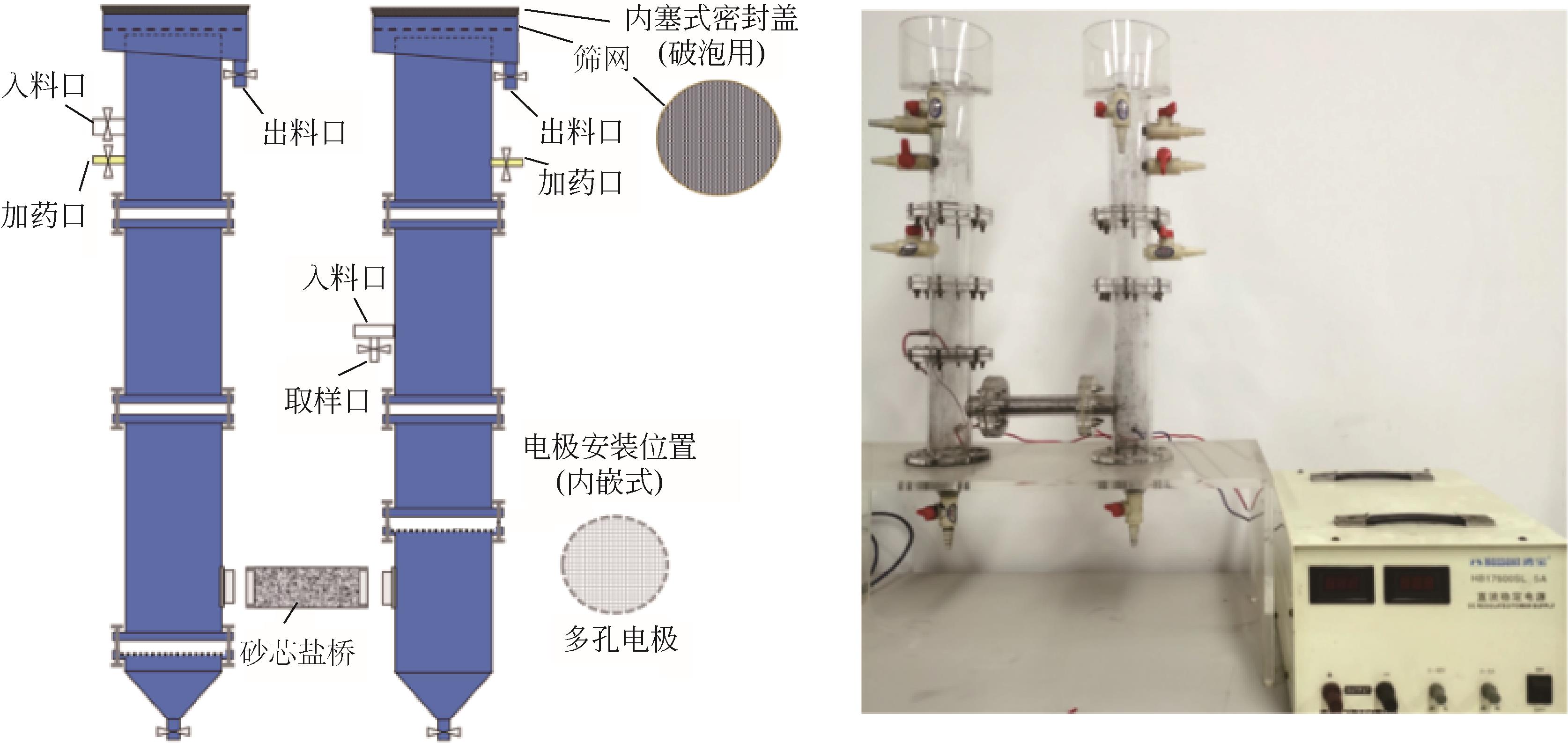
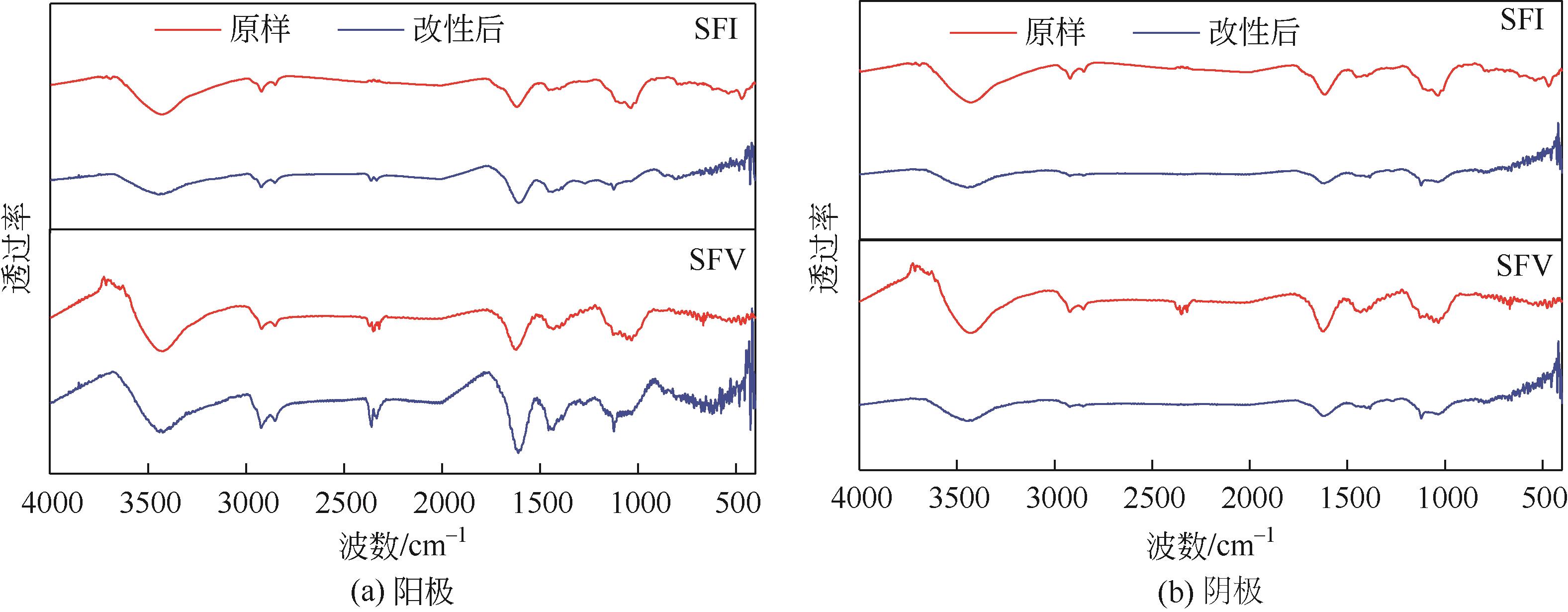
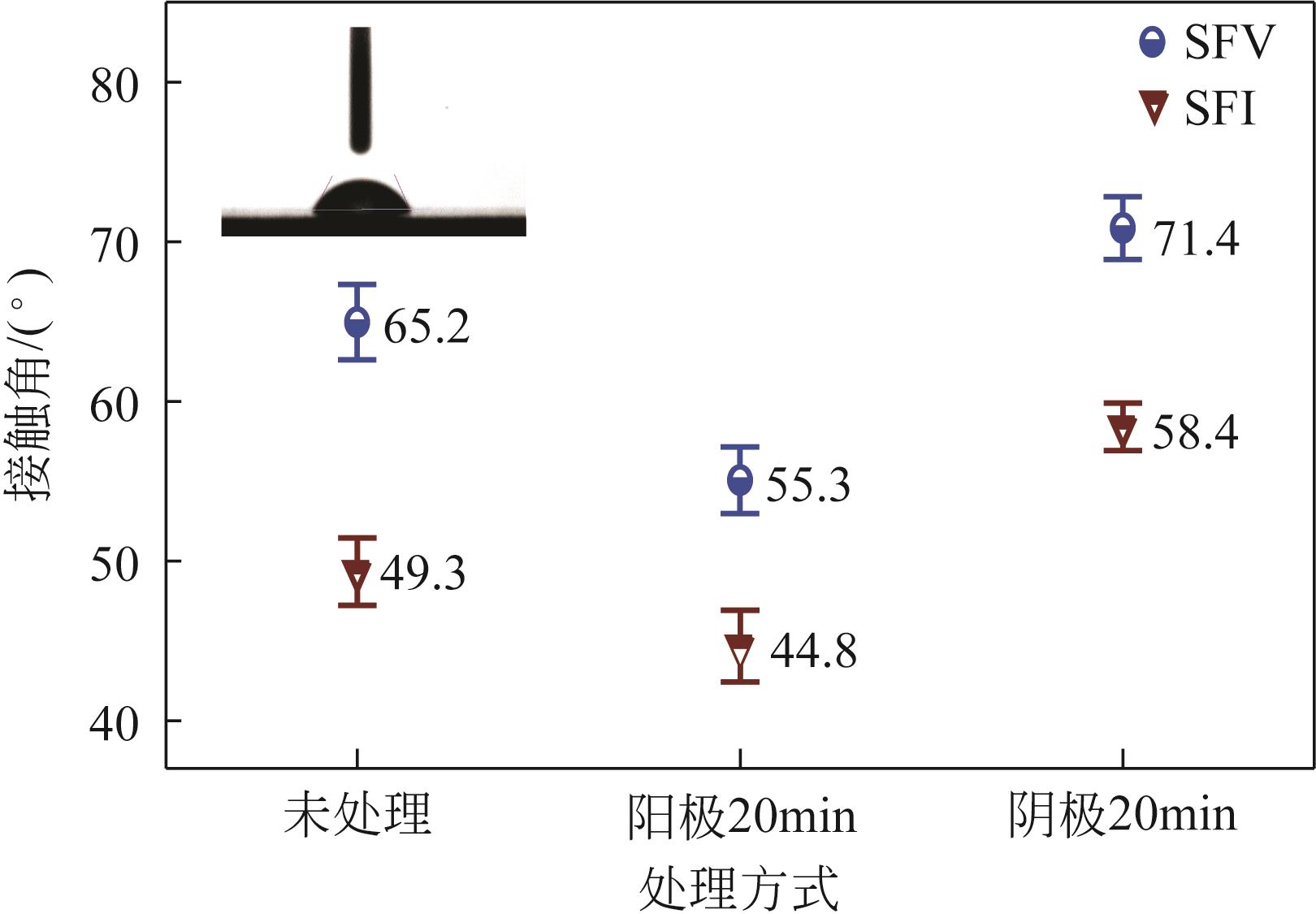
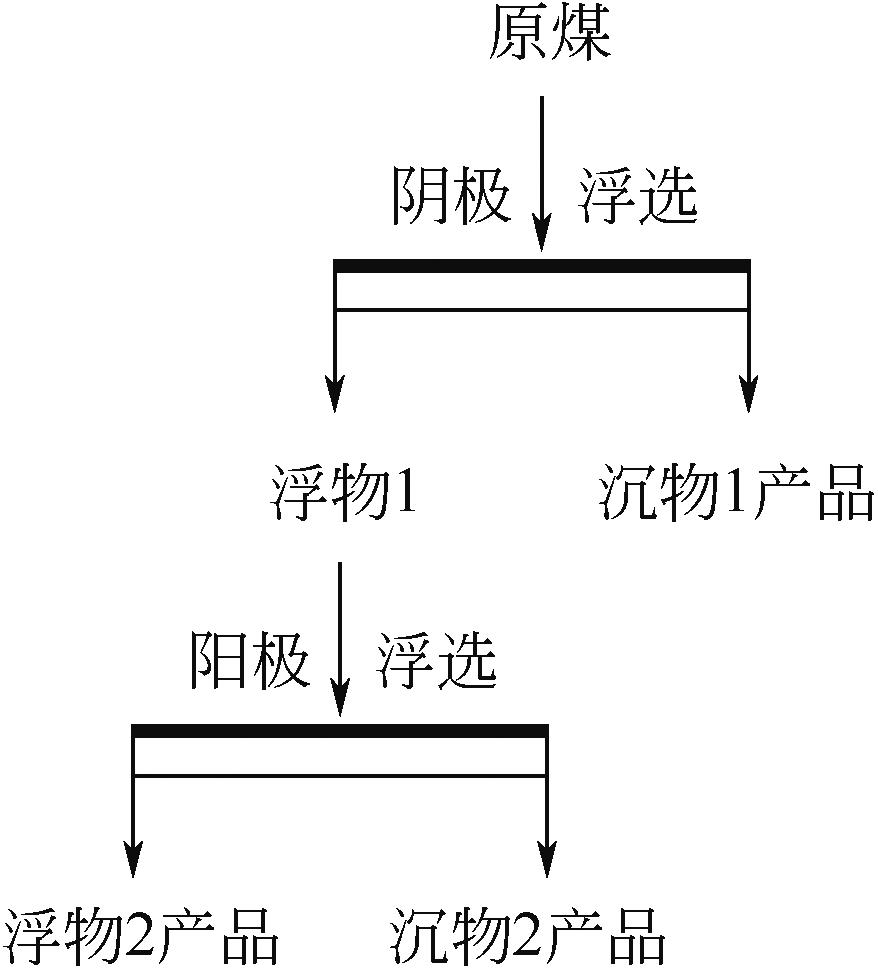
| 1 |
韩德馨. 中国煤岩学[M]. 徐州: 中国矿业大学出版社, 1996.
|
|
HAN Dexin. Coal petrolgy of China[M]. Xuzhou: China University of Mining & Technology Press, 1996.
|
| 2 |
杜芳鹏, 雒铮, 乔军伟, 等. 宁东煤田侏罗纪煤岩煤质特征及清洁高效利用[J]. 煤田地质与勘探, 2020, 48(2): 71-77.
|
|
DU Fangpeng, LUO Zheng, QIAO Junwei, et al. Petrographic, quality characteristics and clean & efficient use of Jurassic coal in Ningdong Coalfield [J]. Coal Geology & Exploration, 2020, 48(2): 71-77.
|
| 3 |
刘颖洲. 电解气泡特性及对煤泥浮选效果影响的研究[D]. 太原: 太原理工大学, 2014.
|
|
LIU Yingzhou. The research of electrolytic bubble characteristics and its effect on slime flotation[D]. Taiyuan: Taiyuan University of Technology, 2014.
|
| 4 |
汪朝晖, 廖振方, 陈德淑. 电浮选过程中气泡行为的研究[J]. 中南大学学报(自然科学版), 2011, 42(3): 658-663.
|
|
WANG Zhaohui, LIAO Zhenfang, CHEN Deshu. Bubble behavior of electro-flotation[J]. Journal of Central South University (Science and Technology), 2011, 42(3): 658-663.
|
| 5 |
PUTRA R S, AIRUN N H. The effect of particle size and dosage on the performance of Papaya seeds (Carica papaya) as biocoagulant on wastewater treatment of batik industry[J]. IOP Conference Series: Materials Science and Engineering, 2021, 1087(1): 012045.
|
| 6 |
TADESSE Bogale, ALBIJANIC Boris, MAKUEI Fidele, et al. Recovery of fine and ultrafine mineral particles by electroflotation—A review[J]. Mineral Processing & Extractive Metallurgy Review, 2018, 5: 108-122.
|
| 7 |
张洲朋, 赵伟, 周安宁, 等 .多孔电极的制备及其在煤岩显微组分电浮选分离中的应用[J]. 中国矿业大学学报, 2023,52(1): 188-198.
|
|
ZHANG Zhoupeng, ZHAO Wei, ZHOU Anning, et al. Preparation of porous electrode and its application in electroflotation separation of coal macerals[J]. Journal of China University of Mining & Technology, 2023,52(1): 188-198.
|
| 8 |
赵伟, 李振, 周安宁, 等. 铝电极电浮选阴极的气泡特征及其影响因素研究[J]. 矿产保护与利用, 2018(3): 87-92.
|
|
ZHAO Wei, LI Zhen, ZHOU Anning, et al. Bubble characteristics and its influence factors in electroflotation with aluminum cathode[J]. Conservation and Utilization of Mineral Resources, 2018(3): 87-92.
|
| 9 |
赵伟, 李振, 杨志远, 等. 煤岩显微组分的电浮选分离及其电化学凝聚特征研究[J]. 中国矿业大学学报, 2018, 47(5): 1104-1112.
|
|
ZHAO Wei, LI Zhen, YANG Zhiyuan, et al. Electroflotation separation of coal macerals and its electro-coagulation characteristics[J]. Journal of China University of Mining & Technology, 2018, 47(5): 1104-1112.
|
| 10 |
徐宏刚. 多孔铜材料的制备工艺研究[D]. 西安: 西安建筑科技大学, 2020.
|
|
XU Honggang. Study on preparation technology of porous copper material[D]. Xi’an: Xi’an University of Architecture and Technology, 2020.
|
| 11 |
庞志成, 罗震宁. 碱性电解水制氢镍合金阴极材料的研究进展[J]. 能源技术, 2004, 25(1): 19-21, 26.
|
|
PANG Zhicheng, LUO Zhenning. Recent development of Ni-base alloy as a cathode materials for the production of hydrogen by alkaline water electrolysis[J]. Energy Technology, 2004, 25(1): 19-21, 26.
|
| 12 |
KYZAS George Z, MATIS Kostas A. Electroflotation process: A review[J]. Journal of Molecular Liquids, 2016, 220: 657-664.
|
| 13 |
LLERENA C, HO J C K, PIRON D L. Effects of pH on electroflotation of sphalerite[J]. Chemical Engineering Communications, 2007, 155(1): 217-228.
|
| 14 |
ABDUL RAHMAN N, ALBANIA LINUS A, PHILIP A, et al. Development of solar power system for Sarawak peat water continuous electrocoagulation treatment process[J]. IOP Conference Series: Materials Science and Engineering, 2021, 1101(1): 012039.
|
| 15 |
LUMANAUW Daniel. Hydrogen bubble characterization in alkaline water electrolysis[D]. Toronto: Department of Metallurgy and Material Science, University of Toronto, 2000.
|
| 16 |
印万忠, 田道来, 谢禹, 等. 浮选过程诱导时间研究进展[J]. 矿产保护与利用,2023,43(3): 1-9.
|
|
YIN Wanzhong, TIAN Daolai, XIE Yu, et al. Research progress of induction time in flotation process[J]. Conservation and Utilization of Mineral Resources, 2023,43(3): 1-9.
|
| 17 |
赵伟, 杨志远, 李振, 等. 电化学处理对神木煤显微组分表面结构及可浮性的影响研究[J]. 燃料化学学报, 2017, 45(4): 400-407.
|
|
ZHAO Wei, YANG Zhiyuan, LI Zhen, et al. Influence of electrochemical treatment on surface structure and flotability of Shenmu coal macerals[J]. Journal of Fuel Chemistry and Technology, 2017, 45(4): 400-407.
|
| 18 |
村田逞诠. 煤的润湿性研究及其应用[M]. 朱春笙, 龚祯祥, 译. 北京: 煤炭工业出版社, 1992.
|
|
MURATA Yuzuru. Research on the wettability of coal and its application[M]. ZHU Chunsheng, GONG Zhenxiang, trans. Beijing: China Coal Industry Publishing House, 1992.
|
| 19 |
CHEN Cong, LIU Jianzhong, WANG Jianbin, et al. Effect of acid concentration on organic structure and small molecule stripping of coal during acid treatment and electrolysis for hydrogen production[J]. Fuel, 2023, 346: 128313.
|
| 20 |
龙江. 神府煤煤岩组分赋存特性及浮选富集工艺研究[D]. 西安: 西安科技大学, 2014.
|
|
LONG Jiang. Study on occurrence characteristics and enrichment by flotation separation of Shenfu macerals[D]. Xi’an: Xi’an University of Science and Technology, 2014.
|
| 21 |
朱凌岳, 王宝辉, 吴红军. 电解水煤浆制氢技术研究进展[J]. 化工进展, 2016, 35(10): 3129-3135.
|
|
ZHU Lingyue, WANG Baohui, WU Hongjun. Review on electrochemical splitting of coal water slurry for hydrogen[J]. Chemical Industry and Engineering Progress, 2016, 35(10): 3129-3135.
|
| 22 |
白清城. 煤浆与废液协同电解制氢的研究[D]. 杭州: 浙江大学, 2020.
|
|
BAI Qingcheng. Study on hydrogen production by synergistic electrolysis of coal slurry and wastewater[D]. Hangzhou: Zhejiang University, 2020.
|
 ), 江雨寒1, 李振1,2, 李毅红3, 周安宁1,2(
), 江雨寒1, 李振1,2, 李毅红3, 周安宁1,2( ), 王宏1
), 王宏1
 ), JIANG Yuhan1, LI Zhen1,2, LI Yihong3, ZHOU Anning1,2(
), JIANG Yuhan1, LI Zhen1,2, LI Yihong3, ZHOU Anning1,2( ), WANG Hong1
), WANG Hong1