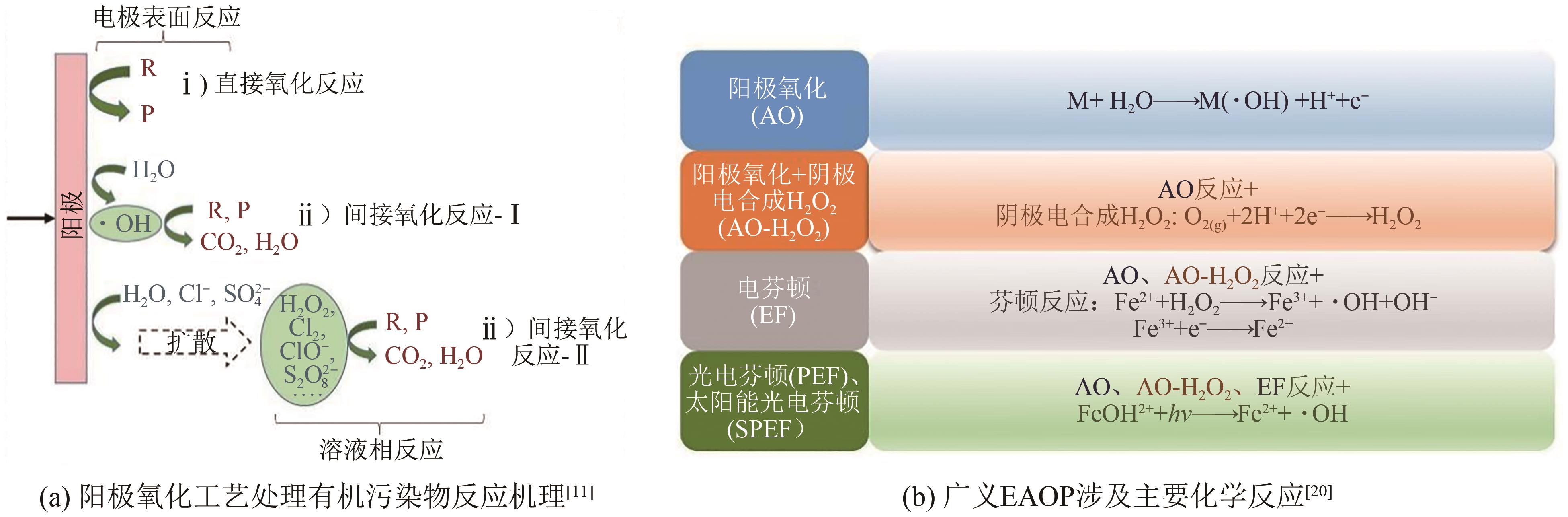
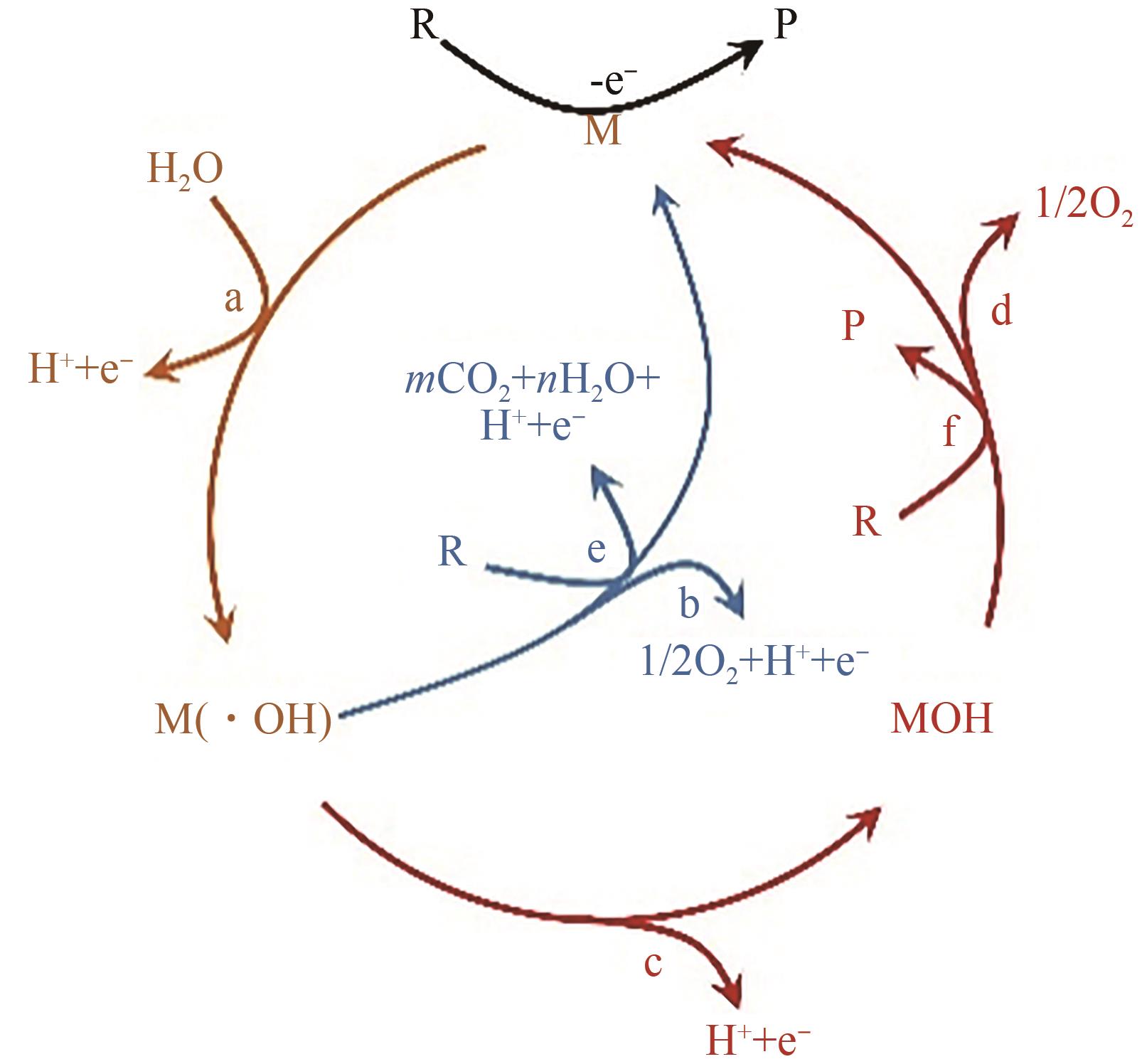
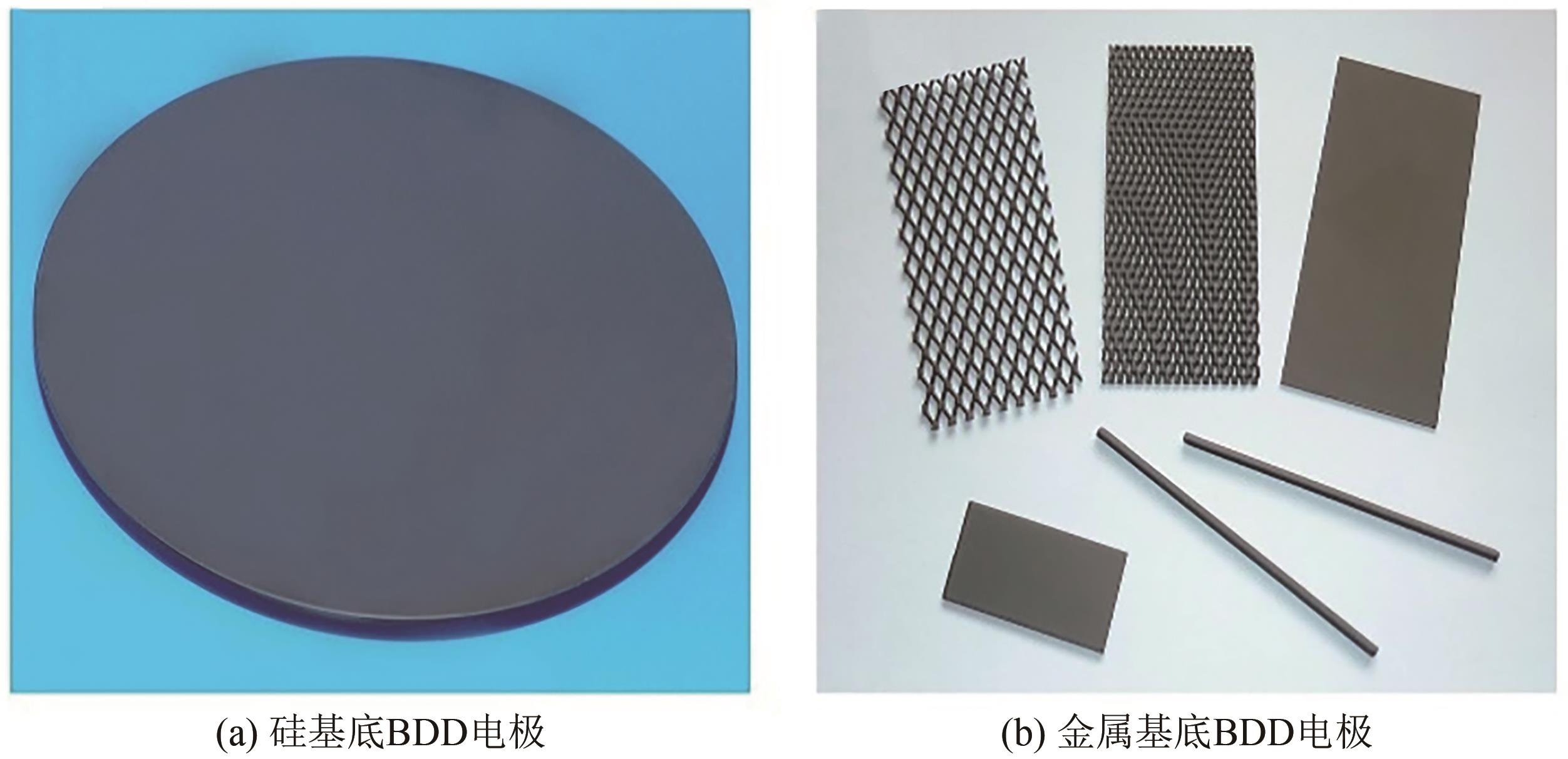
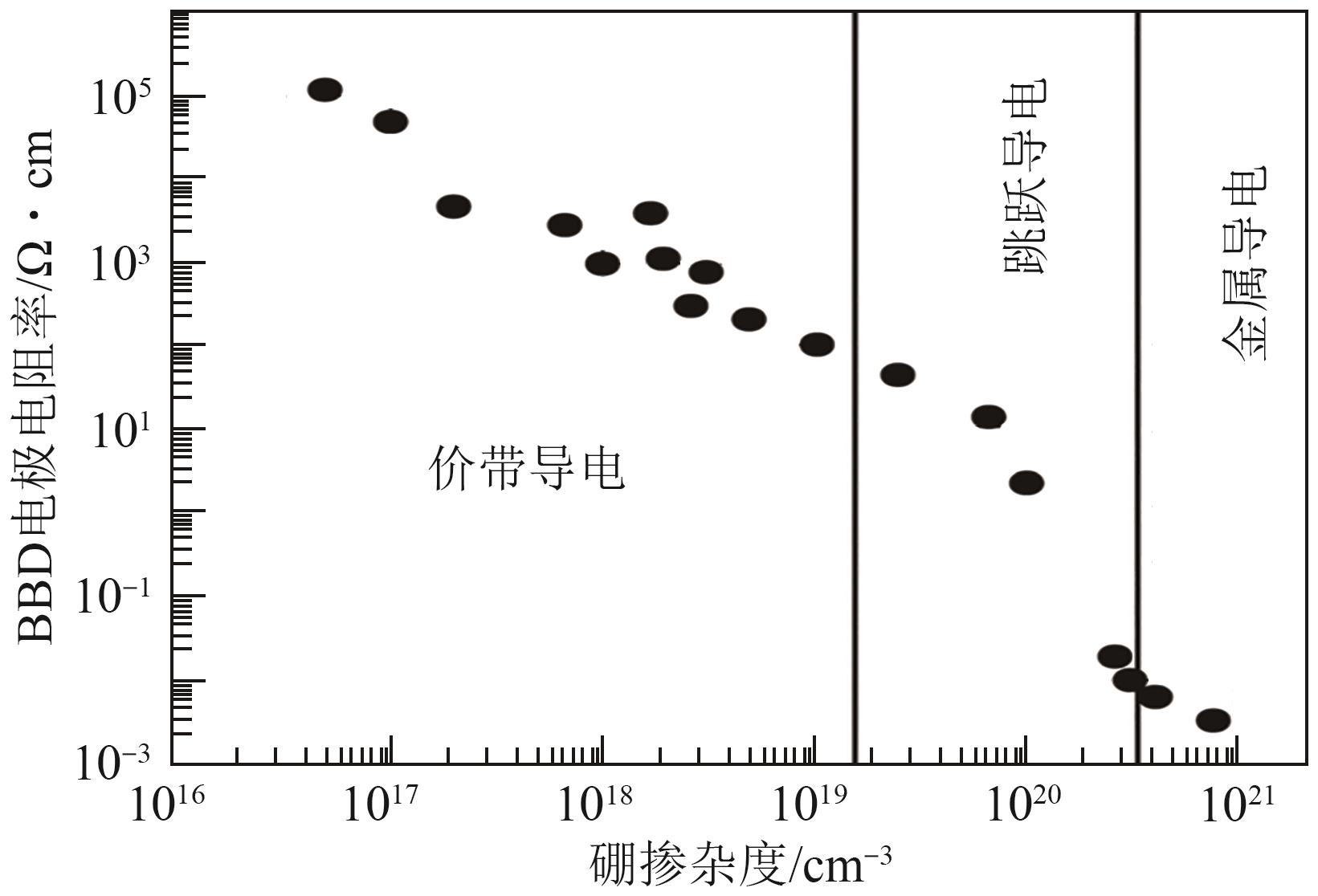
化工进展 ›› 2024, Vol. 43 ›› Issue (1): 501-513.DOI: 10.16085/j.issn.1000-6613.2023-0269
• 资源与环境化工 • 上一篇
基于掺硼金刚石电极的工业废水处理研究进展
- 中石化(大连)石油化工研究院有限公司,辽宁 大连 116045
-
收稿日期:2023-02-27修回日期:2023-07-10出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:张长安 -
作者简介:王博(1994—),男,硕士,助理研究员,研究方向为化学气相沉积。E-mail:wangbo.fshy@sinopec.com。 -
基金资助:中国石化科技项目(院控-142306)
Industrial wastewater treatment technology based on boron-doped diamond electrodes:A review
WANG Bo( ), ZHANG Chang’an(
), ZHANG Chang’an( ), ZHAO Limin, YUAN Jun, SONG Yongyi
), ZHAO Limin, YUAN Jun, SONG Yongyi
- SINOPEC Dalian Research Institute of Petroleum and Petrochemicals Co. , Ltd. , Dalian 116045, Liaoning, China
-
Received:2023-02-27Revised:2023-07-10Online:2024-01-20Published:2024-02-05 -
Contact:ZHANG Chang’an
摘要:
工业废水普遍具有可生化性差、污染物种类多、有机物含量高、难降解等特点,常规处理手段难使其达标排放。以电化学高级氧化工艺为代表的污水深度处理工艺处理污水效果显著,是近年来环境工作者的研究热点之一。掺硼金刚石(boron-doped diamond,BDD)电极理化性质优异,是目前电化学高级氧化处理废水最为理想高效的阳极材料,但关于大尺寸BDD电极的应用及处理真实工业废水的研究情况尚未及时总结归纳。本文以基于BDD电极的电化学高级氧化工艺过程为对象,对该过程涉及到的废水特点、工艺原理、BDD电极特点及制备方法、处理案例和工艺参数优化等方面的研究进展进行综述,重点聚焦不同污染体系下的大型实验室装置、中试装置和处理真实工业废水案例,总结了BDD电极材料开发情况和不同类型工艺的技术特点,探讨了工艺优化方面的研究进展和目前限制该技术大规模工业化应用的主要原因。最后对基于BDD电极的电化学高级氧化工艺应用前景和重点发展方向作出了展望。
中图分类号:
引用本文
王博, 张长安, 赵利民, 袁俊, 宋永一. 基于掺硼金刚石电极的工业废水处理研究进展[J]. 化工进展, 2024, 43(1): 501-513.
WANG Bo, ZHANG Chang’an, ZHAO Limin, YUAN Jun, SONG Yongyi. Industrial wastewater treatment technology based on boron-doped diamond electrodes:A review[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 501-513.
| 阳极材料 | 析氧过电位/V(vs.SHE) |
|---|---|
| 石墨 | 1.7 |
| Pt | 1.6~1.9 |
| RuO2 | 1.4~1.7 |
| IrO2 | 1.5~1.8 |
| Ebonex® (Ti4O7) | 1.7~1.8 |
| PbO2 | 1.8~2.0 |
| SnO2 | 1.9~2.2 |
| BDD | 2.2~2.6 |
表1 常见阳极材料析氧过电位[20]
| 阳极材料 | 析氧过电位/V(vs.SHE) |
|---|---|
| 石墨 | 1.7 |
| Pt | 1.6~1.9 |
| RuO2 | 1.4~1.7 |
| IrO2 | 1.5~1.8 |
| Ebonex® (Ti4O7) | 1.7~1.8 |
| PbO2 | 1.8~2.0 |
| SnO2 | 1.9~2.2 |
| BDD | 2.2~2.6 |
| 参数 | NeoCoat | 湖南新锋 | 参数 | NeoCoat | 湖南新锋 |
|---|---|---|---|---|---|
| 基底 | 硅、金属(铌、钽、钨) | 硅、铌、钽、泡沫金属 | 基体厚度/mm | 1~2 | — |
| 电极形状 | 矩形、网状、定制形状 | 矩形、圆盘、网格、泡沫、定制形状 | 晶体尺寸/μm | — | 1~10 |
| 尺寸/mm | 矩形(100×100) 矩形(100×100) 定制形状(最大400×1200) | 矩形(1×1~350×350) 圆盘(ϕ1~350) 颗粒(2×10-4~30) | BDD涂层厚度/μm | — | 5~50 |
| 膜厚度均匀性 | ±5%(100mm) | — | |||
| 析氧过电位/V | — | 2.4~2.7 | |||
| 掺硼水平/μg·g-1 | 500~10000 | 5000~50000 | |||
| 应用条件 | 阴极/阳极/双极 | 阴极/阳极/双极 | |||
| 电阻率/mΩ·cm | 1~100 | 0.01~10 | |||
| 涂层面 | 单面/双面 | 单面/双面 |
表2 NeoCoat公司和湖南新锋科技有限公司BDD产品参数对比
| 参数 | NeoCoat | 湖南新锋 | 参数 | NeoCoat | 湖南新锋 |
|---|---|---|---|---|---|
| 基底 | 硅、金属(铌、钽、钨) | 硅、铌、钽、泡沫金属 | 基体厚度/mm | 1~2 | — |
| 电极形状 | 矩形、网状、定制形状 | 矩形、圆盘、网格、泡沫、定制形状 | 晶体尺寸/μm | — | 1~10 |
| 尺寸/mm | 矩形(100×100) 矩形(100×100) 定制形状(最大400×1200) | 矩形(1×1~350×350) 圆盘(ϕ1~350) 颗粒(2×10-4~30) | BDD涂层厚度/μm | — | 5~50 |
| 膜厚度均匀性 | ±5%(100mm) | — | |||
| 析氧过电位/V | — | 2.4~2.7 | |||
| 掺硼水平/μg·g-1 | 500~10000 | 5000~50000 | |||
| 应用条件 | 阴极/阳极/双极 | 阴极/阳极/双极 | |||
| 电阻率/mΩ·cm | 1~100 | 0.01~10 | |||
| 涂层面 | 单面/双面 | 单面/双面 |
| 合成方法 | 原理 | 优势 | 劣势 | 最大电极尺寸 | 应用情况 |
|---|---|---|---|---|---|
| 热丝法(HFCVD) | 利用金属丝(Ta、W)通电产生热量,激发气源形成等离子体,并在基底表面沉积成膜 | 工艺、设备简单,投资低,参数范围宽,通过合理的灯丝排布可实现大面积BDD电极制备[ | 成膜均匀性较差,易受灯丝气化污染,沉积速率低(1~10μm/h),对氧化性气体敏感 | 400mm×1200mm | 技术成熟,应用广泛。瑞士NeoCoat公司,德国DiaChem公司均有成熟产品,我国湖南新锋科技有限公司、北京沃尔德金刚石工具有限公司以及中南大学、吉林大学等均有广泛研究和成熟产品 |
| 微波等离子体法(MPCVD) | 利用微波辉光放电等离子体激发气源,在基底表面沉积成膜 | 成膜均匀,质量高,沉积温度低[ | 设备昂贵,有效沉积面积难扩大 | ϕ350mm | 技术成熟,但应用较少,德国Iplas公司具有专利产品(CYRANNUS®),国内武汉工程大学、北京科技大学、西南科技大学等均有相关研究 |
| 直流等离子体 喷射法(DCPJCVD) | 利用直流放电击穿流经阴阳极之间的气体形成电弧,气体被急剧加热后从等离子体炬喷口迅速喷出并在基底表面沉积成膜 | 金刚石沉积速率快[ | 设备昂贵,投资高,工艺控制困难,易对基底造成热损伤 | — | 多以实验室研究为主,尚无成熟产品 |
表3 常见BDD电极CVD合成方法对比及应用情况
| 合成方法 | 原理 | 优势 | 劣势 | 最大电极尺寸 | 应用情况 |
|---|---|---|---|---|---|
| 热丝法(HFCVD) | 利用金属丝(Ta、W)通电产生热量,激发气源形成等离子体,并在基底表面沉积成膜 | 工艺、设备简单,投资低,参数范围宽,通过合理的灯丝排布可实现大面积BDD电极制备[ | 成膜均匀性较差,易受灯丝气化污染,沉积速率低(1~10μm/h),对氧化性气体敏感 | 400mm×1200mm | 技术成熟,应用广泛。瑞士NeoCoat公司,德国DiaChem公司均有成熟产品,我国湖南新锋科技有限公司、北京沃尔德金刚石工具有限公司以及中南大学、吉林大学等均有广泛研究和成熟产品 |
| 微波等离子体法(MPCVD) | 利用微波辉光放电等离子体激发气源,在基底表面沉积成膜 | 成膜均匀,质量高,沉积温度低[ | 设备昂贵,有效沉积面积难扩大 | ϕ350mm | 技术成熟,但应用较少,德国Iplas公司具有专利产品(CYRANNUS®),国内武汉工程大学、北京科技大学、西南科技大学等均有相关研究 |
| 直流等离子体 喷射法(DCPJCVD) | 利用直流放电击穿流经阴阳极之间的气体形成电弧,气体被急剧加热后从等离子体炬喷口迅速喷出并在基底表面沉积成膜 | 金刚石沉积速率快[ | 设备昂贵,投资高,工艺控制困难,易对基底造成热损伤 | — | 多以实验室研究为主,尚无成熟产品 |
| 电流密度/mA·cm-2 | 时间/min | 能耗/kWh·kgCOD-1 | 费用①/EUR·kgCOD-1 |
|---|---|---|---|
| 50 | 60 | 967 | 68 |
| 30 | 60 | 563 | 39 |
| 15 | 120 | 364 | 25 |
| 8 | 120 | 115 | 8 |
| 4 | 120 | 41 | 3 |
表4 不同电流密度条件矿化率达50%时所需的时间、能耗和能源费用对比[73]
| 电流密度/mA·cm-2 | 时间/min | 能耗/kWh·kgCOD-1 | 费用①/EUR·kgCOD-1 |
|---|---|---|---|
| 50 | 60 | 967 | 68 |
| 30 | 60 | 563 | 39 |
| 15 | 120 | 364 | 25 |
| 8 | 120 | 115 | 8 |
| 4 | 120 | 41 | 3 |
| 1 | OKUR Melike Ceren, AKYOL Abdurrahman, NAYIR Tulin Yilmaz, et al. Performance of Ti/RuO2-IrO2 electrodes and comparison with BDD electrodes in the treatment of textile wastewater by electro-oxidation process[J]. Chemical Engineering Research and Design, 2022, 183: 398-410. |
| 2 | SATHINATHAN P, PARAB H M, YUSOFF R, et al. Photobioreactor design and parameters essential for algal cultivation using industrial wastewater: A review[J]. Renewable and Sustainable Energy Reviews, 2023, 173: 113096. |
| 3 | CAI Q Q, LEE B C Y, ONG S L, et al. Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment—Recent advances, challenges and perspective[J]. Water Research, 2021, 190: 116692. |
| 4 | SUN Guangxi, ZHANG Yu, GAO Yingxin, et al. Removal of hard COD from biological effluent of coking wastewater using synchronized oxidation-adsorption technology: Performance, mechanism, and full-scale application[J]. Water Research, 2020, 173: 115517. |
| 5 | 熊富忠, 温东辉. 难降解工业废水高效处理技术与理论的新进展[J]. 环境工程, 2021, 39(11): 1-15, 40. |
| XIONG Fuzhong, WEN Donghui. Advances of highly-efficient technologies and theories for refractory industrial wastewater treatment[J]. Environmental Engineering, 2021, 39(11): 1-15, 40. | |
| 6 | 孙光溪, 田哲, 丁然, 等. 典型行业高浓度难降解工业废水深度处理技术研究进展[J]. 环境工程, 2021, 39(11): 16-27, 134. |
| SUN Guangxi, TIAN Zhe, DING Ran, et al. Review of advanced treatment technologies for high concentration and refractory industrial wastewater from some typical industries[J]. Environmental Engineering, 2021, 39(11): 16-27, 134. | |
| 7 | SARAVANAN A, DEIVAYANAI V C, Senthil KUMAR P, et al. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook[J]. Chemosphere, 2022, 308: 136524. |
| 8 | SUKHATSKIY Y, SHEPIDA M, SOZANSKYI M, et al. Periodate-based advanced oxidation processes for wastewater treatment: A review[J]. Separation and Purification Technology, 2023, 304: 122305. |
| 9 | FU Rui, ZHANG Pengshuang, JIANG Yuanxing, et al. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods[J]. Chemosphere, 2023, 311: 136993. |
| 10 | MOSTAFA Ehab, REINSBERG Philip, Sergi GARCIA-SEGURA, et al. Chlorine species evolution during electrochlorination on boron-doped diamond anodes: In-situ electrogeneration of Cl2, Cl2O and ClO2 [J]. Electrochimica Acta, 2018, 281: 831-840. |
| 11 | 孙怡. 高级氧化技术的阳极强化及耦合生物法处理难降解有机废水[D]. 杭州: 浙江大学, 2020. |
| SUN Yi. Advanced oxidation process anode enhancement and coupling with biological process for refractory organic wastewater treatment[D]. Hangzhou: Zhejiang University, 2020. | |
| 12 | 郭旭, 张永政, 夏厚兵, 等. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| GUO Xu, ZHANG Yongzheng, XIA Houbing, et al. Research progress in the removal of water pollutants by carbon-based materials via electrooxidation[J]. CIESC Journal, 2023, 74(5): 1862-1874. | |
| 13 | HE Yapeng, ZHAO Dandi, LIN Haibo, et al. Design of diamond anodes in electrochemical degradation of organic pollutants[J]. Current Opinion in Electrochemistry, 2022, 32: 100878. |
| 14 | SILVA S W DA, NAVARRO E M O, RODRIGUES M A S, et al. Using p-Si/BDD anode for the electrochemical oxidation of norfloxacin[J]. Journal of Electroanalytical Chemistry, 2019, 832: 112-120. |
| 15 | HE Yapeng, LIN Haibo, GUO Zhongcheng, et al. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants[J]. Separation and Purification Technology, 2019, 212: 802-821. |
| 16 | COMNINELLIS Christos. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment[J]. Electrochimica Acta, 1994, 39(11/12): 1857-1862. |
| 17 | LIU Guoshuai, ZHANG Changyong, ZHOU Yanan, et al. Insight into the overpotential and thermodynamic mechanism of hydroxyl radical formation on diamond anode[J]. Applied Surface Science, 2021, 565: 150559. |
| 18 | MARTÍNEZ-HUITLE Carlos Alberto, PANIZZA Marco. Electrochemical oxidation of organic pollutants for wastewater treatment[J]. Current Opinion in Electrochemistry, 2018, 11: 62-71. |
| 19 | 陈卓. 石墨基BDD涂层电极的制备及其在废水处理中的应用研究[D]. 绵阳: 西南科技大学, 2020. |
| CHEN Zhuo. Preparation and application of BDD coating electrode on graphite substrate in wastewater treatment[D]. Mianyang: Southwest University of Science and Technology, 2020. | |
| 20 | MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters[J]. Applied Catalysis B: Environmental, 2017, 202: 217-261. |
| 21 | GARCIA-SEGURA S, NIENHAUSER A B, FAJARDO A S, et al. Disparities between experimental and environmental conditions: Research steps toward making electrochemical water treatment a reality[J]. Current Opinion in Electrochemistry, 2020, 22: 9-16. |
| 22 | MBAYE Moussa, DIAW Pape Abdoulaye, MBAYE Olivier Maurice Aly, et al. Rapid removal of fungicide thiram in aqueous medium by electro-Fenton process with Pt and BDD anodes[J]. Separation and Purification Technology, 2022, 281: 119837. |
| 23 | PINTO Cláudia, FERNANDES Annabel, MARQUES Albertina, et al. Reuse of wool dyeing wastewater after electrochemical treatment at a BDD anode[J]. Journal of Water Process Engineering, 2022, 49: 102972. |
| 24 | CAREY J J, CHRIST JR C S, LOWERY S N. Method of electrolysis employing a doped diamond anode to oxidize solutes in wastewater: US5399247[P]. 1995-03-21. |
| 25 | 李莲莲, 陈冠钦. 高性能掺硼金刚石电极的研究进展[J]. 金刚石与磨料磨具工程, 2022, 42(5): 543-551. |
| LI Lianlian, CHEN Guanqin. Preparation methods of boron-doped diamond electrode and its research progresses[J]. Diamond & Abrasives Engineering, 2022, 42(5): 543-551. | |
| 26 | 赵丹荻, 贾博, 何亚鹏, 等. 掺硼金刚石阳极电催化降解甲氧苄啶抗生素及其动力学研究[J]. 环境化学, 2022, 41(10): 3425-3434. |
| ZHAO Dandi, JIA Bo, HE Yapeng, et al. Electrochemical degradation of antibiotic trimethoprim on boron doped diamond anode and kinetics[J]. Environmental Chemistry, 2022, 41(10): 3425-3434. | |
| 27 | PLESKOV Y V, KROTOVA M D, ELKIN V V, et al. Electrochemical behaviour of boron-doped diamond compacts—A new electrode material[J]. Electrochimica Acta, 2016, 201: 268-273. |
| 28 | RADJENOVIC J, SEDLAK D L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water[J]. Environmental Science & Technology, 2015, 49(19): 11292-11302. |
| 29 | WEI Qiuping, LIU Guoshuai, ZHU Chengwu, et al. Ordered structures with functional units (OSFU) enabled highly robust diamond anode for electrochemical decomposing of organic pollutants[J]. Chemical Engineering Journal, 2020, 397: 125465. |
| 30 | MIAO Dongtian, LI Zhishen, CHEN Yinhao, et al. Preparation of macro-porous 3D boron-doped diamond electrode with surface micro structure regulation to enhance electrochemical degradation performance[J]. Chemical Engineering Journal, 2022, 429: 132366. |
| 31 | FENG Zhiyuan, GAO Nan, LIU Junsong, et al. Boron-doped diamond electrochemical aptasensors for trace aflatoxin B1 detection[J]. Analytica Chimica Acta, 2020, 1122: 70-75. |
| 32 | YANG Wanlin, TAN Jilin, CHEN Yinhao, et al. Relationship between substrate type and BDD electrode structure, performance and antibiotic tetracycline mineralization[J]. Journal of Alloys and Compounds, 2022, 890: 161760. |
| 33 | KIM Shin, JEONG Yesul, PARK Min-Ouk, et al. Development of boron doped diamond electrodes material for heavy metal ion sensor with high sensitivity and durability[J]. Journal of Materials Research and Technology, 2023, 23: 1375-1385. |
| 34 | 陈卓, 熊鹰, 王兵, 等. 大尺寸石墨基BDD涂层电极的MPCVD法沉积[J]. 稀有金属, 2021, 45(7): 828-835. |
| CHEN Zhuo, XIONG Ying, WANG Bing, et al. Deposition of BDD film on graphite substrate with large size by MPCVD method[J]. Chinese Journal of Rare Metals, 2021, 45(7): 828-835. | |
| 35 | 陈良贤, 邵思武, 刘鹏, 等. 直流电弧等离子体喷射化学气相沉积法制备金刚石膜过程中冷阱的设计及作用[J]. 金刚石与磨料磨具工程, 2022, 42(2): 150-155. |
| CHEN Liangxian, SHAO Siwu, LIU Peng, et al. Design and function of cold trap in the process of preparing diamond films by DC arc plasma jet chemical vapor deposition[J]. Diamond & Abrasives Engineering, 2022, 42(2): 150-155. | |
| 36 | MACPHERSON J V. A practical guide to using boron doped diamond in electrochemical research[J]. Physical Chemistry Chemical Physics, 2015, 17(5): 2935-2949. |
| 37 | 王生艳, 李尚升, 刘书强, 等. p型半导体金刚石研究现状[J]. 硅酸盐通报, 2015, 34(S1): 243-247. |
| WANG Shengyan, LI Shangsheng, LIU Shuqiang, et al. Research status of p type semiconductor diamond[J]. Bulletin of the Chinese Ceramic Society, 2015, 34(S1): 243-247. | |
| 38 | 王志伟, 邹芹, 李艳国, 等. 硼及其协同掺杂金刚石薄膜的研究[J]. 金刚石与磨料磨具工程, 2019, 39(4): 1-8. |
| WANG Zhiwei, ZOU Qin, LI Yanguo, et al. Study on boron and its co-doped diamond films[J]. Diamond & Abrasives Engineering, 2019, 39(4): 1-8. | |
| 39 | YU Peng, ZHANG Jiawei, ZHENG Tong, et al. Influence of boron doped level on the electrochemical behavior of boron doped diamond electrodes and uric acid detection[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 494: 241-247. |
| 40 | 宋来洲, 田彩利, 卢红叶, 等. 掺硼金刚石薄膜电极阳极电催化氧化苯酚废水研究[J]. 燕山大学学报, 2018, 42(5): 458-464. |
| SONG Laizhou, TIAN Caili, LU Hongye, et al. Electrochemical oxidation of phenol wastes with boron-doped diamond anodes[J]. Journal of Yanshan University, 2018, 42(5): 458-464. | |
| 41 | 张韬, 薛喆, 万方, 等. 微米及纳米WC-Co基BDD污水处理电极的制备研究[J]. 人工晶体学报, 2023, 52(2): 354-362. |
| ZHANG Tao, XUE Zhe, WAN Fang, et al. Preparation of micro- and nano-WC-Co/BDD electrodes for wastewater treatment[J]. Journal of Synthetic Crystals, 2023, 52(2): 354-362. | |
| 42 | DE TOLEDO W D M C, PINHEIRO R A, TRAVA-AIROLDI V J, et al. Development of boron-doped diamond (BDD) deposited on carbon nanotubes (CNT) to form BDD/CNT structures relevant for electrochemical degradation[J]. Diamond and Related Materials, 2022, 127: 109159. |
| 43 | 邹广田, 于三, 金曾孙, 等. 半导体金刚石薄膜的制备及性质研究[J]. 功能材料, 1991, 22(2): 70-73, 127. |
| ZOU Guangtian, YU San, JIN Zengsun, et al. The preparation and investigation of properties of semiconductor diamond films[J]. Journal of Functional Materials, 1991, 22(2): 70-73, 127. | |
| 44 | ZOU Guangtian, YU San, JIN Zengsun, et al. Optical properties of boron-doped diamond films[C]. San Diego, CA, USA: Diamond Optics V. 1992, 1759: 224-228. |
| 45 | LE LUU Tran. Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes[J]. Environmental Technology & Innovation, 2020, 20: 101099. |
| 46 | 翟健, 张峰, 孙智宇, 等. 基底材质对于BDD电极电解含溴水时溴酸盐生成的影响[J]. 应用化工, 2020, 49(3): 555-560. |
| ZHAI Jian, ZHANG Feng, SUN Zhiyu, et al. Effect of base material on bromate formation in electrolysis of bromine-containing water at BDD electrodes[J]. Applied Chemical Industry, 2020, 49(3): 555-560. | |
| 47 | SANDOVAL M A, CALZADILLA W, SALAZAR R. Influence of reactor design on the electrochemical oxidation and disinfection of wastewaters using boron-doped diamond electrodes[J]. Current Opinion Electrochemistry, 2022, 33: 100939. |
| 48 | MONTENEGRO-AYO R, PÉREZ T, LANZA M R V, et al. New electrochemical reactor design for emergent pollutants removal by electrochemical oxidation[J]. Electrochimica Acta, 2023, 458: 142551. |
| 49 | YAO Kaili, TAN Xiaojun, DAI Bing, et al. Au nanospheres modified boron-doped diamond microelectrode grown via hydrogen plasma etching solid doping source for dopamine detection[J]. Journal of Materials Science & Technology, 2020, 49: 42-46. |
| 50 | MEI Ruiqiong, WEI Qiuping, ZHU Chengwu, et al. 3D macroporous boron-doped diamond electrode with interconnected liquid flow channels: A high-efficiency electrochemical degradation of RB-19 dye wastewater under low current[J]. Applied Catalysis B: Environmental, 2019, 245: 420-427. |
| 51 | SUO Ni, HUANG Hao, WU Aimin, et al. Porous boron doped diamonds as metal-free catalysts for the oxygen reduction reaction in alkaline solution[J]. Applied Surface Science, 2018, 439: 329-335. |
| 52 | 刘婷, 苗冬田, 魏秋平, 等. 高温刻蚀硼掺杂金刚石电极材料的形貌与电化学性能[J]. 粉末冶金材料科学与工程, 2020, 25(2): 164-170. |
| LIU Ting, MIAO Dongtian, WEI Qiuping, et al. Morphology and electrochemical performance of high temperature etching boron doped diamond electrode materials[J]. Materials Science and Engineering of Powder Metallurgy, 2020, 25(2): 164-170. | |
| 53 | ZHUO Qiongfang, WANG Jinbao, NIU Junfeng, et al. Electrochemical oxidation of perfluorooctane sulfonate (PFOS) substitute by modified boron doped diamond (BDD) anodes[J]. Chemical Engineering Journal, 2020, 379: 122280. |
| 54 | ZHANG Jing, ZHAO Zhiyan, ZHANG Zhiqiang, et al. Construction of flexible fiber-shaped boron-doped diamond film and its supercapacitor application[J]. Journal of Colloid and Interface Science, 2023, 629: 813-821. |
| 55 | 杨志亮, 鲁新如, 徐健, 等. 硼掺杂金刚石厚膜电极对高浓度工业废水的降解实验研究[J]. 表面技术, 2021, 50(3): 212-218. |
| YANG Zhiliang, LU Xinru, XU Jian, et al. Experimental study on degradation of high concentration organic wastewater using thick boron doped diamond film electrodes[J]. Surface Technology, 2021, 50(3): 212-218. | |
| 56 | BANSAL R, VERDUZCO R, WONG M S, et al. Development of nano boron-doped diamond electrodes for environmental applications[J]. Journal of Electroanalytical Chemistry, 2022, 907: 116028. |
| 57 | 庞悦, 楼洪海, 郜睿, 等. 芬顿氧化深度处理实际印染废水研究[J]. 水处理技术, 2023, 49(5): 40-44. |
| PANG Yue, LOU Honghai, GAO Rui, et al. Study on advanced treatment of dyeing wastewater by Fenton oxidation[J]. Technology of Water Treatment, 2023, 49(5): 40-44. | |
| 58 | DÓRIA Aline Resende, PUPO Marilia, DE OLIVEIRA SANTIAGO SANTOS Géssica, et al. Electrochemical oxidation of indanthrene blue dye in a filter-press flow reactor and toxicity analyses with Raphidocelis subcapitata and Lactuca sativa [J]. Ecotoxicology and Environmental Safety, 2020, 198: 110659. |
| 59 | RUIZ E J, HERNÁNDEZ-RAMÍREZ A, PERALTA-HERNÁNDEZ J M, et al. Application of solar photoelectro-Fenton technology to azo dyes mineralization: Effect of current density, Fe2+ and dye concentrations[J]. Chemical Engineering Journal, 2011, 171(2): 385-392. |
| 60 | SALAZAR R, GARCIA-SEGURA S, URETA-ZAÑARTU M S, et al. Degradation of disperse azo dyes from waters by solar photoelectro-Fenton[J]. Electrochimica Acta, 2011, 56(18): 6371-6379. |
| 61 | 任梦娇, 彭永丽, 赵贺芳, 等. 电催化氧化降解高浓度农药废水的研究[J]. 水处理技术, 2022, 48(10): 115-120. |
| REN Mengjiao, PENG Yongli, ZHAO Hefang, et al. Study on the degradation of high-concentration pesticide wastewater by electrocatalytic oxidation[J]. Technology of Water Treatment, 2022, 48(10): 115-120. | |
| 62 | DOMÍNGUEZ J R, GONZÁLEZ T, CORREIA S. BDD electrochemical oxidation of neonicotinoid pesticides in natural surface waters. Operational, kinetic and energetic aspects[J]. Journal of Environmental Management, 2021, 298: 113538. |
| 63 | Orlando GARCÍA, Eloy ISARAIN-CHÁVEZ, Sergi GARCIA-SEGURA, et al. Degradation of 2,4-dichlorophenoxyacetic acid by electro-oxidation and electro-Fenton/BDD processes using a pre-pilot plant[J]. Electrocatalysis, 2013, 4(4): 224-234. |
| 64 | SÁNCHEZ-MONTES I, PÉREZ J F, SÁEZ C, et al. Assessing the performance of electrochemical oxidation using DSA® and BDD anodes in the presence of UVC light[J]. Chemosphere, 2020, 238: 124575. |
| 65 | PIPI A R F, SIRÉS I, DE ANDRADE A R, et al. Application of electrochemical advanced oxidation processes to the mineralization of the herbicide diuron[J]. Chemosphere, 2014, 109: 49-55. |
| 66 | MONTEIL Hélène, PECHAUD Yoan, OTURAN Nihal, et al. Pilot scale continuous reactor for water treatment by electrochemical advanced oxidation processes: Development of a new hydrodynamic/reactive combined model[J]. Chemical Engineering Journal, 2021, 404: 127048. |
| 67 | Eloy ISARAIN-CHÁVEZ, RODRÍGUEZ Rosa María, CABOT Pere Lluís, et al. Degradation of pharmaceutical beta-blockers by electrochemical advanced oxidation processes using a flow plant with a solar compound parabolic collector[J]. Water Research, 2011, 45(14): 4119-4130. |
| 68 | TAWABINI B S, PLAKAS K V, FRAIM M, et al. Assessing the efficiency of a pilot-scale GDE/BDD electrochemical system in removing phenol from high salinity waters[J]. Chemosphere, 2020, 239: 124714. |
| 69 | Sergi GARCIA-SEGURA, BRILLAS Enric. Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode[J]. Water Research, 2011, 45(9): 2975-2984. |
| 70 | HAO Yongyong, MA Hongrui, PROIETTO Federica, et al. Electrochemical treatment of wastewater contaminated by organics and containing chlorides: Effect of operative parameters on the abatement of organics and the generation of chlorinated by-products[J]. Electrochimica Acta, 2022, 402: 139480. |
| 71 | DURÁN Florymar Escalona, DE ARAÚJO Danyelle Medeiros, NASCIMENTO BRITO Chrystiane DO, et al. Electrochemical technology for the treatment of real washing machine effluent at pre-pilot plant scale by using active and non-active anodes[J]. Journal of Electroanalytical Chemistry, 2018, 818: 216-222. |
| 72 | TAWABINI B S, PLAKAS K V, KARABELAS A J. A pilot study of BTEX removal from highly saline water by an advanced electrochemical process[J]. Journal of Water Process Engineering, 2020, 37: 101427. |
| 73 | TSANTAKI Eleni, VELEGRAKI Theodora, KATSAOUNIS Alexandros, et al. Anodic oxidation of textile dyehouse effluents on boron-doped diamond electrode[J]. Journal of Hazardous Materials, 2012, 207/208: 91-96. |
| 74 | PANIZZA M, MICHAUD P A, CERISOLA G, et al. Anodic oxidation of 2-naphthol at boron-doped diamond electrodes[J]. Journal of Electroanalytical Chemistry, 2001, 507(1/2): 206-214. |
| 75 | DOMÍNGUEZ J R, GONZÁLEZ T, PALO P, et al. Electrochemical degradation of a real pharmaceutical effluent[J]. Water, Air, & Soil Pollution, 2012, 223(5): 2685-2694. |
| 76 | MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Remediation of a winery wastewater combining aerobic biological oxidation and electrochemical advanced oxidation processes[J]. Water Research, 2015, 75: 95-108. |
| 77 | SALAZAR Claudio, Ignasi SIRÉS, SALAZAR Ricardo, et al. Treatment of cellulose bleaching effluents and their filtration permeates by anodic oxidation with H2O2 production[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(11): 2017-2026. |
| 78 | ISARAIN-CHÁVEZ E, DE LA ROSA C, GODÍNEZ L A, et al. Comparative study of electrochemical water treatment processes for a tannery wastewater effluent[J]. Journal of Electroanalytical Chemistry, 2014, 713: 62-69. |
| 79 | DU Xiaoming, ZHANG Zhefeng, ZHANG Chunyong, et al. Definitive screening design applied to electrochemical degradation of Chromotrope 2R with BDD anodes[J]. Chemosphere, 2017, 171: 362-369. |
| 80 | ZHANG Chunyong, DU Xiaoming, ZHANG Zhefeng, et al. The peculiar roles of chloride electrolytes in BDD anode cells[J]. RSC Advances, 2016, 6(70): 65638-65643. |
| 81 | ZHANG Chunyong, HE Zhenzhu, WU Jingyu, et al. The peculiar roles of sulfate electrolytes in BDD anode cells[J]. Journal of the Electrochemical Society, 2015, 162(8): 85-89. |
| [1] | 陈瑶姬, 任成瑜, 胡达清, 卢晗锋, 葛春亮, 崔国凯. 离子液体强化一氧化碳转化[J]. 化工进展, 2024, 43(1): 124-134. |
| [2] | 罗芬, 杨晓琪, 段方麟, 李小江, 吴亮, 徐铜文. 双极膜研究进展及应用展望[J]. 化工进展, 2024, 43(1): 145-163. |
| [3] | 于笑笑, 巢艳红, 刘海燕, 朱文帅, 刘植昌. D-A共轭聚合强化光电性能及光催化CO2转化[J]. 化工进展, 2024, 43(1): 292-301. |
| [4] | 于松民, 金洪波, 杨明虎, 余海峰, 江浩. 氟掺杂改性LiMn0.5Fe0.5PO4正极材料及其电化学性能[J]. 化工进展, 2024, 43(1): 302-309. |
| [5] | 夏银萍, 李洲鹏, 汪倩倩. 高载量锂硫电池正极设计优化[J]. 化工进展, 2024, 43(1): 364-375. |
| [6] | 杨雪, 刘可, 张程翔, 李东霖, 王江芹, 杨万亮. 2D层状材料的燃料油氧化脱硫研究进展[J]. 化工进展, 2024, 43(1): 422-436. |
| [7] | 杨成功, 黄蓉, 王冬娥, 田志坚. 氮掺杂二硫化钼纳米催化剂的电催化析氢性能[J]. 化工进展, 2024, 43(1): 465-472. |
| [8] | 杨梦茹, 彭琴, 常玉龙, 邱淑兴, 张溅波, 江霞. 生物炭替代煤粉/焦炭高炉炼铁碳减排技术研究进展[J]. 化工进展, 2024, 43(1): 490-500. |
| [9] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [10] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [11] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [14] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [15] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||