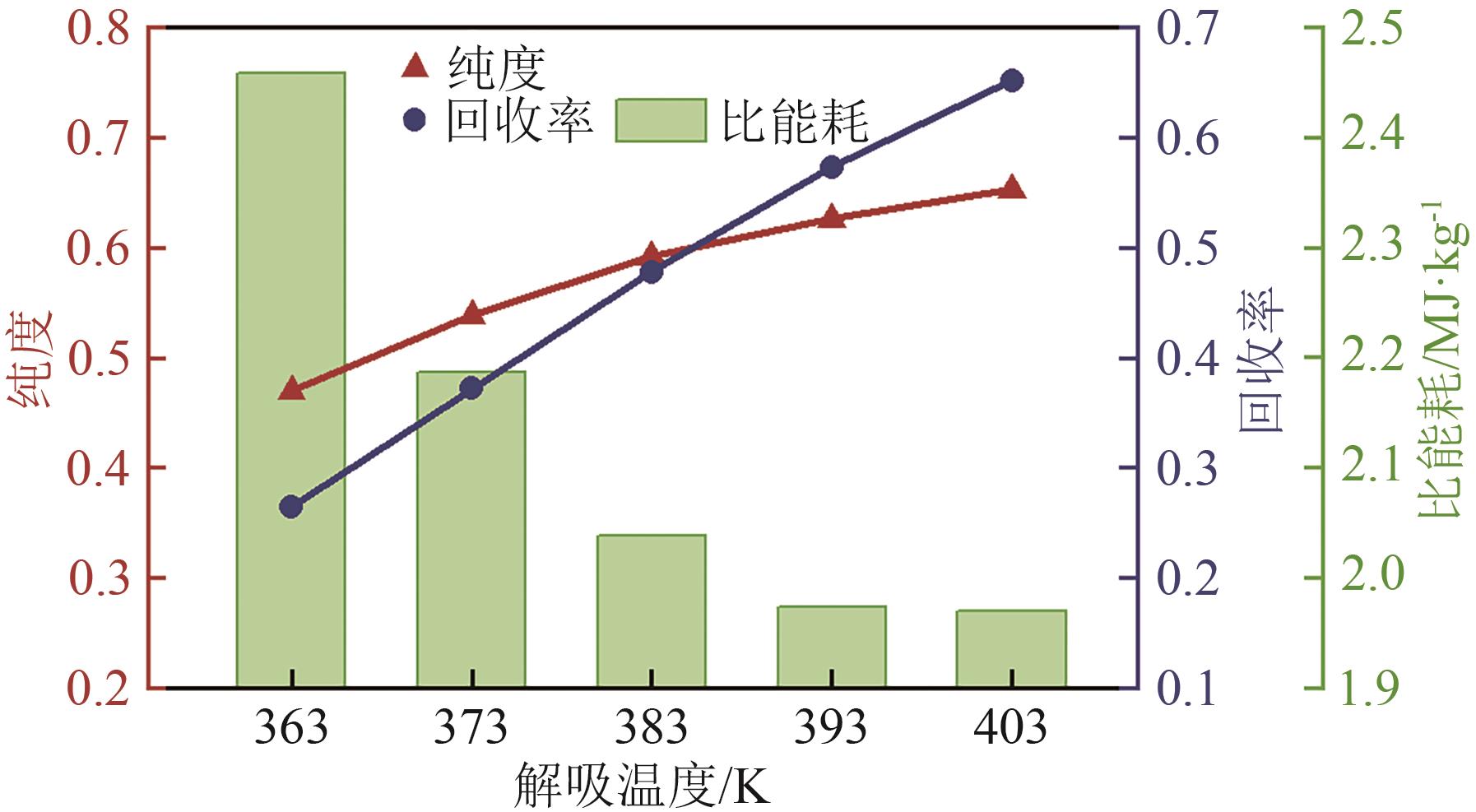
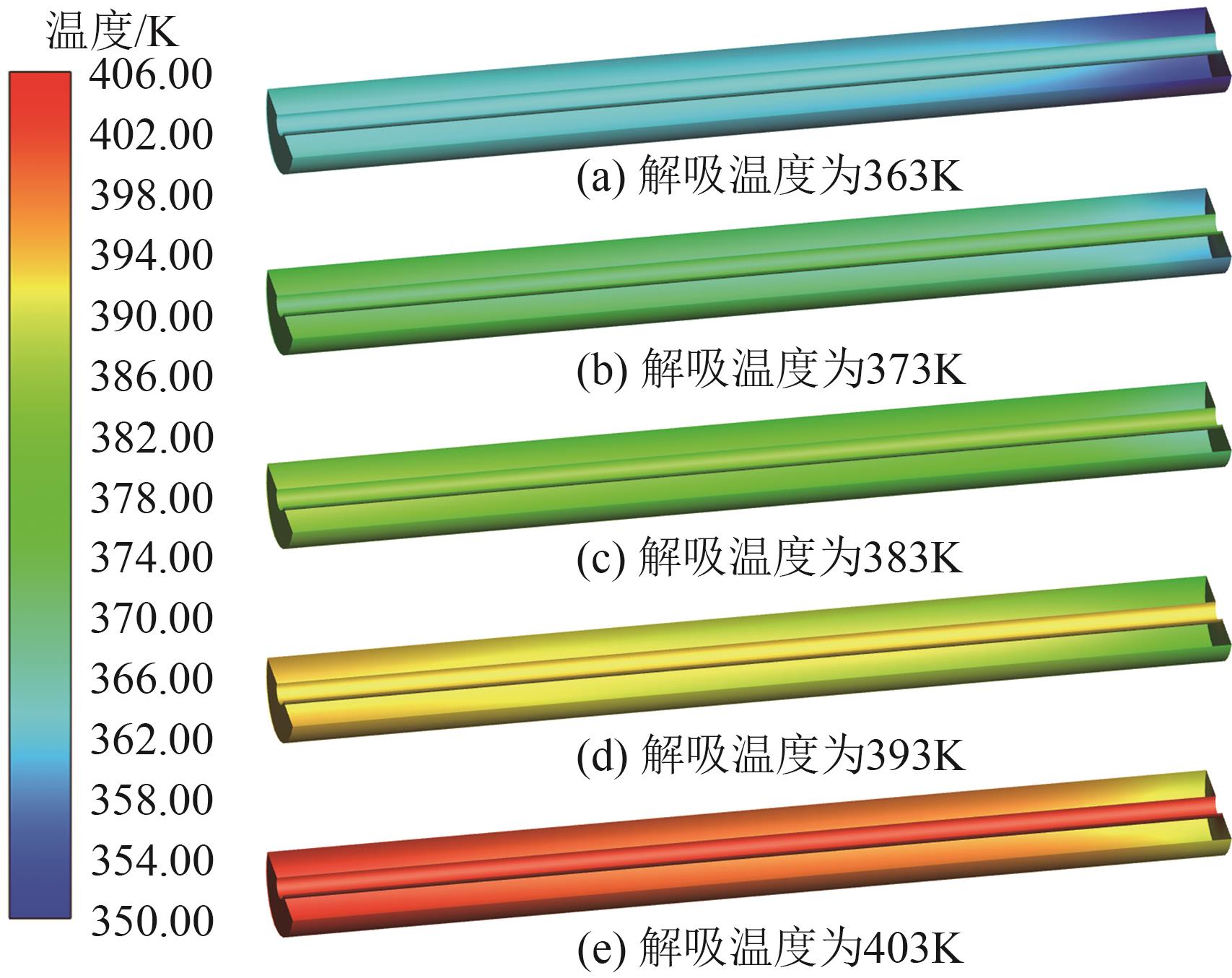
SF6是一种重点控制的强温室气体。为了减少其排放,本文提出使用变温吸附(TSA)循环从混合绝缘气体中(SF6的摩尔分数为15%,N2的摩尔分数为85%)分离回收SF6。采用数值模拟方法建立了TSA循环的物理模型和数学模型,与文献实验数据对比并证明了模型的可靠性。采用3种性能评价指标(纯度、回收率、比能耗),研究不同的吸附剂材料、吸附床几何尺寸和操作条件对TSA循环性能的影响。结果表明,4种材料的循环性能最好的是Mg-MOF-74,然后是AC、UIO-66、13X。吸附剂采用Mg-MOF-74,吸附床当量长径比在6.9~23.6内时,循环性能随长径比增大而提升,纯度和回收率最高分别可达到69.31%和62.90%,比能耗最低为1.89MJ/kg。降低吸附温度和升高解吸温度有利于增大材料的SF6循环工作容量,对混合绝缘气体分离回收SF6有利。
中图分类号:
引用本文
武锦怡, 赵睿恺, 邓帅, 张家麒, 高春霄, 刘葳桦, 赵力. 混合绝缘气体变温吸附分离回收SF6的数值模拟[J]. 化工进展, 2025, 44(S1): 19-28.
WU Jinyi, ZHAO Ruikai, DENG Shuai, ZHANG Jiaqi, GAO Chunxiao, LIU Weihua, ZHAO Li. Numerical simulation of temperature swing adsorption for SF6 recovery from mixed insulating gas[J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 19-28.
| SF6物性参数 | 数值 |
|---|---|
| 密度ρg/kg∙m-3 | 6.07 |
| 摩尔质量M/g∙mol-1 | 146.07 |
| 热导率kg/W∙m-1∙K-1 | 0.014 |
| 比热容cp,g/J∙kg-1∙K-1 | 650 |
| 动力黏度μ/kg∙m-1∙s-1 | 1.58×10-5 |
表1 SF6的物性参数
| SF6物性参数 | 数值 |
|---|---|
| 密度ρg/kg∙m-3 | 6.07 |
| 摩尔质量M/g∙mol-1 | 146.07 |
| 热导率kg/W∙m-1∙K-1 | 0.014 |
| 比热容cp,g/J∙kg-1∙K-1 | 650 |
| 动力黏度μ/kg∙m-1∙s-1 | 1.58×10-5 |
| 参数 | 数值 |
|---|---|
| 壁面厚度δ/m | 0.001 |
| 密度ρw/kg∙m-3 | 8030 |
| 比热容cp,w/J∙kg-1∙K-1 | 502.48 |
表2 圆管材料物理性能
| 参数 | 数值 |
|---|---|
| 壁面厚度δ/m | 0.001 |
| 密度ρw/kg∙m-3 | 8030 |
| 比热容cp,w/J∙kg-1∙K-1 | 502.48 |
| 吸附剂性能 | UIO-66[ | 13X[ | AC[ | Mg-MOF-74[ |
|---|---|---|---|---|
| 密度ρs/kg∙m-3 | 380 | 1099.5 | 592 | 911 |
| 比热容cp,s/J∙kg-1∙K-1 | 750 | 920 | 887 | 900 |
| 孔隙率ε | 0.246 | 0.565 | 0.36 | 0.7417 |
| 传热系数λs/W∙m-1∙K-1 | 0.32 | 0.15 | 0.3 | 0.3 |
| 颗粒直径d/mm | 0.36 | 1 | 2 | 0.2 |
| 黏性阻力系数α-1/m-2 | 4.420×1010 | 1.574×108 | 3.292×108 | 6.132×108 |
| 惯性阻力系数C2/m-1 | 4.924×105 | 8.441×103 | 2.401×104 | 1.108×104 |
表3 不同吸附剂材料的物性参数
| 吸附剂性能 | UIO-66[ | 13X[ | AC[ | Mg-MOF-74[ |
|---|---|---|---|---|
| 密度ρs/kg∙m-3 | 380 | 1099.5 | 592 | 911 |
| 比热容cp,s/J∙kg-1∙K-1 | 750 | 920 | 887 | 900 |
| 孔隙率ε | 0.246 | 0.565 | 0.36 | 0.7417 |
| 传热系数λs/W∙m-1∙K-1 | 0.32 | 0.15 | 0.3 | 0.3 |
| 颗粒直径d/mm | 0.36 | 1 | 2 | 0.2 |
| 黏性阻力系数α-1/m-2 | 4.420×1010 | 1.574×108 | 3.292×108 | 6.132×108 |
| 惯性阻力系数C2/m-1 | 4.924×105 | 8.441×103 | 2.401×104 | 1.108×104 |
| 吸附剂材料 | 气体种类 | qm/mol·kg-1 | k0/Pa-1 | n | ΔH/J·mol-1 |
|---|---|---|---|---|---|
| UIO-66 | SF6 | 1.81 | 2.92×10-10 | 0.581 | -38906 |
| N2 | 12.03 | 4.74×10-10 | 0.57 | -14582 | |
| 13X | SF6 | 1.546 | 9.402×10-10 | 1.053 | -26173 |
| N2 | 1.014 | 2.26×10-8 | 0.4376 | -13360 | |
| AC | SF6 | 4.765 | 6.995×10-12 | 0.440 | -42193 |
| N2 | 9.74 | 6.91×10-10 | 0.518 | -16310 | |
| Mg-MOF-74 | SF6 | 6.565 | 3.206×10-11 | 1.849 | -34444 |
| N2 | 6.7072 | 9.36×10-10 | 1 | -18000 |
表4 不同吸附剂材料的Toth模型参数
| 吸附剂材料 | 气体种类 | qm/mol·kg-1 | k0/Pa-1 | n | ΔH/J·mol-1 |
|---|---|---|---|---|---|
| UIO-66 | SF6 | 1.81 | 2.92×10-10 | 0.581 | -38906 |
| N2 | 12.03 | 4.74×10-10 | 0.57 | -14582 | |
| 13X | SF6 | 1.546 | 9.402×10-10 | 1.053 | -26173 |
| N2 | 1.014 | 2.26×10-8 | 0.4376 | -13360 | |
| AC | SF6 | 4.765 | 6.995×10-12 | 0.440 | -42193 |
| N2 | 9.74 | 6.91×10-10 | 0.518 | -16310 | |
| Mg-MOF-74 | SF6 | 6.565 | 3.206×10-11 | 1.849 | -34444 |
| N2 | 6.7072 | 9.36×10-10 | 1 | -18000 |
| 参数 | UIO-66 | 13X | AC | Mg-MOF-74 |
|---|---|---|---|---|
| SF6吸附时间常数/s-1 | 0.1 | 0.1 | 0.1 | 0.1 |
| N2吸附时间常数/s-1 | 0.15[ | 0.6[ | 0.2[ | 0.313[ |
表5 SF6和N2在不同材料床层的吸附时间常数
| 参数 | UIO-66 | 13X | AC | Mg-MOF-74 |
|---|---|---|---|---|
| SF6吸附时间常数/s-1 | 0.1 | 0.1 | 0.1 | 0.1 |
| N2吸附时间常数/s-1 | 0.15[ | 0.6[ | 0.2[ | 0.313[ |
| [27] | DING Zhaoyang, HAN Zhiyang, SHI Wenrong, et al. Analysis of dynamic effective mass transfer coefficients of rapid pressure swing adsorption process for oxygen production[J]. CIESC Journal, 2018, 69(2): 759-768. |
| [28] | 彭荣. 多孔材料吸附储氢的CFD模拟与优化[D]. 武汉: 武汉理工大学, 2012. |
| PENG Rong. CFD modeling and optimization of adsorptive hydrogen storage in porous materials[D]. Wuhan: Wuhan University of Technology, 2012. | |
| [29] | Rached BEN-MANSOUR, QASEM Naef A A, ANTAR Mohammed A. Carbon dioxide adsorption separation from dry and humid CO2/N2 mixture[J]. Computers & Chemical Engineering, 2018, 117: 221-235. |
| [1] | 孙强, 杨典, 王芳, 等. 基于ASPEN PLUS的六氟化硫提纯工艺研究[J]. 云南化工, 2020, 47(9): 41-45. |
| SUN Qiang, YANG Dian, WANG Fang, et al. Research on distillation of pure sulfur hexafluoride based on ASPEN PLUS[J]. Yunnan Chemical Technology, 2020, 47(9): 41-45. | |
| [2] | REN Jiahao, CHANG Miao, ZENG Wenjiang, et al. Computer-aided discovery of MOFs with calixarene-analogous microenvironment for exceptional SF6 capture[J]. Chemistry of Materials, 2021, 33(13): 5108-5114. |
| [3] | FANG Xuekun, HU Xia, Greet JANSSENS-MAENHOUT, et al. Sulfur hexafluoride (SF6) emission estimates for China: An Inventory for 1990—2010 and a projection to 2020[J]. Environmental Science & Technology, 2013, 47(8): 3848-3855. |
| [4] | 中国气象局气候变化中心. 中国温室气体公报[R]. 北京: 中国气象局, 2023. |
| China Meteorological Administration Climate Center. China greenhouse gas bulletin[R]. Beijing: China Meteorological Administration, 2023. | |
| [5] | 刘红志, 彭柯. 六氟化硫回收回充净化处理系统的应用研究[J]. 四川电力技术, 2011, 34(1): 52-55. |
| LIU Hongzhi, PENG Ke. Research on the application of sulfur hexafluoride recovery and purification treatment system[J]. Sichuan Electric Power Technology. 2011, 34(1): 52-55. | |
| [6] | ZHENG Xianqiang, SHEN Yanlong, WANG Shitao, et al. Selective adsorption of SF6 in covalent- and metal-organic frameworks[J]. Chinese Journal of Chemical Engineering, 2021, 39: 88-95. |
| [7] | MOSLEH Soleiman, KHAKSAR Hadis. Cu-BDC MOF/CNFs hybrids for rapid CO2 capture in a circulating fluidized bed via temperature swing adsorption process[J]. Chemical Engineering Science, 2024, 287:119773. |
| [8] | 谢任禹, 雍觐源, 江龙, 等. 变湿吸附三维循环构建及能效分析[J]. 工程热物理学报, 2024, 45(9): 2593-2598. |
| XIE Renyu, YONG Jinyuan, JIANG Long, et al. Construction of three-dimensional moisture swing adsorption cycle and energy efficiency analysis[J]. Journal of Engineering Thermophysics, 2024, 45(9): 2593-2598. | |
| [9] | ZHAO Qinghu, WU Fan, HE Yingdian, et al. Impact of operating parameters on CO2 capture using carbon monolith by electrical swing adsorption technology (ESA)[J]. Chemical Engineering Journal, 2017, 327: 441-453. |
| [10] | 闫江文. 用于SF6/N2吸附分离的微孔MOFs的合成及其光响应研究[D]. 保定: 河北大学, 2024. |
| YAN Jiangwen. Synthesis microporous MOFs for SF6/N2 adsorption separation and photoresponse[D]. Baoding: Hebei University, 2024. | |
| [11] | CHEN Sirui, SHEN Yuanhui, GUAN Zhongbo, et al. Adsorption properties of SF6 on zeolite NaY, 13X, activated carbon, and silica gel[J]. Journal of Chemical & Engineering Data, 2020, 65(8): 4044-4051. |
| [12] | 高春霄, 赵睿恺, 邓帅, 等. 混合绝缘气体变温吸附分离回收SF6优化研究[J]. 低碳化学与化工, 2025, 50: 95-100. |
| GAO Chunxiao, ZHAO Ruikai, DENG Shuai,et al. Optimization study of SF6 recovery from mixed insulating gases using temperatureswing adsorption[J]. Low-Carbon Chemistry and Chemical Engineering, 2025, 50: 95-100. | |
| [13] | LIU Lei, JIN Seongmin, Kwangjun KO, et al. Alkyl-functionalization of (3-aminopropyl) triethoxysilane-grafted zeolite beta for carbon dioxide capture in temperature swing adsorption[J]. Chemical Engineering Journal, 2020, 382: 122834. |
| [14] | HAN Bo, CHAKRABORTY Anutosh. Advanced cooling heat pump and desalination employing functional UiO-66 (Zr) metal-organic frameworks [J]. Energy Conversion and Management, 2020, 213: 112825. |
| [15] | 任可欣, 鲁军辉, 王随林, 等. 低湿CO2/H2O混合气体吸附特性实验[J]. 化工进展, 2022, 41(12): 6698-6710. |
| REN Kexin, LU Junhui, WANG Suilin, et al. Adsorption characteristics of CO2/H2O with low humidity[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6698-6710. | |
| [16] | Ammar ALI ABD, OTHMAN Mohd Roslee, SHAMSUDIN Ili Khairunnisa, et al. Biogas upgrading to natural gas pipeline quality using pressure swing adsorption for CO2 separation over UiO-66: Experimental and dynamic modelling assessment[J]. Chemical Engineering Journal, 2023, 453: 139774. |
| [17] | CHEN Lijin, DENG Shuai, ZHAO Ruikai, et al. Temperature swing adsorption for CO2 capture: Thermal design and management on adsorption bed with single-tube/three-tube internal heat exchanger[J]. Applied Thermal Engineering, 2021, 199: 117538. |
| [18] | 周圆圆, 杨华伟, 张东辉. 甲烷/氮气变压吸附分离的实验与模拟[J]. 天然气化工, 2011, 36(5): 21-27. |
| ZHOU Yuanyuan, YANG Huawei, ZHANG Donghui. Simulation and experiment for the pressure swing adsorption separation of methane and nitrogen[J]. Natural Gas Chemical Industry, 2011, 36(5): 21-27. | |
| [19] | LIAN Yahui, DENG Shuai, LI Shuangjun, et al. Numerical analysis on CO2 capture process of temperature swing adsorption (TSA): Optimization of reactor geometry[J]. International Journal of Greenhouse Gas Control, 2019, 85: 187-198. |
| [20] | QASEM Naef A A, Rached BEN-MANSOUR. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation[J]. Applied Energy, 2018, 209: 190-202. |
| [21] | Jaehoon CHA, Seongbin GA, LEE Seung-Jun, et al. Integrated material and process evaluation of metal-organic frameworks database for energy-efficient SF6/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 131787. |
| [22] | CHO Wan-Seon, LEE Kwang-Hoon, CHANG Hyang-Ja, et al. Evaluation of pressure-temperature swing adsorption for sulfur hexafluoride (SF6) recovery from SF6 and N2 gas mixture[J]. Korean Journal of Chemical Engineering, 2011, 28(11): 2196-2201. |
| [23] | KIM Min-Bum, LEE Seung-Joon, LEE Chang Yeon, et al. High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Microporous and Mesoporous Materials, 2014, 190: 356-361. |
| [24] | YANG Xiaoxian, Arash ARAMI-NIYA, Jiafei LYU, et al. Net, excess, and absolute adsorption of N2, CH4, and CO2 on metal-organic frameworks of ZIF-8, MIL-101(Cr), and UiO-66 at 282—361K and up to 12MPa[J]. Journal of Chemical & Engineering Data, 2020, 66(1): 404-414. |
| [25] | ZHAO Ruikai, LIU Longcheng, ZHAO Li, et al. A comprehensive performance evaluation of temperature swing adsorption for post-combustion carbon dioxide capture[J]. Renewable and Sustainable Energy Reviews, 2019, 114: 109285. |
| [26] | Rached BEN-MANSOUR, QASEM Naef A A. An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74[J]. Energy Conversion and Management, 2018, 156: 10-24. |
| [27] | 丁兆阳, 韩治洋, 石文荣, 等. 快速变压吸附制氧动态传质系数模拟分析[J]. 化工学报, 2018, 69(2): 759-768. |
| [1] | 尹晓云, 朱进, 刘春艳, 张锦涛, 徐源, 朱英如, 苏明, 孙月, 孙杰, 袁颖. 基于PB设计和响应面法的CPS硫黄回收装置能量优化[J]. 化工进展, 2025, 44(S1): 124-133. |
| [2] | 符红梅, 刘定华, 刘晓勤. MOF材料在芳烃同分异构体分离中的研究进展[J]. 化工进展, 2025, 44(9): 5006-5017. |
| [3] | 张文静, 黄致新, 李士腾, 邓帅, 李双俊. 生物质碳气凝胶CO2吸附剂研究进展[J]. 化工进展, 2025, 44(9): 5018-5032. |
| [4] | 洪凯, 樊欢, 田佳, 张荥斐. 硫化沉淀法处理铜砷多金属酸性废水研究进展[J]. 化工进展, 2025, 44(9): 5301-5314. |
| [5] | 杜璇, 王战宏, 郑彬, 许卫, 王硕, 石鹏, 高国. 钴铁酸浸液分离及电池级磷酸铁回收技术进展[J]. 化工进展, 2025, 44(9): 5327-5338. |
| [6] | 孙梦圆, 陆诗建, 刘玲, 薛艳阳, 张云蓉, 董琦, 康国俊. 金属有机框架及衍生物在碳捕集领域的研究进展[J]. 化工进展, 2025, 44(9): 5339-5350. |
| [7] | 王晓光, 董青, 郎文丽, 洪翔鑫, 黄振祥, 谭凤玉, 雷以柱, 余子夷. 超低浓度甲烷减排与资源化利用研究进展[J]. 化工进展, 2025, 44(9): 5363-5376. |
| [8] | 段先哲, 毕文婷, 李南, 豆佳乐, 邵冰清, 汪佳伟, 吴鹏, 黄欢, 唐振平. 数值模拟在高放废物处置中的应用:放射性核素迁移机制及其影响因素[J]. 化工进展, 2025, 44(9): 5391-5405. |
| [9] | 鲁玲, 俞磊, 顾霞, 赖敏明, 周凯, 王亚鹏, 李响. 制药废盐的高效热催化处理及资源化利用[J]. 化工进展, 2025, 44(9): 5432-5441. |
| [10] | 张光辉, 江金旭, 黄磊, 陈士祥, 马天添. 市政污泥富氧燃烧特性影响因素分析及预测[J]. 化工进展, 2025, 44(9): 5460-5470. |
| [11] | 周敬皓, 张朝阳, 胡昊星, 王思茗, 刘静远, 魏光华. 基于格子玻尔兹曼方法的PEMFC微孔层气体传质分析[J]. 化工进展, 2025, 44(9): 4898-4907. |
| [12] | 王吉龙, 何磊, 苏毅, 唐昭帆. 基于尾气焚烧炉膛天然气无焰燃烧(MILD)数值模拟[J]. 化工进展, 2025, 44(9): 4928-4936. |
| [13] | 翟宇航, 丛立新, 韩冰, 王启林, 邹慧传. 大尺度氢气云爆燃压力波形成机制及灾害效应判定[J]. 化工进展, 2025, 44(8): 4709-4719. |
| [14] | 汪国超, 丁晖殿, 师丽, 李强, 夏涛, 苑杨. 复合精馏序列的温度推断控制[J]. 化工进展, 2025, 44(8): 4720-4731. |
| [15] | 杨勇, 张钊, 王东亮, 周怀荣, 赵子豪, 李煜坤. 二甲苯异构体不同分离策略的技术经济评价[J]. 化工进展, 2025, 44(8): 4732-4740. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||