化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5498-5516.DOI: 10.16085/j.issn.1000-6613.2023-1705
• 工业催化 • 上一篇
甲醇水蒸气重整制氢催化剂的研究进展
- 北京化工大学化学学院,化工资源有效利用国家重点实验室,北京 100029
-
收稿日期:2023-09-26修回日期:2024-03-21出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:孟浩,杨宇森 -
作者简介:冯凯(2000—),男,硕士研究生,研究方向为甲醇重整制氢和丙烷脱氢。E-mail:2022201055@buct.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22172006)
Research progress on catalysts for hydrogen production by methanol steam reforming
FENG Kai( ), MENG Hao(
), MENG Hao( ), YANG Yusen(
), YANG Yusen( ), WEI Min
), WEI Min
- State Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2023-09-26Revised:2024-03-21Online:2024-10-15Published:2024-10-29 -
Contact:MENG Hao, YANG Yusen
摘要:
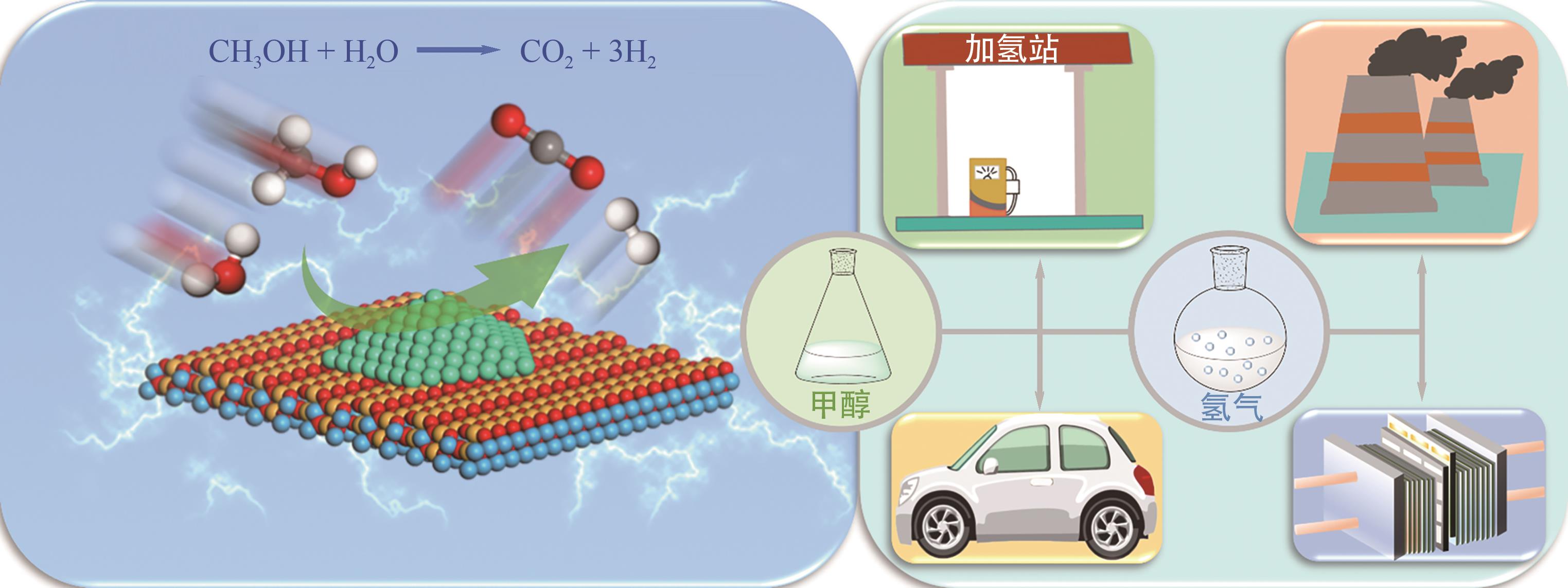
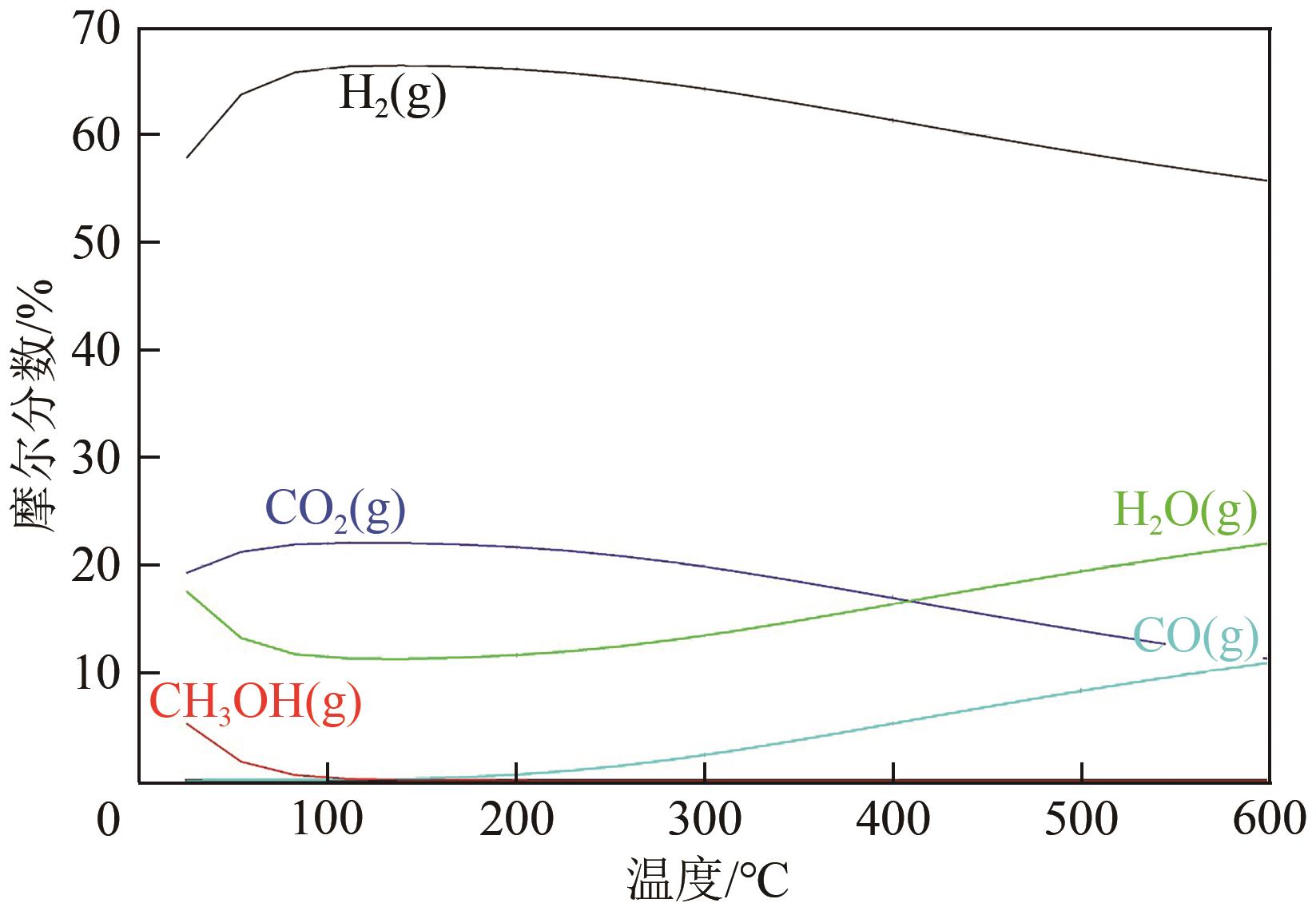
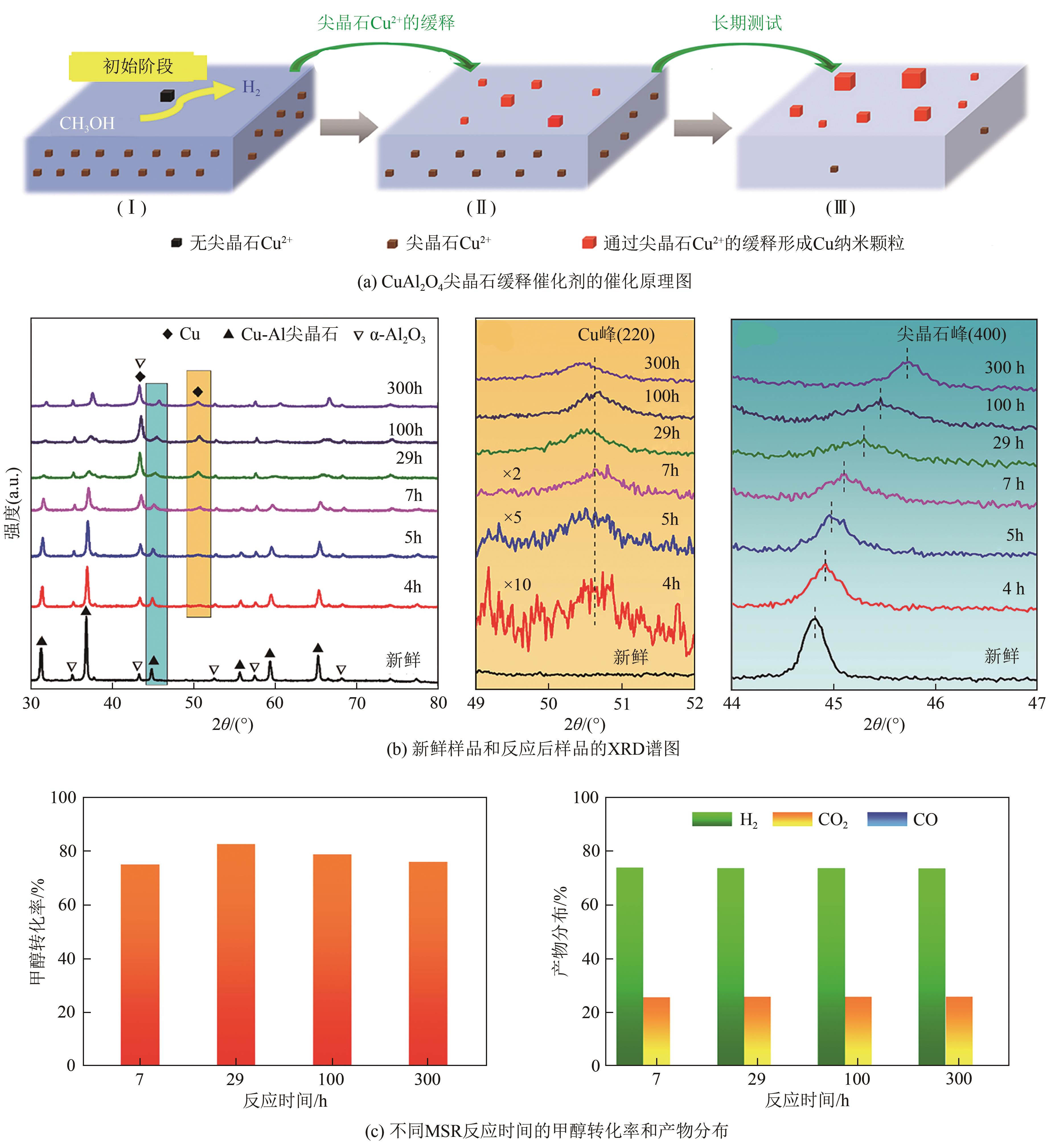
甲醇作为一种常温常压下稳定的液相储氢介质,具有高的氢碳比、价格低廉、储运方便等优势。通过甲醇重整制氢来替代传统碳氢化合物的催化重整过程是实现氢能绿色制取和高效储运的重要手段。本文首先介绍了甲醇重整制氢反应的机理及特点;然后从单金属、双金属以及金属价态调控方面综述了金属活性位点的结构优化策略;接着从载体元素掺杂、缺陷位点调控以及载体晶相控制方面阐述了金属-载体界面结构调控策略;进一步从载体诱导活化以及金属位点缓释方面论述了活性位点重构策略;最后对未来开发高性能催化剂的制备策略及其揭示构效关系所采用的表征技术和理论计算方法进行了展望。
中图分类号:
引用本文
冯凯, 孟浩, 杨宇森, 卫敏. 甲醇水蒸气重整制氢催化剂的研究进展[J]. 化工进展, 2024, 43(10): 5498-5516.
FENG Kai, MENG Hao, YANG Yusen, WEI Min. Research progress on catalysts for hydrogen production by methanol steam reforming[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5498-5516.
| 甲醇重整产氢 | 反应示意图 | 反应方程 | 优势 | 劣势 |
|---|---|---|---|---|
| 甲醇水蒸气重整 | 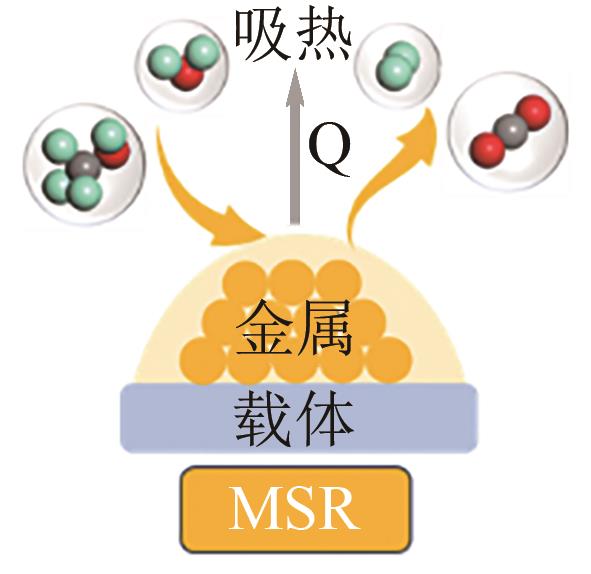 | H2产率最高;不需要提供氧气;CO含量较低 | 需要外部较高的能量供应 | |
| 甲醇部分氧化 | 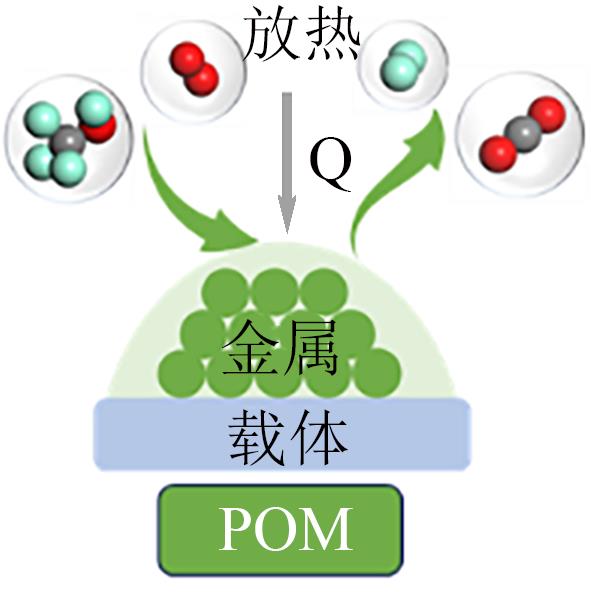 | 快速启动和响应时间;碳积累少;无需较高的热供应 | 较低的H2产率;H2容易过氧化;CO含量高 | |
| 甲醇自热重整 | 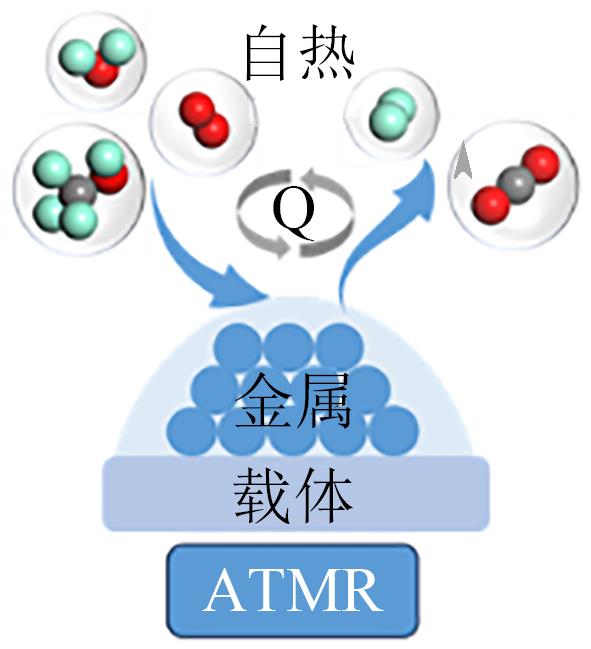 | 放热和吸热反应的耦合;简化热量管理;工作温度低;快速启动 | H2产率低;需要控制系统平衡放热和吸热过程;H2容易过氧化 | |
| 甲醇分解 |  | 反应简便;无需额外原料的引入 | H2产率最低;CO含量过高;催化剂容易积炭失活 |
表1 多种甲醇重整制氢方法示意图及其优缺点
| 甲醇重整产氢 | 反应示意图 | 反应方程 | 优势 | 劣势 |
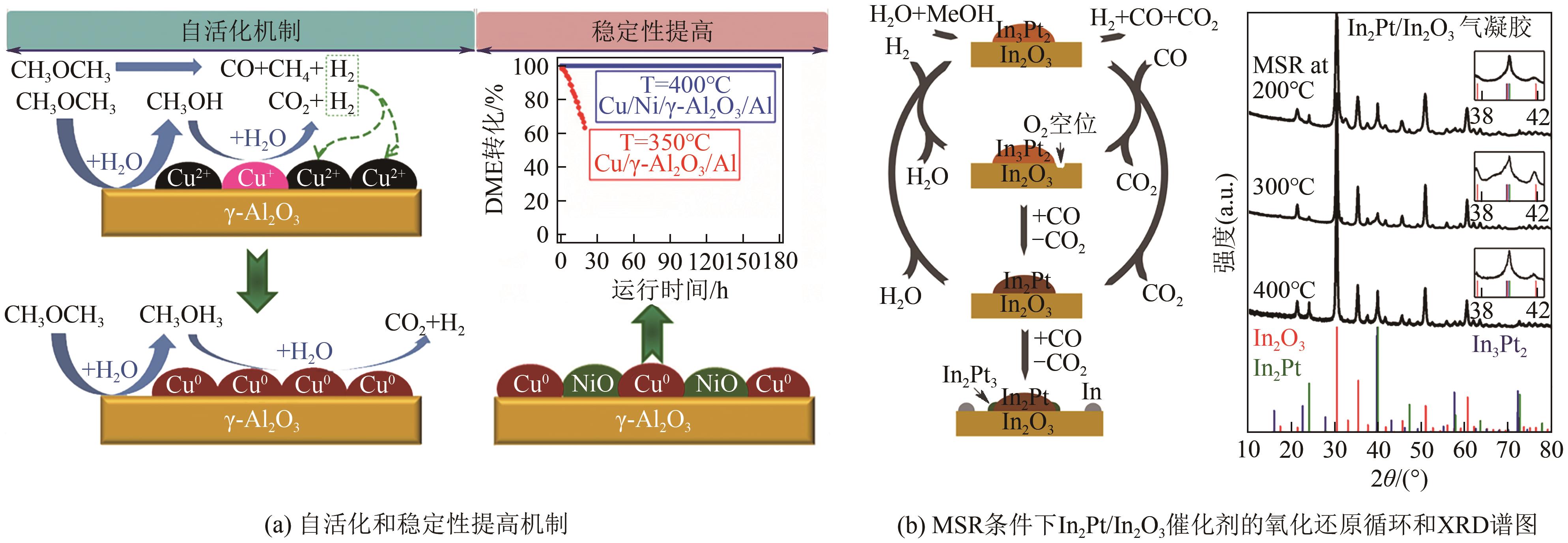
|---|---|---|---|---|
| 甲醇水蒸气重整 |  | H2产率最高;不需要提供氧气;CO含量较低 | 需要外部较高的能量供应 | |
| 甲醇部分氧化 |  | 快速启动和响应时间;碳积累少;无需较高的热供应 | 较低的H2产率;H2容易过氧化;CO含量高 | |
| 甲醇自热重整 |  | 放热和吸热反应的耦合;简化热量管理;工作温度低;快速启动 | H2产率低;需要控制系统平衡放热和吸热过程;H2容易过氧化 | |
| 甲醇分解 |  | 反应简便;无需额外原料的引入 | H2产率最低;CO含量过高;催化剂容易积炭失活 |
| 催化剂 | 反应温度/℃ | 水碳比 | 转化率/% | CO2选择性/% | CO选择性/% | H2生成速率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 0.2%Pt/α-MoC | 190 | 1 | — | — | 0.14 | 18046 | [ |
| PdO/In2O3 | 260 | 1 | 50 | 93 | — | — | [ |
| CuPd/ZrO2 | 220 | 1.5 | 65 | — | 5 | 86.3mmol·h-1·gcat-1 | [ |
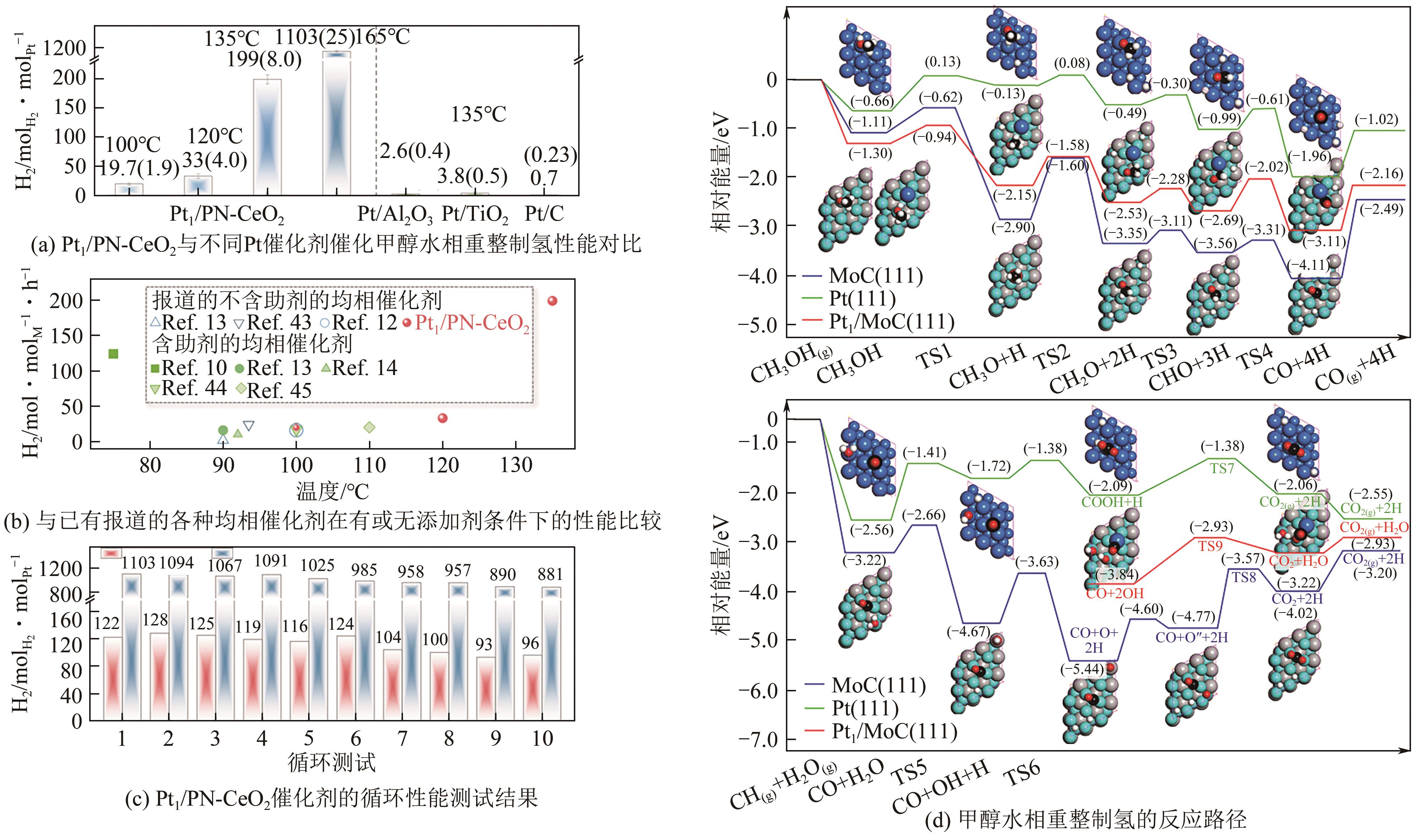
| Pt1/PN-CeO2 | 135 | 1 | — | — | 0.05 | 199mol | [ |
| 2% Ni/α-MoC | 240 | 1 | — | — | 0.7 | 1805mol | [ |
| Pt1/ZnO | 390 | 1.5 | 43 | — | — | — | [ |
| Ru1/CeO2 | 350 | 3 | 26 | 98 | — | 139.6mL | [ |
| CuFe(50∶50) | 350 | 3 | 48 | 0 | 200mmol·kgcat-1·s-1 | [ | |
| Cu-Fe/硅酸盐 | 200 | — | 99 | — | 0 | 1.64μmol·s-1·gcat-1 | [ |
| Cu/Ni/γ-Al2O3 | 400 | 2 | 100 | 83.3 | 11 | — | [ |
| Ni0.2Cu0.8/BN | 320 | 1 | 100 | — | — | 1.8mol | [ |
| PtCo/MoS2 | 220 | 3 | — | — | — | 37142mol | [ |
| Cu-Zn/CeAlO3 | 320 | 6 | 98.9 | 96.1 | — | — | [ |
| Cu-In2O3 | 350 | 2 | 84 | — | 5 | 3.8µmol·gCu-1·s-1 | [ |
| Pt/In2O3/Al2O3 | 350 | 4 | 100 | 96.1 | 3.2 | 0.6mol·h-1·gcat-1 | [ |
| Pt/In2O3 | 300 | 1 | — | 99.5 | — | 1500mol | [ |
| In7Pt3 | 400 | 1 | — | 99.2 | — | 6mol·molpt-1·h-1 | [ |
| InPd/In2O3 | 300 | 1 | 26 | 99 | — | 50mmol | [ |
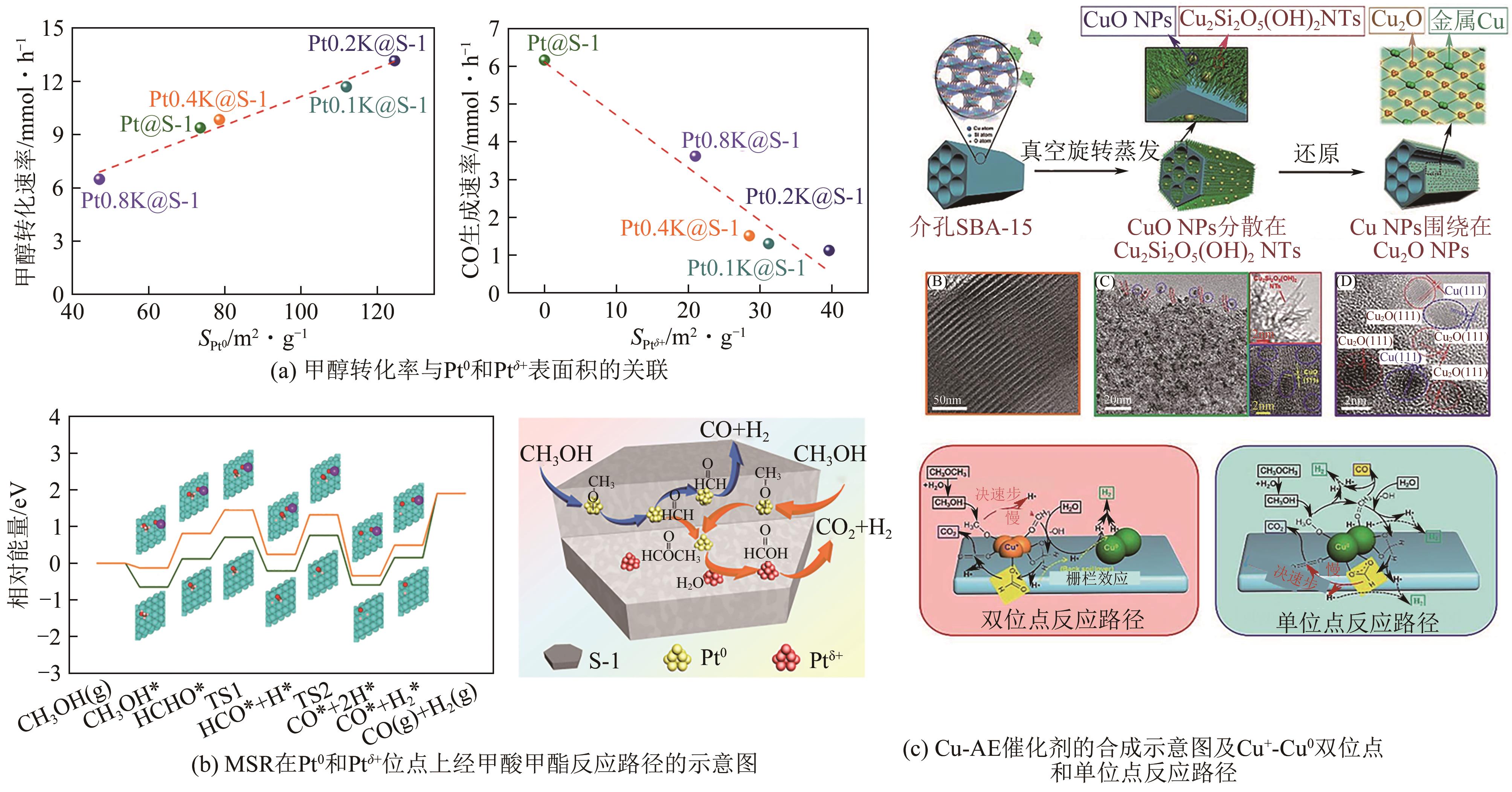
| Pt0.2K@S-1 | 400 | 3 | 45 | — | 1.9 | 61.12mmol | [ |
| 25Cu-AE | 400 | 2 | 100 | 89.3 | 10.4 | 1145mol·kgcat-1·h-1 | [ |
| CuZnO/γ-Al2O3/Al | 225 | 2 | 60 | 90 | — | — | [ |
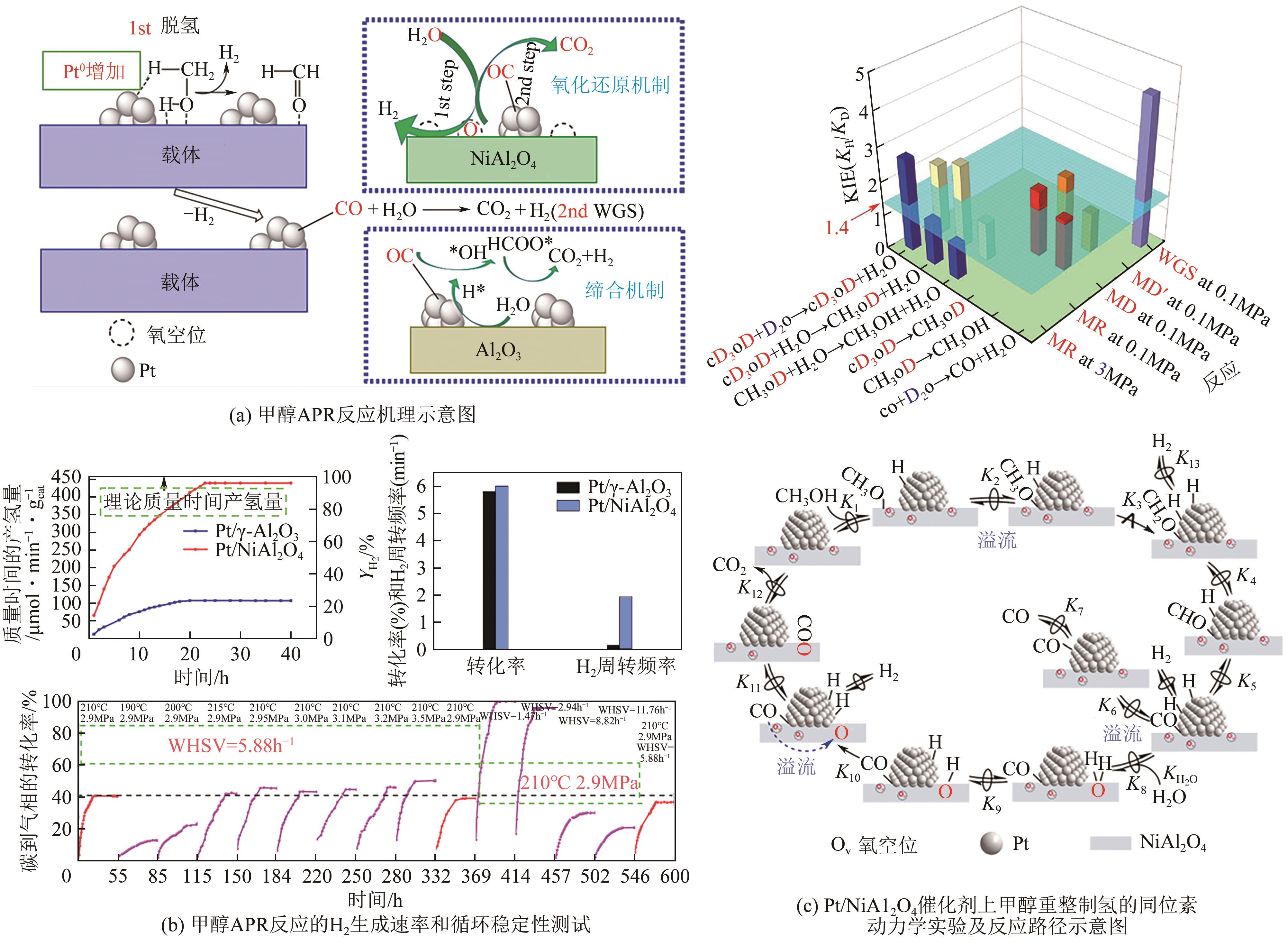
| Pt/NiAl2O4 | 210 | 16 | 100 | 99.7 | 0.05 | 439.2μmol·min-1·gcat-1 | [ |
| Pd/ZnAl2O4 | 250 | 1.1 | 60 | 97 | 3 | 11.4μmol | [ |
| 30Cu/CeO2 | 200 | 1.3 | 4.5 | 99.5 | — | 0.21mol | [ |
| Cu/Sc2O3-ZnO | 400 | 1.5 | 100 | — | 11 | 140μmol·g-1·s-1 | [ |
| CuZrAl0.4 | 220 | 1.5 | 70 | — | 0.1 | 460.1mmol·gmet-1·h-1 | [ |
| 10Pr-NA | 300 | 1.5 | 95 | — | — | — | [ |
| 18GaCuMg | 200 | 1.5 | 90 | — | 0.4 | — | [ |
| Cu/ZrO2 | 260 | 1.3 | 87 | 100 | — | 260mmol·gcat-1·h-1 | [ |
| Cu/ZrO2 | 270 | 2 | 40 | 99 | — | 14μmol·gCu-1·s-1 | [ |
| Cu/ZnO/Al2O3 | 225 | 1.3 | 67 | — | — | — | [ |
| CuAl2O4 | 320 | 1.5 | 30 | 98.95 | 1.05 | 52mol·min-1·molCu-1 | [ |
| CuHAl-Ac-950 | 255 | 2.3 | 80 | — | 0.4 | — | [ |
| CuNi0.05/Al2O3 | 255 | 2.3 | 90 | — | 0.8 | — | [ |
| 1.7Mg/Cu/Al2O3 | 255 | 2.3 | 96.5 | 96.2 | 3.8 | — | [ |
| CuAl2O4 | 300 | 2.3 | 95 | — | 1.0 | — | [ |
表2 甲醇水蒸气重整制氢催化性能列表
| 催化剂 | 反应温度/℃ | 水碳比 | 转化率/% | CO2选择性/% | CO选择性/% | H2生成速率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 0.2%Pt/α-MoC | 190 | 1 | — | — | 0.14 | 18046 | [ |
| PdO/In2O3 | 260 | 1 | 50 | 93 | — | — | [ |
| CuPd/ZrO2 | 220 | 1.5 | 65 | — | 5 | 86.3mmol·h-1·gcat-1 | [ |
| Pt1/PN-CeO2 | 135 | 1 | — | — | 0.05 | 199mol | [ |
| 2% Ni/α-MoC | 240 | 1 | — | — | 0.7 | 1805mol | [ |
| Pt1/ZnO | 390 | 1.5 | 43 | — | — | — | [ |
| Ru1/CeO2 | 350 | 3 | 26 | 98 | — | 139.6mL | [ |
| CuFe(50∶50) | 350 | 3 | 48 | 0 | 200mmol·kgcat-1·s-1 | [ | |
| Cu-Fe/硅酸盐 | 200 | — | 99 | — | 0 | 1.64μmol·s-1·gcat-1 | [ |
| Cu/Ni/γ-Al2O3 | 400 | 2 | 100 | 83.3 | 11 | — | [ |
| Ni0.2Cu0.8/BN | 320 | 1 | 100 | — | — | 1.8mol | [ |
| PtCo/MoS2 | 220 | 3 | — | — | — | 37142mol | [ |
| Cu-Zn/CeAlO3 | 320 | 6 | 98.9 | 96.1 | — | — | [ |
| Cu-In2O3 | 350 | 2 | 84 | — | 5 | 3.8µmol·gCu-1·s-1 | [ |
| Pt/In2O3/Al2O3 | 350 | 4 | 100 | 96.1 | 3.2 | 0.6mol·h-1·gcat-1 | [ |
| Pt/In2O3 | 300 | 1 | — | 99.5 | — | 1500mol | [ |
| In7Pt3 | 400 | 1 | — | 99.2 | — | 6mol·molpt-1·h-1 | [ |
| InPd/In2O3 | 300 | 1 | 26 | 99 | — | 50mmol | [ |
| Pt0.2K@S-1 | 400 | 3 | 45 | — | 1.9 | 61.12mmol | [ |
| 25Cu-AE | 400 | 2 | 100 | 89.3 | 10.4 | 1145mol·kgcat-1·h-1 | [ |
| CuZnO/γ-Al2O3/Al | 225 | 2 | 60 | 90 | — | — | [ |
| Pt/NiAl2O4 | 210 | 16 | 100 | 99.7 | 0.05 | 439.2μmol·min-1·gcat-1 | [ |
| Pd/ZnAl2O4 | 250 | 1.1 | 60 | 97 | 3 | 11.4μmol | [ |
| 30Cu/CeO2 | 200 | 1.3 | 4.5 | 99.5 | — | 0.21mol | [ |
| Cu/Sc2O3-ZnO | 400 | 1.5 | 100 | — | 11 | 140μmol·g-1·s-1 | [ |
| CuZrAl0.4 | 220 | 1.5 | 70 | — | 0.1 | 460.1mmol·gmet-1·h-1 | [ |
| 10Pr-NA | 300 | 1.5 | 95 | — | — | — | [ |
| 18GaCuMg | 200 | 1.5 | 90 | — | 0.4 | — | [ |
| Cu/ZrO2 | 260 | 1.3 | 87 | 100 | — | 260mmol·gcat-1·h-1 | [ |
| Cu/ZrO2 | 270 | 2 | 40 | 99 | — | 14μmol·gCu-1·s-1 | [ |
| Cu/ZnO/Al2O3 | 225 | 1.3 | 67 | — | — | — | [ |
| CuAl2O4 | 320 | 1.5 | 30 | 98.95 | 1.05 | 52mol·min-1·molCu-1 | [ |
| CuHAl-Ac-950 | 255 | 2.3 | 80 | — | 0.4 | — | [ |
| CuNi0.05/Al2O3 | 255 | 2.3 | 90 | — | 0.8 | — | [ |
| 1.7Mg/Cu/Al2O3 | 255 | 2.3 | 96.5 | 96.2 | 3.8 | — | [ |
| CuAl2O4 | 300 | 2.3 | 95 | — | 1.0 | — | [ |
| 1 | FALCONE Pasquale Marcello, HIETE Michael, SAPIO Alessandro. Hydrogen economy and sustainable development goals: Review and policy insights[J]. Current Opinion in Green and Sustainable Chemistry, 2021, 31: 100506. |
| 2 | HOSSEINI Seyed Ehsan, WAHID Mazlan Abdul. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy[J]. International Journal of Energy Research, 2020, 44(6): 4110-4131. |
| 3 | WANG Qianru, GUO Jianping, CHEN Ping. Recent progress towards mild-condition ammonia synthesis[J]. Journal of Energy Chemistry, 2019, 36: 25-36. |
| 4 | YANG Bing, DING Weilu, ZHANG Honghua, et al. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity[J]. Energy & Environmental Science, 2021, 14(2): 672-687. |
| 5 | BEPARI Sujoy, KUILA Debasish. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts—A review[J]. International Journal of Hydrogen Energy, 2020, 45(36): 18090-18113. |
| 6 | 迟军, 俞红梅. 基于可再生能源的水电解制氢技术[J]. 催化学报, 2018, 39(3): 390-394. |
| CHI Jun, YU Hongmei. Water electrolysis based on renewable energy for hydrogen production[J]. Chinese Journal of Catalysis, 2018, 39(3): 390-394. | |
| 7 | EBERLE Ulrich, FELDERHOFF Michael, Ferdi SCHÜTH. Chemical and physical solutions for hydrogen storage[J]. Angewandte Chemie (International Ed in English), 2009, 48(36): 6608-6630. |
| 8 | VON HELMOLT Rittmar, EBERLE Ulrich. Fuel cell vehicles: Status 2007[J]. Journal of Power Sources, 2007, 165(2): 833-843. |
| 9 | CHEN Luning, HOU Kaipeng, LIU Yisheng, et al. Efficient hydrogen production from methanol using a single-site Pt1/CeO2 catalyst[J]. Journal of the American Chemical Society, 2019, 141(45): 17995-17999. |
| 10 | LIN Lili, ZHOU Wu, GAO Rui, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648): 80-83. |
| 11 | NEUMANN Matthias, TESCHNER Detre, Axel KNOP-GERICKE, et al. Controlled synthesis and catalytic properties of supported In-Pd intermetallic compounds[J]. Journal of Catalysis, 2016, 340: 49-59. |
| 12 | Cátia AZENHA, LAGARTEIRA Tiago, Cecilia MATEOS-PEDRERO, et al. Production of hydrogen from methanol steam reforming using CuPd/ZrO2 catalysts-Influence of the catalytic surface on methanol conversion and CO selectivity[J]. International Journal of Hydrogen Energy, 2021, 46(33): 17490-17499. |
| 13 | RANJEKAR Apoorva M, YADAV Ganapati D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope[J]. Industrial & Engineering Chemistry Research, 2021, 60(1): 89-113. |
| 14 | SCHUBERT Teresa. Production routes of advanced renewable C1 to C4 alcohols as biofuel components-a review[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(4): 845-878. |
| 15 | MEI Deqing, QIU Xingye, LIU Haiyu, et al. Progress on methanol reforming technologies for highly efficient hydrogen production and applications[J]. International Journal of Hydrogen Energy, 2022, 47(84): 35757-35777. |
| 16 | XU Xinhai, SHUAI Kaipeng, XU Ben. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen[J]. Catalysts, 2017, 7(6): 183. |
| 17 | ZHANG Sai, LIU Yuxuan, ZHANG Mingkai, et al. Sustainable production of hydrogen with high purity from methanol and water at low temperatures[J]. Nature Communications, 2022, 13(1): 5527. |
| 18 | LIN Lili, YU Qiaolin, PENG Mi, et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water[J]. Journal of the American Chemical Society, 2021, 143(1): 309-317. |
| 19 | KANG Jeongmee, SONG Youjung, KIM Taejun, et al. Recent trends in the development of reactor systems for hydrogen production via methanol steam reforming[J]. International Journal of Hydrogen Energy, 2022, 47(6): 3587-3610. |
| 20 | LYTKINA A A, OREKHOVA N V, YAROSLAVTSEV A B. Catalysts for the steam reforming and electrochemical oxidation of methanol[J]. Inorganic Materials, 2018, 54(13): 1315-1329. |
| 21 | LUO Hui, BARRIO Jesús, SUNNY Nixon, et al. Progress and perspectives in photo- and electrochemical-oxidation of biomass for sustainable chemicals and hydrogen production[J]. Advanced Energy Materials, 2021, 11(43): 2101180. |
| 22 | ACURIO CERDA Karen, KATHOL Mark, PUROHIT Gunjan, et al. Cationic lignin as an efficient and biorenewable antimicrobial material[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(28): 10364-10379. |
| 23 | YE Runping, XIAO Shuwei, LAI Qinghua, et al. Advances in enhancing the stability of Cu-based catalysts for methanol reforming[J]. Catalysts, 2022, 12(7): 747. |
| 24 | GU Xiangkui, QIAO Botao, HUANG Chuanqi, et al. Supported single Pt1/Au1 atoms for methanol steam reforming[J]. ACS Catalysis, 2014, 4(11): 3886-3890. |
| 25 | QI Zhiyuan, CHEN Luning, ZHANG Shuchen, et al. Mechanism of methanol decomposition over single-site Pt1/CeO2 catalyst: A DRIFTS study[J]. Journal of the American Chemical Society, 2021, 143(1): 60-64. |
| 26 | CHEN Luning, QI Zhiyuan, PENG Xinxing, et al. Insights into the mechanism of methanol steam reforming tandem reaction over CeO2 supported single-site catalysts[J]. Journal of the American Chemical Society, 2021, 143(31): 12074-12081. |
| 27 | LIU Lichen, CORMA Avelino. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 28 | ZHANG Leilei, ZHOU Maoxiang, WANG Aiqin, et al. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 29 | DONG Chunyang, LI Yinlong, CHENG Danyang, et al. Supported metal clusters: Fabrication and application in heterogeneous catalysis[J]. ACS Catalysis, 2020, 10(19): 11011-11045. |
| 30 | SHARMA Richa, KUMAR Amit, UPADHYAY Rajesh K. Bimetallic Fe-promoted catalyst for CO-free hydrogen production in high-temperature-methanol steam reforming[J]. ChemCatChem, 2019, 11(18): 4568-4580. |
| 31 | KUO Meite, CHEN Yunying, HUNG Weiying, et al. Synthesis of mesoporous Cu-Fe/silicates catalyst for methanol steam reforming[J]. International Journal of Hydrogen Energy, 2019, 44(28): 14416-14423. |
| 32 | FAN Feiyue, ZHANG Qi, WANG Xing, et al. A structured Cu-based/γ-Al2O3/Al plate-type catalyst for steam reforming of dimethyl ether: Self-activation behavior investigation and stability improvement[J]. Fuel, 2016, 186: 11-19. |
| 33 | KOVALSKII Andrey M, MATVEEV Andrei T, POPOV Zakhar I, et al. (Ni, Cu)/hexagonal BN nanohybrids—New efficient catalysts for methanol steam reforming and carbon monoxide oxidation[J]. Chemical Engineering Journal, 2020, 395: 125109. |
| 34 | WANG Ruiyi, LIU Huan, ZHENG Zhanfeng. Low temperature light-assisted hydrogen production from aqueous reforming ethylene glycol over Pt/Al2O3 and Pd/Al2O3 catalysts[J]. Journal of Fuel Chemistry and Technology, 2019, 47(12):1486-1494. |
| 35 | RANJEKAR Apoorva M, YADAV Ganapati D. Hydrogen production by steam reforming of methanol by Cu-Zn/CeAlO3 perovskite[J]. New Journal of Chemistry, 2023, 47(10): 4860-4870. |
| 36 | PLONER Kevin, SCHLICKER Lukas, GILI Albert, et al. Reactive metal-support interaction in the Cu-In2O3 system: Intermetallic compound formation and its consequences for CO2-selective methanol steam reforming[J]. Science and Technology of Advanced Materials, 2019, 20(1): 356-366. |
| 37 | LIU Di, Yong MEN, WANG Jinguo, et al. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming[J]. International Journal of Hydrogen Energy, 2016, 41(47): 21990-21999. |
| 38 | HODGSON A, HAQ S. Water adsorption and the wetting of metal surfaces[J]. Surface Science Reports, 2009, 64(9): 381-451. |
| 39 | PHATAK Abhijit A, Nicholas DELGASS W, RIBEIRO Fabio H, et al. Density functional theory comparison of water dissociation steps on Cu, Au, Ni, Pd, and Pt[J]. The Journal of Physical Chemistry C, 2009, 113(17): 7269-7276. |
| 40 | Nicolas KÖWITSCH, THONI Lukas, KLEMMED Benjamin, et al. Unprecedented catalytic activity and selectivity in methanol steam reforming by reactive transformation of intermetallic In-Pt compounds[J]. The Journal of Physical Chemistry C, 2021, 125(18): 9809-9817. |
| 41 | Nicolas KÖWITSCH, BARTH Stefan, PLONER Kevin, et al. Properties of bulk In-Pt intermetallic compounds in methanol steam reforming[J]. ChemPhysChem, 2022(8):23. |
| 42 | RAMESHAN Christoph, LORENZ Harald, MAYR Lukas, et al. CO2-selective methanol steam reforming on In-doped Pd studied by in situ X-ray photoelectron spectroscopy[J]. Journal of Catalysis, 2012, 295(2/3): 186-194. |
| 43 | HAGHOFER Andreas, FERRI Davide, Karin FÖTTINGER, et al. Who is doing the job? Unraveling the role of Ga2O3 in methanol steam reforming on Pd2Ga/Ga2O3 [J]. ACS Catalysis, 2012, 2(11): 2305-2315. |
| 44 | SHAO Zilong, ZHANG Shunan, LIU Xiaofang, et al. Maximizing the synergistic effect between Pt0 and Pt δ + in a confined Pt-based catalyst for durable hydrogen production[J]. Applied Catalysis B: Environmental, 2022, 316: 121669. |
| 45 | YANG Huanhuan, CHEN Yanyan, CUI Xiaojing, et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation[J]. Angewandte Chemie, 2018, 130(7): 1854-1858. |
| 46 | MA Kui, TIAN Ye, ZHAO Zhijian, et al. Achieving efficient and robust catalytic reforming on dual-sites of Cu species[J]. Chemical Science, 2019, 10(9): 2578-2584. |
| 47 | ZHANG Guiru, ZHAO Jiali, YANG Taotao, et al. In-situ self-assembled Cu2O/ZnO core-shell catalysts synergistically enhance the durability of methanol steam reforming[J]. Applied Catalysis A: General, 2021, 616: 118072. |
| 48 | CUI Zhonghui, SONG Song, LIU Huibin, et al. Synergistic effect of Cu+ single atoms and Cu nanoparticles supported on alumina boosting water-gas shift reaction[J]. Applied Catalysis B: Environmental, 2022, 313: 121468. |
| 49 | RUANO Daniel, CORED Jorge, Cátia AZENHA, et al. Dynamic structure and subsurface oxygen formation of a working copper catalyst under methanol steam reforming conditions: An in situ time-resolved spectroscopic study[J]. ACS Catalysis, 2019, 9(4): 2922-2930. |
| 50 | MAYR Lukas, Bernhard KlÖTZER, ZEMLYANOV Dmitry, et al. Steering of methanol reforming selectivity by zirconia-copper interaction[J]. Journal of Catalysis, 2015, 321: 123-132. |
| 51 | LI Didi, LI Yi, LIU Xiaohui, et al. NiAl2O4 spinel supported Pt catalyst: High performance and origin in aqueous-phase reforming of methanol[J]. ACS Catalysis, 2019, 9(10): 9671-9682. |
| 52 | WANG Xiuyi, LI Didi, GAO Zirui, et al. The nature of interfacial catalysis over Pt/NiAl2O4 for hydrogen production from methanol reforming reaction[J]. Journal of the American Chemical Society, 2023, 145(2): 905-918. |
| 53 | LIU Liang, LIN Yangjian, HU Yanru, et al. ZnAl2O4 spinel-supported PdZnβ catalyst with parts per million Pd for methanol steam reforming[J]. ACS Catalysis, 2022, 12(4): 2714-2721. |
| 54 | JIN Shiqing, LI Didi, WANG Zhen, et al. Dynamics of the Cu/CeO2 catalyst during methanol steam reforming[J]. Catalysis Science & Technology, 2022, 12(23): 7003-7009. |
| 55 | NING Jing, ZHOU Yan, SHEN Wenjie. Atomically dispersed copper species on ceria for the low-temperature water-gas shift reaction[J]. Science China Chemistry, 2021, 64(7): 1103-1110. |
| 56 | LYKHACH Yaroslava, KOZLOV Sergey M, Tomáš SKÁLA, et al. Counting electrons on supported nanoparticles[J]. Nature Materials, 2016, 15(3): 284-288. |
| 57 | ARAIZA Daniel G, Antonio GÓMEZ-CORTÉS, Gabriela DÍAZ. Reactivity of methanol over copper supported on well-shaped CeO2: A TPD-DRIFTS study[J]. Catalysis Science & Technology, 2017, 7(22): 5224-5235. |
| 58 | CHEN Fei, ZHANG Peipei, ZENG Yan, et al. Vapor-phase low-temperature methanol synthesis from CO2-containing syngas via self-catalysis of methanol and Cu/ZnO catalysts prepared by solid-state method[J]. Applied Catalysis B: Environmental, 2020, 279: 119382. |
| 59 | PLONER Kevin, WATSCHINGER Maximilian, NEZHAD Parastoo Delir Kheyrollahi, et al. Mechanistic insights into the catalytic methanol steam reforming performance of Cu/ZrO2 catalysts by in situ and operando studies[J]. Journal of Catalysis, 2020, 391: 497-512. |
| 60 | PU Yunchuan, LI Shuirong, YAN Shuai, et al. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming[J]. Fuel, 2019, 241: 607-615. |
| 61 | MATEOS-PEDRERO C, AZENHA C, D-A Pacheco Tanaka, et al. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming[J]. Applied Catalysis B: Environmental, 2020, 277: 119243. |
| 62 | LU Jichang, LEI Yanqiu, WAN Gengping, et al. Weakening the metal-support strong interaction to enhance catalytic performances of alumina supported Ni-based catalysts for producing hydrogen[J]. Applied Catalysis B: Environmental, 2020, 263: 118177. |
| 63 | SUN Zhao, LIU Junpeng, ZHANG Rongjun, et al. Fabricating Ga doped and MgO embedded nanomaterials for sorption-enhanced steam reforming of methanol[J]. Journal of Materials Chemistry A, 2022, 10(13): 7300-7313. |
| 64 | WANG Lucun, LIU Qian, CHEN Miao, et al. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique[J]. The Journal of Physical Chemistry C, 2007, 111(44): 16549-16557. |
| 65 | RHODES Michael D, Bell Alexis T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies[J]. Journal of Catalysis, 2005, 233(1): 198-209. |
| 66 | RHODEs Michael D, Pokrovski Konstantin A, Bell Alexis T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part Ⅱ. Transient-response infrared studies[J]. Journal of Catalysis, 2005, 233(1): 210-220. |
| 67 | PLONER Kevin, NEZHAD Parastoo Delir Kheyrollahi, GILI Albert, et al. The sol-gel autocombustion as a route towards highly CO2-selective, active and long-term stable Cu/ZrO2 methanol steam reforming catalysts[J]. Materials Chemistry Frontiers, 2021, 5(13): 5093-5105. |
| 68 | Eva-Maria KÖCK, KOGLER Michaela, BIELZ Thomas, et al. In situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3, ZrO2, and yttria-stabilized ZrO2 [J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2013, 117(34): 17666-17673. |
| 69 | Eva-Maria KÖCK, KOGLER Michaela, Bernhard KLÖTZER, et al. Structural and electrochemical properties of physisorbed and chemisorbed water layers on the ceramic oxides Y2O3, YSZ, and ZrO2 [J]. ACS Applied Materials & Interfaces, 2016, 8(25): 16428-16443. |
| 70 | Eva-Maria KÖCK, KOGLER Michaela, Thomas GÖTSCH, et al. Surface chemistry of pure tetragonal ZrO2 and gas-phase dependence of the tetragonal-to-monoclinic ZrO2 transformation[J]. Dalton Transactions, 2017, 46(14): 4554-4570. |
| 71 | HENDERSON Michael A. Complexity in the decomposition of formic acid on the TiO2(110) surface[J]. The Journal of Physical Chemistry B, 1997, 101(2): 221-229. |
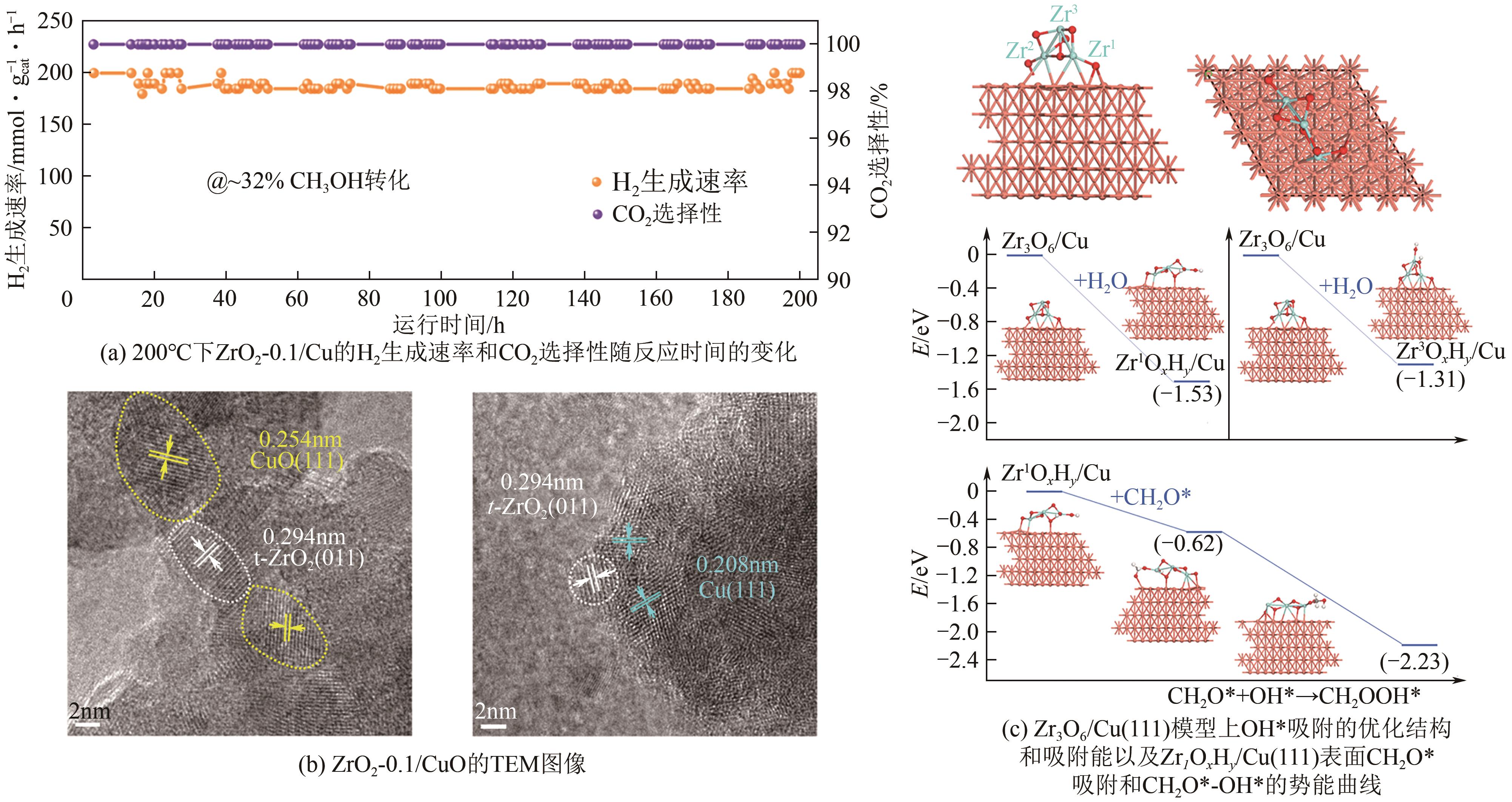
| 72 | XU Xinyi, LAN Tian, ZHAO Guofeng, et al. Interface-hydroxyl enabling methanol steam reforming toward CO-free hydrogen production over inverse ZrO2/Cu catalyst[J]. Applied Catalysis B: Environmental, 2023, 334: 122839. |
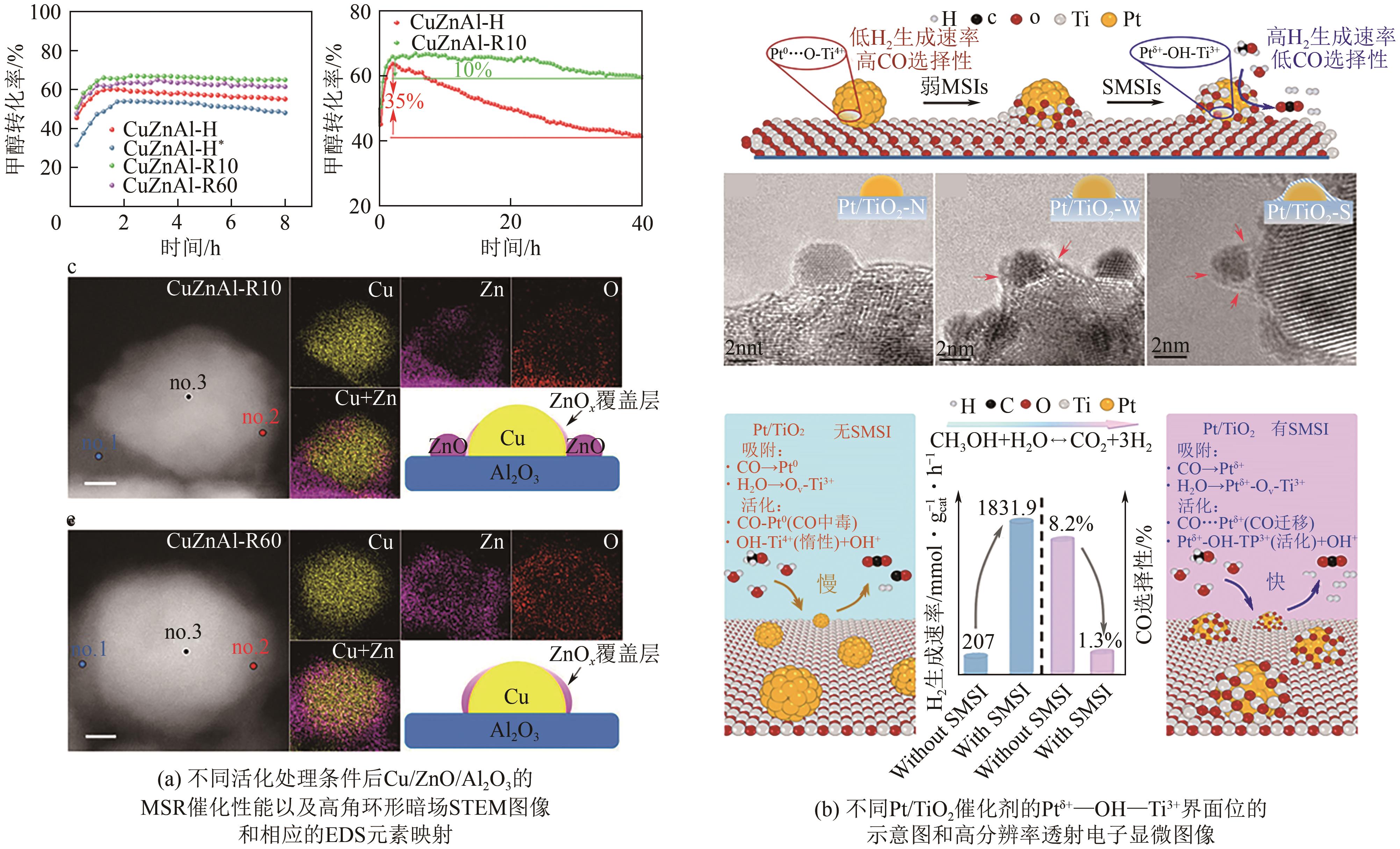
| 73 | LI Didi, XU Fang, TANG Xuan, et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol[J]. Nature Catalysis, 2022, 5(2): 99-108. |
| 74 | JIN Shiqing, ZHANG Zekai, LI Didi, et al. Alcohol-induced strong metal-support interactions in a supported copper/ZnO catalyst[J]. Angewandte Chemie International Edition, 2023, 62(21): e202301563. |
| 75 | WANG Hai, HUI Yu, NIU Yiming, et al. Construction of Ptδ+-O(H)-Ti3+ species for efficient catalytic production of hydrogen[J]. ACS Catalysis, 2023, 13(15): 10500-10510. |
| 76 | TABAKOVA T, IDAKIEV V, AVGOUROPOULOS G, et al. Highly active copper catalyst for low-temperature water-gas shift reaction prepared via a Cu-Mn spinel oxide precursor[J]. Applied Catalysis A: General, 2013, 451: 184-191. |
| 77 | KIM Nam Dong, PARK Jae Ryul, PARK Dae Sung, et al. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1, 2-propanediol[J]. Green Chemistry, 2012, 14(9): 2638-2646. |
| 78 | 李光俊, 郗宏娟, 张素红, 等. 尖晶石CuM2O4(M=Al、Fe、Cr)催化甲醇重整反应的特性[J]. 燃料化学学报, 2012, 40(12): 1466-1471. |
| LI Guangjun, XI Hongjuan, ZHANG Suhong, et al. Catalytic characteristics of spinel CuM2O4(M=Al, Fe, Cr) for the steam reforming of methanol[J]. Journal of Fuel Chemistry and Technology, 2012, 40(12): 1466-1471. | |
| 79 | HUANG Yung-Han, WANG Sea-Fue, TSAI An-Pang, et al. Reduction behaviors and catalytic properties for methanol steam reforming of Cu-based spinel compounds CuX2O4 (X=Fe, Mn, Al, La)[J]. Ceramics International, 2014, 40(3): 4541-4551. |
| 80 | LIU Yajie, QIN Fajie, HOU Xiaoning, et al. Effects of ball milling medium on Cu-Al spinel sustained release catalyst for H2 generation from methanol steam reforming[J]. Journal of Fuel Chemistry and Technology, 2023, 51(5): 665-671. |
| 81 | XI Hongjuan, HOU Xiaoning, LIU Yajie, et al. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angewandte Chemie International Edition, 2014, 53(44): 11886-11889. |
| 82 | LIU Yajie, QING Shaojun, HOU Xiaoning, et al. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catalysis Science & Technology, 2017, 7(21): 5069-5078. |
| 83 | QING Shaojun, HOU Xiaoning, LIU Yajie, et al. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming[J]. Chemical Communications, 2018, 54(86): 12242-12245. |
| 84 | LIU Yajie, QING Shaojun, HOU Xiaoning, et al. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming[J]. ChemCatChem, 2018, 10(24): 5698-5706. |
| 85 | HOU Xiaoning, QING Shaojun, LIU Yajie, et al. Cu1- x Mg x Al3 spinel solid solution as a sustained release catalyst: One-pot green synthesis and catalytic performance in methanol steam reforming[J]. Fuel, 2021, 284: 119041. |
| 86 | HOU Xiaoning, QING Shaojun, LIU Yajie, et al. Enhancing effect of MgO modification of Cu-Al spinel oxide catalyst for methanol steam reforming[J]. International Journal of Hydrogen Energy, 2020, 45(1): 477-489. |
| 87 | LIU Yajie, KANG Hefei, HOU Xiaoning, et al. Sustained release catalysis: Dynamic copper releasing from stoichiometric spinel CuAl2O4 during methanol steam reforming[J]. Applied Catalysis B: Environmental, 2023, 323: 122043. |
| [1] | 陈良, 罗冬梅, 王正豪, 钟山, 唐思扬, 梁斌. 工业副产气化学链回收氢气技术研究进展[J]. 化工进展, 2024, 43(7): 3729-3746. |
| [2] | 王嘉锐, 刘大伟, 邓耀, 徐瑾, 马晓迅, 徐龙. 载氧体在甲烷化学链重整反应中的研究进展[J]. 化工进展, 2024, 43(5): 2235-2253. |
| [3] | 赵伟, 江雨寒, 李振, 李毅红, 周安宁, 王宏. 煤岩显微组分电浮选分离与制氢过程中氢/氧气泡的影响机制[J]. 化工进展, 2024, 43(5): 2428-2435. |
| [4] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [5] | 曾悦, 王月, 张学瑞, 宋玺文, 夏博文, 陈梓颀. 可再生能源合成绿氨研究进展及氢-氨储运经济性分析[J]. 化工进展, 2024, 43(1): 376-389. |
| [6] | 孙崇正, 李玉星, 许洁, 韩辉, 宋光春, 卢晓. 浮式氢能储运过程中FLH2通道管外降膜流动的海上适应性强化机理[J]. 化工进展, 2024, 43(1): 338-352. |
| [7] | 孙崇正, 樊欣, 李玉星, 许洁, 韩辉, 刘亮. 海上多孔介质通道内氢气换热与正仲氢转化的耦合特性[J]. 化工进展, 2023, 42(3): 1281-1290. |
| [8] | 张巍, 王锐, 缪平, 田戈. 全球可再生能源电转甲烷的应用[J]. 化工进展, 2023, 42(3): 1257-1269. |
| [9] | 杨程瑞雪, 黄琪媛, 冉建速, 崔耘通, 王健健. 磷酸修饰二氧化硅负载钯催化剂用于木质素衍生物高效水相低温加氢脱氧[J]. 化工进展, 2023, 42(10): 5179-5190. |
| [10] | 刘艳辉, 周明芳, 马铭, 王凯, 谭天伟. 可再生能源驱动的生物催化固定CO2的研究进展[J]. 化工进展, 2023, 42(1): 1-15. |
| [11] | 王红霞, 徐婉怡, 张早校. 可再生电力电解制绿色氢能的发展现状与建议[J]. 化工进展, 2022, 41(S1): 118-131. |
| [12] | 胡兵, 徐立军, 何山, 苏昕, 汪继伟. 碳达峰与碳中和目标下PEM电解水制氢研究进展[J]. 化工进展, 2022, 41(9): 4595-4604. |
| [13] | 陈波, 刘爱贤, 孙强, 王逸伟, 郭绪强, 杨庆伟, 龙有, 肖树萌, 马绍坤. 柴油加氢尾气中氢气的水合物法回收工业侧线试验[J]. 化工进展, 2022, 41(6): 2924-2930. |
| [14] | 朱庆山. 超低碳炼铁技术路径分析[J]. 化工进展, 2022, 41(3): 1391-1398. |
| [15] | 苗青青, 石春艳, 张香平. 碳中和目标下的光伏发电技术[J]. 化工进展, 2022, 41(3): 1125-1131. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||