化工进展 ›› 2023, Vol. 42 ›› Issue (9): 4677-4691.DOI: 10.16085/j.issn.1000-6613.2022-2004
质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展
- 中国石化石油化工科学研究院有限公司,北京 100083
-
收稿日期:2022-10-27修回日期:2023-01-20出版日期:2023-09-15发布日期:2023-09-28 -
通讯作者:荣峻峰 -
作者简介:张启(1998—),男,硕士研究生,研究方向为氢燃料电池。E-mail:andy1106@vip.qq.com。 -
基金资助:中国石油化工股份有限公司合同项目(ST14071);中国石油化工股份有限公司课题(222067)
Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC
ZHANG Qi( ), ZHAO Hong, RONG Junfeng(
), ZHAO Hong, RONG Junfeng( )
)
- SINOPEC Research Institute of Petroleum Processing Co. , Ltd. , Beijing 100083, China
-
Received:2022-10-27Revised:2023-01-20Online:2023-09-15Published:2023-09-28 -
Contact:RONG Junfeng
摘要:
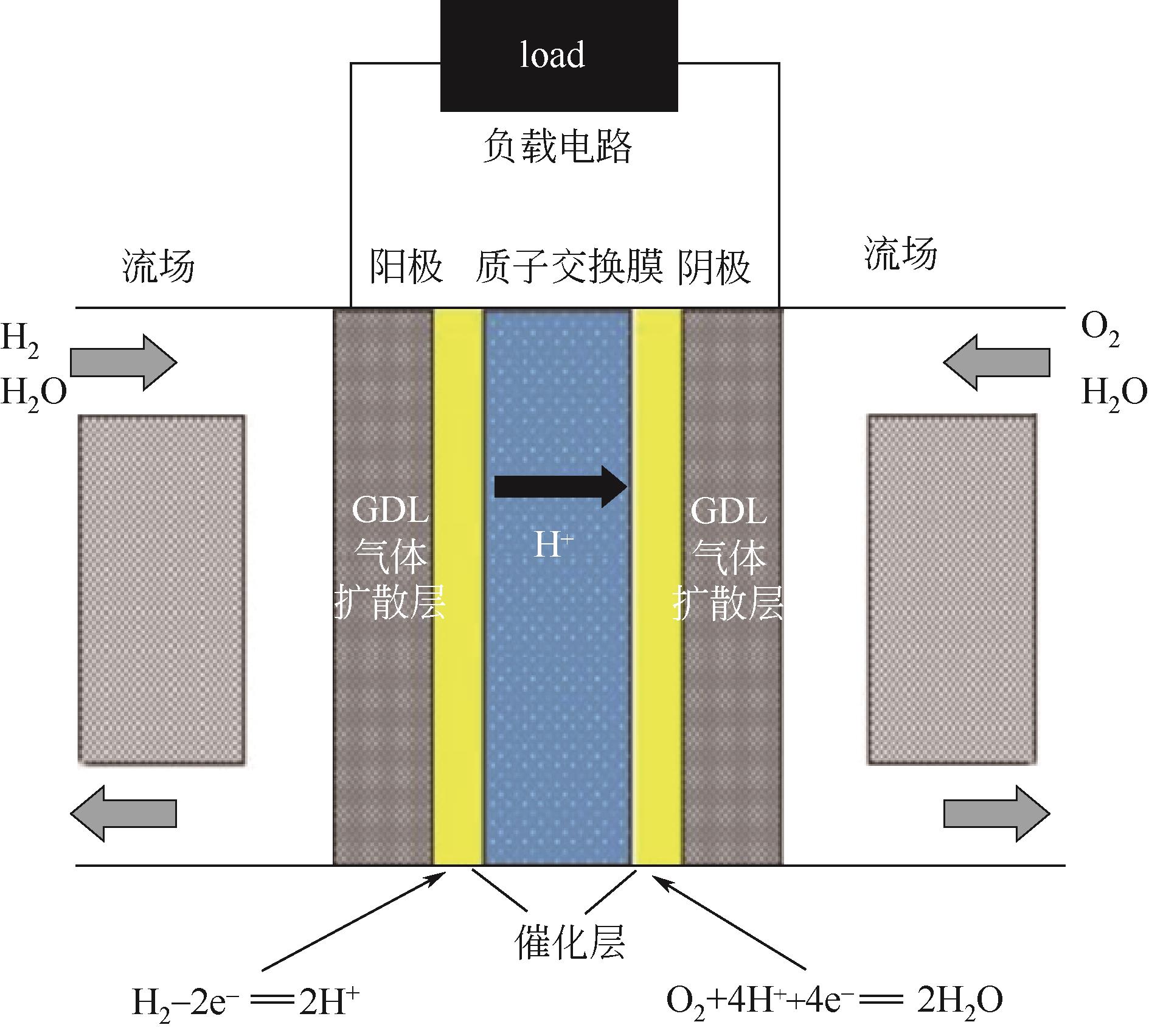
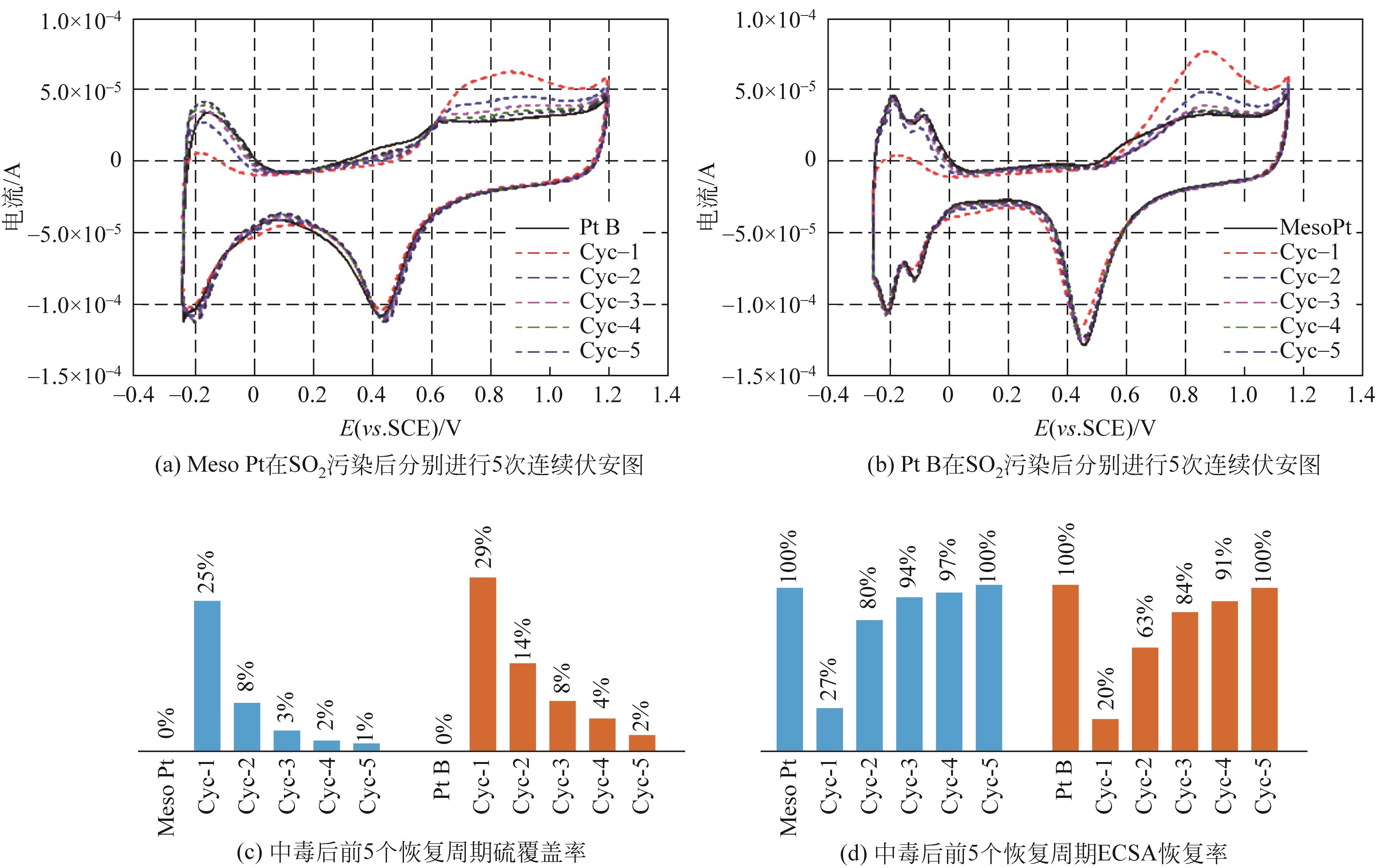
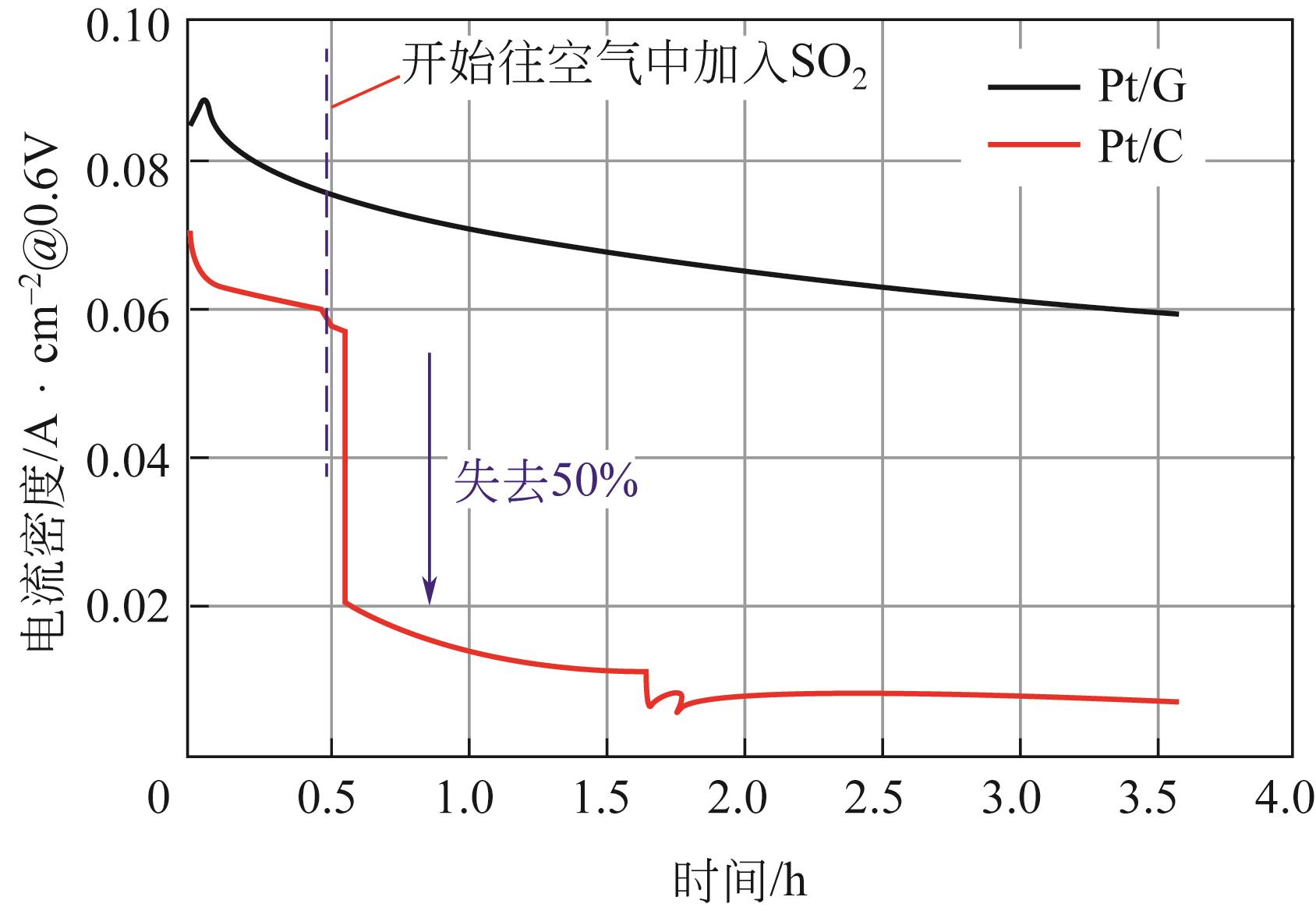
在质子交换膜燃料电池(proton exchange membrane fuel cell,PEMFC)中,出于成本和实用性等方面的考虑,氧还原反应所需氧气基本都来自于空气。而空气中存在的污染物,如SO2、NO2等都会导致阴极Pt催化剂中毒。因此,提高催化剂的抗毒性就成为推动PEMFC规模化实际应用的关键问题之一。本文介绍了PEMFC氧还原催化剂抗毒性的研究进展,并分析其抗中毒机理。目前,抗毒性阴极催化剂已取得一定的突破,其主要可分为铂基改性催化剂、非贵金属催化剂和非金属碳基催化剂,通过抑制毒化物与铂之间的电子作用、增强溢出效应、锚定金属原子、引入杂原子(如氮、磷)、采用不易中毒的金属等方式可增强催化剂的抗毒性。与此同时,抗毒性催化剂也面临一定的问题,如催化剂导电性下降、活性降低、制备复杂等。如何在保持催化剂活性和稳定性的同时,开发出具有良好抗毒性的催化剂已成为研究人员的重点研究方向。
中图分类号:
引用本文
张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691.
ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691.
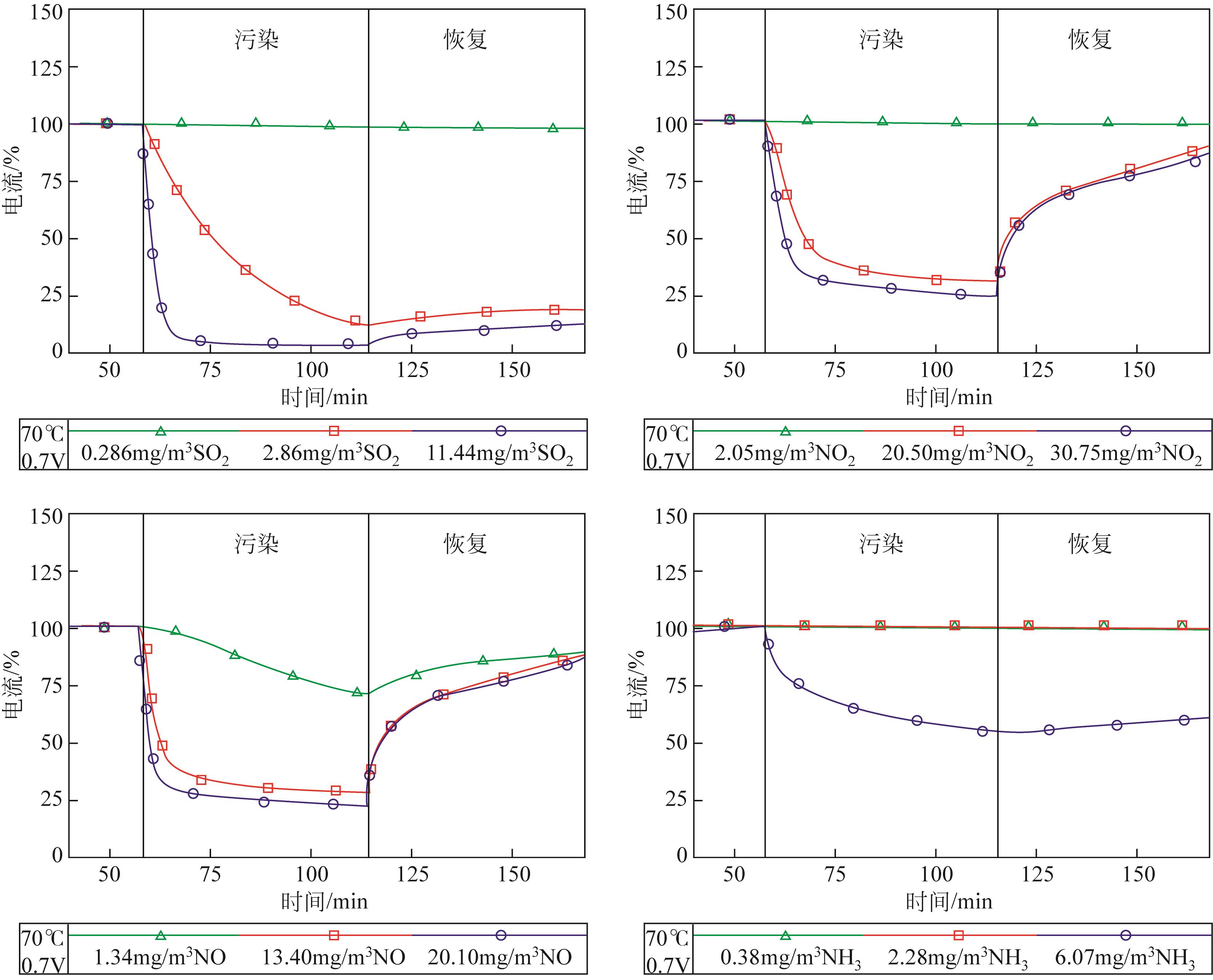
| 污染物 | 浓度/mg·m-3 | 最大电流降/% | 最小电流降/% | 平均电流降/% |
|---|---|---|---|---|
| SO2 | 0.286 | 6 | 0 | 2 |
| 2.86 | 88 | 53 | 62 | |
| 11.44 | 95 | 67 | 85 | |
| NO2 | 2.05 | 13 | 1 | 4 |
| 20.50 | 91 | 31 | 66 | |
| 30.75 | 95 | 42 | 69 | |
| NO | 1.34 | 63 | 11 | 33 |
| 13.40 | 96 | 36 | 67 | |
| 20.10 | 97 | 43 | 72 | |
| NH3 | 0.759 | 4 | 0 | 1 |
| 2.28 | 34 | 0 | 9 | |
| 6.072 | 75 | 30 | 51 |
表1 污染物浓度对电流的影响[14]
| 污染物 | 浓度/mg·m-3 | 最大电流降/% | 最小电流降/% | 平均电流降/% |
|---|---|---|---|---|
| SO2 | 0.286 | 6 | 0 | 2 |
| 2.86 | 88 | 53 | 62 | |
| 11.44 | 95 | 67 | 85 | |
| NO2 | 2.05 | 13 | 1 | 4 |
| 20.50 | 91 | 31 | 66 | |
| 30.75 | 95 | 42 | 69 | |
| NO | 1.34 | 63 | 11 | 33 |
| 13.40 | 96 | 36 | 67 | |
| 20.10 | 97 | 43 | 72 | |
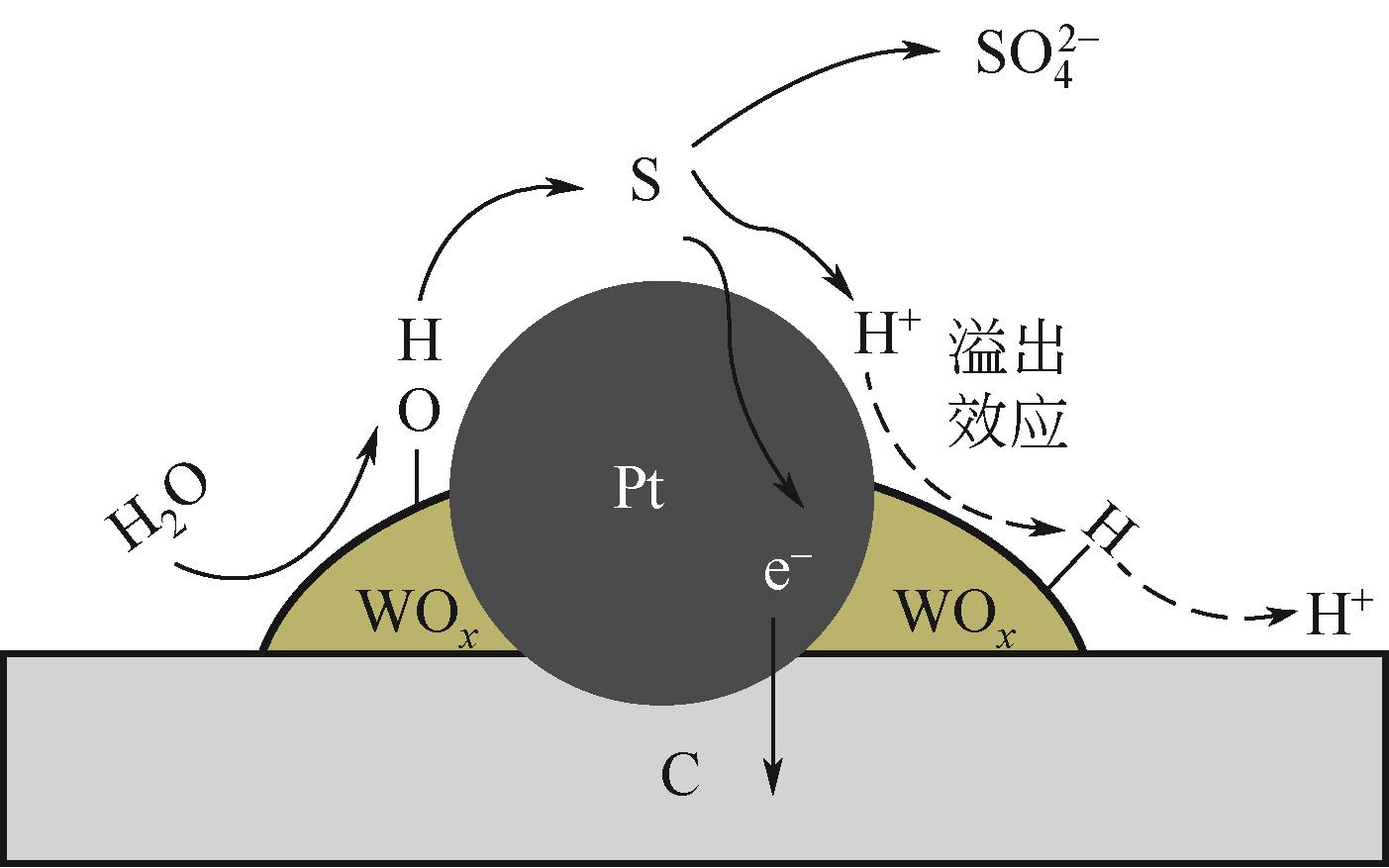
| NH3 | 0.759 | 4 | 0 | 1 |
| 2.28 | 34 | 0 | 9 | |
| 6.072 | 75 | 30 | 51 |
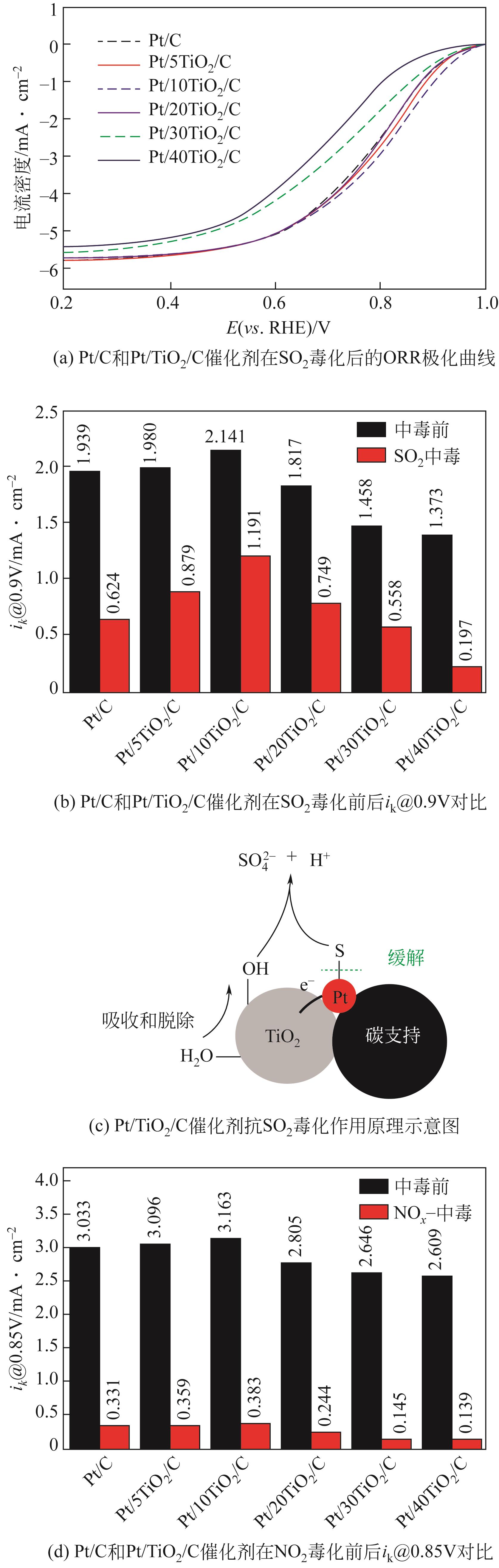
| 结构 | 吸附能 /kJ·mol-1 | 距离/nm | ||
|---|---|---|---|---|
| S/Pt(111) | -604.0 | 0.2310 | 0.2311 | 0.2312 |
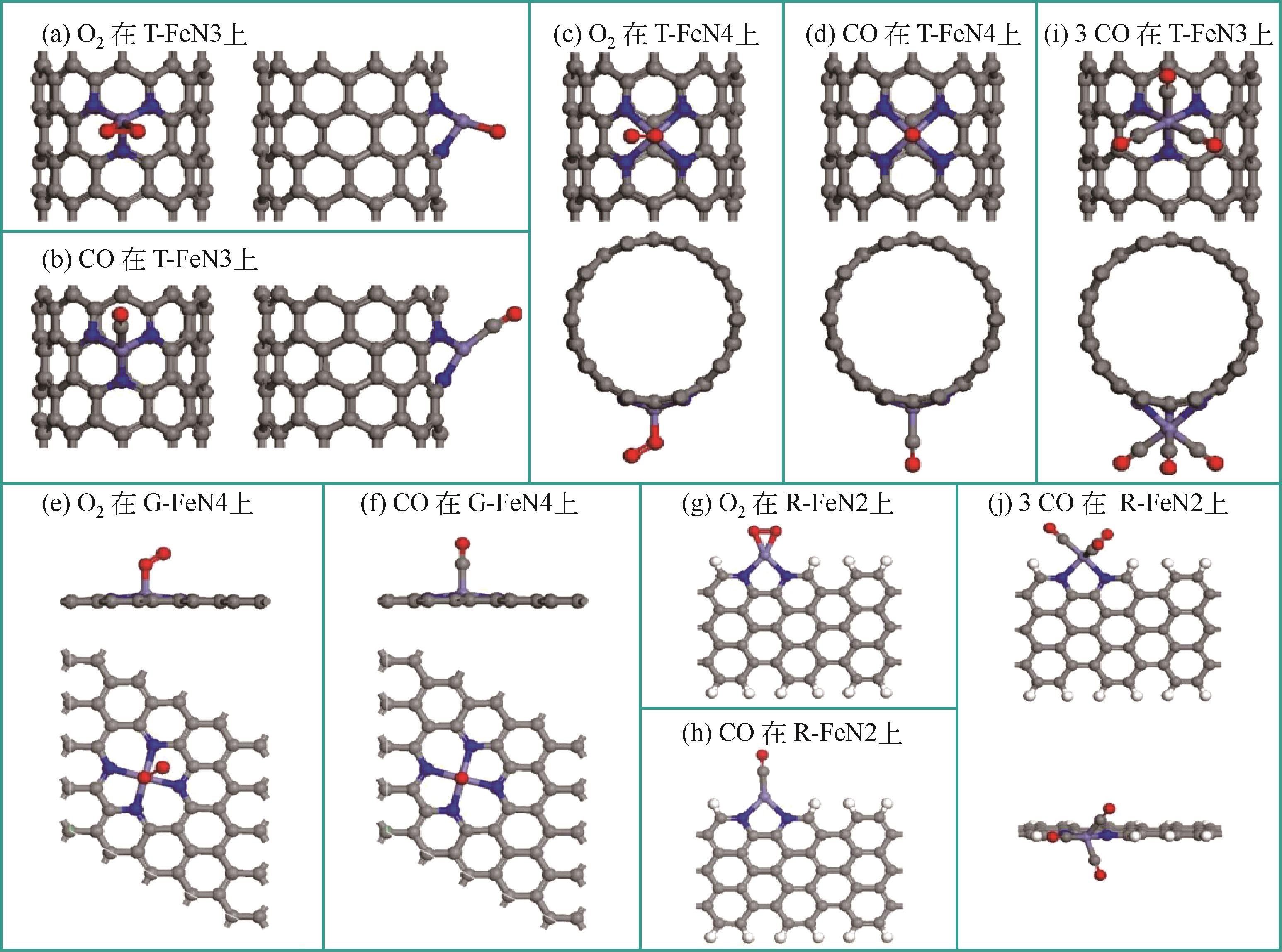
| S/PtMo(111) | -466.8 | 0.2353 | 0.2355 | 0.2354(S—Mo) |
| SO3/Pt(111) | -282.8 | 0.2291 | 0.2153(O—Pt) | 0.2160(O—Pt) |
| SO3/PtMo(111) | -114.6 | 0.2222 | 0.2130(O—Pt) | 0.1964(O—Mo) |
表2 S和SO3在Pt(111)和PtMo(111)上的吸附能和距离[42]
| 结构 | 吸附能 /kJ·mol-1 | 距离/nm | ||
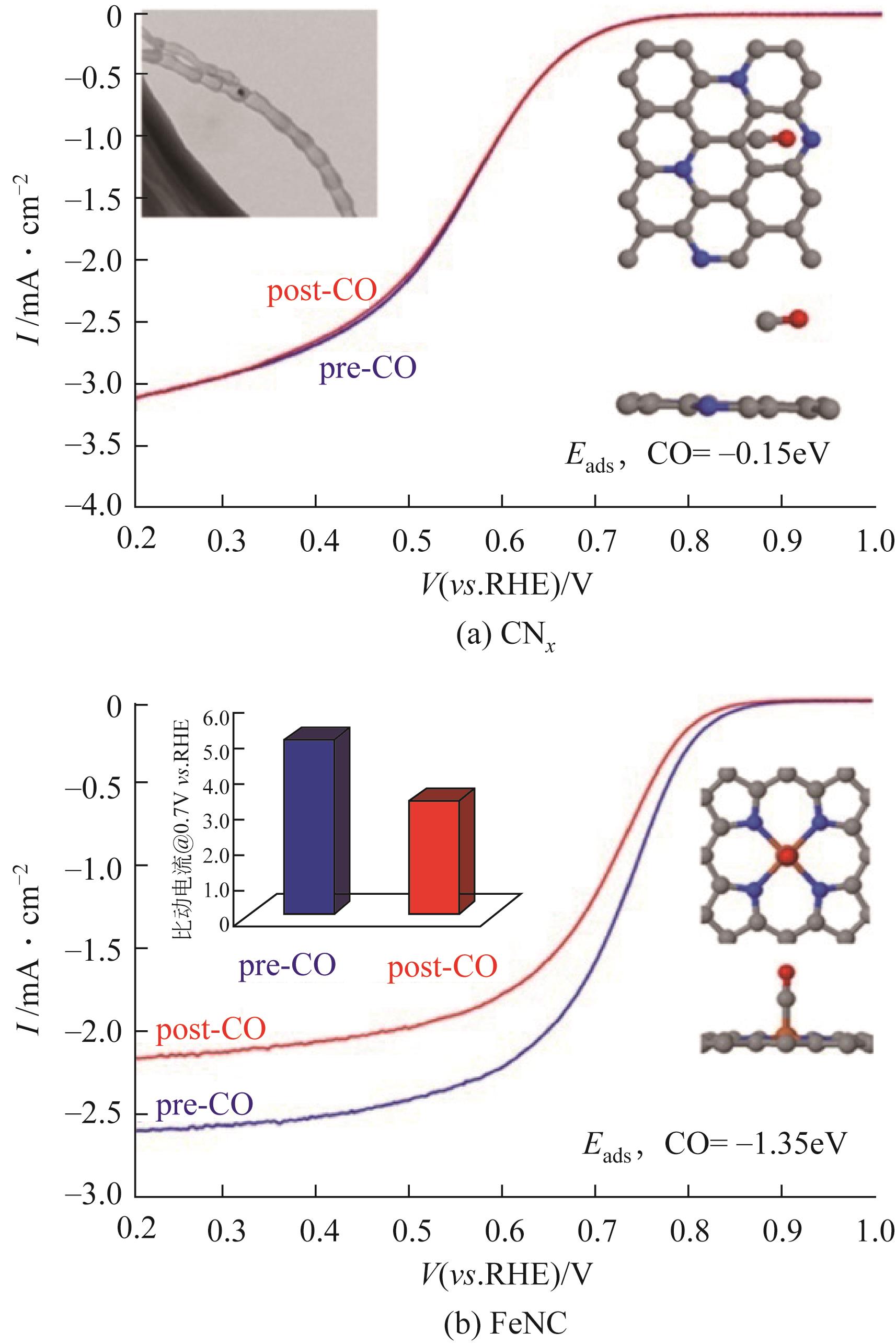
|---|---|---|---|---|
| S/Pt(111) | -604.0 | 0.2310 | 0.2311 | 0.2312 |
| S/PtMo(111) | -466.8 | 0.2353 | 0.2355 | 0.2354(S—Mo) |
| SO3/Pt(111) | -282.8 | 0.2291 | 0.2153(O—Pt) | 0.2160(O—Pt) |
| SO3/PtMo(111) | -114.6 | 0.2222 | 0.2130(O—Pt) | 0.1964(O—Mo) |
毒化物浓度 /mol·L-1 | Pt/C | 5% WO x -Pt/C | ||
|---|---|---|---|---|
| 初始覆盖率 | 损失程度/% | 初始覆盖率 | 损失程度/% | |
| 0.001 | 0.50±0.04 | 37±2 | 0.53±0.0.4 | 45±2 |
| 0.01 | 0.84±0.06 | 45±3 | 0.85±0.05 | 50±2 |
| 0.1 | 1.00±0.07 | 43±3 | 1.00±0.06 | 47±3 |
表3 Pt/C与质量分数5% WO x -Pt/C在经过一定浓度毒化物毒化后的SO x 初始覆盖率和损失程度[48]
毒化物浓度 /mol·L-1 | Pt/C | 5% WO x -Pt/C | ||
|---|---|---|---|---|
| 初始覆盖率 | 损失程度/% | 初始覆盖率 | 损失程度/% | |
| 0.001 | 0.50±0.04 | 37±2 | 0.53±0.0.4 | 45±2 |
| 0.01 | 0.84±0.06 | 45±3 | 0.85±0.05 | 50±2 |
| 0.1 | 1.00±0.07 | 43±3 | 1.00±0.06 | 47±3 |
| 吸附物 | 吸附能/eV | ||
|---|---|---|---|
| Fe-N4 | Fe-N2C2 | N掺杂石墨烯 | |
| O2 | -1.01 | -2.34 | -0.15 |
| SO2 | -0.81 | -1.63 | -0.17 |
| NO | -1.97 | -3.37 | -0.09 |
| NO2 | -1.45 | -2.63 | -0.43 |
| CO | -1.61 | -2.09 | -0.01 |
表4 Fe-N4、Fe-N2C2和N掺杂石墨烯对O2、NO、NO2、CO和SO2的吸附能[54]
| 吸附物 | 吸附能/eV | ||
|---|---|---|---|
| Fe-N4 | Fe-N2C2 | N掺杂石墨烯 | |
| O2 | -1.01 | -2.34 | -0.15 |
| SO2 | -0.81 | -1.63 | -0.17 |
| NO | -1.97 | -3.37 | -0.09 |
| NO2 | -1.45 | -2.63 | -0.43 |
| CO | -1.61 | -2.09 | -0.01 |
| 1 | 毛宗强. 燃料电池[M]. 北京: 化学工业出版社, 2005: 11-12. |
| MAO Zongqiang. Fuel cell[M]. Beijing: Chemical Industry Press, 2005: 11-12. | |
| 2 | 衣宝廉. 燃料电池的原理、技术状态与展望[J]. 电池工业, 2003, 8(1): 16-22. |
| YI Baolian. Fuel cell: Fundamental, technology and prospect[J]. Battery Industry, 2003, 8(1): 16-22. | |
| 3 | LIU Hansan, SONG Chaojie, ZHANG Lei, et al. A review of anode catalysis in the direct methanol fuel cell[J]. Journal of Power Sources, 2006, 155(2): 95-110. |
| 4 | Jung-Ho WEE, LEE Kwan-Young, KIM Sung Hyun. Fabrication methods for low-Pt-loading electrocatalysts in proton exchange membrane fuel cell systems[J]. Journal of Power Sources, 2007, 165(2): 667-677. |
| 5 | BOCKRIS J O M, SRINIVASAN S. Fuel cells: Their electrochemistry[M]. New York: McGraw Hill, 1969:469. |
| 6 | 郭琳. 高性能铂基燃料电池催化剂研究[D]. 重庆: 重庆大学, 2015. |
| GUO Lin. Studies on high performance Pt-based catalysts for PEMFC[D]. Chongqing: Chongqing University, 2015. | |
| 7 | 杨博龙, 韩清, 向中华. 质子交换膜燃料电池膜电极结构与设计研究进展[J]. 化工进展, 2021, 40(9): 4882-4893. |
| YANG Bolong, HAN Qing, XIANG Zhonghua. Progress of membrane electrode structure and its design for proton exchange membrane fuel cell[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4882-4893. | |
| 8 | ZHONG Chuanjian, LUO Jin, NJOKI P N, et al. Fuelcell technology: Nano-engineered multimetallic catalysts[J]. Energy & Environmental Science, 2008, 1(4): 454-466. |
| 9 | GASTEIGER H A, MARKOVIC N M, ROSS P N JR. H2 and CO electrooxidation on well-characterized Pt, Ru, and Pt-Ru. 1. rotating disk electrode studies of the pure gases including temperature effects[J]. The Journal of Physical Chemistry, 1995, 99(20): 8290-8301. |
| 10 | DE BRUIJN F. The Current status of fuel cell technology for mobile and stationary applications[J]. Green Chemistry, 2005, 7(3): 132-150. |
| 11 | DE BRUIJN F A, PAPAGEORGOPOULOS D C, SITTERS E F, et al. The influence of carbon dioxide on PEM fuel cell anodes[J]. Journal of Power Sources, 2002, 110(1): 117-124. |
| 12 | NAGAHARA Y, SUGAWARA S, SHINOHARA K. The impact of air contaminants on PEMFC performance and durability[J]. Journal of Power Sources, 2008, 182(2): 422-428. |
| 13 | PILLAY D, JOHANNES M D, GARSANY Y, et al. Poisoning of Pt3Co electrodes: A combined experimental and DFT study[J]. The Journal of Physical Chemistry C, 2010, 114(17): 7822-7830. |
| 14 | MISZ U, TALKE A, HEINZEL A, et al. Sensitivity analyses on the impact of air contaminants on automotive fuel cells[J]. Fuel Cells, 2016, 16(4): 444-462. |
| 15 | ZHANG Ling, LI Li, CHEN Hongmei, et al. Recent progress in precious metal-free carbon-based materials towards the oxygen reduction reaction: Activity, stability, and anti-poisoning[J]. Chemistry, 2020, 26(18): 3973-3990. |
| 16 | JING Fenning, HOU Ming, SHI Weiyu, et al. The effect of ambient contamination on PEMFC performance[J]. Journal of Power Sources, 2007, 166(1): 172-176. |
| 17 | CONTRACTOR A Q, LAL H. The nature of species adsorbed on platinum from SO2 solutions[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1978, 93(2): 99-107. |
| 18 | CONTRACTOR A Q, LAL H. Two forms of chemisorbed sulfur on platinum and related studies[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1979, 96(2): 175-181. |
| 19 | QUIJADA C, RODES A, HUERTA F, et al. In situ FT-IRRAS study of SO2 adlayers formed on Pt(111) electrodes from open-circuit adsorption in acidic media[J]. Electrochimica Acta, 1998, 44(6/7): 1091-1096. |
| 20 | LOUČKA T. Adsorption and oxidation of sulphur and of sulphur dioxide at the platinum electrode[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1971, 31(2): 319-332. |
| 21 | SZKLARCZYK M, CZERWINSKI A, SOBKOWSKI J. The study of electrode processes of sulphur dioxide on platinized electrode by the radiochemical method[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1982, 132: 263-271. |
| 22 | CONTRACTOR A Q, LAL H. Formic acid oxidation at platinized platinum electrodes[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1979, 103(1): 103-117. |
| 23 | GARSANY Y, BATURINA O A, SWIDER-LYONS K E. Impact of sulfur dioxide on the oxygen reduction reaction at Pt/Vulcan carbon electrocatalysts[J]. Journal of the Electrochemical Society, 2007, 154(7): B670. |
| 24 | NAJDEKER E, BISHOP E. The formation and behaviour of platinum sulphide on platinum electrodes[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1973, 41(1): 79-87. |
| 25 | HORÁNYI G, RIZMAYER E M. Radiotracer study of the adsorption of H2S at a platinized platinum electrode[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1986, 206(1/2): 297-305. |
| 26 | LAMY-PITARA E, TAINON Y, BEDEN B, et al. Nature and effects of sulphur adsorbed on platinum in acid medium[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1990, 279(1/2): 291-303. |
| 27 | LOUČKA T. Adsorption and oxidation of organic compounds on a platinum electrode partly covered by adsorbed sulphur[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1972, 36(2): 355-367. |
| 28 | LOUČKA T. The adsorption of sulphur and of simple organic substances on platinum electrodes[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1972, 36(2): 369-381. |
| 29 | M-V MATHIEU, PRIMET Michel. Sulfurization and regeneration of platinum[J]. Applied Catalysis, 1984, 9(3): 361-370. |
| 30 | QUIJADA C, RODES A, VÁZQUEZ J L, et al. Electrochemical behaviour of aqueous sulphur dioxide at polycrystalline Pt electrodes in acidic medium. A voltammetric and in situ FT-IR study Part Ⅱ. Promoted oxidation of sulphur dioxide. Reduction of sulphur dioxide[J]. Journal of Electroanalytical Chemistry, 1995, 398(1/2): 105-115. |
| 31 | QUIJADA C, RODES A, VÁZQUEZ J L, et al. Electrochemical behaviour of aqueous SO2 at Pt electrodes in acidic medium. A voltammetric and in situ Fourier transform IR study Part Ⅰ. Oxidation of SO2 on Pt electrodes with sulphur-oxygen adsorbed species[J]. Journal of Electroanalytical Chemistry, 1995, 394(1/2): 217-227. |
| 32 | FORAL M J, LANGER S H. Characterization of sulfur layers from reduced sulfur dioxide on porous platinum black/teflon electrodes[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1988, 246(1): 193-205. |
| 33 | RAMASUBRAMANIAN N. Anodic behavior of platinum electrodes in sulfide solutions and the formation of platinum sulfide[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1975, 64(1): 21-37. |
| 34 | HE Chunzhi, KUNZ H R, FENTON J M. Electro-oxidation of hydrogen with carbon monoxide on Pt/Ru-based ternary catalysts[J]. Journal of the Electrochemical Society, 2003, 150(8): A1017. |
| 35 | IGARASHI H, FUJINO T, WATANABE M. Hydrogen electro-oxidation on platinum catalysts in the presence of trace carbon monoxide[J]. Journal of Electroanalytical Chemistry, 1995, 391(1/2): 119-123. |
| 36 | RUSH B M, REIMER J A, CAIRNS E J. Nuclear magnetic resonance and voltammetry studies of carbon monoxide adsorption and oxidation on a carbon-supported platinum fuel cell electrocatalyst[J]. Journal of the Electrochemical Society, 2001, 148(2): A137. |
| 37 | WATANABE M, MOTOO S. Chemisorbed CO on a polycrystalline platinum electrode The effect of conditioning of the surface and of partial pressure of CO[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1986, 206(1/2): 197-208. |
| 38 | 徐峰. 质子交换膜燃料电池抗SO2/CO中毒催化剂的制备及其性能研究[D]. 武汉: 武汉理工大学, 2013. |
| XU Feng. Study on synthesis and characterization of SO2/CO poisoning resistant catalysts for proton exchange membrane fuel cell[D]. Wuhan: Wuhan University of Technology, 2013. | |
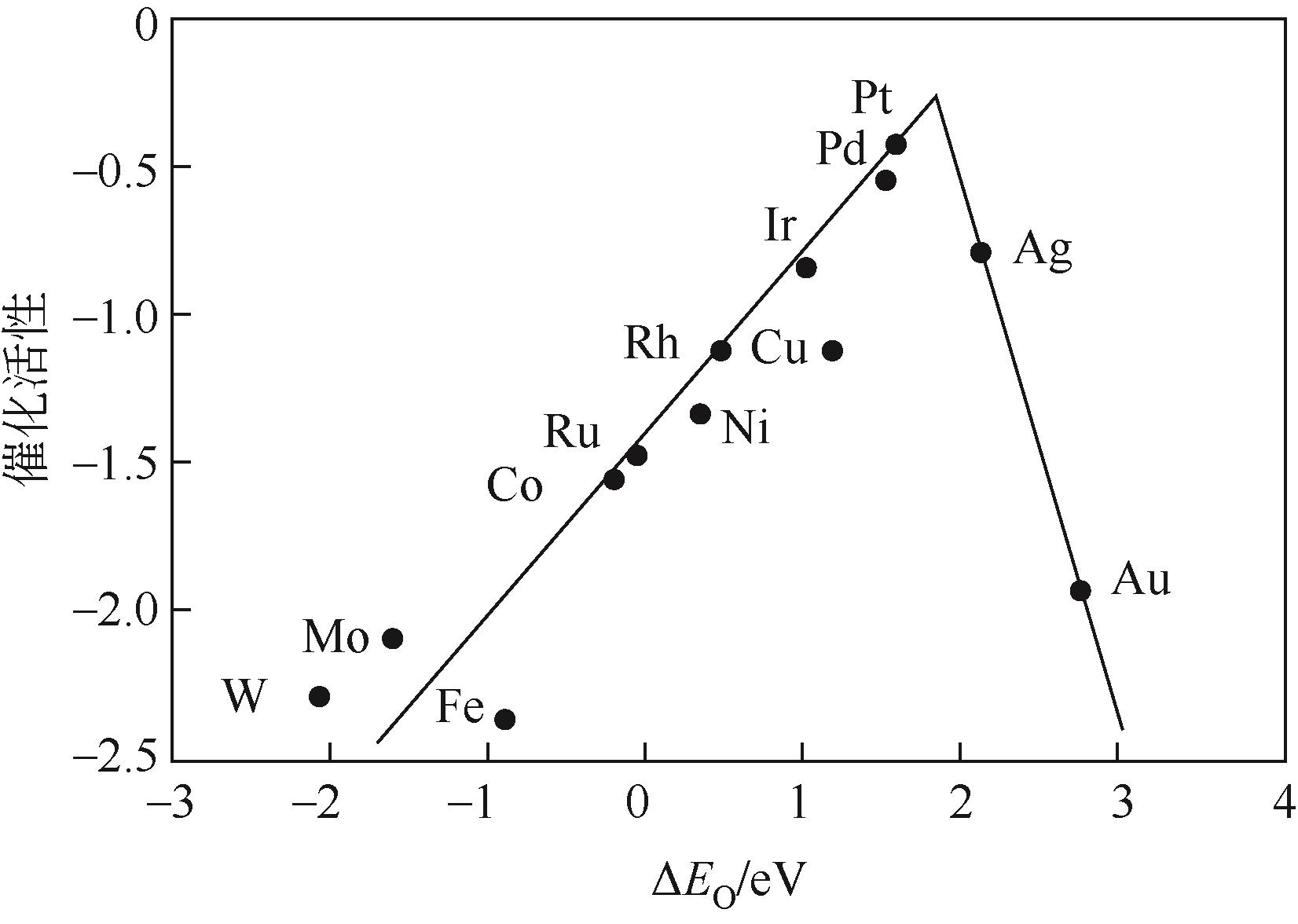
| 39 | NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. The Journal of Physical Chemistry B, 2004, 108(46): 17886-17892. |
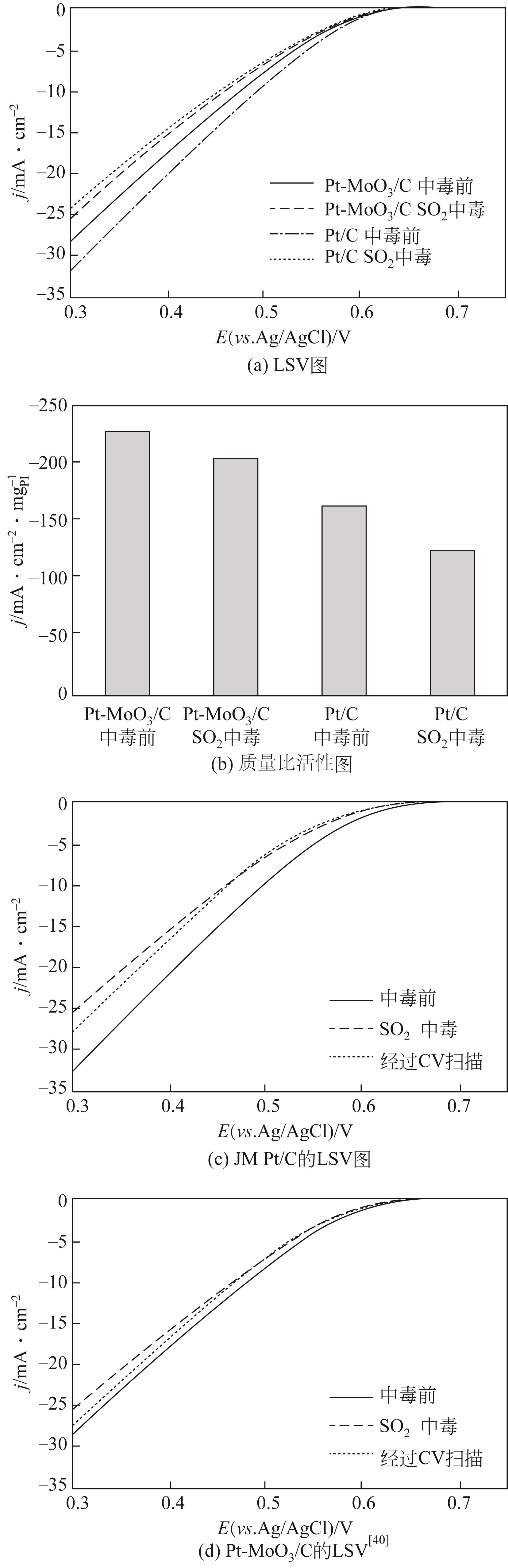
| 40 | LIU Xiao, WANG Hongmin, CHEN Siguo, et al. SO2-tolerant Pt-MoO3/C catalyst for oxygen reduction reaction[J]. Journal of Energy Chemistry, 2014, 23(3): 358-362. |
| 41 | 柳晓. 抗硫化物中毒及甲醇氧化燃料电池催化剂的研究[D]. 重庆: 重庆大学, 2012. |
| LIU Xiao. Sulfide-tolerant catalysts and methanol oxidation catalysts for fuel cells[D]. Chongqing: Chongqing University, 2012. | |
| 42 | XIA Meirong, LIU Ying, LI Li, et al. A DFT study on PtMo resistance to SO2 poisoning[J]. Science China Chemistry, 2013, 56(7): 1004-1008. |
| 43 | XU Feng, XU Ran, MU Shichun. Enhanced SO2 and CO poisoning resistance of CeO2 modified Pt/C catalysts applied in PEM fuel cells[J]. Electrochimica Acta, 2013, 112: 304-309. |
| 44 | PRITHI J A, RAJALAKSHMI N, DHATHATHEREYAN K S. Mesoporous platinum as sulfur-tolerant catalyst for PEMFC cathodes[J]. Journal of Solid State Electrochemistry, 2017, 21(12): 3479-3485. |
| 45 | KAHNG Yung Ho, TOBIN R G, LOLOEE R, et al. Sulfur surface chemistry on the platinum gate of a silicon carbide based hydrogen sensor[J]. Journal of Applied Physics, 2007, 102(6): 064505. |
| 46 | AWAD M I, SALEH M M, OHSAKA T. Electroactivity regeneration of sulfur-poisoned platinum nanoparticle-modified glassy carbon electrode at low anodic potentials[J]. Journal of Solid State Electrochemistry, 2015, 19(5): 1331-1340. |
| 47 | JAYARAJ P, KARTHIKA P, RAJALAKSHMI N, et al. Mitigation studies of sulfur contaminated electrodes for PEMFC[J]. International Journal of Hydrogen Energy, 2014, 39(23): 12045-12051. |
| 48 | XU Ran, XU Feng, PAN Mu, et al. Improving sulfur tolerance of noble metal catalysts by tungsten oxide-induced effects[J]. RSC Advances, 2013, 3(3): 764-773. |
| 49 | GARSANY Y, BATURINA O A, SWIDER-LYONS K E. Oxygen reduction reaction kinetics of SO2-contaminated Pt3Co and Pt/vulcan carbon electrocatalysts[J]. Journal of the Electrochemical Society, 2009, 156(7): B848. |
| 50 | RAMAKER D E, GATEWOOD D, KOROVINA A, et al. Resolving sulfur oxidation and removal from Pt and Pt3Co electrocatalysts using in situ X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry C, 2010, 114(27): 11886-11897. |
| 51 | LIU Yuxin, YE Jing, KONG Fanpeng, et al. Pt/C-TiO2 as oxygen reduction electrocatalysts against sulfur poisoning[J]. Catalysts, 2022, 12(5): 571. |
| 52 | 叶菁. 抗SO2/NO x 中毒的氧还原催化剂制备及性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| YE Jing. Synthesis and characterization of SO2/NO x poisoning resistance catalysts for oxygen reduction reaction[D]. Harbin: Harbin Institute of Technology, 2019. | |
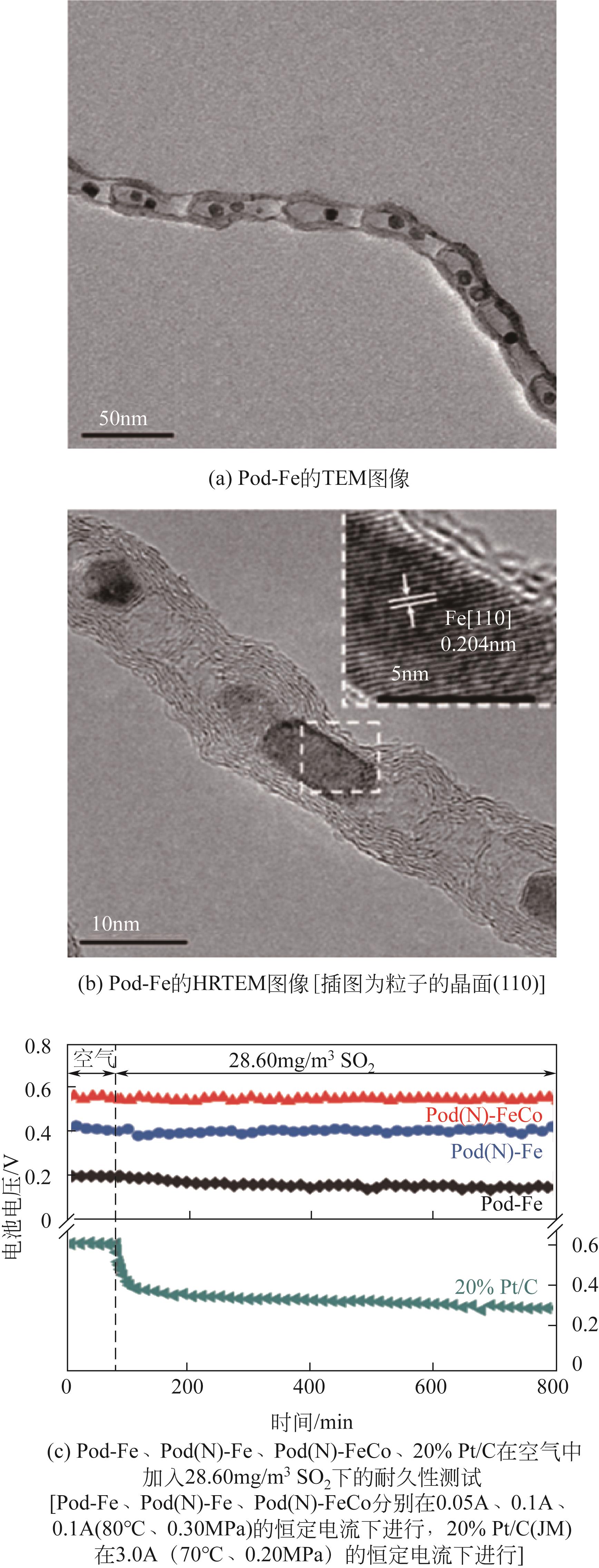
| 53 | DENG Dehui, YU Liang, CHEN Xiaoqi, et al. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction[J]. Angewandte Chemie (International Ed in English), 2013, 52(1): 371-375. |
| 54 | RESHETENKO T, SEROV A, ARTYUSHKOVA K, et al. Tolerance of non-platinum group metals cathodes proton exchange membrane fuel cells to air contaminants[J]. Journal of Power Sources, 2016, 324: 556-571. |
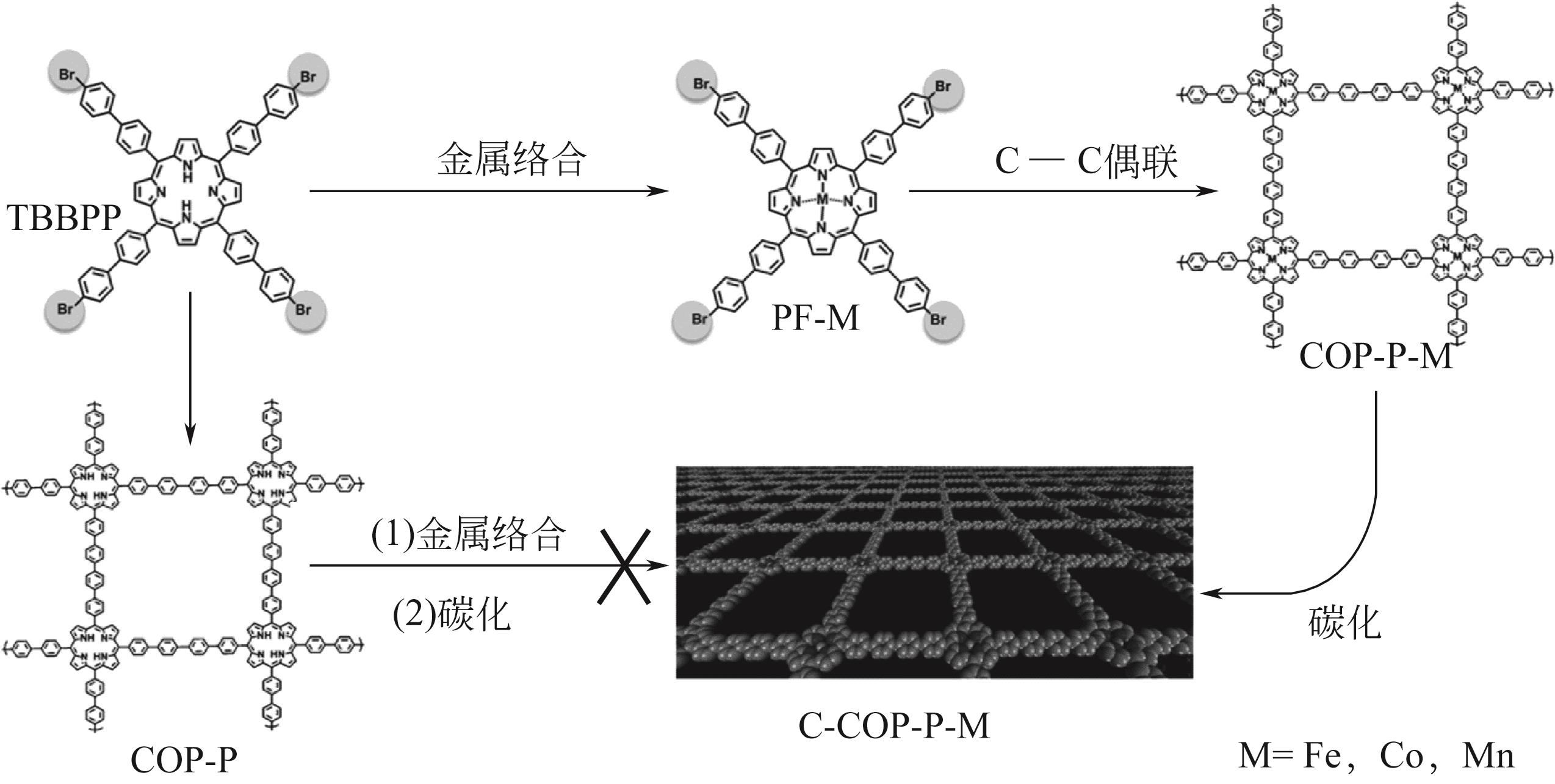
| 55 | XIANG Zhonghua, XUE Yuhua, CAO Dapeng, et al. Highly efficient electrocatalysts for oxygen reduction based on 2D covalent organic polymers complexed with non-precious metals[J]. Angewandte Chemie (International Ed in English), 2014, 53(9): 2433-2437. |
| 56 | ZHANG P, CHEN X F, LIAN J S, et al. Structural selectivity of CO oxidation on Fe/N/C catalysts[J]. The Journal of Physical Chemistry C, 2012, 116(33): 17572-17579. |
| 57 | HE Chunyong, ZHANG Jiujun, SHEN Peikang. Nitrogen-self-doped graphene-based non-precious metal catalyst with superior performance to Pt/C catalyst toward oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(9): 3231-3236. |
| 58 | LIU Zong, XUE Jia, YU Huaguang, et al. Thermal annealing effect of Co-N-C/carbon nanotube on the electrochemical oxygen reduction reaction[J]. Energy Technology, 2018, 6(12): 2394-2398. |
| 59 | ZHANG Qiang, MAMTANI K, JAIN D, et al. CO poisoning effects on FeNC and CN x ORR catalysts: A combined experimental-computational study[J]. The Journal of Physical Chemistry C, 2016, 120(28): 15173-15184. |
| 60 | VON DEAK D, SINGH D, BIDDINGER E J, et al. Investigation of sulfur poisoning of CN x oxygen reduction catalysts for PEM fuel cells[J]. Journal of Catalysis, 2012, 285(1): 145-151. |
| 61 | CHEN Sheng, BI Jiyu, ZHAO Yu, et al. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction[J]. Advanced Materials, 2012, 24(41): 5593-5597. |
| 62 | QU Liangti, LIU Yong, BAEK Jong-Beom, et al. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells[J]. ACS Nano, 2010, 4(3): 1321-1326. |
| 63 | LI Rong, WEI Zidong, GOU Xinglong, et al. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts[J]. RSC Advances, 2013, 3(25): 9978-9984. |
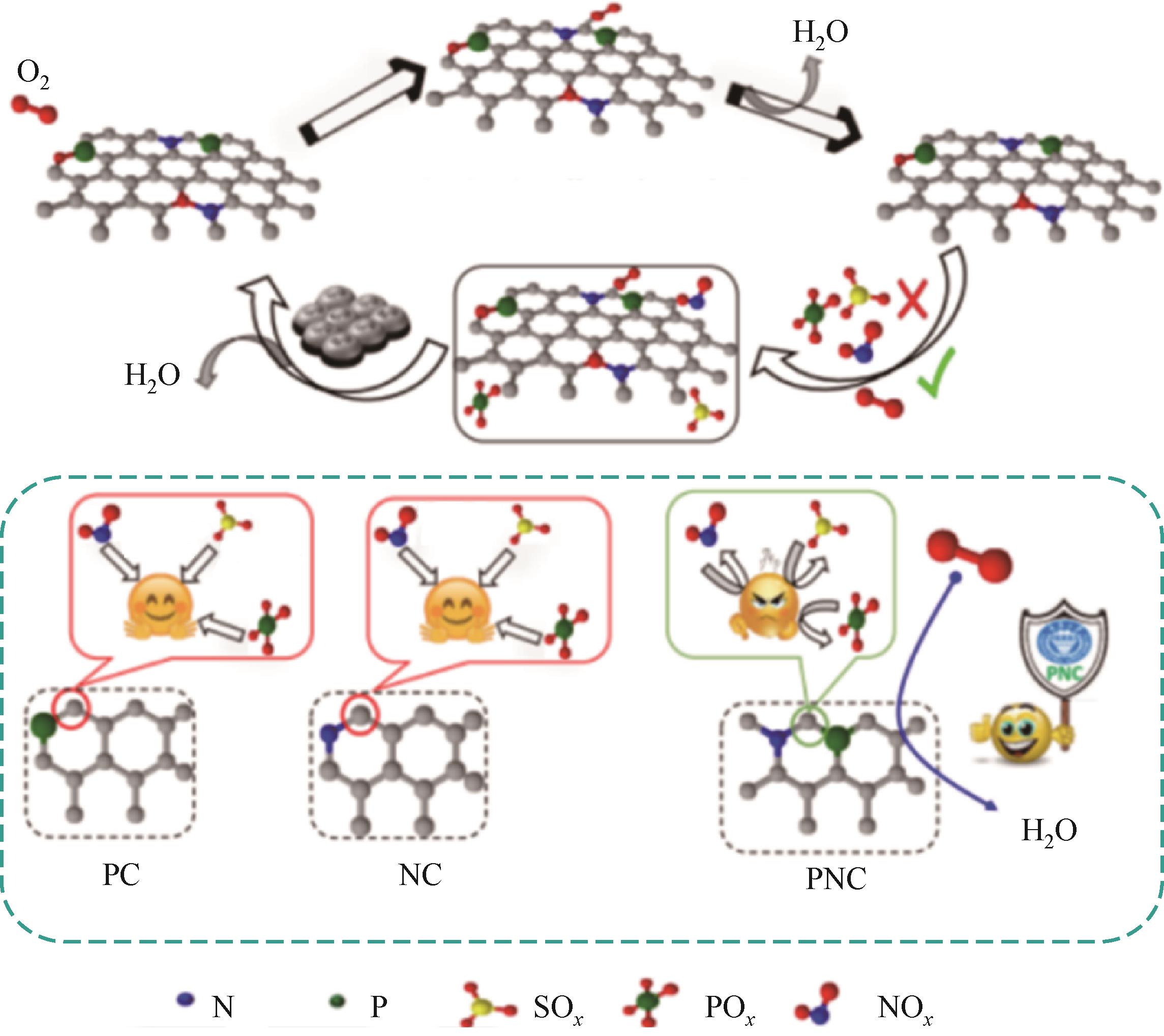
| 64 | NAJAM T, SHAH S S A, DING Wei, et al. An efficient anti-poisoning catalyst against SO x, NO x, and PO x : P, N-doped carbon for oxygen reduction in acidic media[J]. Angewandte Chemie International Edition, 2018, 57(46): 15101-15106. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||