化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4630-4641.DOI: 10.16085/j.issn.1000-6613.2023-1036
• 资源与环境化工 • 上一篇
钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理
刘玉灿1( ), 高中鲁1, 徐心怡1, 纪现国1, 张岩1, 孙洪伟2, 王港2(
), 高中鲁1, 徐心怡1, 纪现国1, 张岩1, 孙洪伟2, 王港2( )
)
- 1.烟台大学土木工程学院,山东 烟台 264005
2.烟台大学环境与材料工程学院,山东 烟台 264005
-
收稿日期:2023-06-25修回日期:2023-08-17出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:刘玉灿,王港 -
作者简介:刘玉灿(1986—),男,博士,副教授,研究方向为水处理理论与技术。E-mail:liuyucan@ytu.edu.cn。 -
基金资助:山东省自然科学基金(ZR2021ME119);西南林业大学生物质材料国际联合研究中心开放基金(2023-GH04);山东省水土保持与环境保育重点实验室项目(STKF202311);烟台大学科技项目(TM17B19)
Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar
LIU Yucan1( ), GAO Zhonglu1, XU Xinyi1, JI Xianguo1, ZHANG Yan1, SUN Hongwei2, WANG Gang2(
), GAO Zhonglu1, XU Xinyi1, JI Xianguo1, ZHANG Yan1, SUN Hongwei2, WANG Gang2( )
)
- 1.School of Civil Engineering, Yantai University, Yantai 264005, Shandong, China
2.School of Environmental and Material Engineering, Yantai University, Yantai 264005, Shandong, China
-
Received:2023-06-25Revised:2023-08-17Online:2024-08-15Published:2024-09-02 -
Contact:LIU Yucan, WANG Gang
摘要:
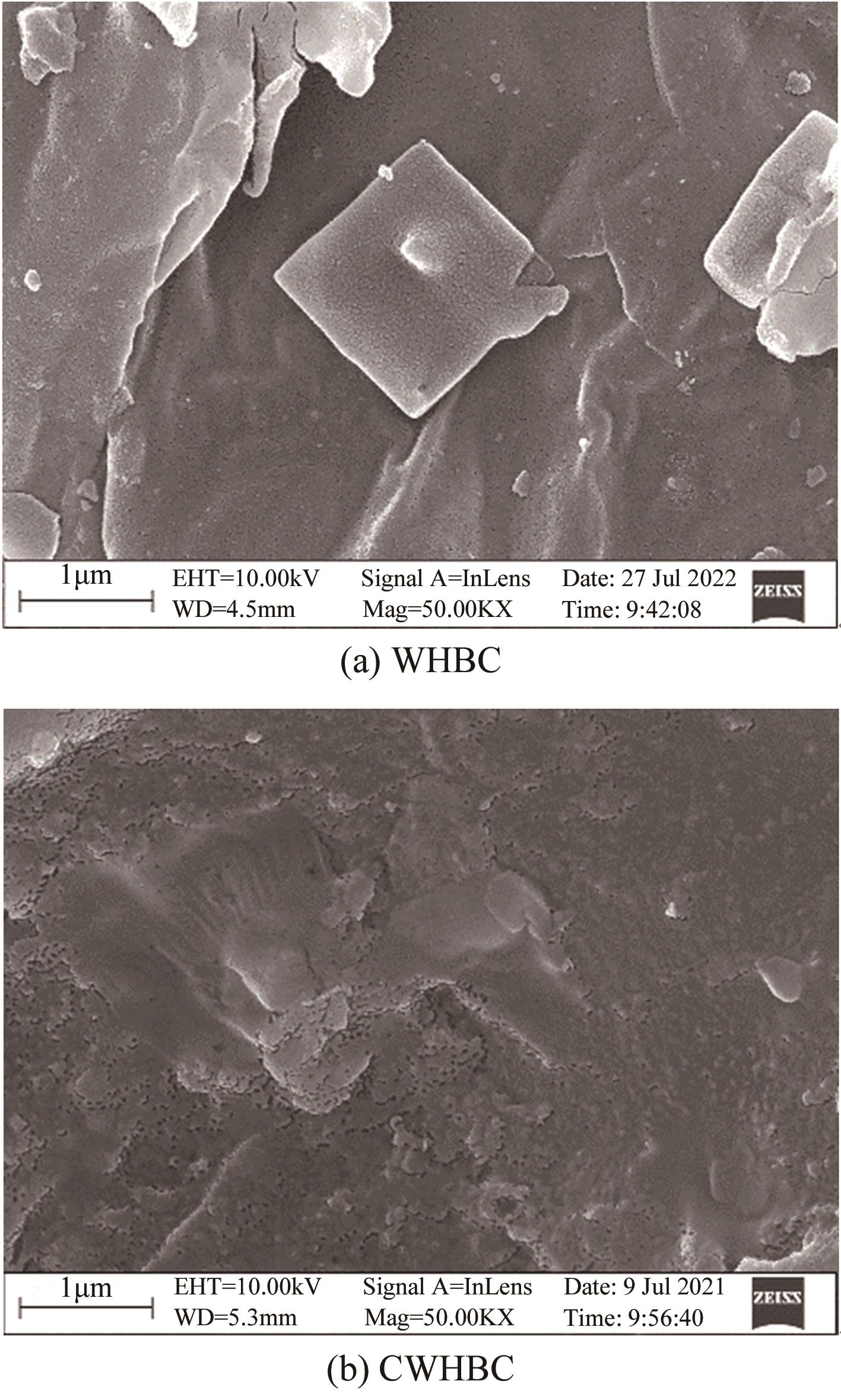
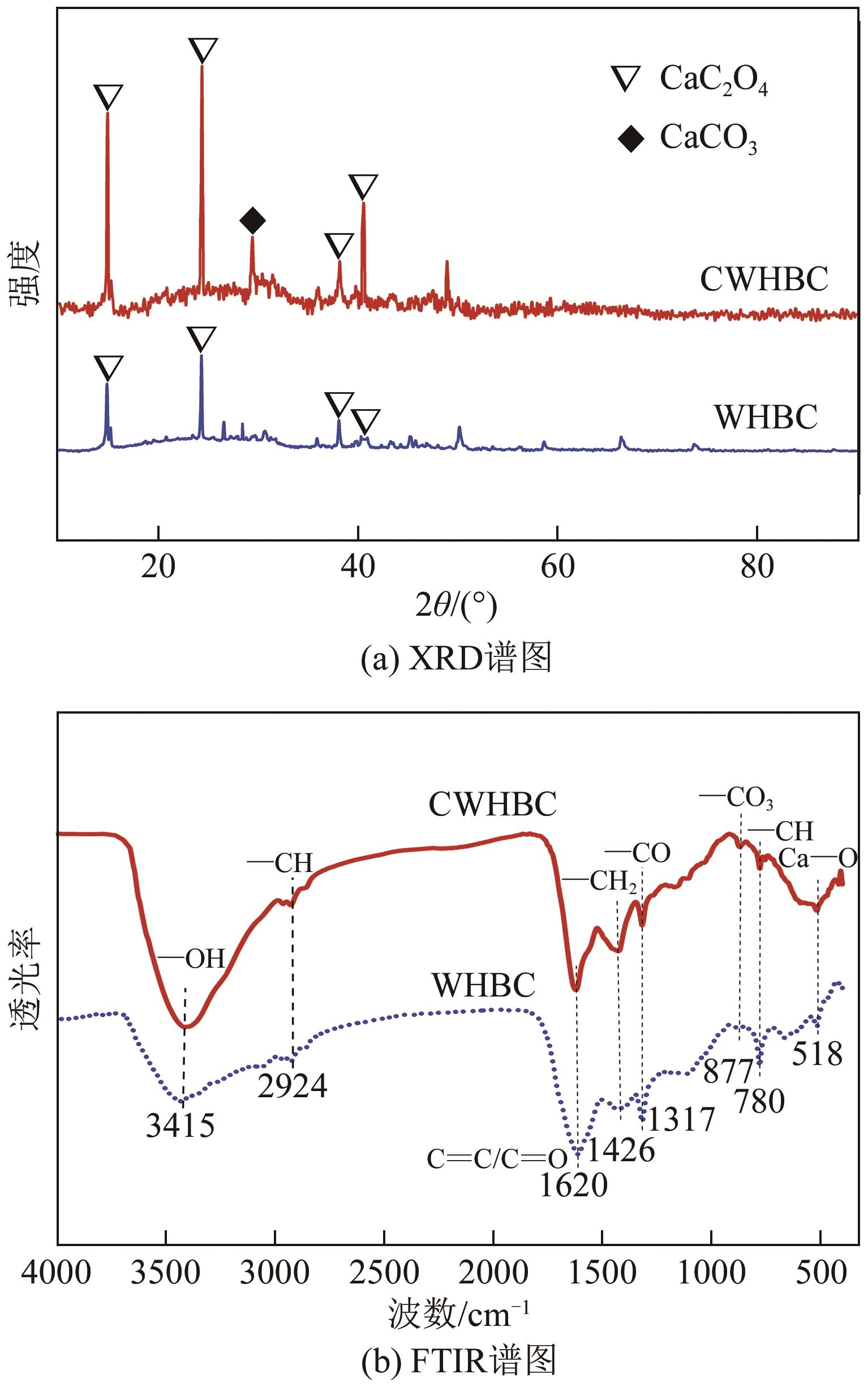
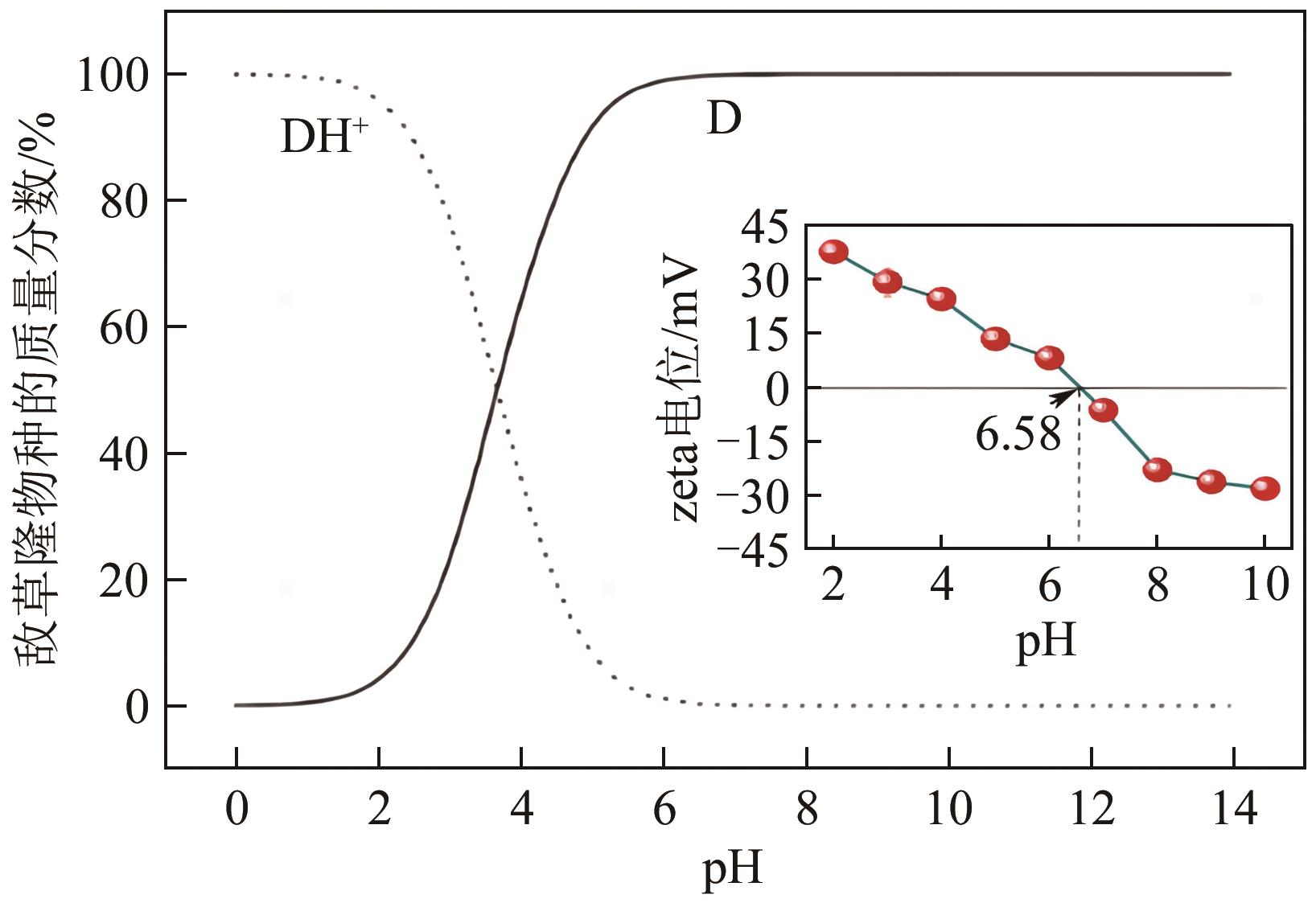
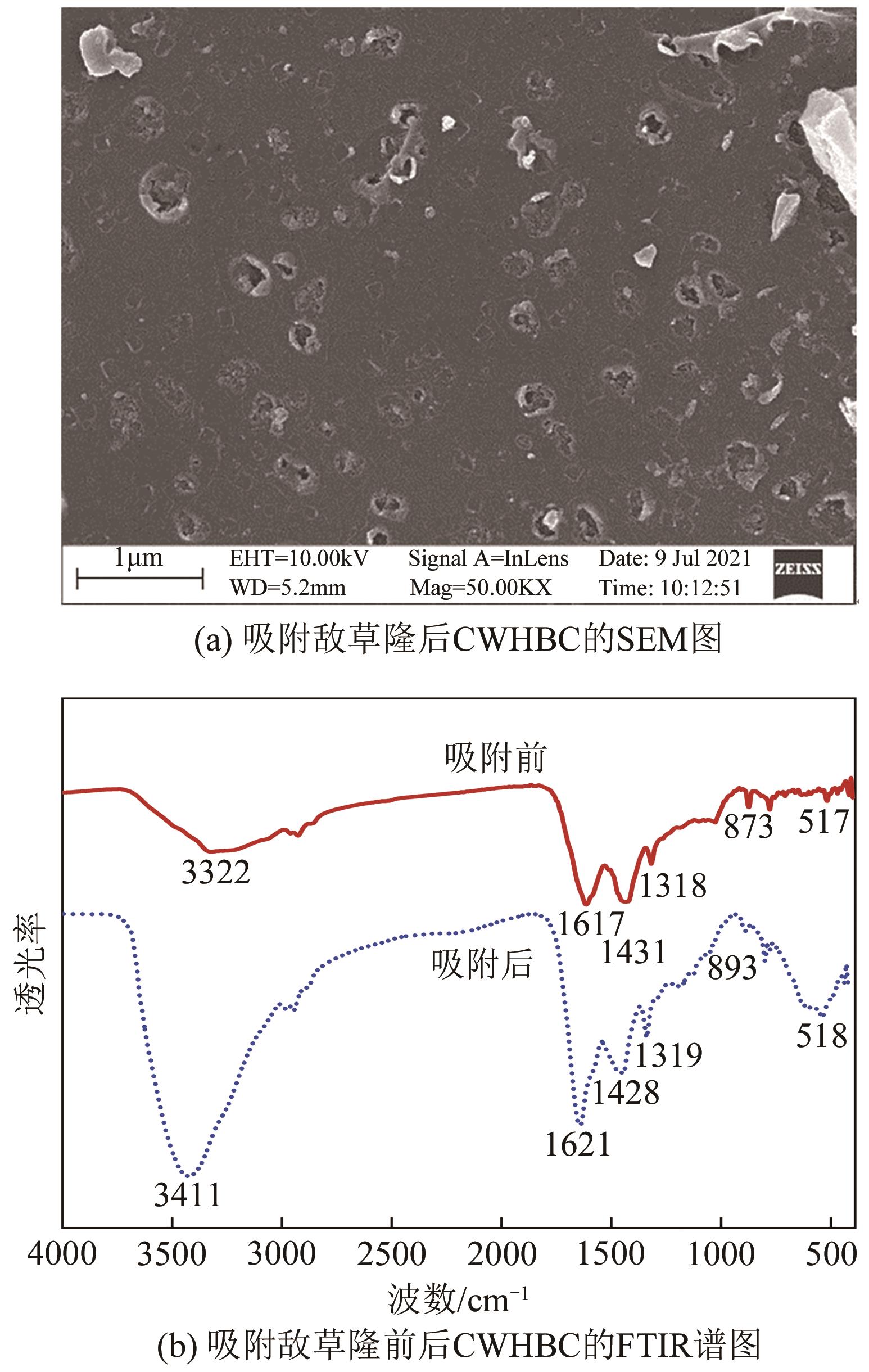
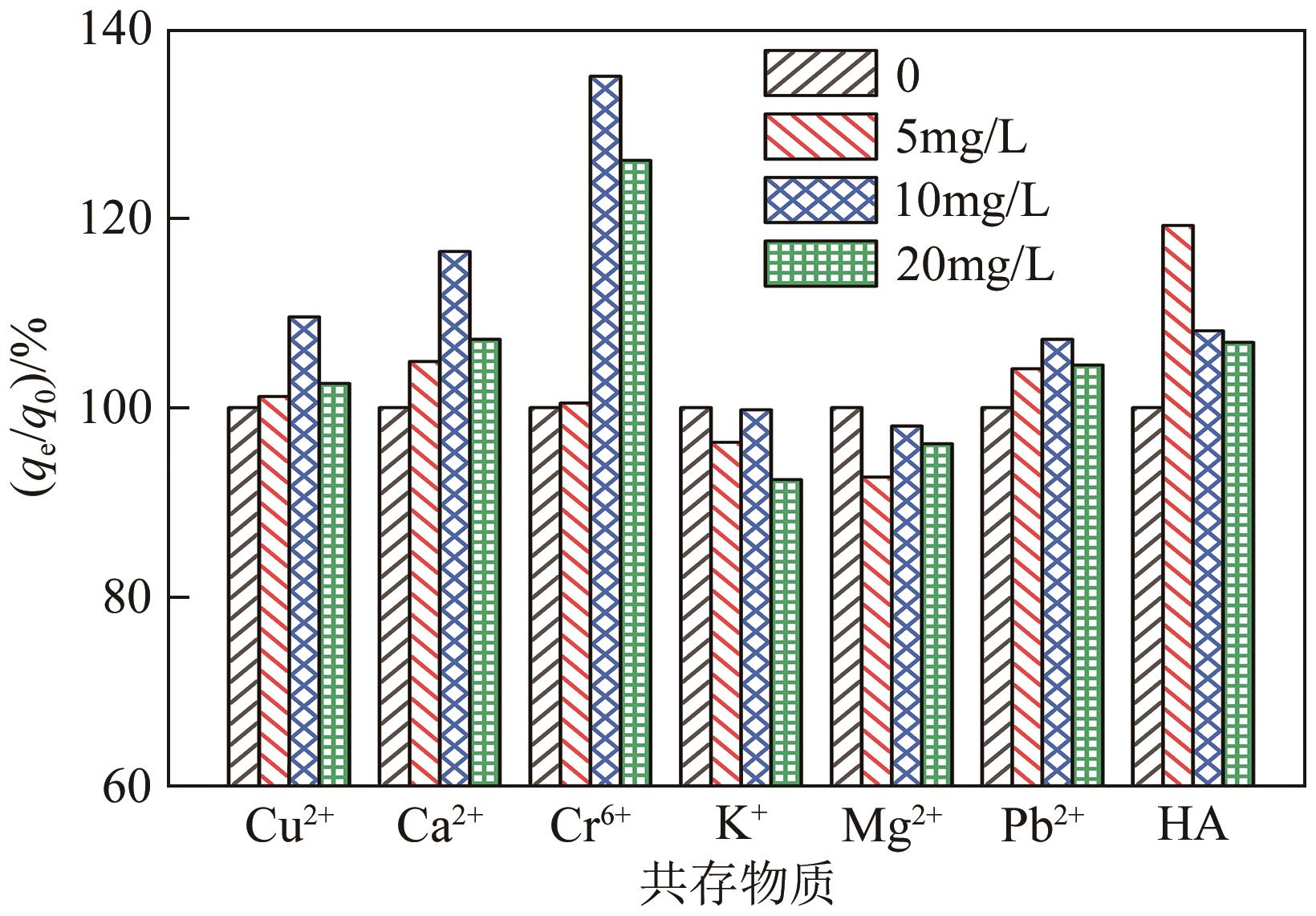
以水葫芦为原料、CaCl2为改性剂,通过一步热解法制备了钙改性水葫芦基生物炭(CWHBC),基于表征技术分析了其表面形貌、比表面积、孔径分布、官能团组成等物化性能,并探究了其吸附去除水中敌草隆的效能与机理。结果表明,CWHBC比未改性生物炭(WHBC)具有更大的比表面积、更丰富的孔隙结构、更多的含氧官能团、更强的亲水性,这些物化性能的改变增强了生物炭的吸附能力。CWHBC对水中敌草隆的吸附符合准二级吸附动力学模型和Langmuir吸附等温线模型,表明该吸附以单层化学吸附为主,主要吸附机理为氢键作用、π-π作用和表面络合。单因素试验结果表明,CWHBC在各种条件下均具有良好的吸附性能,采用0.2mol/L HCl对其进行5次吸附/解吸循环后的吸附容量仍高达初次吸附容量的94.62%。因此,使用一步热解法制备的CWHBC可有效去除水中敌草隆,且具有较好的环境适应能力和重复使用性能。该研究提供了一种低成本、高效的吸附材料,能有效实现水葫芦的资源化利用,具有良好的工程应用前景与潜力。
中图分类号:
引用本文
刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641.
LIU Yucan, GAO Zhonglu, XU Xinyi, JI Xianguo, ZHANG Yan, SUN Hongwei, WANG Gang. Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4630-4641.
| 生物炭 | C | O | N | P | Si | Cl | Ca | O/C | (N+O)/C |
|---|---|---|---|---|---|---|---|---|---|
| WHBC | 66.17 | 9.60 | 1.70 | 4.23 | 0.66 | 15.4 | 1.73 | 0.145 | 0.171 |
| CWHBC | 40.10 | 21.66 | 1.31 | 1.04 | 0.01 | 14.85 | 20.43 | 0.540 | 0.572 |
表1 WHBC和CWHBC的元素组成 (质量分数/%)
| 生物炭 | C | O | N | P | Si | Cl | Ca | O/C | (N+O)/C |
|---|---|---|---|---|---|---|---|---|---|
| WHBC | 66.17 | 9.60 | 1.70 | 4.23 | 0.66 | 15.4 | 1.73 | 0.145 | 0.171 |
| CWHBC | 40.10 | 21.66 | 1.31 | 1.04 | 0.01 | 14.85 | 20.43 | 0.540 | 0.572 |
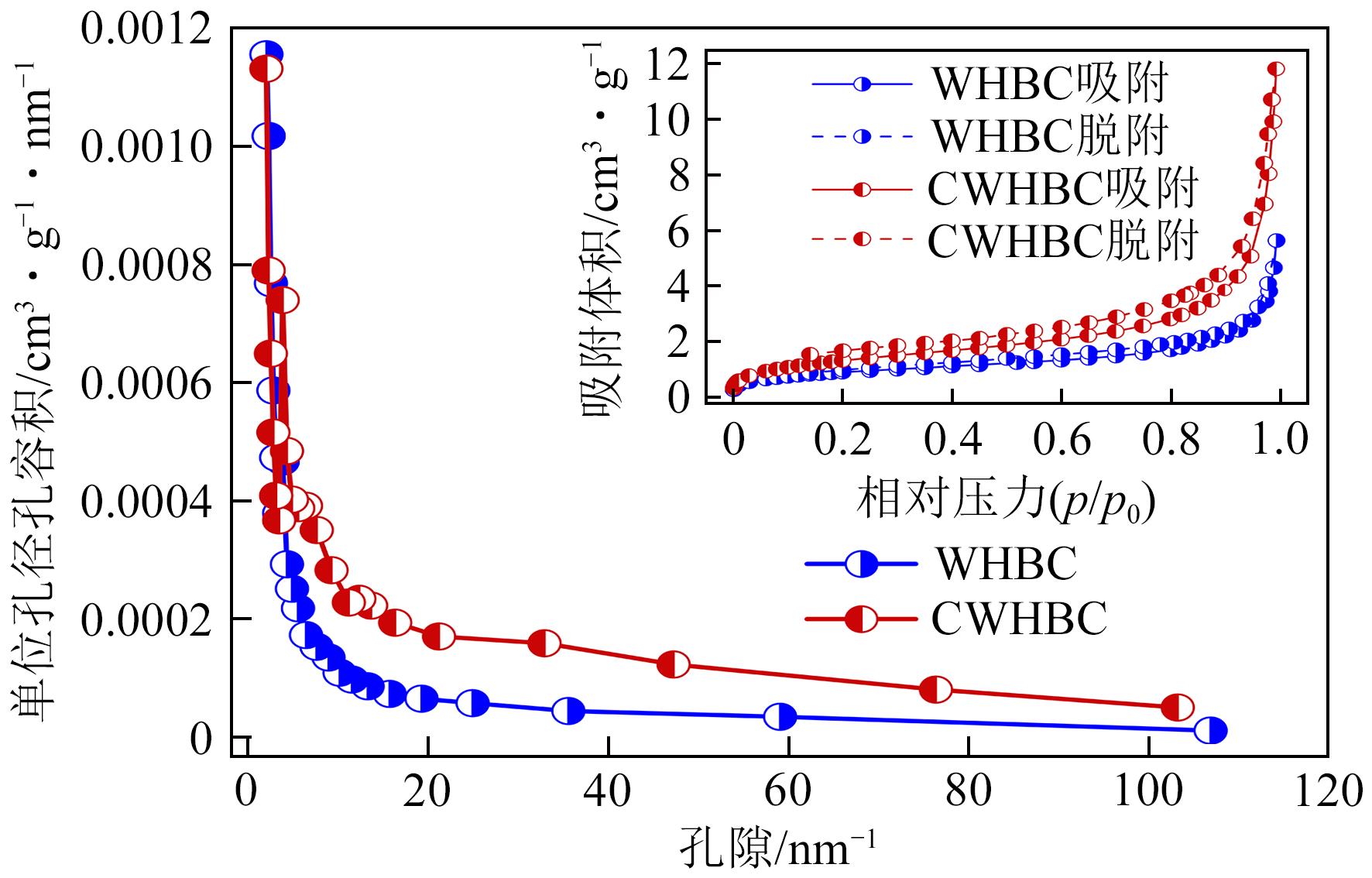
| 生物炭 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| WHBC | 3.30 | 0.00430 | 5.22 |
| CWHBC | 5.29 | 0.00812 | 6.63 |
表2 WHBC和CWHBC的比表面积、孔容和平均孔径
| 生物炭 | 比表面积/m2·g-1 | 总孔体积/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| WHBC | 3.30 | 0.00430 | 5.22 |
| CWHBC | 5.29 | 0.00812 | 6.63 |
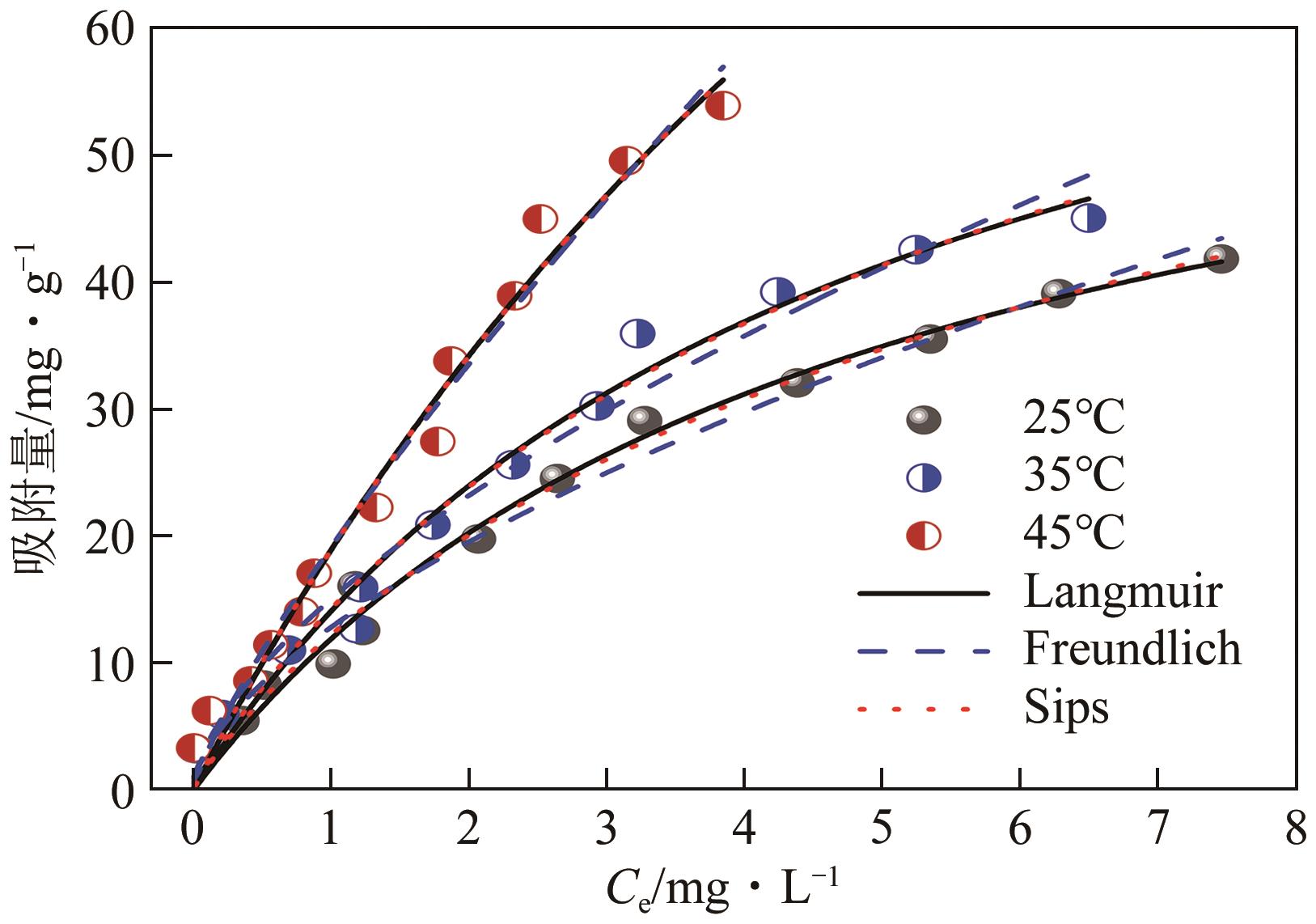
| 参数 | 25℃ | 35℃ | 45℃ |
|---|---|---|---|
| Langmuir等温线模型 | |||
| qm/mg·g-1 | 67.7 | 80.1 | 179.4 |
| KL/mg·g-1 | 0.213 | 0.213 | 0.118 |
| R2 | 0.985 | 0.979 | 0.980 |
| Freundlich等温线模型 | |||
| KF/mg·g-1·L-1/n ·mg-1/n | 12.8 | 15.0 | 19.1 |
| n | 1.65 | 1.60 | 1.23 |
| R2 | 0.981 | 0.972 | 0.979 |
| Sips等温线模型 | |||
| qm/mg·g-1 | 83.1 | 88.0 | 186 |
| KS/L·mg-1 | 0.137 | 0.175 | 0.110 |
| m | 0.886 | 0.944 | 0.991 |
| R2 | 0.984 | 0.977 | 0.978 |
表3 敌草隆在CWHBC上的吸附等温线模型拟合参数
| 参数 | 25℃ | 35℃ | 45℃ |
|---|---|---|---|
| Langmuir等温线模型 | |||
| qm/mg·g-1 | 67.7 | 80.1 | 179.4 |
| KL/mg·g-1 | 0.213 | 0.213 | 0.118 |
| R2 | 0.985 | 0.979 | 0.980 |
| Freundlich等温线模型 | |||
| KF/mg·g-1·L-1/n ·mg-1/n | 12.8 | 15.0 | 19.1 |
| n | 1.65 | 1.60 | 1.23 |
| R2 | 0.981 | 0.972 | 0.979 |
| Sips等温线模型 | |||
| qm/mg·g-1 | 83.1 | 88.0 | 186 |
| KS/L·mg-1 | 0.137 | 0.175 | 0.110 |
| m | 0.886 | 0.944 | 0.991 |
| R2 | 0.984 | 0.977 | 0.978 |
| 参数 | WHBC | CWHBC |
|---|---|---|
| 实验吸附量 | ||
| qm/mg·g-1 | 3.61 | 12.56 |
| 准一级动力学模型 | ||
| qm/mg·g-1 | 3.46 | 11.40 |
| K1/min-1 | 0.120 | 0.149 |
| R2 | 0.981 | 0.936 |
| 准二级动力学模型 | ||
| qm/mg·g-1 | 3.58 | 12.40 |
| K2/g·mg-1·min-1 | 0.0381 | 0.0160 |
| R2 | 0.991 | 0.963 |
| Elovich模型 | ||
| α/g·mg-1·min-1 | 0.931 | 8.590 |
| β/g·mg-1 | 1.620 | 0.520 |
| R2 | 0.999 | 0.883 |
| kid1/g·mg-1·min-1/2 | 0.544 | 2.910 |
| c1/mg·g-1 | 0.102 | 0.919 |
| R12 | 0.996 | 0.860 |
| kid2/g·mg-1·min-1/2 | 0.144 | 0.398 |
| c2/mg·g-1 | 1.71 | 7.62 |
| R22 | 0.905 | 0.724 |
| kid3/g·mg-1·min-1/2 | 0.0734 | 0.1820 |
| c3/mg·g-1 | 2.47 | 9.78 |
| R32 | 1.000 | 0.979 |
| Kfd | 0.004 | 0.745 |
| R2 | 0.902 | 0.878 |
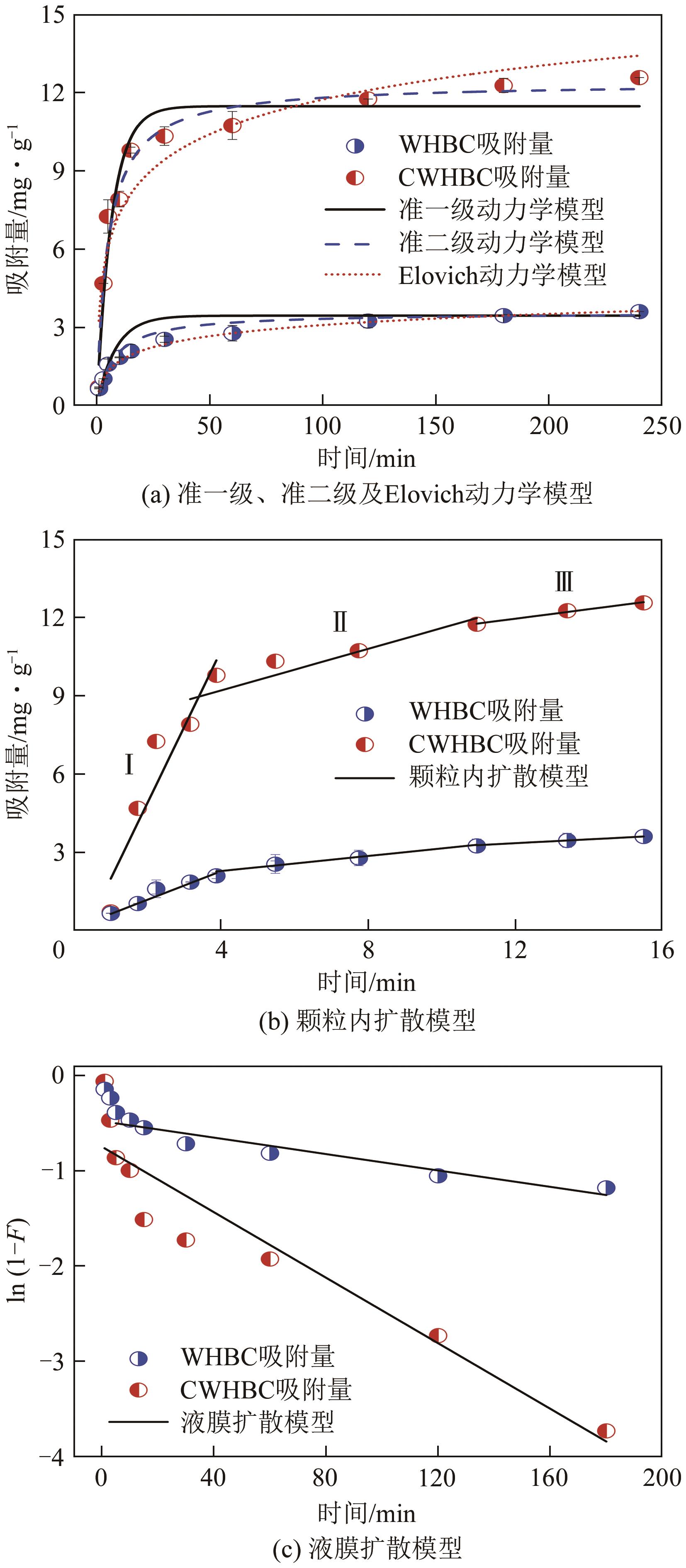
表4 CWHBC吸附去除水中敌草隆的动力学参数
| 参数 | WHBC | CWHBC |
|---|---|---|
| 实验吸附量 | ||
| qm/mg·g-1 | 3.61 | 12.56 |
| 准一级动力学模型 | ||
| qm/mg·g-1 | 3.46 | 11.40 |
| K1/min-1 | 0.120 | 0.149 |
| R2 | 0.981 | 0.936 |
| 准二级动力学模型 | ||
| qm/mg·g-1 | 3.58 | 12.40 |
| K2/g·mg-1·min-1 | 0.0381 | 0.0160 |
| R2 | 0.991 | 0.963 |
| Elovich模型 | ||
| α/g·mg-1·min-1 | 0.931 | 8.590 |
| β/g·mg-1 | 1.620 | 0.520 |
| R2 | 0.999 | 0.883 |
| kid1/g·mg-1·min-1/2 | 0.544 | 2.910 |
| c1/mg·g-1 | 0.102 | 0.919 |
| R12 | 0.996 | 0.860 |
| kid2/g·mg-1·min-1/2 | 0.144 | 0.398 |
| c2/mg·g-1 | 1.71 | 7.62 |
| R22 | 0.905 | 0.724 |
| kid3/g·mg-1·min-1/2 | 0.0734 | 0.1820 |
| c3/mg·g-1 | 2.47 | 9.78 |
| R32 | 1.000 | 0.979 |
| Kfd | 0.004 | 0.745 |
| R2 | 0.902 | 0.878 |
| 生物炭 | C | O | N | P | S | Cl | Ca |
|---|---|---|---|---|---|---|---|
| CWHBC | 73.25 | 18.65 | 0 | 0.76 | 0.57 | 1.59 | 5.17 |
表5 吸附敌草隆后CWHBC的元素组成 (质量分数/%)
| 生物炭 | C | O | N | P | S | Cl | Ca |
|---|---|---|---|---|---|---|---|
| CWHBC | 73.25 | 18.65 | 0 | 0.76 | 0.57 | 1.59 | 5.17 |
| 1 | Sang-Eun NAM, HAQUE Md Niamul, Seong Duk DO, et al. Chronic effects of environmental concentrations of antifoulant diuron on two marine fish: Assessment of hormone levels, immunity, and antioxidant defense system[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2023, 263: 109510. |
| 2 | MOHAMMED Ali Mustafa, HUOVINEN Marjo, VÄHÄKANGAS Kirsi H. Toxicity of diuron metabolites in human cells[J]. Environmental Toxicology and Pharmacology, 2020, 78: 103409. |
| 3 | MIKLOS David B, REMY Christian, JEKEL Martin, et al. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review[J]. Water Research, 2018, 139: 118-131. |
| 4 | PLAKAS Konstantinos V, KARABELAS Anastasios J. Removal of pesticides from water by NF and RO membranes—A review[J]. Desalination, 2012, 287: 255-265. |
| 5 | SOLÍS Rafael R, Javier RIVAS F, Ana MARTÍNEZ-PIERNAS, et al. Ozonation, photocatalysis and photocatalytic ozonation of diuron. Intermediates identification[J]. Chemical Engineering Journal, 2016, 292: 72-81. |
| 6 | INYANG Mandu, DICKENSON Eric. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review[J]. Chemosphere, 2015, 134: 232-240. |
| 7 | 吴阳, 刘振中, 江文, 等. 生物炭对几类常见新兴污染物去除的研究进展[J]. 化工进展, 2021, 40(5): 2839-2851. |
| WU Yang, LIU Zhenzhong, JIANG Wen, et al. Research progress on removal of several common emerging pollutants by biochar[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2839-2851. | |
| 8 | 王申宛, 郑晓燕, 校导, 等. 生物炭的制备、改性及其在环境修复中应用的研究进展[J]. 化工进展, 2020, 39(S2): 352-361. |
| WANG Shenwan, ZHENG Xiaoyan, XIAO Dao, et al. Research progress of production, modification and application in environment remediation of biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 352-361. | |
| 9 | 范方方, 仝仲凯, 左卫元. 钙改性花生壳生物炭对废水中四环素的吸附研究[J]. 无机盐工业, 2023, 55(6): 109-115. |
| FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar[J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. | |
| 10 | SHRESTHA Priya, CHUN Dave D, KANG Kang, et al. Role of metals in biochar production and utilization in catalytic applications: A review[J]. Waste and Biomass Valorization, 2022, 13(2): 797-822. |
| 11 | ZHUO Shengnan, DAI Tianchi, REN Hongyu, et al. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism[J]. Bioresource Technology, 2022, 359: 127477. |
| 12 | AMALINA Farah, RAZAK Abdul Syukor Abd, KRISHNAN Santhana, et al. Water hyacinth (Eichhornia crassipes) for organic contaminants removal in water—A review[J]. Journal of Hazardous Materials Advances, 2022, 7: 100092. |
| 13 | VISWANATHAN Shanthi Prabha, NJAZHAKUNNATHU Gopika Vijayakumar, NEELAMURY Sreekanth Prakasan, et al. The efficiency of aquatic weed-derived biochar in enhanced removal of cationic dyes from aqueous medium[J]. Biomass Conversion and Biorefinery, 2024, 14(12): 12895-12910. |
| 14 | FENG Qianwei, CHEN Miao, WU Pan, et al. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms[J]. Chemical Engineering Journal, 2022, 429: 132166. |
| 15 | LIU Wujun, JIANG Hong, YU Hanqing. Development of biochar-based functional materials: Toward a sustainable platform carbon material[J]. Chemical Reviews, 2015, 115(22): 12251-12285. |
| 16 | LIU Yucan, JI Xianguo, GAO Zhonglu, et al. Adsorption characteristics and removal mechanism of malathion in water by high and low temperature calcium-modified water hyacinth-based biochar[J]. Journal of Cleaner Production, 2023, 411: 137258. |
| 17 | CHEN Zhihao, WU Yonghong, HUANG Yingping, et al. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 651: 129728. |
| 18 | ZHANG Haibo, SU Long, CHENG Caiping, et al. A new type of calcium-rich biochars derived from spent mushroom substrates and their efficient adsorption properties for cationic dyes[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1007630. |
| 19 | PI Liu, JIANG Rui, ZHOU Wangchi, et al. g-C3N4 Modified biochar as an adsorptive and photocatalytic material for decontamination of aqueous organic pollutants[J]. Applied Surface Science, 2015, 358: 231-239. |
| 20 | FANG Qile, CHEN Baoliang, LIN Yajie, et al. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups[J]. Environmental Science & Technology, 2014, 48(1): 279-288. |
| 21 | 姜记威, 张诗轩, 曾文炉, 等. 生物炭基材料在抗生素废水处理中的研究进展[J]. 化工进展, 2021, 40(S2): 389-401. |
| JIANG Jiwei, ZHANG Shixuan, ZENG Wenlu, et al. Research progress on biochar-based materials for the treatment of antibiotic wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 389-401. | |
| 22 | AL-GHOUTI Mohammad A, DA'ANA Dana A. Guidelines for the use and interpretation of adsorption isotherm models: A review[J]. Journal of Hazardous Materials, 2020, 393: 122383. |
| 23 | ZENG Zhuotong, YE Shujing, WU Haipeng, et al. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution[J]. Science of the Total Environment, 2019, 648: 206-217. |
| 24 | LIU Yucan, JI Xianguo, WANG Ying, et al. A stable Fe-Zn modified sludge-derived biochar for diuron removal: Kinetics, isotherms, mechanism, and practical research[J]. Molecules, 2023, 28(6): 2868. |
| 25 | GEORGIN Jordana, FRANCO Dison S P, NETTO Matias S, et al. Effective adsorption of harmful herbicide diuron onto novel activated carbon from Hovenia dulcis [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 654: 129900. |
| 26 | HADDAD Khouloud, JELLALI Salah, JEGUIRIM Mejdi, et al. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust[J]. Journal of Environmental Management, 2018, 216: 305-314. |
| 27 | ZUO Weiqi, CHEN Chen, CUI Haojie, et al. Enhanced removal of Cd(Ⅱ) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar[J]. RSC Advances, 2017, 7(26): 16238-16243. |
| 28 | WU Zhibin, ZHONG Hua, YUAN Xingzhong, et al. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater[J]. Water Research, 2014, 67: 330-344. |
| 29 | CHAO Yanhong, YANG Long, JI Haiyan, et al. Graphene-analogue molybdenum disulfide for adsorptive removal of tetracycline from aqueous solution: Equilibrium, kinetic, and thermodynamic studies[J]. Environmental Progress & Sustainable Energy, 2017, 36(3): 815-821. |
| 30 | WANG Bing, LIAN Guoqi, LEE Xinqing, et al. Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water[J]. Chemosphere, 2020, 238: 124684. |
| 31 | WANG Bing, MA Yuena, LEE Xinqing, et al. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer[J]. Science of the Total Environment, 2021, 758: 143664. |
| 32 | ALMASI Ali, OMIDI Mehdi, KHODADADIAN Mehdi, et al. Lead(Ⅱ) and cadmium(Ⅱ) removal from aqueous solution using processed walnut shell: Kinetic and equilibrium study[J]. Toxicological & Environmental Chemistry, 2012, 94(4): 660-671. |
| 33 | LIU Xiaoning, SHEN Feng, SMITH Richard L, et al. Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions[J]. Bioresource Technology, 2019, 294: 122198. |
| 34 | DENG Jing, SHAO Yisheng, GAO Naiyun, et al. Multiwalled carbon nanotubes as adsorbents for removal of herbicide diuron from aqueous solution[J]. Chemical Engineering Journal, 2012, 193/194: 339-347. |
| 35 | WANG Wei, GAO Ming, CAO Mengbo, et al. Self-propagating synthesis of Zn-loaded biochar for tetracycline elimination[J]. Science of the Total Environment, 2021, 759: 143542. |
| 36 | DAI Yingjie, LI Jingjing, SHAN Dexin. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs[J]. Chemosphere, 2020, 238: 124432. |
| 37 | ZHOU Yaoyu, LIU Xiaocheng, XIANG Yujia, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-273. |
| 38 | Xiaoshu LYU, HU Yunjun, TANG Jie, et al. Effects of co-existing ions and natural organic matter on removal of chromium (Ⅵ) from aqueous solution by nanoscale zero valent iron (nZVI)-Fe3O4 nanocomposites[J]. Chemical Engineering Journal, 2013, 218: 55-64. |
| 39 | XU Yan, LIU Yunguo, LIU Shaobo, et al. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes)[J]. Environmental Science and Pollution Research, 2016, 23(23): 23606-23618. |
| 40 | LI Xiaoyun, XIE Yanhua, JIANG Fei, et al. Enhanced phosphate removal from aqueous solution using resourceable nano-CaO2/BC composite: Behaviors and mechanisms[J]. Science of the Total Environment, 2020, 709: 136123. |
| 41 | HUANG Weihao, WU Rome-Ming, CHANG Jo-Shu, et al. Manganese ferrite modified agricultural waste-derived biochars for copper ions adsorption[J]. Bioresource Technology, 2023, 367: 128303. |
| [1] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [2] | 焦文磊, 刘震, 陈俊先, 张天钰, 姬忠礼. 叶片式分离元件结构及性能影响因素研究进展[J]. 化工进展, 2024, 43(8): 4187-4202. |
| [3] | 何海霞, 万亚萌, 李帆帆, 牛心雨, 张静雯, 李涛, 任保增. 盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺[J]. 化工进展, 2024, 43(8): 4230-4245. |
| [4] | 殷晨阳, 刘永峰, 陈睿哲, 张璐, 宋金瓯, 刘海峰. 基于量子化学计算的正己烷热解反应动力学模拟[J]. 化工进展, 2024, 43(8): 4273-4282. |
| [5] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [6] | 谢娟, 贺文, 赵勖丞, 李帅辉, 卢真真, 丁哲宇. 分子动力学模拟在沥青体系中的应用研究进展[J]. 化工进展, 2024, 43(8): 4432-4449. |
| [7] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [8] | 丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612. |
| [9] | 曹景沛, 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇. 煤热解研究进展及其发展历程[J]. 化工进展, 2024, 43(7): 3620-3636. |
| [10] | 胡锐, 李先如, 朴玮玲, 冯盼, 罗磊, 罗刚, 卫皇曌, 刘振刚, 张士成. 有机废物水热转化设备与技术研究进展[J]. 化工进展, 2024, 43(7): 3672-3691. |
| [11] | 龚德成, 沈倩, 朱贤青, 黄云, 夏奡, 张敬苗, 朱恂, 廖强. 微藻超临界水气化制取富氢合成气的研究进展[J]. 化工进展, 2024, 43(7): 3709-3728. |
| [12] | 顾颂琦, 孙凡飞, 韦尧, 宋兴飞, 南兵, 李丽娜, 黄宇营. 时间分辨热化学原位XAFS方法[J]. 化工进展, 2024, 43(7): 3747-3755. |
| [13] | 黄军, 张应娟, 林茵童, 韦雪纯, 吴雨桐, 毋高博, 莫钧麟, 赵祯霞, 赵钟兴. 蚕沙基生物多孔炭的制备及对杀虫单/呋虫胺的协同吸附与缓释性能[J]. 化工进展, 2024, 43(7): 3964-3971. |
| [14] | 张昊, 陆小明. 纳米钛酸钡前体热分解反应动力学及颗粒演化机理[J]. 化工进展, 2024, 43(7): 3987-3995. |
| [15] | 于立爽, 李青云, 刘兆明, 张淑茹, 刘幽燕, 唐爱星. 油菜花粉生物炭固定化脂肪酶催化蒎烯环氧化[J]. 化工进展, 2024, 43(7): 3996-4004. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||