化工进展 ›› 2025, Vol. 44 ›› Issue (8): 4732-4740.DOI: 10.16085/j.issn.1000-6613.2025-0154
• 过程系统工程的模拟与仿真 • 上一篇
二甲苯异构体不同分离策略的技术经济评价
杨勇1,2( ), 张钊1, 王东亮1,2, 周怀荣1,2, 赵子豪1, 李煜坤1
), 张钊1, 王东亮1,2, 周怀荣1,2, 赵子豪1, 李煜坤1
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃省低碳能源化工重点实验室,甘肃 兰州 730050
-
收稿日期:2025-02-07修回日期:2025-05-12出版日期:2025-08-25发布日期:2025-09-08 -
通讯作者:杨勇 -
作者简介:杨勇(1986—),男,博士,副教授,研究方向为化工系统工程。E-mail:yangy@lut.edu.cn。 -
基金资助:国家自然科学基金(22468030);甘肃省科技重大专项(23ZDGF002)
Technical-economic evaluation for different separation strategies of xylene isomers
YANG Yong1,2( ), ZHANG Zhao1, WANG Dongliang1,2, ZHOU Huairong1,2, ZHAO Zihao1, LI Yukun1
), ZHANG Zhao1, WANG Dongliang1,2, ZHOU Huairong1,2, ZHAO Zihao1, LI Yukun1
- 1.School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Laboratory of Low Carbon Energy and Chemical Engineering of Gansu Province, Lanzhou 730050, Gansu, China
-
Received:2025-02-07Revised:2025-05-12Online:2025-08-25Published:2025-09-08 -
Contact:YANG Yong
摘要:
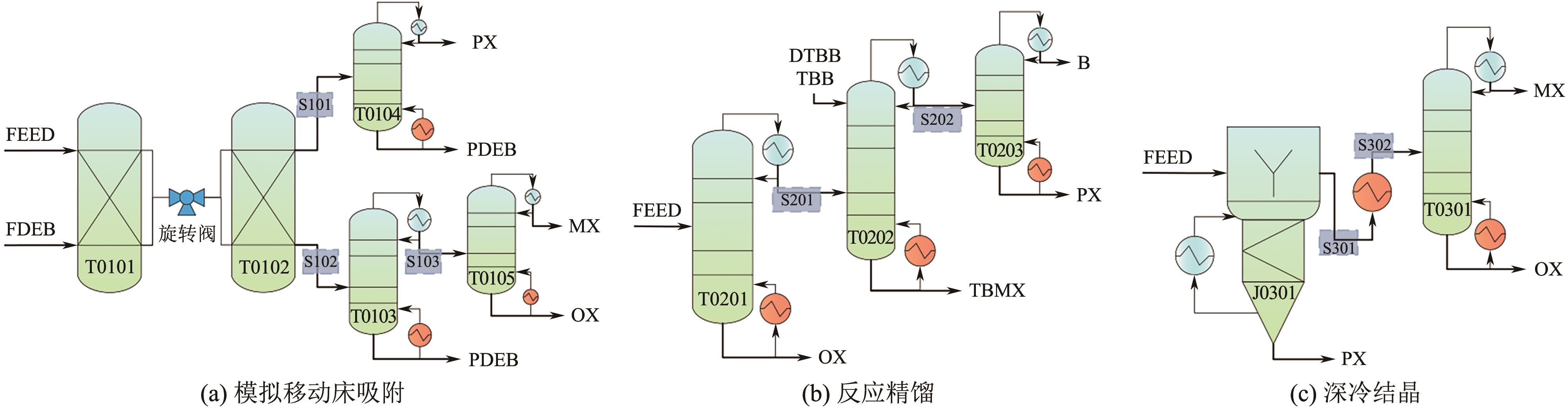
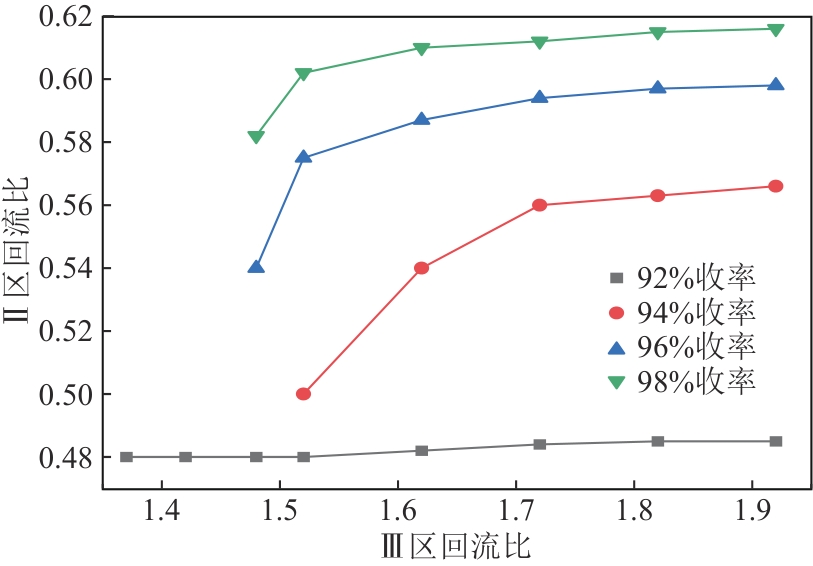
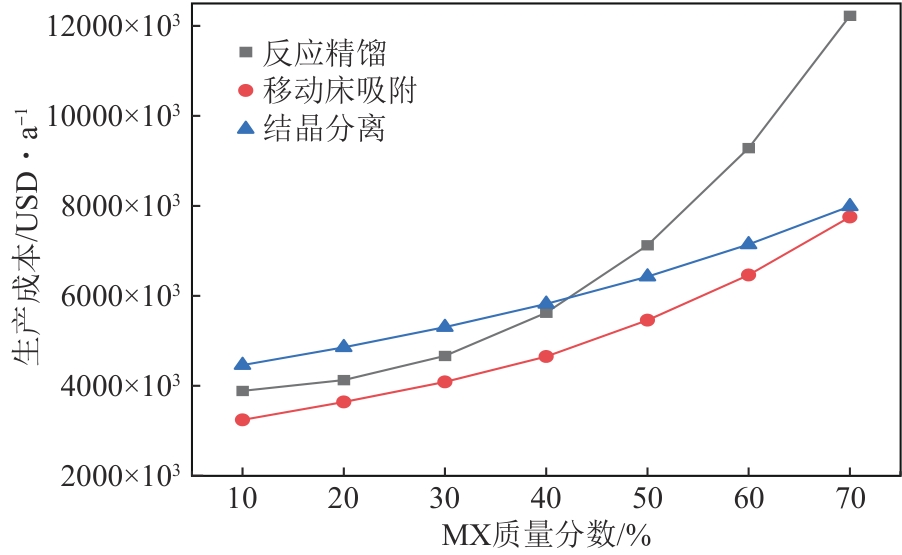
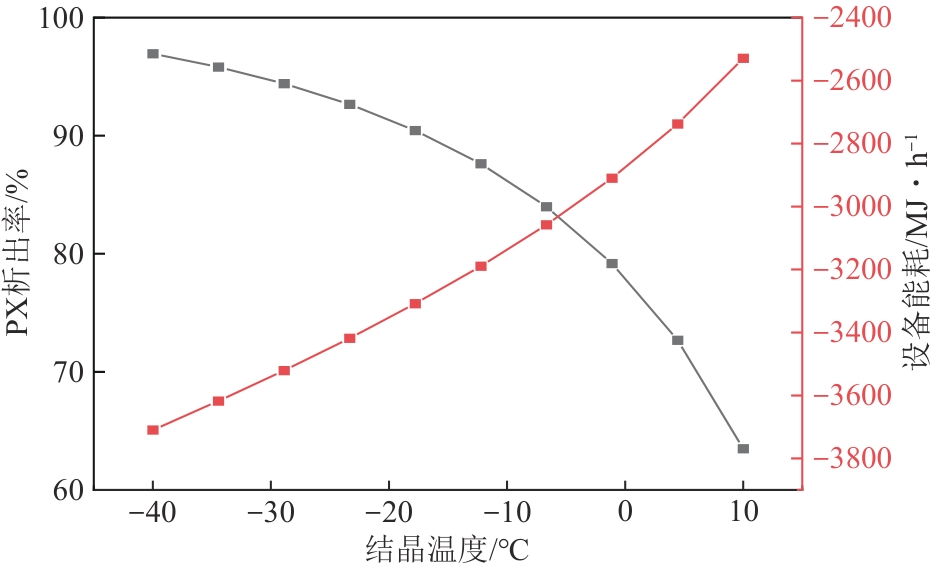
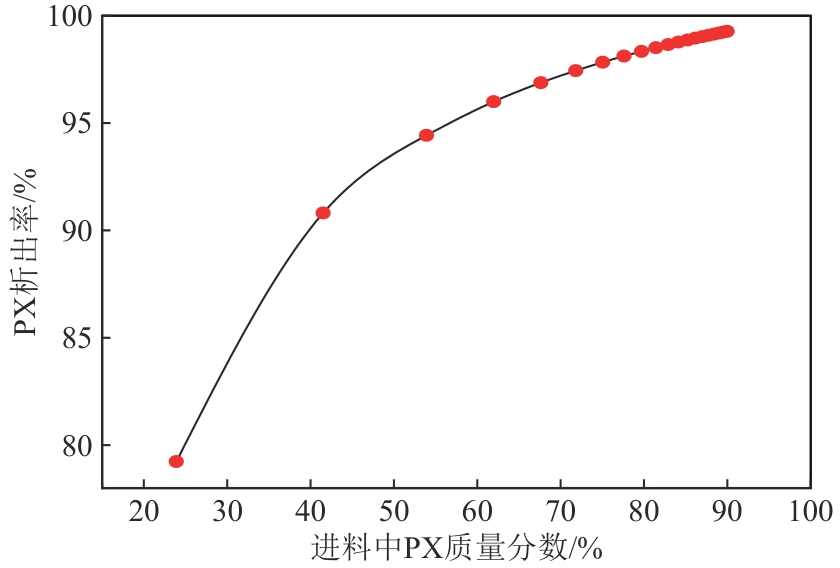
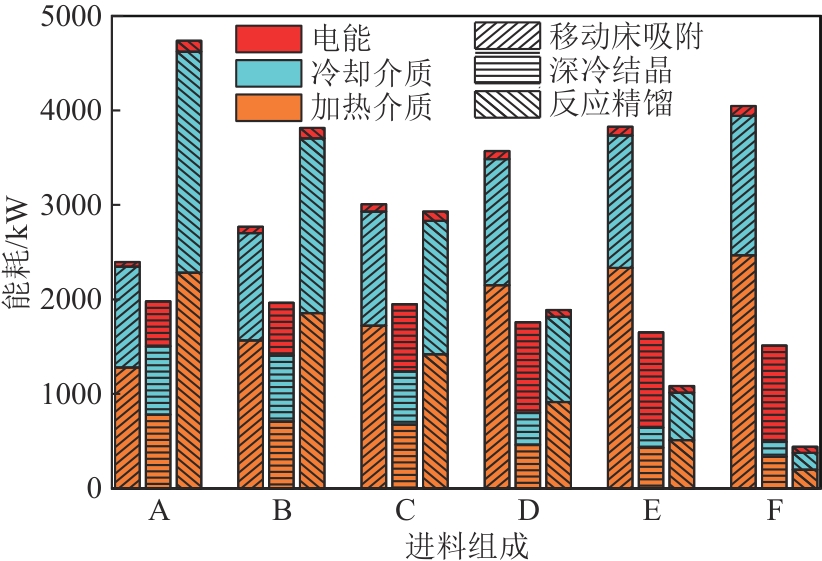
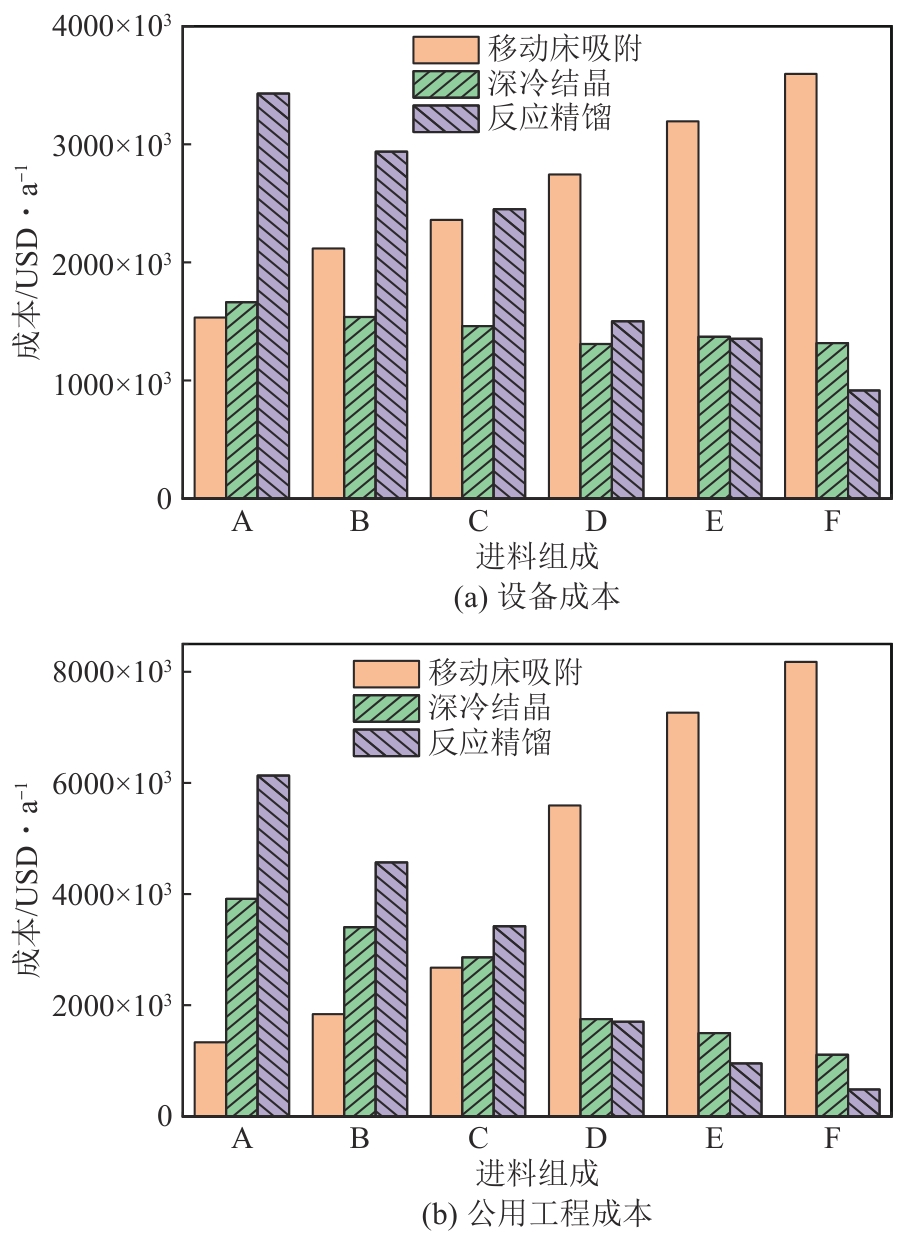
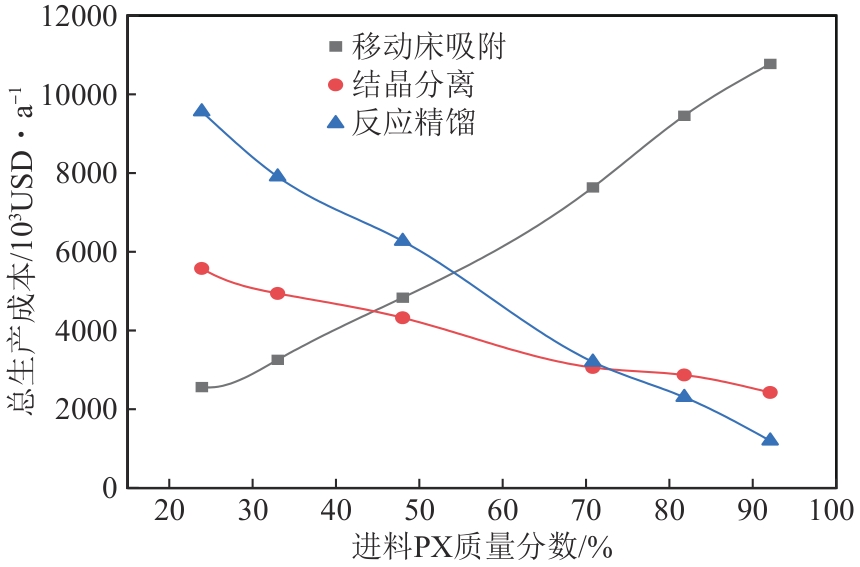
二甲苯异构体是重要的化工中间体产品,但传统生产方法和增产技术获得的异构体含量差异较大,对二甲苯(PX)的含量可由热力学平衡组成(约23.8%)上升至90%以上,对低碳高效的分离策略提出了巨大挑战。针对不同工艺来源二甲苯异构体含量差异,重点探究模拟移动床吸附、深冷结晶、反应精馏3种分离策略的分离效率、能耗和技术经济性,对3种分离策略的关键因素进行灵敏度分析。结果表明,当异构体中PX含量由热力学平衡组成增加至90%以上时,模拟移动床分离效率先降低后增加,过程能耗、设备成本和公用工程都在显著增加,经济性逐渐变差;而反应精馏和结晶分离的分离效率逐渐升高,能耗降低、经济性增加。当二甲苯异构体中PX含量较低时,模拟移动床具有显著的技术经济性;PX含量较高时,反应精馏分离效率较高;当PX含量位于45.49%~72.57%时,结晶分离工艺具有较低的能耗和较优的技术经济性。
中图分类号:
引用本文
杨勇, 张钊, 王东亮, 周怀荣, 赵子豪, 李煜坤. 二甲苯异构体不同分离策略的技术经济评价[J]. 化工进展, 2025, 44(8): 4732-4740.
YANG Yong, ZHANG Zhao, WANG Dongliang, ZHOU Huairong, ZHAO Zihao, LI Yukun. Technical-economic evaluation for different separation strategies of xylene isomers[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4732-4740.
| 类别 | 对二甲苯 | 间二甲苯 | 邻二甲苯 | 催化剂 | 参考文献 |
|---|---|---|---|---|---|
| A | 23.89 | 53.26 | 22.84 | [ | |
| B | 33.00 | 48.00 | 19.00 | ZnZrO x /4Z5 | [ |
| C | 48.00 | 26.85 | 25.15 | ZnZrO x /2Z5 | [ |
| D | 70.81 | 20.10 | 9.06 | ZnZrO x /2Z5 | [ |
| E | 81.79 | 12.26 | 5.95 | ZrCuO0.1-Z50.5(25)-Si(2) | [ |
| F | 92.09 | 3.98 | 3.93 | ZSM-5 | [ |
表1 不同工艺来源二甲苯异构体的进料组成
| 类别 | 对二甲苯 | 间二甲苯 | 邻二甲苯 | 催化剂 | 参考文献 |
|---|---|---|---|---|---|
| A | 23.89 | 53.26 | 22.84 | [ | |
| B | 33.00 | 48.00 | 19.00 | ZnZrO x /4Z5 | [ |
| C | 48.00 | 26.85 | 25.15 | ZnZrO x /2Z5 | [ |
| D | 70.81 | 20.10 | 9.06 | ZnZrO x /2Z5 | [ |
| E | 81.79 | 12.26 | 5.95 | ZrCuO0.1-Z50.5(25)-Si(2) | [ |
| F | 92.09 | 3.98 | 3.93 | ZSM-5 | [ |
| SMB结构参数 | 吸附操作条件 | 吸附模型参数 | ||
|---|---|---|---|---|
| 吸附剂参数 | 吸附平衡常数 | 最大吸附量 | ||
| HSMB=122.7cm | t=70s | ρ=876kg/m3 | KPX=1.0750 | qmPX=0.168kg/kg |
| DSMB=600cm | T=177℃ | ε=0.39 | KOX=0.2850 | qmOX=0.168kg/kg |
| 床层数分配7-9-5-3 | p=0.88MPa | dp=0.56mm | KMX=0.2645 | qmMX=0.168kg/kg |
| Pe=2000 | KPDEB=1.3125 | qmPDEB=0.138kg/kg | ||
| KL=9min-1 | ||||
表2 模拟移动床吸附操作工况及模型参数[33]
| SMB结构参数 | 吸附操作条件 | 吸附模型参数 | ||
|---|---|---|---|---|
| 吸附剂参数 | 吸附平衡常数 | 最大吸附量 | ||
| HSMB=122.7cm | t=70s | ρ=876kg/m3 | KPX=1.0750 | qmPX=0.168kg/kg |
| DSMB=600cm | T=177℃ | ε=0.39 | KOX=0.2850 | qmOX=0.168kg/kg |
| 床层数分配7-9-5-3 | p=0.88MPa | dp=0.56mm | KMX=0.2645 | qmMX=0.168kg/kg |
| Pe=2000 | KPDEB=1.3125 | qmPDEB=0.138kg/kg | ||
| KL=9min-1 | ||||
| 流股 | 模拟移动床 | 反应精馏 | 结晶 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDEB | S101 | PX | MX | OX | DTBB,TBB | S202 | PX | OX | TBMX | S301 | PX | MX | OX | |||
| 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量 /kmol·h-1 | 物流量 /kmol·h-1 | |||
| A | 156.64 | 96.44 | 98.38 | 52.19 | 22.38 | 79.89 | 45.34 | 94.94 | 22.38 | 50.60 | 10.67 | 89.33 | 52.25 | 22.41 | ||
| B | 167.80 | 95.36 | 97.42 | 47.04 | 18.62 | 72.00 | 50.08 | 95.32 | 18.70 | 46.08 | 8.68 | 91.32 | 47.14 | 18.66 | ||
| C | 194.43 | 93.88 | 96.87 | 26.31 | 24.65 | 40.28 | 71.15 | 96.77 | 24.75 | 26.04 | 6.22 | 93.78 | 26.39 | 24.72 | ||
| D | 235.55 | 92.43 | 96.27 | 19.70 | 8.88 | 30.15 | 77.90 | 97.43 | 8.93 | 19.70 | 3.14 | 96.86 | 19.80 | 8.92 | ||
| E | 274.96 | 95.69 | 95.16 | 12.01 | 5.83 | 18.39 | 85.44 | 97.94 | 5.89 | 12.14 | 2.57 | 97.43 | 12.09 | 5.87 | ||
| F | 310.32 | 98.26 | 94.22 | 3.90 | 3.85 | 5.97 | 94.46 | 98.38 | 3.92 | 3.94 | 0.09 | 99.91 | 3.93 | 3.88 | ||
表3 不同进料组成的产品纯度和收率
| 流股 | 模拟移动床 | 反应精馏 | 结晶 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDEB | S101 | PX | MX | OX | DTBB,TBB | S202 | PX | OX | TBMX | S301 | PX | MX | OX | |||
| 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量 /kmol·h-1 | 物流量 /kmol·h-1 | |||
| A | 156.64 | 96.44 | 98.38 | 52.19 | 22.38 | 79.89 | 45.34 | 94.94 | 22.38 | 50.60 | 10.67 | 89.33 | 52.25 | 22.41 | ||
| B | 167.80 | 95.36 | 97.42 | 47.04 | 18.62 | 72.00 | 50.08 | 95.32 | 18.70 | 46.08 | 8.68 | 91.32 | 47.14 | 18.66 | ||
| C | 194.43 | 93.88 | 96.87 | 26.31 | 24.65 | 40.28 | 71.15 | 96.77 | 24.75 | 26.04 | 6.22 | 93.78 | 26.39 | 24.72 | ||
| D | 235.55 | 92.43 | 96.27 | 19.70 | 8.88 | 30.15 | 77.90 | 97.43 | 8.93 | 19.70 | 3.14 | 96.86 | 19.80 | 8.92 | ||
| E | 274.96 | 95.69 | 95.16 | 12.01 | 5.83 | 18.39 | 85.44 | 97.94 | 5.89 | 12.14 | 2.57 | 97.43 | 12.09 | 5.87 | ||
| F | 310.32 | 98.26 | 94.22 | 3.90 | 3.85 | 5.97 | 94.46 | 98.38 | 3.92 | 3.94 | 0.09 | 99.91 | 3.93 | 3.88 | ||
| 参数 | 模拟移动床 | |||||||
|---|---|---|---|---|---|---|---|---|
| 流股 | FEED | PDEB | S101 | S102 | S103 | PX | MX | OX |
| 温度/℃ | 150.00 | 25.00 | 138.00 | 138.00 | 153.00 | 132.00 | 139.00 | 174.00 |
| 压力/MPa | 0.30 | 0.10 | 0.20 | 0.20 | 0.15 | 0.15 | 0.12 | 0.15 |
| 摩尔流量/kmol·h-1 | 100.00 | 235.55 | 254.43 | 81.10 | 29.54 | 66.05 | 20.41 | 9.13 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 69.86 | 0.95 | 0.80 | 65.85 | 0.71 | 0.09 |
| MX | 20.10 | 0 | 0.24 | 19.86 | 19.86 | 0.20 | 19.70 | 0.16 |
| OX | 9.06 | 0 | 0.16 | 8.90 | 8.88 | 0 | 0 | 8.88 |
| PDEB | 0 | 235.55 | 184.17 | 51.39 | 0 | 0 | 0 | 0 |
| 参数 | 反应精馏 | |||||||
| 流股 | FEED | DTBB,TBB | S201 | S202 | B | PX | TBMX | OX |
| 温度/℃ | 150.00 | 150.00 | 132.00 | 97.00 | 120.00 | 184.00 | 188.00 | 178.00 |
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.20 | 0.18 | 0.20 | 0.25 | 0.30 |
| 摩尔流量/kmol·h-1 | 100.00 | 30.15 | 90.71 | 86.43 | 15.87 | 70.31 | 19.42 | 9.26 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 70.56 | 70.56 | 0 | 70.31 | 0 | 0.25 |
| MX | 20.10 | 0 | 20.02 | 0 | 0 | 0 | 0 | 0.08 |
| OX | 9.06 | 0 | 0.13 | 0 | 0 | 0 | 0 | 8.93 |
| DTBB | 0 | 20.10 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBB | 0 | 10.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 0 | 15.87 | 15.87 | 0 | 0 | 0 |
| TBMX | 0 | 0 | 0 | 0 | 0 | 0 | 19.42 | 0 |
| 参数 | 深冷结晶 | |||||||
| 流股 | FEED | S301 | S302 | PX | MX | OX | ||
| 温度/℃ | 150.00 | -30.00 | 138.00 | -30.00 | 132.00 | 153.00 | ||
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.30 | 0.23 | 0.25 | ||
| 摩尔流量/kmol·h-1 | 100.00 | 30.97 | 30.97 | 69.00 | 21.24 | 9.73 | ||
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 1.81 | 1.81 | 69.00 | 1.30 | 0.51 | ||
| MX | 20.10 | 20.10 | 20.10 | 0 | 19.80 | 0.30 | ||
| OX | 9.06 | 9.06 | 9.06 | 0 | 0.14 | 8.92 | ||
表4 D组成进料不用分离工艺的物料平衡
| 参数 | 模拟移动床 | |||||||
|---|---|---|---|---|---|---|---|---|
| 流股 | FEED | PDEB | S101 | S102 | S103 | PX | MX | OX |
| 温度/℃ | 150.00 | 25.00 | 138.00 | 138.00 | 153.00 | 132.00 | 139.00 | 174.00 |
| 压力/MPa | 0.30 | 0.10 | 0.20 | 0.20 | 0.15 | 0.15 | 0.12 | 0.15 |
| 摩尔流量/kmol·h-1 | 100.00 | 235.55 | 254.43 | 81.10 | 29.54 | 66.05 | 20.41 | 9.13 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 69.86 | 0.95 | 0.80 | 65.85 | 0.71 | 0.09 |
| MX | 20.10 | 0 | 0.24 | 19.86 | 19.86 | 0.20 | 19.70 | 0.16 |
| OX | 9.06 | 0 | 0.16 | 8.90 | 8.88 | 0 | 0 | 8.88 |
| PDEB | 0 | 235.55 | 184.17 | 51.39 | 0 | 0 | 0 | 0 |
| 参数 | 反应精馏 | |||||||
| 流股 | FEED | DTBB,TBB | S201 | S202 | B | PX | TBMX | OX |
| 温度/℃ | 150.00 | 150.00 | 132.00 | 97.00 | 120.00 | 184.00 | 188.00 | 178.00 |
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.20 | 0.18 | 0.20 | 0.25 | 0.30 |
| 摩尔流量/kmol·h-1 | 100.00 | 30.15 | 90.71 | 86.43 | 15.87 | 70.31 | 19.42 | 9.26 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 70.56 | 70.56 | 0 | 70.31 | 0 | 0.25 |
| MX | 20.10 | 0 | 20.02 | 0 | 0 | 0 | 0 | 0.08 |
| OX | 9.06 | 0 | 0.13 | 0 | 0 | 0 | 0 | 8.93 |
| DTBB | 0 | 20.10 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBB | 0 | 10.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 0 | 15.87 | 15.87 | 0 | 0 | 0 |
| TBMX | 0 | 0 | 0 | 0 | 0 | 0 | 19.42 | 0 |
| 参数 | 深冷结晶 | |||||||
| 流股 | FEED | S301 | S302 | PX | MX | OX | ||
| 温度/℃ | 150.00 | -30.00 | 138.00 | -30.00 | 132.00 | 153.00 | ||
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.30 | 0.23 | 0.25 | ||
| 摩尔流量/kmol·h-1 | 100.00 | 30.97 | 30.97 | 69.00 | 21.24 | 9.73 | ||
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 1.81 | 1.81 | 69.00 | 1.30 | 0.51 | ||
| MX | 20.10 | 20.10 | 20.10 | 0 | 19.80 | 0.30 | ||
| OX | 9.06 | 9.06 | 9.06 | 0 | 0.14 | 8.92 | ||
| 参数 | 公用工程 | 原料 | 产品 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 急冷水 | 低压蒸汽 | 中压蒸汽 | 工业用电 | 混合二甲苯 | DTBB/TBB | PX,MX,OX | TBMX | B | |
| 价格 | 5CNY/t | 120CNY/t | 180CNY/t | 0.38CNY/kWh | 5900CNY/t | 7700CNY/t | 7600CNY/t | 7700CNY/t | 7600CNY/t |
表5 物价
| 参数 | 公用工程 | 原料 | 产品 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 急冷水 | 低压蒸汽 | 中压蒸汽 | 工业用电 | 混合二甲苯 | DTBB/TBB | PX,MX,OX | TBMX | B | |
| 价格 | 5CNY/t | 120CNY/t | 180CNY/t | 0.38CNY/kWh | 5900CNY/t | 7700CNY/t | 7600CNY/t | 7700CNY/t | 7600CNY/t |
| [4] | MINCEVA Mirjana, GOMES Pedro Sá, MESHKO Vera, et al. Simulated moving bed reactor for isomerization and separation of p-xylene[J]. Chemical Engineering Journal, 2008, 140(1/2/3): 305-323. |
| [5] | GONÇALVES Jonathan C, FARIA Rui P V, FERREIRA Alexandre F P, et al. Optimization of a simulated moving bed unit within an existing and revamped aromatics complex with crystallization and toluene methylation units[J]. Industrial & Engineering Chemistry Research, 2020, 59(25): 11570-11581. |
| [6] | 刘莹, 郑芳, 杨启炜, 等. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| LIU Ying, ZHENG Fang, YANG Qiwei, et al. Recent progress in adsorption and separation of xylene isomers[J]. CIESC Journal, 2024, 75(4): 1081-1095. | |
| [7] | ASHRAF Muhammad Tahir, CHEBBI Rachid, DARWISH Naif A. Process of p-xylene production by highly selective methylation of toluene[J]. Industrial & Engineering Chemistry Research, 2013, 52(38): 13730-13737. |
| [8] | SAITO Shozaburo, MICHISHITA Tsuguo, MAEDA Siro. Separation of meta- and para-xylene mixture by distillation accompanied by chemical reactions[J]. Journal of Chemical Engineering of Japan, 1971, 4(1): 37-43. |
| [9] | 张方坤, 王云龙, 徐啟蕾, 等. 一种萃取精馏分离邻/间/对二甲苯混合物的方法: CN117285405A[P]. 2023-12-26. |
| ZHANG Fangkun, Wang Yunlong, Xu Qilei, et al. A method for separating o/m/p-xylene mixture by extractive distillation: CN117285405A[P] 2023-12-26. | |
| [10] | 赵同复, 李斌, 王振龙, 等. BaX分子筛的阳离子分布及其吸附分离对二甲苯的机理[J]. 催化学报, 2005, 26(8): 655-659. |
| ZHAO Tongfu, LI Bin, WANG Zhenlong, et al. Distribution of cation in BaX zeolite and mechanism of adsorption and separation for paraxylene[J]. Chinese Journal of Catalysis, 2005, 26(8): 655-659. | |
| [11] | RASOULI Milad, YAGHOBI Nakisa, MOVASSAGHI GILANI Seyedeh Zahra, et al. Influence of monovalent alkaline metal cations on binder-free nano-zeolite X in para-xylene separation[J]. Chinese Journal of Chemical Engineering, 2015, 23(1): 64-70. |
| [12] | CHEN Weiye, YAO Tuo, LIU Jian, et al. Modeling and validation of multi-objective optimization for mixed xylene hybrid distillation/crystallization process[J]. Separation and Purification Technology, 2025, 354: 128778. |
| [13] | 杨勇, 张钊, 王东亮, 等. 基于CO2加氢耦合甲苯甲基化选择催化的PX生产工艺对比[J]. 清华大学学报(自然科学版), 2024, 64(3): 538-544. |
| YANG Yong, ZHANG Zhao, WANG Dongliang, et al. Production technology of p-xylene production by toluene methylation with selective carbon dioxide hydrogenation[J]. Journal of Tsinghua University (Science and Technology), 2024, 64(3): 538-544. | |
| [14] | 夏敦焰, 彭莉, 吴政奇, 等. MFI型分子筛膜的两段变温合成及对二甲苯异构体的分离性能[J]. 高等学校化学学报, 2020, 41(12): 2813-2821. |
| XIA Dunyan, PENG Li, WU Zhengqi, et al. Two-stage varying-temperature synthesis of MFI zeolite membrane and their separation performance for xylene isomers[J]. Chemical Journal of Chinese Universities, 2020, 41(12): 2813-2821. | |
| [15] | ZHOU Jian, LIU Zhicheng, WANG Yangdong, et al. Shape selective catalysis in methylation of toluene: Development, challenges and perspectives[J]. Frontiers of Chemical Science and Engineering, 2018, 12(1): 103-112. |
| [16] | SHARMA Poonam, SEBASTIAN Joby, GHOSH Sreetama, et al. Recent advances in hydrogenation of CO2 into hydrocarbons via methanol intermediate over heterogeneous catalysts[J]. Catalysis Science & Technology, 2021, 11(5): 1665-1697. |
| [17] | CHAKINALA Nandana, CHAKINALA Anand G. Process design strategies to produce p-xylene via toluene methylation: A review[J]. Industrial & Engineering Chemistry Research, 2021, 60(15): 5331-5351. |
| [18] | LIU Jing, YANG Yu, WEI Shunan, et al. Intensified p-xylene production process through toluene and methanol alkylation[J]. Industrial & Engineering Chemistry Research, 2018, 57(38): 12829-12841. |
| [19] | WANG Dongliang, ZHANG Junqiang, YANG Yong, et al. Process simulation for enhanced p-xylene production via aromatics complex integrated toluene methylation with low-cost methanol feedstock[J]. Chemical Engineering Research and Design, 2023, 191: 184-195. |
| [20] | SHANG Xin, ZHUO Hongying, HAN Qiao, et al. Xylene synthesis through tandem CO2 hydrogenation and toluene methylation over a composite ZnZrO zeolite catalyst[J]. Angewandte Chemie International Edition, 2023, 62(37): e202309377. |
| [21] | ZUO Jiachang, CHEN Weikun, LIU Jia, et al. Selective methylation of toluene using CO2 and H2 to para-xylene[J]. Science Advances, 2020, 6(34): eaba5433. |
| [22] | MIAO Dengyun, PAN Xiulian, JIAO Feng, et al. Selective synthesis of para-xylene and light olefins from CO2/H2 in the presence of toluene[J]. Catalysis Science & Technology, 2021, 11(13): 4521-4528. |
| [1] | BUSCA Guido. Production of gasolines and monocyclic aromatic hydrocarbons: From fossil raw materials to green processes[J]. Energies, 2021, 14(13): 4061. |
| [2] | PENG Bo, WANG Shaoyan. Separation of p-xylene and m-xylene by simulated moving bed chromatography with MIL-53(Fe) as stationary phase[J]. Journal of Chromatography A, 2022, 1673: 463091. |
| [3] | GONÇALVES Jonathan C, RODRIGUES Alírio E. Simulated moving bed reactor for p-xylene production: Adsorbent and catalyst homogeneous mixture[J]. Chemical Engineering Journal, 2014, 258: 194-202. |
| [23] | SHI Qian, GONÇALVES Jonathan C, FERREIRA Alexandre F P, et al. A review of advances in production and separation of xylene isomers[J]. Chemical Engineering and Processing: Process Intensification, 2021, 169: 108603. |
| [24] | ZHANG Peipei, TAN Li, YANG Guohui, et al. One-pass selective conversion of syngas to para-xylene[J]. Chemical Science, 2017, 8(12): 7941-7946. |
| [25] | HAN Xiaoqin, ZUO Jiachang, WEN Danlu, et al. Toluene methylation with syngas to para-xylene by bifunctional ZnZrO x -HZSM-5 catalysts[J]. Chinese Journal of Catalysis, 2022, 43(4): 1156-1164. |
| [26] | TIAN Haifeng, HE Huanhuan, JIAO Jiapeng, et al. Tandem catalysts composed of different morphology HZSM-5 and metal oxides for CO2 hydrogenation to aromatics[J]. Fuel, 2022, 314: 123119. |
| [27] | LI Wen, WANG Kuncan, HUANG Junjie, et al. M x O y -ZrO2 (M=Zn, Co, Cu) solid solutions derived from schiff base-bridged UiO-66 composites as high-performance catalysts for CO2 hydrogenation[J]. ACS Applied Materials & Interfaces, 2019, 11(36): 33263-33272. |
| [28] | XIAO Zhikai, HUANG Hai, CAO Chenxi, et al. Designing a bifunctional ZrCuO x /HZSM-5 catalyst for selective methylation of toluene with carbon dioxide to para-xylene[J]. Fuel, 2022, 319: 123848. |
| [29] | NI Youming, CHEN Zhiyang, FU Yi, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nature Communications, 2018, 9(1): 3457. |
| [30] | Mahdi ABDI-KHANGHAH, ALRASHED Abdullah A A A, HAMOULE Touba,et al. Toluene methylation to para-xylene[J]. Journal of Thermal Analysis and Calorimetry, 2018, 135(3): 1723-1732. |
| [31] | 杨明磊, 魏民, 胡蓉, 等. 二甲苯模拟移动床分离过程建模与仿真[J]. 化工学报, 2013, 64(12): 4335-4341. |
| YANG Minglei, WEI Min, HU Rong, et al. Modeling of simulated moving bed for xylene separation[J]. CIESC Journal, 2013, 64(12): 4335-4341. | |
| [32] | 张东辉, 沈圆辉, 吴丽梅, 等. 对二甲苯模拟移动床分离过程的模拟与优化[J]. 天津大学学报(自然科学与工程技术版), 2016, 49(3): 279-286. |
| ZHANG Donghui, SHEN Yuanhui, WU Limei, et al. Simulation and optimization of simulated moving bed for the separation of p-xylene[J]. Journal of Tianjin University (Science and Technology), 2016, 49(3): 279-286. | |
| [33] | WANKAT P C. Simulated moving bed cascades for ternary separations[J]. Industrial & Engineering Chemistry Research, 2001, 40(26): 6185-6193. |
| [34] | JIN Weihua, WANKAT Phillip C. Hybrid simulated moving bed processes for the purification of p‐xylene[J]. Separation Science and Technology, 2007, 42(4): 669-700. |
| [35] | Cristofer BRAVO-BRAVO, SEGOVIA-HERNÁNDEZ Juan Gabriel, Salvador HERNÁNDEZ, et al. Hybrid distillation/melt crystallization process using thermally coupled arrangements: Optimization with evolutive algorithms[J]. Chemical Engineering and Processing: Process Intensification, 2013, 67: 25-38. |
| [36] | KONG Lingxun, WU Yaqing, MARAVELIAS Christos T. Simultaneous utility and heat exchanger area targeting for integrated process synthesis and heat integration[J]. Industrial & Engineering Chemistry Research, 2017, 56(41): 11847-11859. |
| [37] | IBRAHIM Dauda, JOBSON Megan, Gonzalo GUILLÉN-GOSÁLBEZ. Optimization-based design of crude oil distillation units using rigorous simulation models[J]. Industrial & Engineering Chemistry Research, 2017, 56(23): 6728-6740. |
| [1] | 纵华健, 李英, 张香平. CO2化学转化碳酸二甲酯/乙二醇的能量集成和碳流分析[J]. 化工进展, 2024, 43(10): 5369-5380. |
| [2] | 陈乐, 种海玲, 张致慧, 何明阳, 陈群. CTAB改性Cu-BTC材料的合成及其吸附分离二甲苯异构体的性能[J]. 化工进展, 2024, 43(1): 455-464. |
| [3] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [4] | 韩文韬, 韩振为, 李洪, 高鑫, 李鑫钢. 乙酰丙酸乙酯的反应精馏模型及隔壁塔节能优化设计[J]. 化工进展, 2022, 41(4): 1759-1769. |
| [5] | 赖佳宁, 高鑫, 从海峰, 李洪, 李鑫钢. 丙酮缩甘油反应精馏工艺全流程模拟与优化[J]. 化工进展, 2021, 40(7): 3584-3590. |
| [6] | 王晓达, 陈宇, 王清莲, 黄智贤, 杨臣, 王红星, 邱挺. 醚化反应精馏研究进展[J]. 化工进展, 2021, 40(4): 1797-1811. |
| [7] | 王洪海, 尹依, 刘星, 魏斯文, 苏伟怡, 李春利. 载酶塑料填料的制备与优化[J]. 化工进展, 2021, 40(12): 6829-6838. |
| [8] | 王红星, 李海勇, 周庆, 张路. 隔壁反应精馏合成碳酸甲乙酯节能新工艺[J]. 化工进展, 2020, 39(S2): 66-72. |
| [9] | 陆佳伟, 孔倩, 汤吉海, 张竹修, 崔咪芬, 陈献, 乔旭. “背包式”反应精馏集成过程研究进展[J]. 化工进展, 2020, 39(12): 4940-4953. |
| [10] | 耿雪丽, 孟莹, 从海峰, 李洪, 高鑫, 李鑫钢. 聚甲氧基二甲醚合成工艺及产业化述评[J]. 化工进展, 2020, 39(12): 4993-5008. |
| [11] | 刘家琪, 刘连永, 王双瑜, 李志会, 张艳华, 丁晓墅, 张东升, 赵新强, 王延吉. 酮肟水解反应及其羟胺产品分离的研究进展[J]. 化工进展, 2020, 39(10): 4147-4154. |
| [12] | 黄建松,许松林. 制取无水叔丁醇的精馏工艺优化和对比[J]. 化工进展, 2019, 38(11): 5181-5188. |
| [13] | 董颜箔, 何瑞宁, 牟春霞, 邹昀, 童张法. 离子液体催化反应精馏合成乙酸乙酯工艺模拟[J]. 化工进展, 2018, 37(02): 468-474. |
| [14] | 凌笑媚, 郑伟跃, 王晓达, 邱挺. 隔壁反应精馏技术进展[J]. 化工进展, 2017, 36(08): 2776-2786. |
| [15] | 李洪, 孟莹, 李鑫钢, 高鑫. 乙酸戊酯酯化反应精馏过程系统控制模拟及分析[J]. 化工进展, 2015, 34(12): 4165-4171. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||